Abstract
Lung diffusing capacity for nitric oxide (DlNO) is used to measure alveolar membrane conductance (DmNO), but disagreement remains as to whether DmNO = DlNO, and whether blood conductance (θNO) = ∞. Our previous in vitro and in vivo studies suggested that θNO < ∞. We now show in a membrane oxygenator model perfused with whole blood that addition of a cell-free bovine hemoglobin (Hb) glutamer-200 solution increased diffusing capacity of the circuit (D) for NO (Dno) by 39%, D for carbon monoxide (Dco) by 24%, and the ratio of Dno to Dco by 12% (all P < 0.001). In three anesthetized dogs, DlNO and DlCO were measured by a rebreathing technique before and after three successive equal volume-exchange transfusions with bovine Hb glutamer-200 (10 ml/kg each, total exchange 30 ml/kg). At baseline, DlNO/DlCO = 4.5. After exchange transfusion, DlNO rose 57 ± 16% (mean ± SD, P = 0.02) and DlNO/DlCO = 7.1, whereas DlCO remained unchanged. Thus, in vitro and in vivo data directly demonstrate a finite θNO. We conclude that the erythrocyte and/or its immediate environment imposes considerable resistance to alveolar-capillary NO uptake. DlNO is sensitive to dynamic hematological factors and is not a pure index of conductance of the alveolar tissue membrane. With successive exchange transfusion, the estimated in vivo θNO [5.1 ml NO·(ml blood·min·Torr)−1] approached 4.5 ml NO·(ml blood·min·Torr)−1, which was derived from in vitro measurements by Carlsen and Comroe (J Gen Physiol 42: 83–107, 1958). Therefore, we suggest use of θNO = 4.5 ml NO·(min·Torr·ml blood)−1 for calculation of DmNO and pulmonary capillary blood volume from DlNO and DlCO.
Keywords: lung diffusing capacity, membrane diffusing capacity, carbon monoxide, lung diffusing capacity for carbon monoxide, lung diffusing capacity for nitric oxide, gas exchange, membrane oxygenator, exchange transfusion, bovine hemoglobin glutamer, blood substitute
nitric oxide (NO), in combination with carbon monoxide (CO), has been used to measure gas transfer in the lung since the 1980s (5, 11, 30, 31). Similar to CO, NO combines with high affinity to hemoglobin (Hb), but, in contrast to CO, the rate of combination is ∼250 times faster (5, 12), so that the lung diffusing capacity for NO (DlNO) is a measure of diffusive gas conductance independent of chemical reaction. Lung transfer of a gas that combines with Hb can be described by the equation of Roughton and Forster (25), which was originally applied to CO
| (1) |
where Dl is conductance across the lung and Dm is alveolar membrane conductance [ml gas·(min·Torr)−1], θ is the rate of combination of gas (ligand) with erythrocyte [ml gas·(ml blood·min·Torr)−1], and Vc is the pulmonary capillary blood volume (in ml). The left-hand term (1/Dl) represents the overall resistance to lung gas transfer, and the right-hand terms represent the resistance of the membrane (1/Dm) and the resistance of pulmonary capillary blood (1/θVc). The equation for 1/θ contains a term for diffusion resistance (a) and a term for chemical reaction resistance (b); both terms are hematocrit-dependent. Roughton and Forster exploited this and calculated Dm and Vc by measuring Dl at several Po2 levels and applying known values for θCO derived from in vitro measurements using a rapid-reaction apparatus (25, 26). On the basis of their experiments in healthy adult men, resistance of the membrane was approximately equal to resistance of blood. Guenard et al. (11) assumed that since the reaction of NO with Hb is effectively infinite, θNO must be infinite; therefore, DlNO = DmNO ≈ 2 DmCO, since the ratio of diffusion of NO to diffusion of CO is directly proportional to the ratio of their respective solubility coefficients (α) and inversely proportional to the ratio of the square root of their molecular weights (MW)
| (2) |
Implicit in their assumption is that resistance of the erythrocyte membrane, its interior, and the immediately surrounding plasma is negligible. With this assumption, Eq. 1 can be solved from simultaneous measurements of DlCO and DlNO in a single maneuver and with a known value of θCO. The values for Dm and Vc obtained in this way were close to those obtained via DlCO measured at two levels of Po2 (24, 30). Combined DlNO-DlCO measurement offered a rapid way of obtaining Dm and Vc in a variety of clinical situations, obviating the need for multiple DlCO measurements.
However, disagreement soon arose as to whether DlNO = DmNO (1, 4). The in vitro maximum rate constant of NO binding is 500–1,000 times faster with free Hb solution (2 × 105 M−1·s−1) than with red blood cells (12, 33), suggesting the presence of intrinsic transmembrane or intracellular properties of the erythrocyte that limit NO uptake. In 1958, using a rapid-reaction apparatus, Carlsen and Comroe (6) measured in vitro NO reaction with human erythrocytes and found a second-order rate constant for erythrocytes (j′c) of 167 mM−1·s−1, from which θNO = 4.5 ml NO·(min·Torr·ml blood)−1 at Hb = 14.6 g/dl can be derived (3, 4), i.e., substantially less than infinity. To address this issue, we used a membrane oxygenator model primed with horse blood to measure in vitro NO transfer (Dno) and eliminated red cell resistance by water-induced hemolysis (2). In this model, hemolysis was accompanied by a rise in Dno, suggesting that red cell resistance to NO transfer was finite, i.e., Dno < DmNO. Water-induced hemolysis is impracticable in laboratory animals or isolated lung preparations, inasmuch as it disrupts the endothelium and epithelium, immediately altering in vivo Dm. However, a cell-free heme-based blood substitute (bovine Hb glutamer-200) is approved for canine clinical use (13, 14). In this study, we first progressively added bovine Hb glutamer-200 in the membrane oxygenator and examined its effect on Dno, comparing it with in situ hemolysis and with hemolyzed red blood cells. Then we progressively replaced whole blood in anesthetized dogs with bovine Hb glutamer-200 and examined the effect on DlNO. We reasoned that if resistance of the blood to NO were significant, in vitro Dno and in vivo DlNO would increase progressively as erythrocytes are “bypassed” by replacement with cell-free Hb.
METHODS
In Vitro Experiments
Membrane oxygenator circuit.
Our circuit is described in detail elsewhere (2, 9). Briefly, two membrane oxygenators (Cobe Duo CML, Cobe Cardiovascular, Arvada, CO) were connected in series to form a continuous circuit perfused with 1–1.5 liters of horse blood (TCS Biosciences, Buckingham, UK) with hematocrit of 40–50% depending on batch and flowing at 2.5 l/min and 37°C. One oxygenator received a gas flow of 2.5 liters of a mixture of 22% O2, 0.02% CO, 5 parts per million (ppm) NO, and the remainder N2; the second oxygenator scrubbed the oxygenated blood with 8.3% CO2 in N2. In this way, near-physiological levels of O2, CO2, and pH were maintained over several hours in a steady state without apparent hemolysis (9).
Diffusing capacity measurements.
Dno and Dco were measured from the difference in gas concentrations leaving and entering the first oxygenator as follows (2)
| (3) |
where Temp is ambient temperature (°C), Pb is barometric pressure (Torr), Ph2o is saturated water vapor pressure (Torr), RH is relative humidity, and NOin and NOout are NO concentrations at the inlet and outlet ports. For CO, the calculations were identical, except COin and COout were substituted for Nin and Nout.
Addition of blood substitute.
Bovine Hb glutamer-200, a cell-free heme-based blood substitute licensed for canine clinical use (Oxyglobin, Dechra, Overland Park, KS), was added in 25-ml aliquots at 5-min intervals to a total of 250 ml via the oxygenator blood-sampling port, and Dno and Dco were measured after each addition.
Addition of free Hb.
For preparation of Hb, the cells were allowed to separate in a 1-liter bag of horse blood overnight; then the plasma was expressed by a separating clamp. Tap water was added to a total volume of 1 liter to achieve complete hemolysis.
Comparison with hemolysis.
Oxyglobin was added to a total of 250 ml in 25-ml aliquots, and hemolysis was induced by addition of 100-ml aliquots to a total of 700 ml and a potassium concentration >9.5 mmol/l.
Statistical analysis.
Dno was entered as a dependent variable in ANOVA (SPSS version 17.0, SPSS, Chicago, IL). Solution added (Hb or Oxyglobin) was entered as an independent variable, and volume added was entered as a covariate.
Animal Experiments
The Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center approved all protocols and procedures. Three purpose-bred adult male foxhounds [28.3 ± 1.0 kg body wt (mean ± SD)] were studied. Circulating blood, plasma, and red cell volumes were determined by the Evans blue dye-dilution method (22) on a separate day before the main study while the animal was awake and at rest.
Anesthesia and catheterization.
The animal was fasted overnight and premedicated with acepromazine (0.2 mg/kg) and glycopyrrolate (0.01 ml/kg) by subcutaneous injection. A peripheral intravenous catheter was inserted. Anesthesia was induced with intravenous propofol and maintained by an infusion of ketamine and diazepam titrated to effect. Additional boluses of propofol (0.3–0.5 mg/kg iv) were administered as needed to maintain deep anesthesia and minimize fluid load. A urinary catheter was placed to monitor fluid balance. The animal was intubated with a cuffed endotracheal tube, placed supine, and mechanically ventilated (model 607, Harvard Apparatus, Millis, MA) at a tidal volume (10–12 ml/kg) and rate (18–24 breaths/min) sufficient to suppress spontaneous ventilation. Transcutaneous O2 saturation and heart rate were monitored continuously. One external jugular vein was catheterized with an 8.5-French introducer and one femoral artery with a 5-French catheter. Through the jugular introducer, a pulmonary artery catheter was placed, and its position was verified by pressure tracing. The catheters were flushed with heparinized saline and sutured to skin. A balloon-tipped polyethylene catheter was positioned in the distal one-third of the esophagus, and the balloon was inflated with 0.5–1.0 ml of air for measurement of esophageal pressure.
Blood analyses.
Arterial and mixed venous blood samples (3 ml) were drawn, at baseline and on completion of rebreathing maneuvers following each exchange transfusion, for measurement of Hb concentration, conventional blood gases, and O2 saturation by blood analyzers (models ABL720 and OSM3, Radiometer America, Westlake, OH). The instruments were calibrated for dog blood. Hematocrit was determined using a microcapillary centrifuge.
Pressure-volume curves.
Static pressure-volume curves were measured at the beginning and end of the experiment by stepwise increments and then decrements (15, 30, 45, and 60 ml/kg) of inflation volume delivered using a calibrated syringe from end-expiratory lung volume. Transpulmonary pressure was calculated as the difference between mouth and esophageal pressures. Paired measurements at each volume were averaged.
Rebreathing measurements.
Rebreathing was measured as described elsewhere (19). Briefly, a known volume (45 ml/kg) of test gas mixture containing 0.3% CO, 0.3% methane, 0.8% acetylene, and 40–55 ppm NO in balance of 21% or 99% O2 was delivered from end-expiratory lung volume via a calibrated syringe and mixed with resident gas in the lung by rebreathing maneuvers for 20 s. Gas concentrations were measured at the end of the endotracheal tube. Concentrations of methane, acetylene, and CO were measured using rapid-response infrared analyzers (Act II S, Sensors, Saline, MI). NO concentration was measured by chemiluminescence (model NOA 280, GE Analytical Instruments, Boulder, CO). Duplicate measurements at each inspired O2 fraction were averaged. On completion of these procedures, a separate rebreathing maneuver (20 s) was performed while the animal breathed 100% O2 followed by slow (over 7 s) exhalation, during which alveolar NO concentration was measured. In addition, CO backpressure was measured.
Exchange transfusion.
After baseline measurements, 10 ml/kg of blood was removed via the femoral artery catheter into a collection bag containing citrate-phosphate-dextrose-adenine (CPDA-1, Fenwal, Lake Zurich, IL) and replaced with an equal volume of Oxyglobin solution warmed in a water bath to 37°C and infused via the central venous port. The exchange transfusion took ∼1 h. Then arterial and venous blood analyses and rebreathing measurements were repeated. The exchange transfusion-and-measurement procedure was performed twice more for a total exchange volume of 30 ml/kg. The removed blood was refrigerated.
Recovery.
The catheters were removed, and pressure was applied until bleeding stopped. On the next day, a venous blood sample was collected for measurement of hematocrit and Hb concentration, and a portion (10 ml/kg) of the removed blood was warmed to body temperature and reinfused into the dog via a peripheral vein with use of a blood filter (Baxter, Deerfield, IL). This procedure was performed twice more on consecutive days until all the removed blood had been reinfused.
Data analysis.
Lung volumes were calculated from methane dilution. Pulmonary blood flow was calculated from the logarithmic disappearance of acetylene relative to methane, corrected for the intercept of CO disappearance. DlCO and DlNO were calculated from end-tidal logarithmic disappearance of CO and NO, respectively, relative to methane (27, 30). Gas exchange septal (tissue + blood) volume was calculated from the extrapolated intercept of the acetylene disappearance curve (28). DmCO and Vc were estimated by three methods, each with the assumption of different values of θCO or θNO (Table 1): 1) by the Roughton-Forster (RF) method (Eq. 1) from DLCO measured at two alveolar Po2 (PaO2) levels and the θCO value given by Holland (18) for canine whole blood incorporating a temperature correction for 39°C and made at pH 7.4 using a stopped-flow apparatus (17), calculated from the mean PaO2 during rebreathing and Hb concentration ([Hb])
| (4) |
2) from simultaneously measured DlNO and DlCO (NO-CO method) (5, 11) with the assumption that θNO = ∞ (DlNO = DmNO) and an empirical DlNO-to-DmCO ratio = 2.34 measured in 69 dogs cumulatively studied in our laboratory (unpublished observation), and 3) by the NO-CO method as described above, but with the assumption of a finite θNO = 4.5 ml NO·(ml blood·min·Torr)−1 derived from the j′c of Carlsen and Comroe (6) and DmCO = DmNO/2. Equation 1 was solved for CO and NO in turn using Mathematica software (Wolfram Research, Champaign, IL). This approach gives a solution identical to that previously published (3). These data were expressed as means ± SD and compared before and after exchange transfusion by repeated-measures ANOVA. P < 0.05 was considered significant.
Table 1.
A summary of methods and assumed values for estimating DmCO and Vc
| Method | 1/θCO | DmCO | 1/θNO |
|---|---|---|---|
| 1) Roughton-Forster (RF) | (0.93 + 0.0052 alveolar Po2) · (14.6/Hb) | Calculated using θCO at 2 alveolar Po2 levels | Not used |
| 2) NO-CO | (0.93 + 0.0052 alveolar Po2) · (14.6/Hb) | = DlNO/2.34 | 0 |
| 3) NO-CO | (0.93 + 0.0052 alveolar Po2) · (14.6/Hb) | = DlNO/2 | 0.222 |
Vc, pulmonary capillary blood volume; θ, rate at which gas [carbon monoxide (CO) or nitric oxide (NO)] combines with erythrocytes; DmCO, alveolar membrane conductance of CO.
RESULTS
In Vitro Experiments
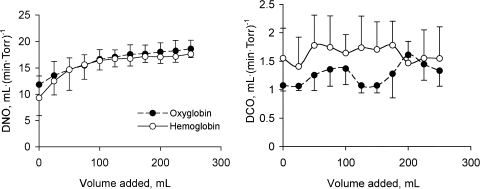
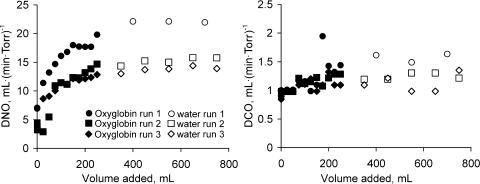
Addition of Oxyglobin to our oxygenator circuit increased Dno from 13.4 ± 0.115 to 18.6 ± 1.659 (SD) ml·(min·Torr)−1 (n = 3, P < 0.001), while Dco increased from 1.075 ± 0.05 to 1.33 ± 0.139 ml·(min·Torr)−1 (n = 3, P = 0.006; Fig. 1). The increase in Dno after addition of Oxyglobin was not significantly different (P = 0.44) from that after addition of hemolyzed free Hb, whereas the mean Dco was significantly greater with Oxyglobin than with free Hb (P = 0.001). No significant further increase in Dno or Dco was observed after hemolysis was induced in the oxygenator circuit (Fig. 2). A highly significant between-run effect was observed for Dno (P < 0.001) and Dco (P = 0.002).
Fig. 1.
In vitro diffusing capacity for nitric oxide (Dno) and carbon monoxide (Dco) measured in the membrane oxygenator model after successive addition of free Hb or bovine Hb glutamer-200 (Oxyglobin) solution. Values are means ± SD of 3 replicate runs.
Fig. 2.
In vitro Dno and Dco measured in the membrane oxygenator model with successive addition of bovine Hb glutamer-200 (Oxyglobin) solution followed by water hemolysis. Individual runs are shown.
In Vivo Experiments
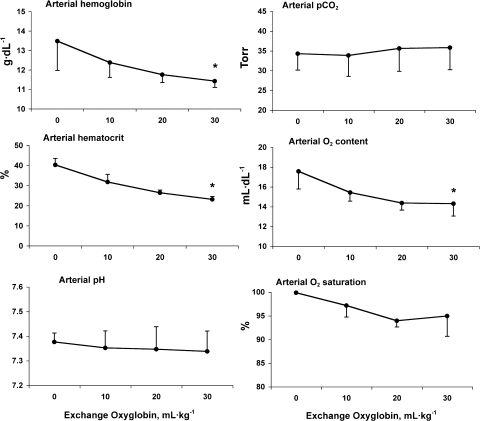
Baseline hematological parameters measured during wakefulness at rest were normal in all three animals [hematocrit = 0.45 ± 0.02, plasma volume = 74.5 ± 6.3 ml/kg, red cell volume = 60.0 ± 6.6 ml/kg, and total blood volume = 134.6 ± 11.5 ml/kg (mean ± SD)]. All animals recovered from exchange transfusion without adverse effects, except for temporary darkening of the mucous membrane and the urine. From baseline to postexchange, pressure-volume curves and systemic and pulmonary arterial blood pressures were unaltered (data not shown). Hematological data and arterial blood gases are shown in Fig. 3. DlCO was not corrected for reduction in Hb, since the anticipated alteration was <10%.
Fig. 3.
Blood parameters before and after successive in vivo exchange transfusion with Oxyglobin solution. Values are means ± SD. *P < 0.05 vs. baseline.
As expected, exchange transfusion reduced arterial hematocrit from 40.3 ± 3.3% to 23.2 ± 1.4%. There were also absolute declines in postexchange Hb concentration from 13.5 ± 1.5 to 11.4 ± 0.3 g/dl and in arterial O2 saturation from 100 ± 0% to 95 ± 4% (P < 0.05) without significant changes in arterial Pco2 or pH.
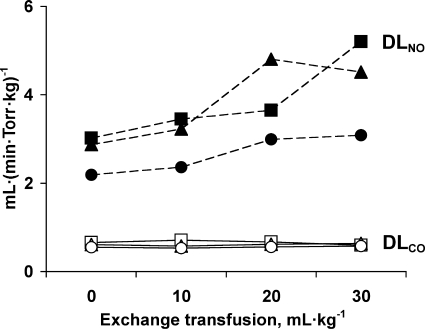
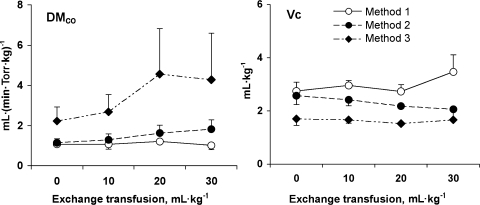
Rebreathing data obtained during 21% O2 breathing are summarized in Table 2 and Fig. 4. Compared with baseline, DlNO increased 59% after exchange transfusion (P = 0.021), while mean PaO2, lung volumes, and DlCO did not change. With use of Eq. 4 and the assumption that θNO = ∞, estimates of DmCO by the RF or NO-CO method (methods 1 and 2, respectively, in Table 1) were similar at baseline. As a result of a rising DlNO with each exchange transfusion, DmCO estimated by method 2 progressively increased, whereas the corresponding DmCO estimated by method 1 did not change (Fig. 5, left). In a reciprocal relationship, Vc estimated by method 2 progressively declined with exchange transfusion compared with the corresponding Vc estimated by method 1 (Fig. 5, right).
Table 2.
In vivo data
| Exchange Transfusion, ml/kg |
|||||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | P | |
| Hb, g/dl | 13.5 ± 1.5 | 12.4 ± 0.8 | 11.8 ± 0.4 | 11.4 ± 0.3 | * |
| Hematocrit, % | 40.3 ± 3.3 | 31.8 ± 3.8 | 26.5 ± 1.3 | 23.2 ± 1.4 | * |
| Mean alveolar Po2, Torr | 141 ± 3 | 141 ± 6 | 137 ± 7 | 135 ± 2 | |
| Heart rate, beats/min | 119 ± 5 | 107 ± 11 | 100 ± 22 | 106 ± 10 | |
| Pulmonary blood flow, ml · (min · kg)−1 | 121 ± 5 | 114 ± 20 | 119 ± 18 | 109 ± 10 | |
| End-expiratory lung volume, ml/kg | 32 ± 5 | 32 ± 5 | 31 ± 5 | 31 ± 8 | |
| End-inspiratory lung volume, ml/kg | 84 ± 5 | 84 ± 4 | 83 ± 5 | 83 ± 8 | |
| Septal tissue volume, ml/kg | 5.10 ± 2.15 | 4.88 ± 1.84 | 3.80 ± 1.45 | 4.30 ± 1.92 | |
| DlCO, ml · (min · Torr · kg)−1 | 0.61 ± 0.05 | 0.61 ± 0.10 | 0.61 ± 0.06 | 0.60 ± 0.03 | |
| DlNO, ml · (min · Torr · kg)−1 | 2.69 ± 0.44 | 3.01 ± 0.57 | 3.81 ± 0.92 | 4.27 ± 1.08 | * |
| Method 1 (RF) | |||||
| DmCO, ml · (min · Torr · kg)−1 | 1.08 ± 0.11 | 1.07 ± 0.23 | 1.21 ± 0.10 | 1.01 ± 0.21 | |
| Vc, ml/kg | 2.75 ± 0.33 | 2.96 ± 0.19 | 2.73 ± 0.26 | 3.47 ± 0.64 | * |
| Method 2 (NO-CO, θNO = ∞) | |||||
| DmCO, ml · (min · Torr · kg)−1 | 1.15 ± 0.19 | 1.29 ± 0.29 | 1.63 ± 0.39 | 1.82 ± 0.46 | *† |
| Vc, ml/kg | 2.57 ± 0.33 | 2.42 ± 0.22 | 2.18 ± 0.05 | 2.06 ± 0.35 | *† |
| Method 3 (NO-CO, θNO = 4.5) | |||||
| DmCO, ml · (min · Torr · kg)−1 | 2.23 ± 0.70 | 2.68 ± 0.86 | 4.55 ± 2.28 | 4.28 ± 2.31 | † |
| Vc, ml/kg | 1.70 ± 0.25 | 1.66 ± 0.12 | 1.52 ± 0.06 | 1.66 ± 0.03 | † |
Values are means ± SD. RF, Roughton-Forster method using θCO from Eq. 4. NO-CO method assumes DlNO/DmCO = 2.34 and θNO = ∞ or θNO = 4.5. Repeated-measures ANOVA and post hoc Fisher's protected least significant difference:
P < 0.05 vs. exchange transfusion;
P < 0.05 vs. method 1.
Fig. 4.
In vivo lung diffusing capacity for NO (DlNO) in individual animals (closed symbols, dashed lines) rose significantly after successive exchange transfusion with Oxyglobin solution compared with baseline (P < 0.05 by repeated-measures ANOVA), while DLCO in each animal (open symbols, solid lines) remained unchanged.
Fig. 5.
Membrane diffusing capacity for CO (DmCO) and pulmonary capillary blood volume (Vc) estimated by the Roughton-Forster method (method 1), the NO-CO method with the assumption that specific transfer conductance of blood per milliliter for NO (θNO) = ∞ (method 2), and the NO-CO method with the assumption that θNO = 4.5 ml NO·(ml blood·min·Torr)−1. Values are means ± SD. Estimates by all 3 methods were significantly different from one another by repeated-measures ANOVA (all P < 0.05).
DmCO and Vc calculated using different values of θNO and θCO are given in Table 2, and their comparisons are shown in Table 3. At baseline, methods 1 and 2 (NO-CO assuming θNO = ∞) yielded similar estimates of DmCO and Vc. Method 3 [NO-CO assuming θNO = 4.5 ml NO·(ml blood·min·Torr)−1] approximately doubled the estimated DmCO and halved the estimated Vc compared with method 2. With successive exchange transfusions, estimates of DmCO and Vc by method 1 remained relatively stable until 30 ml/kg of blood had been exchanged. In contrast, method 2 yielded progressively increasing values of DmCO and declining values of Vc with each exchange transfusion. Method 3 also yielded increasing values of DmCO with exchange transfusion, while the estimates of Vc were more stable.
Table 3.
Comparison of methods
| Exchange Transfusion, ml/kg |
||||
|---|---|---|---|---|
| 0 | 10 | 20 | 30 | |
| DlNO/DlCO | 4.44 ± 0.40 | 4.94 ± 0.54 | 6.22 ± 1.38 | 7.07 ± 1.68*‡ |
| NO-CO method using different θNO (method 3/method 2) | ||||
| DmCO θNO4.5/θNO∞ | 1.90 ± 0.33 | 2.04 ± 0.31 | 2.68 ± 0.71 | 2.53 ± 0.75 |
| Vc θNO4.5/θNO∞ | 0.66 ± 0.03 | 0.69 ± 0.03 | 0.72 ± 0.04 | 0.74 ± 0.02*† |
Values are means ± SD. Repeated-measures ANOVA and post hoc Fisher's protected least significant difference:
P < 0.05 vs. 0 ml/kg;
P < 0.05 vs. 20 ml/kg;
P < 0.05 vs. 10 ml/kg.
Using the in vivo data (Table 2) and applying Eq. 1 to NO and CO individually (rearranged in Eq. 5), we may estimate the likely range of θNO values
| (5) |
At baseline, DlCO = 0.61 and DlNO = 2.69 ml·(min·Torr)−1. Since DmCO = 0.5 DmNO, 1/DmCO = 2/DmNO. θCO was calculated at 141 Torr arterial Po2 (Table 2) = 0.6 ml CO·(ml blood·min·Torr)−1. With the assumption that with each successive exchange transfusion the measured DlNO (line 10, Table 2) more closely approximates true conductance of the tissue membrane (DlNO → DmNO), in vivo θNO values were as follows: 0.6 × (1/0.61 − 2/3.01)/(1/2.69 − 2/3.01) = 14.8, 0.6 × (1/0.61 − 2/3.81)/(1/2.69 − 2/3.81) = 6.1, and 0.6 × (1/0.61 − 2/4.27)/(1/2.69 − 2/4.27) = 5.1 ml NO·(ml blood·min·Torr)−1 after 10, 20, and 30 ml/kg exchange, respectively; i.e., with each exchange, the in vivo θNO declined in a nonlinear fashion toward the in vitro value of 4.5 ml NO·(ml blood·min·Torr)−1 (4).
DISCUSSION
Summary of Findings
In vitro and in vivo replacement of whole blood with hemolyzed blood and a cell-free Hb solution (Oxyglobin) caused DlNO to increase progressively, suggesting that DmNO exceeds DlNO. The final value achieved using Oxyglobin in vitro is identical to that after hemolysis. The cell-free Hb solution penetrates the plasma surrounding the erythrocytes and between the erythrocytes and the endothelial layer; it reduces blood resistance by “bypassing” the erythrocyte membrane and interior and increases the effective surface area of tissue membrane available for gas exchange by allowing all perfused tissue surfaces to directly contact Hb. On the basis of our data, it is almost certain that θNO in vitro and in vivo is substantially less than infinity; the true in vivo θNO is probably close to the in vitro estimate of 4.5 ml NO·(ml blood·min·Torr)−1 given the results from Eq. 5. The only (and unlikely) alternative explanation would be the presence of a periendothelial plasma layer that offered the sole blood resistance to NO transfer. Estimates of DmCO and Vc obtained by the combined NO-CO methods (methods 2 and 3) are highly sensitive to the addition of cell-free Hb, while estimates obtained by the RF method (method 1) are relatively insensitive, because the former use DlNO in their calculation. DlNO increases with exchange transfusion.
Critique of Methods
Although the in vitro post-Oxyglobin, post-hemolyzed blood, and post-Oxyglobin posthemolysis values of Dno are similar, they are appreciably lower than those reported in our previous work (2). The marked between-run variation observed on the same day with blood from a single horse shows that the variation is between oxygenators.
Oxyglobin does not cause hemolysis (14), and repeated administration over 1 yr caused no adverse physiological effects in dogs (13). The Hb content of Oxyglobin (13 g/dl) is similar to that of baseline canine blood under anesthesia (13.5 g/dl). After equal volume exchanges of blood with the Hb solution, arterial Hb concentration progressively declined to 11.4 g/dl, likely because of the known plasma-expanding effect of Oxyglobin. We followed the clinical slow-infusion protocol (10 mg/kg over 1 h) up to the recommended maximum (30 mg/kg total) to minimize, but not eliminate, the effect. Hemodilution would have reduced DlCO more than DlNO, because the blood resistance to CO transfer is greater than that to NO transfer.
A small decrease in DlCO due to hemodilution could have offset a small expected increase in DlCO because of reduced erythrocyte diffusion resistance with Oxyglobin, thereby explaining why in vitro Dco increased with exchange transfusion but in vivo DlCO did not. In the oxygenator, Dco increased more with blood replacement by Oxyglobin than with blood replacement by lysed blood; given the predominant reaction dependence of Dco, this likely reflects a slower reaction rate of CO with free Hb in lysed blood than with Oxyglobin. We have no in vitro data for the reaction kinetics of CO or NO with Oxyglobin. Using the oxygenator, we observed a considerably higher Dno-to-Dco ratio than was observed in vivo (DlNO-to-DlCO ratio); the difference has been discussed previously (2) and attributed to the design of the multipore membrane, where gas directly contacts a thin boundary layer of blood, resulting in a higher Dm-to-Vc ratio in the oxygenator than in the lung.
There was a modest reduction in arterial and mixed venous O2 saturation with exchange transfusion, consistent with a rightward shift of the O2 dissociation curve caused by Oxyglobin [Po2 necessary to obtain 50% O2 saturation of Hb (P50) = 34 Torr] (8). We observed a similar rightward shift when using the oxygenator (Dunningham and Borland, unpublished observations).
Estimation of Dm and Vc by the NO-CO method requires knowledge of the DmNO-to-DmCO ratio, which theoretically is ∼2 (Eq. 2). Experimentally, we observed DlNO/DmCO = 2.42 in healthy human subjects and 2.24 in patients with pulmonary sarcoidosis (24, 30). The empirical value in dogs (2.34) is within this range. This variability in the DlNO-to-DmCO ratio per se makes little difference to method 2, which assumes DlNO = DmNO. If we assumed a ratio of 2.42, instead of 2.34, the estimated DmCO would be only 3.4% lower and the estimated Vc would be 3.1% higher. With the assumption that θNO is infinite, the empirical DmNO-to-DmCO ratio (2.24–2.42) is modestly (12–20%) higher than the theoretical value. This difference may be explained by the range of θCO values given in the literature, which easily causes a 20% variation in the estimated DmCO. Other possible explanations have been discussed previously (24). 1) The NO solubility coefficient may be higher in plasma than in water. 2) Facilitated NO diffusion may occur in plasma; NO interacts with sulfhydryl groups in plasma lipids and proteins and can be transported as S-nitrosothiols.
Relative Resistance of Tissue and Erythrocyte Membranes
From Table 2, the baseline and postexchange transfusion DlCO values [0.61 and 0.60 ml·(min·Torr·kg)−1, respectively] may be used in conjunction with DmCO [1.08 and 1.01 ml·(min·Torr·kg)−1, respectively] obtained by method 1 to estimate the percentage of in vivo resistance to CO uptake (1/DlCO) due to resistance of the tissue-plasma-erythrocyte diffusion barrier (1/DmCO), i.e., 100 × DlCO/DmCO. At baseline, the combined barrier resistance was (100 × 0.61/1.08) = 56% of total CO resistance. After exchange transfusion, the barrier resistance was (100 × 0.60/1.01) = 59% of total CO resistance. These values agree with previous estimates showing about equal diffusion and reaction limitation for CO uptake (20). Exchange of erythrocytes for cell-free Hb contributed little (3%) to overall CO resistance. For NO uptake, 1/DlNO pre = membrane + red cell resistance, while 1/DLNO post = membrane resistance only; mean values of DlNO before and after exchange transfusion were 2.69 and 4.27 ml·(min·Torr·kg)−1, respectively (Table 2). Thus
| (6) |
This value (37%) represents a lower-limit underestimation of true erythrocyte-related barrier resistance in alveolar capillaries, since only a fraction of erythrocytes was removed.
Values for θCO
The initial justification for the veracity of the NO-CO method (method 2) used by Guenard et al. (11) was the apparent agreement between values for Dm and Vc obtained by their method and by the traditional RF method (method 1) using the θCO values given by Roughton and Forster (25) as 1/θCO = a + b·PaO2 = 0.34 + 0.0061·PaO2. Until now, the greatest challenge to their method came from Robert E. Forster himself (10). He acknowledged that the earlier θCO value had been obtained at pH 7.8–8.0 and, in 1987, repeated the measurements in red blood cells from five normal individuals at pH 7.4 and 37°C obtaining a new relationship: 1/θCO = a + b·PaO2 = 1.3 + 0.0041·PaO2. Using the 1987 value, instead of the 1957 value, of θCO gives a 36% lower value for Vc and a twofold greater DmCO in normal adult subjects (20), an observation also made by others (35). These later values no longer agree with those obtained from the NO-CO method (method 2) with the assumption that θNO = ∞.
DLNO and Hb
One way to determine whether θNO is infinity or less might be to examine the effect of altering Hb concentration, which might alter θNO, were it less than infinity, while leaving Dm and Vc unchanged. However, clinical studies involving patients with anemia have yielded conflicting results (1, 23, 32) at least partly attributed to the difficulty of selectively altering θ without altering Dm and Vc in pathological states. For example, patients with renal failure, especially those undergoing dialysis, will have permanent or temporary alteration in Dm and Vc. Because Dm is intrinsically “coupled” to Vc (20), it will vary as Vc varies (3). Furthermore, clinical alterations in Hb may not be sufficient to allow the effect of θ to be dissected out from other changes. Because the membrane oxygenator is a rigid structure, we were not only able to alter Hb without changing Vc or Dm, but we were also able to alter Hb 1,000% between 1 and 10 g/dl (2).
There is also a fundamental chemical kinetic reason why θNO may be less than infinity and yet independent of hematocrit. θ derives from the rate constant for the reactions of Hb and ligands. For NO, the reaction is second order: d[NO]/dt = j′[NO][HbO2]. Roughton and Forster and colleagues extended chemical kinetic theory to red blood cells in vitro and in vivo, so that the reaction for NO becomes d[NO]/dt = j′c[NO][HbO2], where j′c is the second-order rate constant for red blood cells (167 l·mmol−1·s−1), which equates to θNO = 4.5 ml NO·(ml blood·min·Torr)−1 (4). There are good grounds for thinking that this reaction could be zero-order with respect to Hb and, therefore, just pseudo-first-order for NO. If the Hb concentration is great compared with NO concentration, then so few binding sites would be used in the reaction that altering Hb concentration would have little effect on reaction rate. Inhaling 40 ppm NO gives a plasma concentration of 2.5 × 10−9 M compared with 9 × 10−3 M for Hb concentration; i.e., Hb is greatly in excess. This argument assumes that the Hb is well mixed in solution. If Hb is packaged in red blood cells and there is an advancing front effect (20), then the effective Hb concentration would be much smaller. Nevertheless, pseudo-first-order kinetics could explain why some clinical studies (32) found no effect of Hb on DlNO.
True Values for θNO
An apparent inconsistency in the approach of Guenard et al. (11) (method 2) was to accept the earlier θCO value in the RF method (25) but reject the data of Carlsen and Comroe (6), which gave a finite value for θNO obtained in the same laboratory using the same continuous-flow rapid-reaction apparatus. Their approach may not be as illogical as it first appears: the values for θCO and θNO may be underestimated unpredictably by diffusion limitation in stagnant layers in the apparatus. This effect is likely to be greater for NO, since it is exclusively diffusion limited, in contrast to CO, which is equally reaction and diffusion limited. The stagnant layer effects are likely to be less in the continuous-flow than in the stopped-flow apparatus. In support of the approach of Guenard et al. (method 2), indirect estimates of θNO using thin layers of blood are significantly higher than that reported by Carlsen and Comroe; Heller and Schuster (16) used a value of 14 ml NO·(ml blood·min·Torr)−1, which appears to have been calculated from (θO2 = 9.2) obtained from Heidelberger and Reeves (15) by multiplying by the ratio of water solubilities (αNO/αO2) and Krogh diffusion constants (kNO/kO2), although we obtain a higher value: 9.2 × 0.041/0.021 × 1.76 = 27.7 ml NO·(ml blood·min·Torr)−1. Chakraborty et al. (7), using computer simulation to eliminate the stagnant layer effect, report θNO = 4.2 × 106 ml NO·(ml blood·min·Torr)−1! This raises the counterargument that stagnant layers could well be present in alveolar capillaries in vivo, a possibility that Hughes and Bates (20) emphasize strongly. Indeed, if, as suggested by this and our previous work (2, 4), θNO approximates 4–5 ml NO·(ml blood·min·Torr)−1, then the thin film work (15) supports the argument that the major resistance to red cell-associated NO diffusion lies immediately adjacent to, rather than within, the cell or its membrane. This fits in neatly with recent work examining Hb and red cell effect on vasoconstriction and the effect of chemically and physically altering the red cell membrane and observing red cell NO uptake (21). These experiments point to diffusion limitation of NO in vivo by a cell-free zone along the endothelium, an unstirred layer around the red blood cell, and, finally, the red cell membrane itself (21). The respective contribution of these three barriers remains in dispute.
Taken together, our in vitro experiments favor the red cell membrane and its surrounding layer as a major source of the diffusion resistance to NO. Reducing hematocrit will proportionately increase plasma and reduce red cell surface area by approximately (hematocrit)2/3. Although we found a statistically significant relationship between 1/Hb and 1/Dno in vitro, there was little change in Dno above Hb ∼4 g/dl. By contrast, the effect of hemolysis and bypassing the red cell membrane using Oxyglobin is striking. One way to circumvent some of these difficulties is to consider the entire diffusion barrier from air-tissue-plasma-erythrocyte to intracellular Hb as a single dynamic resistance (1/Db), measured as 1/Dno, separate from the chemical reaction rate with Hb; then the latter becomes the empirically measured gas uptake by 1 ml of free Hb solution at a given concentration (θHb), i.e., 1/Dl = 1/Db + 1/(θHb·Vc). This approach assumes similar chemical reaction kinetics between free extra- and intraerythrocyte Hb; this assumption may or may not be true (29) and requires further examination.
Use of Blood Substitutes
It is increasingly clear that the “encapsulation” of Hb within erythrocytes markedly slows NO scavenging by heme-binding sites, allowing NO to act as a vasodilator “downstream.” Cell-free heme-based blood substitutes accelerate removal of NO, risking local vasoconstriction and potentially limiting their application (34). This work should encourage the development of encapsulated heme-based blood substitutes, and our oxygenator circuit could be used to test the NO-scavenging properties of blood substitutes.
Implication for Clinical Use of DlNO
All the recent reports of DlNO have assumed θNO to be infinite and used DlNO as a pure and direct measure of membrane conductance, i.e., unaffected by any diffusive resistance associated with capillary erythrocytes. Our findings showed this assumption to be untrue: 1/DlNO is 37% erythrocyte resistance and 63% nonerythrocyte resistance. Caution is warranted when interpreting isolated DlNO measurements. Reconciling the different methods and θ values remains challenging. Method 1 (the RF method) yields stable estimates of Dm and Vc during exchange transfusion until 30 ml/kg of blood has been exchanged. Method 2 yields Dm and Vc that change in opposite directions with exchange transfusion. Method 3 yields relatively stable estimates of Vc, whereas Dm increases with exchange transfusion. The important point is to ensure internal consistency within a given study. On the basis of current data, when calculating DmNO and Vc from DlNO and DlCO, we recommend the use of θNO = 4.5 ml NO·(min·Torr·ml blood)−1.
Red cell dependence also raises the following question: Should DlNO be adjusted for Hb concentration? With use of the finite θNO = 4.5, DmNO = 4.27 ml·(min·Torr·kg)−1, and Vc = 1.7 ml/kg, substituting in Eq. 1 the reduction in DlNO is ∼0.1 ml·(min·Torr·kg)−1 per (g/dl) until Hb <8 g/dl. Therefore, a Hb correction is not needed in routine clinical practice.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants R01 HL-040070, HL-054060, and HL-062873.
DISCLAIMER
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute or of the National Institutes of Health.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank the staff of the University of Texas Southwestern Animal Resources Center for veterinary assistance.
REFERENCES
- 1. Borland C. NO and CO transfer. Eur Respir J 3: 977–978, 1990 [PubMed] [Google Scholar]
- 2. Borland C, Dunningham H, Bottrill F, Vuylsteke A. Can a membrane oxygenator be a model for lung NO and CO transfer? J Appl Physiol 100: 1527–1538, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Borland C, Mist B, Zammit M, Vuylsteke A. Steady-state measurement of NO and CO lung diffusing capacity on moderate exercise in men. J Appl Physiol 90: 538–544, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Borland CD, Cox Y. Effect of varying alveolar oxygen partial pressure on diffusing capacity for nitric oxide and carbon monoxide, membrane diffusing capacity and lung capillary blood volume. Clin Sci (Lond) 81: 759–765, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Borland CD, Higenbottam TW. A simultaneous single breath measurement of pulmonary diffusing capacity with nitric oxide and carbon monoxide. Eur Respir J 2: 56–63, 1989 [PubMed] [Google Scholar]
- 6. Carlsen E, Comroe JH. The rate of uptake of carbon monoxide and of nitric oxide by normal erythrocytes and experimentally produced spherocytes. J Gen Physiol 42: 83–107, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chakraborty S, Balakotaiah V, Bidani A. Diffusing capacity reexamined: relative roles of diffusion and chemical reaction in red cell uptake of O2, CO, CO2, and NO. J Appl Physiol 97: 2284–2302, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Driessen B, Jahr JS, Lurie F, Griffey SM, Gunther RA. Effects of haemoglobin-based oxygen carrier hemoglobin glutamer-200 (bovine) on intestinal perfusion and oxygenation in a canine hypovolaemia model. Br J Anaesth 86: 683–692, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Dunningham H, Borland C, Bottrill F, Gordon D, Vuylsteke A. Modelling lung and tissue diffusion using a membrane oxygenator circuit. Perfusion 22: 231–238, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Forster RE. Diffusion of gases across the alveolar membrane. In: Handbook of Physiology. The Respiratory System. Gas Exchange. Bethesda, MD: Am. Physiol. Soc., 1987, sect. 3, vol. IV, chapt. 5, p. 71–88 [Google Scholar]
- 11. Guenard H, Varene N, Vaida P. Determination of lung capillary blood volume and membrane diffusing capacity in man by the measurements of NO and CO transfer. Respir Physiol 70: 113–120, 1987 [DOI] [PubMed] [Google Scholar]
- 12. Hakim TS, Sugimori K, Camporesi EM, Anderson G. Half-life of nitric oxide in aqueous solutions with and without haemoglobin. Physiol Meas 17: 267–277, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Hamilton RG, Kelly N, Gawryl MS, Rentko VT. Absence of immunopathology associated with repeated IV administration of bovine Hb-based oxygen carrier in dogs. Transfusion 41: 219–225, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Hamilton RG, Kickler TS. Bovine hemoglobin (glutamer-250, Hemopure)-specific immunoglobulin G antibody cross-reacts with human hemoglobin but does not lyse red blood cells in vitro. Transfusion 47: 723–728, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Heidelberger E, Reeves RB. Factors affecting whole blood O2 transfer kinetics: implications for θO2. J Appl Physiol 68: 1865–1874, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Heller H, Schuster K. Role of reaction resistance in limiting carbon monoxide uptake in rabbit lungs. J Appl Physiol 84: 2066–2069, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Holland RA, Forster RE. The effect of size of red cells on the kinetics of their oxygen uptake. J Gen Physiol 49: 727–742, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holland RAB. Rate at which CO replaces O2 from O2Hb in red cells of different species. Respir Physiol 7: 43–63, 1969 [DOI] [PubMed] [Google Scholar]
- 19. Hsia CCW, Herazo LF, Ramanathan M, Johnson RL., Jr Cardiopulmonary adaptations to pneumonectomy in dogs. IV. Membrane diffusing capacity and capillary blood volume. J Appl Physiol 77: 998–1005, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Hughes JM, Bates DV. The carbon monoxide diffusing capacity (DlCO) and its membrane (Dm) and red cell (θVc) components. Respir Physiol Neurobiol 138: 115–142, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol 26: 697–705, 2006 [DOI] [PubMed] [Google Scholar]
- 22. McDonough P, Dane DM, Hsia CC, Yilmaz C, Johnson RL., Jr Long-term enhancement of pulmonary gas exchange after high-altitude residence during maturation. J Appl Physiol 100: 474–481, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Moinard J, Guenard H. Membrane diffusion of the lungs in patients with chronic renal failure. Eur Respir J 6: 225–230, 1993 [PubMed] [Google Scholar]
- 24.Phansalkar AR, Hanson CM, Shakir AR, Johnson RL, Jr, Hsia CC. Nitric oxide diffusing capacity and alveolar microvascular recruitment in sarcoidosis. Am J Respir Crit Care Med 169: 1034–1040, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Roughton FJW, Forster RE. Relative importance of diffusion and chemical reaction rates in determining the rate of exchange of gases in the human lung, with special reference to true diffusing capacity of the pulmonary membrane and volume of blood in lung capillaries. J Appl Physiol 11: 290–302, 1957 [DOI] [PubMed] [Google Scholar]
- 26. Roughton FJW, Forster RE, Cander L. Rate at which carbon monoxide replaces oxygen from combination with human hemoglobin in solution and in the red cell. J Appl Physiol 11: 269–276, 1957 [DOI] [PubMed] [Google Scholar]
- 27. Sackner MA, Greenletch D, Heiman M, Epstein S, Atkins N. Diffusing capacity, membrane diffusing capacity, capillary blood volume, pulmonary tissue volume and cardiac output by a rebreathing technique. Am Rev Respir Dis 111: 157–165, 1975 [DOI] [PubMed] [Google Scholar]
- 28. Sackner MA, Markwell G, Atkins N, Birch SJ, Fernandez RJ. Rebreathing techniques for pulmonary capillary blood flow and tissue volume. J Appl Physiol 49: 910–915, 1980 [DOI] [PubMed] [Google Scholar]
- 29. Sirs JA. The kinetics of the reaction of carbon monoxide with fully oxygenated haemoglobin in solution and erythrocytes. J Physiol 236: 387–401, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest 120: 1850–1856, 2001 [DOI] [PubMed] [Google Scholar]
- 31. van der Lee I, Zanen P. Diffusion capacity for nitric oxide and carbon monoxide. Chest 126: 1708–1710, 2004 [DOI] [PubMed] [Google Scholar]
- 32. van der Lee I, Zanen P, Biesma DH, van den Bosch JM. The effect of red cell transfusion on nitric oxide diffusing capacity. Respiration 72: 512–516, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem 275: 2342–2348, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Winslow RM. Red cell substitutes. Semin Hematol 44: 51–59, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Zavorsky GS, Cao J, Murias JM. Reference values of pulmonary diffusing capacity for nitric oxide in an adult population. Nitric Oxide 18: 70–79, 2008 [DOI] [PubMed] [Google Scholar]