Abstract
Previous studies in our laboratory established that reactive oxygen species (ROS) generated by NADPH oxidase (NOX) facilitate the open state of a subset of K+ channels in oxygen-sensitive type I cells of the carotid body. Thus pharmacological inhibition of NOX or deletion of a NOX gene resulted in enhanced chemoreceptor sensitivity to hypoxia. The present study tests the hypothesis that chronic hypoxia (CH)-induced hypersensitivity of chemoreceptors is modulated by increased NOX activity and elevated levels of ROS. Measurements of dihydroethidium fluorescence in carotid body tissue slices showed that increased ROS production following CH (14 days, 380 Torr) was blocked by the specific NOX inhibitor 4-(2-amino-ethyl)benzenesulfonyl fluoride (AEBSF, 3 μM). Consistent with these findings, in normal carotid body AEBSF elicited a small increase in the chemoreceptor nerve discharge evoked by an acute hypoxic challenge, whereas after 9 days of CH the effect of the NOX inhibitor was some threefold larger (P < 0.001). Evaluation of gene expression after 7 days of CH showed increases in the isoforms NOX2 (∼1.5-fold) and NOX4 (∼3.8-fold) and also increased presence of the regulatory subunit p47phox (∼4.2-fold). Involvement of p47phox was further implicated in studies of isolated type I cells that demonstrated an ∼8-fold and an ∼11-fold increase in mRNA after 1 and 3 days, respectively, of hypoxia in vivo. These findings were confirmed in immunocytochemical studies of carotid body tissue that showed a robust increase of p47phox in type I cells after 14 days of CH. Our findings suggest that increased ROS production by NOX enzymes in type I cells dampens CH-induced hypersensitivity in carotid body chemoreceptors.
Keywords: dihydroethidium, p47phox, reactive oxygen species
hypoxia in mammals increases ventilatory drive principally via excitation of peripheral oxygen chemoreceptors in the carotid body, a small, highly vascularized organ lying near the bifurcation of the carotid artery. Carotid body parenchyma consists of groups of oxygen-sensitive type I glomus cells that form synapses with afferent axon terminals of the carotid sinus nerve (CSN), a branch of the ninth cranial nerve. Type I cells are neurochemically pluripotent, containing high levels of the catecholamine dopamine, in addition to lesser amounts of norepinephrine, acetylcholine, and multiple neuroactive and vasoactive peptides (16, 17, 19). Numerous studies have shown that increased impulse traffic in the CSN elicited by lowered arterial Po2 is correlated with the release of multiple chemical agents from type I cells (28, 38, 40).
Oxygen chemotransduction appears to be mediated by specialized K+ channels. Studies within the last decade have revealed background leak currents conducted by TASK-like voltage-insensitive K+ channels in rat type I cells (6, 7). Low O2 closes these channels and initiates membrane depolarization and increased intracellular Ca2+ concentration. Earlier studies in rat and rabbit demonstrated that type I cells also express a separate set of K+ channels that are both voltage- and O2 sensitive (19, 26, 31, 47). Like those mediating background leak currents, these latter channels tend to close in hypoxia. However, the function of these channels may be species dependent because in rat their current-voltage curve indicates that they are closed at rest and open when the cell membrane depolarizes, suggesting participation in recovery of membrane potential (12), whereas in rabbit a dominant-negative Kv4 construct suppresses the O2-sensitive K+ current (37).
Studies of O2-sensitive cells from other tissues have shown that specific subsets of K+ channels are regulated by reactive oxygen species (ROS) produced by NADPH oxidase (NOX) (18, 29, 32, 44), a 91-kDa glycoprotein that was first described in phagocytes (gp91phox; phox = phagocytic oxidase), where it produces high levels of ROS as part of an extracellular killing mechanism in response to invading microorganisms (15). Homologs of the oxidase (NOX1–5) have been cloned and sequenced from a variety of cell types, and ROS have subsequently been recognized as important intracellular signaling molecules (2, 23). Specific NOX isoforms have been shown to participate in selected signaling pathways that mediate O2 sensitivity, including the expression of the transcription factor hypoxia-inducible factor-1 (HIF-1), and modulation of ion channel activity (2). Of particular interest are studies that documented the generation of ROS by NOX in the lung, where hypoxia in chemosensory cells derived from neuroepithelial bodies lowers ROS levels and inhibits voltage-dependent K+ current (29). In these cells, K+ channels are activated by low concentrations of H2O2, and hypoxia-evoked depression of channel activity is occluded in the presence of oxidase inhibitors, further suggesting that ROS production is a key factor coupling local Po2 to cell activity (32). Moreover, expression of NOX4 in HEK293 cells activates coexpressed TASK-1 channels, providing further evidence of involvement of NOX-generated ROS in O2 signaling cascades (24).
In addition, recent studies in our laboratory (20, 21) demonstrated that H2O2 enhances the voltage-sensitive K+ current in mouse and rat type I cells, indicating that these channels are likewise sensitive to the cellular redox potential. Consistent with this conclusion is the finding that manipulations that lower the cellular production of ROS also reduce the magnitude of voltage-evoked K+ current in type I cells (21). A role for ROS in chemotransduction is further suggested by the finding that hypoxia increases oxygen radical production in mouse and rat type I cells (20, 21). Moreover, hypoxia-evoked chemoreceptor nerve activity is increased in gene-deleted mice lacking the protein p47phox, a key regulatory subunit of NOX (11, 21). Taken together, these findings are consistent with the notion that increased ROS production facilitates recovery of membrane potential in depolarized type I cells.
Another indicator of ROS involvement in carotid body function comes from recent studies of chronic intermittent hypoxia (CIH), a model for sleep apnea. Prabhakar and colleagues have documented that CIH elicits sensory long-term facilitation (sLTF) in chemoreceptor afferents, similar to changes previously observed in efferent respiratory motor neurons after several days' exposure to recurrent short hypoxic episodes (1, 41). A role for ROS in carotid chemosensitivity is indicated by the fact that concurrent treatment with oxygen scavenger molecules blocks the development of sLTF (35). Also, ROS production in carotid body tissue is increased after CIH, as is the expression of NOX2 and NOX4 in type I glomus cells. NOX involvement is further supported by the finding that NOX2 gene-deleted animals fail to develop sLTF after CIH (33).
In contrast to CIH, distinctly different functional changes occur in the carotid body after chronic hypoxia (CH), a condition that simulates chronic obstructive pulmonary disease (COPD) and chronic heart failure. While both CH and CIH elicit increased basal sensory nerve discharge and an increased neural sensitivity to hypoxia, only CIH elicits sLTF, which is manifest as an increased sensory neural discharge after repeated acute hypoxic exposures (39). In addition, while CH induces carotid body enlargement with type I cell hyperplasia, no such changes result from CIH (34).
A role for ROS in CH-induced hypersensitivity has never been investigated. Given the apparent modulatory role of ROS in the chemotransduction cascade (11), it is conceivable that CH alters the expression of NOX and the production of O2 radicals in type I cells. With respect to K+-channel function, increased chemoreceptor excitability is consistent with decreased NOX expression and lowered production of ROS. On the other hand, elevated ROS levels could serve to limit excess chemoreceptor activity via activation of voltage-sensitive K+ channels. In the present study, we examined these possibilities by comparing the effects of pharmacological inhibition of NOX on hypoxia-evoked CSN activity in normal versus CH preparations and quantifying the effects of CH on ROS production in carotid body tissue slices. In addition, we studied the effect of CH on mRNA expression of NOX2 and NOX4 and on expression of the regulatory subunit p47phox in type I cells. Our results indicate that there are distinctly different mechanisms for ROS involvement in the changes in chemosensitivity associated with CH versus CIH.
METHODS
Animals and exposure to chronic hypoxia.
Twenty-six rats were exposed in a hypobaric chamber while housed in standard rodent cages with food and water. Pressures were reduced from ambient barometric pressure (Pb) at the University of Utah (i.e., Pb ∼630 Torr, 1,500 m) until a selected pressure equivalent to ∼5,500 m (380 Torr) was reached and then maintained for a selected period (up to 14 days). The chamber was opened every 2 days to replenish food and water and change litter. Normal age-matched control animals (16 rats) were maintained outside the chamber under ambient conditions. Animal protocols were approved by the University of Utah Institutional Animal Care and Use Committee.
Quantitative reverse transcriptase-polymerase chain reaction.
Carotid bodies were harvested from rats anesthetized with a mixture of ketamine (10 mg/100 g) plus xylazine (0.9 mg/100 g). Carotid artery bifurcations were located, excised, and placed in a Lucite chamber containing 100% O2-equilibrated modified Tyrode solution at 0–4°C (in mM: 112 NaCl, 4.7 KCl, 2.2 CaCl2, 1.1 MgCl2, 42 sodium glutamate, 5 HEPES buffer, 5.6 glucose; pH = 7.4). Each carotid body was carefully dissected from the artery and cleaned of surrounding connective tissue. Tissues were then immediately frozen on Al-foil on dry ice. In accord with the kit instructions (RNAqueous-Micro, Ambion, Austin, TX), total RNA was extracted from homogenized tissue samples pooled from groups of five rats for each experiment. After removal of contaminating DNA (DNase treatment), first-strand complementary DNA (cDNA) was synthesized from 1 μg of total RNA (quantified with a NanoDrop ND-1000 spectrophotometer) with RETROscript (Ambion). Aliquots of cDNA corresponding to 2 ng of total RNA were introduced into a SYBR Green reaction mix (25 μl; Qiagen) containing “upstream” and “downstream” primers for selected NADPH components (NOX2, NOX4, or p47phox). All primer pairs were “blasted” against known rat gene sequences. Real-time quantitative reverse transcriptase-polymerase chain reaction (qPCR) was conducted in an MJ Research PTC-200 equipped with a Chromo4 detector. From each pooled group of cDNA three to five PCR reactions were initiated at 95°C for 15 min, followed by 40 cycles consisting of 30 s at 94°C, 30 s at 58°C, and 30 s at 72°C, with the final cycle extended to 5 min at 72°C. Product purity was evaluated by determination of the melting curve, after which samples were stabilized at 4°C. Sample comparisons were based on the relative standard curve method (42), and data are normalized to 18S rRNA expression. In preliminary studies using cDNA aliquots equivalent to equal amounts of total RNA we found that 18S rRNA varies <10% in CH versus normal samples, with P > 0.24. Amplifications of samples not treated with RETROscript were performed to exclude possible contamination with genomic DNA.
Amplified RNA.
Carotid bodies were dissected free of surrounding connective tissue and transferred to Ham's F-12 medium (Ca2+ and Mg2+ free) containing 0.2% collagenase and 0.2% trypsin. Each organ was cut into 6–12 pieces and incubated for 40 min in a CO2 incubator (5% CO2, 95% air) at 36.5°C. Tissue fragments were rinsed (2 × 10 min, room temperature) in F-12 medium (Ca2+ and Mg2+ free), transferred to poly-l-lysine-coated glass coverslips, and triturated in a small volume of medium plus 10% fetal calf serum and 5 μg/ml insulin. Dissociated single type I cells collected with a patch-clamp pipette were immersed in buffer provided in a PicoPure RNA isolation kit (Microgenomics/Arcturus), and total RNA was isolated according to kit directions. The RNA was further purified with the RNeasy MinElute Cleanup kit (Qiagen). mRNA was amplified according to directions in the MessageBOOSTER cDNA Synthesis kit (Epicentre Biotechnologies).
Dihydroethidium assessment of NOX activity.
Carotid bodies and superior cervical ganglia from normal or 14-day CH rats were embedded in agar at 4°C and mounted in a Leica VT-1000S vibratome. Tissue slices (∼100 μm) were collected in buffered modified Tyrode solution. Sections were incubated in 5 μM dihydroethidium (DHE) for 10 min, followed by fixation in 2% phosphate-buffered paraformaldehyde for 15 min in accordance with the method of Bindokas et al. (3). In some sections, the specific NOX inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF, 3 μM) was introduced 20 min before DHE. Sections were viewed in a Zeiss M30 laser scanning confocal microscope. Image intensity was quantified with ImageTool software.
Immunocytochemistry.
Anesthetized rats were perfused intracardially with ice-cold 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Carotid bodies were removed, cleaned of surrounding connective tissue, immersed in the same fixative for 1 h, rinsed in 20% sucrose-PBS for 2 h, and stored at 4°C in 30% sucrose-PBS for 1 h. Cryostat sections (6 μm) were thaw-mounted onto gelatin-subbed slides. Sections were treated for 20 min with 5% goat serum in PBS plus 0.1% Triton X-100 and then incubated at 4°C overnight in primary antibodies for tyrosine hydroxylase (TH; STI-Signal Transduction Products, catalog no. A-2029, mouse monoclonal, diluted 1:2,000) and p47phox (Santa Cruz Biotechnology, catalog no. SC-14015, rabbit polyclonal, diluted 1:100) in PBS containing 2% goat serum and 0.1% Triton X-100. Sections were then rinsed in PBS at room temperature, incubated for 1 h with selected secondary antibodies (diluted 1:200 to 1:400) conjugated with fluorescein or rhodamine in 2% goat serum plus 0.1% Triton X-100, and then rinsed in PBS for 20 min. In all experiments, normal versus experimental tissue samples and frozen sections were processed simultaneously, and all incubation and reaction conditions were identical. In selected sections the primary antibody was omitted to assess nonspecific staining of the secondary fluorescent antibodies. Specimens were viewed in a Zeiss Model M30 laser scanning confocal microscope.
Electrophysiological recording of carotid sinus nerve activity.
As has been described previously (8), the carotid bifurcations were excised from rats under ketamine-xylazine anesthesia and placed in a Lucite chamber containing 100% O2-equilibrated modified Tyrode solution at 0–4°C. Each carotid body along with its attached nerve was carefully dissected from the artery and cleaned of surrounding connective tissue. Preparations were then placed in a conventional flow chamber (volume ∼300 μl), where the carotid body was continuously superfused (up to 4 h) with modified Tyrode solution maintained at 37°C and equilibrated with a selected gas mixture. To achieve mechanically stable recording conditions, the flow rate of superfusate was maintained at <1 ml/min. The CSN was drawn up into the tip (∼100-μm ID) of a glass suction electrode for monopolar recording of chemoreceptor activity. Because of the low flow rate and the avascular nature of the preparations, basal neural activity was established in superfusates equilibrated at Po2 = 450 Torr. Po2 was lowered to 120 Torr in superfusates equilibrated with air to provide a moderate hypoxic stimulus. Neural activity was led to an AC-coupled preamplifier, filtered, and transferred to a window discriminator and a frequency-to-voltage converter. Signals were processed by an AD/DA converter for display of frequency histograms on a PC computer monitor. Data were expressed as impulses per second and analyzed with Student's t-test and ANOVA.
RESULTS
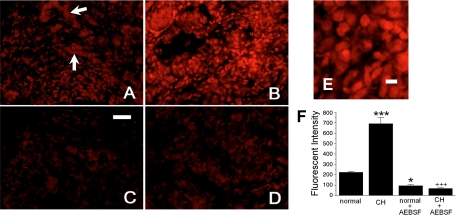
In accord with previous demonstrations that oxidized DHE is retained in fixed cells and tissues, we found robust fluorescence in carotid body tissue slices treated with paraformaldehyde (3, 5). In normal tissue (Fig. 1A) ROS production is concentrated in ovoid cells, some of which form groups (Fig. 1A, arrows) characteristic of the lobular formations of chemoreceptor type I cells. ROS production also appears to occur in many isolated cells distributed throughout the slice. After 14 days of hypoxia (Fig. 1B), fluorescence is substantially increased. The enlarged image in Fig. 1E shows cellular detail and the varying fluorescent intensity among the cells. Fluorescent activity in some cells is consistent with ROS production in the nucleus as well as the cytoplasm. Figure 1, C and D, show that treatment of slices with the specific NOX inhibitor AEBSF (3 μM) suppresses ROS production in both normal and CH preparations, respectively. Quantitative summary data in Fig. 1F obtained from four or five slices from each experimental group indicate that CH elicits a threefold increase in ROS production following CH and that treatment of normal or CH slices with AEBSF depresses production of the superoxide anion to levels that are significantly below normal. Data from control experiments in the superior cervical ganglion (not shown) indicated that CH did not alter ROS production; furthermore, AEBSF did not significantly change basal ROS levels in this tissue.
Fig. 1.
Chronic hypoxia (CH) increases NADPH oxidase (NOX)-dependent reactive oxygen species (ROS) production in carotid body tissue slices incubated in dihydroethidium (DHE; 5 μM, 10 min at 37°C), followed by fixation (30 min) in buffered 2% paraformaldehyde. A: fluorescence in normal carotid body; arrows indicate putative lobules of type I cells. B: tissue from a rat exposed to CH (380 Torr, 14 days). C and D: fluorescence is noticeably less in slices coincubated with DHE + 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF, 3 mM; normal, C; CH, D). Scale bar, 50 μm. E: enlarged area from B showing cellular detail. Scale bar, 10 μm. F: summary data of fluorescent intensity from 5 or 6 slices in each group. *P < 0.05, ***P < 0.001 vs. normal; +++P < 0.001 vs. CH.
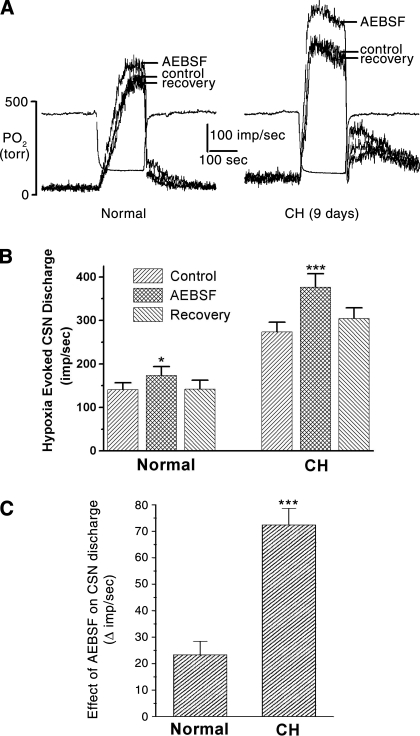
Figure 2 demonstrates the effect of AEBSF on hypoxia-evoked chemoreceptor activity. Superimposed records of integrated CSN activity in Fig. 2A, left, show that AEBSF (3 μM) applied to normal preparations (superfused in vitro) elicits a modest increase in the nerve discharge. After 9 days of CH (Fig. 2A, right), the basal (resting) and the hypoxia-evoked nerve discharge are elevated above normal. AEBSF evokes a robust enhancement of the hypoxia-evoked nerve activity. In both preparations the drug application began 2.5 min before the hypoxic stimulus, and during this time basal nerve discharge was unaffected, which is consistent with our previous findings with mouse type I cells, where ROS production was low at rest but increased substantially during hypoxia (21).
Fig. 2.
NOX-dependent ROS production modulates chemoreceptor sensitivity. A: superimposed traces of carotid sinus nerve (CSN) activity evoked by a standardized acute hypoxic stimulus; in vitro superfused carotid bodies. In normal carotid body the NOX inhibitor AEBSF elicits a modest enhancement of the nerve discharge. After 9 days of CH, CSN activity is enhanced, and treatment with AEBSF results in an additional robust increase in activity. B: summary data from 5 normal and 5 CH preparations. C: the absolute increase in impulses per second in the presence of AEBSF is >3-fold greater after CH. *P < 0.05, ***P < 0.001 vs. normal.
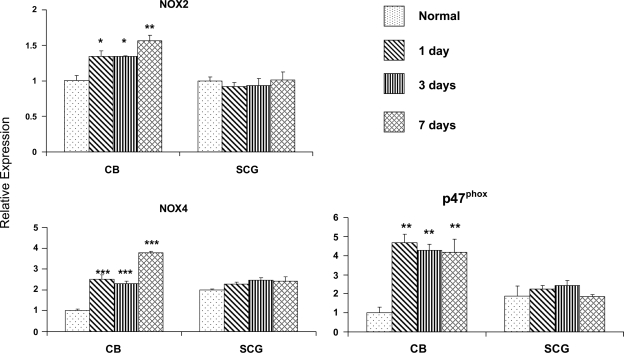
Figure 3 shows the effect of CH on gene expression of NOX2, NOX4, and the p47phox regulatory subunit of NOX in carotid body. An increase in the expression of these genes is detectable after only 1 day of hypoxia: the level of NOX2 transcript is increased ∼30%, while NOX4 is elevated some 2.6-fold. Further changes in the expression of these genes was not detectable after 3 days of hypoxia, but at day 7 NOX2 was increased 1.5-fold and NOX4 ∼3.8-fold above that inn control animals. Changes in p47phox were even more robust after 1 day of hypoxia (∼4.7-fold), and this high level of expression was maintained throughout the period from day 3 to day 7 of exposure. It is noteworthy that CH did not alter the expression of NOX genes in the superior cervical ganglion, which contains a variety of cells, including catecholaminergic postganglionic sympathetic neurons, Schwann cells, fibroblasts, and small intensely fluorescent cells. This finding suggests a tissue-specific effect of the hypoxic exposure and is consistent with the absence of increased DHE fluorescence in the ganglion following CH.
Fig. 3.
Effect of CH on gene expression of selected NOX subunits in rat carotid body (CB) and superior cervical ganglion (SCG). Real-time PCR data obtained from rats exposed to 0 (normal), 1, 3, and 7 days of hypoxia. Note that low O2 elevates transcript levels for NOX2 (gp91phox), NOX4, and p47phox in CB but not in SCG. *P < 0.05, **P < 0.01, ***P < 0.001 vs. normal.
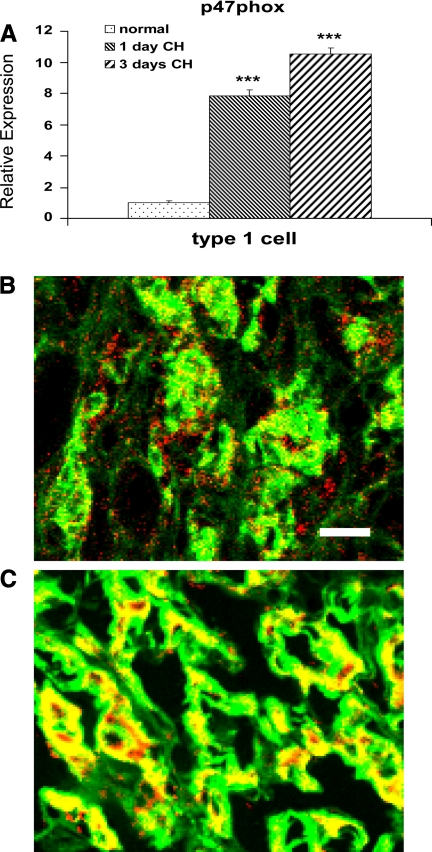
Evidence for the involvement of oxygen-sensitive type I cells in the NOX gene response is shown in Fig. 4. Data from amplified RNA (aRNA)-qPCR studies (Fig. 4A) show that p47phox expression is elevated in cultured type I cells harvested from rats after 1 and 3 days of hypoxia. This robust response is consistent with findings shown in Fig. 3, which indicate a greater than fourfold increase in p47phox in the carotid body. Previous studies in our laboratory (25) using aRNA-qPCR techniques demonstrated that 1 day of in vivo hypoxia elicited an increased expression of TH, consistent with the known effects of hypoxia on this gene in type I cells. The ∼10-fold increase in p47phox in cells isolated after 3 days of in vivo CH is confirmed by immunofluorescence data presented in Fig. 4, B and C, which show that 14 days of CH elicits an equally robust increase in p47phox protein in type I cells. In normal tissue (Fig. 4B) p47phox (red) is evident in cells surrounding chemoreceptor cell lobules. This localization is consistent with the presence of resident immune cells, as has been demonstrated previously (14, 25). Also evident in normal tissue is the colocalization (indicated by yellow in Fig. 4B) of p47phox with TH in some type I cells. After CH, colocalization of the two antigens (Fig. 4C) is substantially increased, indicating elevated expression of p47phox in type I cells.
Fig. 4.
A: results from single-cell real-time PCR assays of isolated type I cells showing effect of in vivo hypoxia at 380 Torr for 1 and 3 days on expression of p47phox transcript. ***P < 0.001 vs. normal. B and C: immunofluorescence images of p47phox (red) and tyrosine hydroxylase (green) in rat carotid body; yellow indicates antigen colocalization. B: normal carotid body. C: after 14 days of CH at 380 Torr.
DISCUSSION
CH-induced chemoreceptor hypersensitivity is an adaptive mechanism that enhances ventilatory drive both at high altitude and in pathophysiological conditions where lung function is chronically compromised. Numerous studies have focused attention on cellular mechanisms that are putatively involved in increasing type I cell sensitivity to hypoxia (4, 45). The present data demonstrate that ROS generated by NOX enzymes inhibit chemosensitivity, thus documenting a mechanism that functions to dampen impulse traffic in the CSN. Moreover, NOX expression is induced by CH, thus increasing ROS production in type I cells. Consistent with increased ROS production, our data demonstrate that blocking NOX with AEBSF elicits a more robust elevation of CSN activity following CH. The specific mechanism for modulation of CH-induced hypersensitivity is not revealed by these data. However, previous studies in our laboratory (21) demonstrated that ROS facilitate the open state of a subset of voltage-sensitive K+ channels, indicating that ROS promote recovery of the resting membrane potential during hypoxia. ROS-induced inhibition of type I cell activity during CH may protect cells from perturbation of Ca2+ homeostasis, thus lessening the probability of apoptosis associated with excess intracellular Ca2+ (30).
The oxidized form of DHE is a vital fluorescent dye that is retained in cells after fixation (5). Thus the degree of fluorescence in our tissue slice preparations indicates an inherent potential for ROS production. Our data in paraformaldehyde-fixed slices suggest that ROS production is widespread among cells in carotid body. In these studies we also showed that the CH-induced increase in fluorescent signal was completely eliminated in slices treated with the NOX inhibitor AEBSF. This drug has been shown to inhibit the activation of NOX by blocking the membrane translocation of soluble NOX enzyme subunits p47phox and p67phox (10). However, AEBSF is also a known serine protease inhibitor (10), and it has been shown to promote the induction of heme oxygenase-1 in human and mouse monocytes (46). Although we cannot eliminate possible alternate actions, AEBSF did not inhibit the fluorescent signal in slices of superior cervical ganglion, where CH likewise did not increase the expression of NOX isoforms (see Fig. 3). Moreover, in a previous study we showed (21) that AEBSF potently inhibited hypoxia-evoked ROS production in normal mouse carotid body type I cells, but not in cells from p47phox gene-deleted animals. An additional concern is the potential role of mitochondria in ROS production. In view of the fact that AEBSF blocks increased fluorescence following CH, it seems unlikely that mitochondria are a significant source of ROS. Previous studies in other tissues have shown that acute hypoxia produces ROS via a reaction at enzyme complex III, but this effect was observed at Po2 levels substantially lower than those used in our experiments (13).
Earlier studies suggested that multiple NOX subunits were present in type I cells (22). However, Dvorakova et al. (14) used a highly specific monoclonal antibody to show that high levels of NOX2 are present primarily in ED1-positive macrophages in normal carotid body. These findings differ with a recent report by Peng et al. (33), who used a polyclonal antibody to show that NOX2 is present in normal type I cells and that increased ROS production following CIH involves increased expression of NOX2 as well as NOX4. These authors localized NOX2 to the cytoplasm and NOX4 to the cell nucleus, consistent with our present study suggesting both cytoplasmic and nuclear ROS production in carotid body tissue slices. The specific NOX inhibitor AEBSF reduced fluorescence intensity in all visible cell types in the carotid body, indicating a multicellular distribution of NOX enzymes. Studies in other tissues have documented the presence of NOX in numerous cell types, including vascular endothelial cells, fibroblasts, and neurons, in addition to immune cells (2), all of which are present in rat carotid body (27).
Our results using qPCR assays on carotid body tissue samples suggest that CH elevates transcript levels for NOX2 and NOX4. Importantly, no such changes in NOX expression were found in the superior cervical ganglion. Peng et al. (33) showed that CIH likewise induces NOX2 and NOX4 isoforms in carotid body, but whereas our data indicate that CH preferentially increases NOX4, CIH appears to favor the induction of NOX2. These divergent results are consistent with previously documented differences in the effects of CH versus CIH on carotid body adaptation. Thus, while CH is known to elicit enlargement of the carotid body, including vascular expansion and type I cell hyperplasia (9, 36, 45), CIH does not involve any such morphological adaptations (34). In addition, CIH induces long-term facilitation of the sensory nerve discharge (sLTF), which is not present after CH (33, 34).
Our single-cell qPCR and immunocytochemical findings show that CH elevates expression of the p47phox regulatory subunit in type I cells. These data further confirm a role for NOX enzymes in chemoreceptor adaptation. Classical studies have demonstrated that full activation of NOX2 requires assembly of multiple subunits including phosphorylated p47phox (2). In contrast, the NOX4 isoform is activated by Ca2+ influx, and ROS production occurs independent of p47phox (2). Thus our findings may indicate an important role for both isoforms in adaptation to CH. It is particularly noteworthy that in a previous study we showed (43) that hypoxia-evoked CSN activity is elevated in p47phox gene-deleted mice, confirming a vital role for this subunit in modulation of chemoreceptor activity.
Consistent with the effect of p47phox deletion, we now observe that the NOX inhibitor AEBSF also enhances hypoxia-evoked CSN activity. Moreover, the effect of this drug is increased after CH, consistent with ROS modulation of receptor sensitivity. In contrast to these findings, Prabhakar and colleagues (33, 35) have shown that sLTF following CIH is absent in NOX2 gene-deleted mice and that ROS scavenger molecules block the development of sLTF. These very different results suggest that ROS engage highly specific cellular mechanisms: in CH they primarily function to inhibit, whereas in CIH they enhance chemoreceptor sensitivity. As noted above, our earlier studies demonstrated (21) that ROS generated in type I cells target a subset of voltage-sensitive K+ channels. The recent report by Peng et al. (33) indicates that sLTF is dependent on the release of 5-HT and the activation of PKC via 5-HT2 receptors. The contrasting effects of CH versus CIH nonetheless share in common an increase in NOX expression and ROS production in type I cells. These distinctly novel findings may indicate a high degree of compartmentalization of cell signaling, which might arise in part from the observed differential localization of a cytoplasmic NOX2 versus a nuclear NOX4 (33).
GRANTS
This study was supported by National Institutes of Health Grants NS-12636, NS-07938, and HL-086508.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol 94: 399–409, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci 16: 1324–1336, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bisgard GE. Carotid body mechanisms in acclimatization to hypoxia. Respir Physiol 121: 237–246, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Bucana C, Saiki I, Nayar R. Uptake and accumulation of the vital dye hydroethidine in neoplastic cells. J Histochem Cytochem 34: 1109–1115, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Buckler KJ. Background leak K+-currents and oxygen sensing in carotid body type 1 cells. Respir Physiol 115: 179–187, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Buckler KJ, Williams BA, Orozco RV, Wyatt CN. The role of TASK-like K+ channels in oxygen sensing in the carotid body. Novartis Found Symp 272: 73–85, 2006 [PubMed] [Google Scholar]
- 8. Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in physiological adaptation of the carotid body during chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 282: L1314–L1323, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Chen J, He L, Liu X, Dinger B, Stensaas L, Fidone S. Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am J Physiol Lung Cell Mol Physiol 292: L1257–L1262, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Diatchuk V, Lotan O, Koshkin V, Wikstroem P, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem 272: 13292–13301, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Dinger B, He L, Chen J, Liu X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol 157: 45–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donnelly DF. Are oxygen dependent K+ channels essential for carotid body chemo-transduction? Respir Physiol 110: 211–218, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumaker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273: 11619–11624, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Dvorakova M, Hohler B, Vollerthun R, Fischbach T, Kummer W. Macrophages: a major source of cytochrome b558 in the rat carotid body. Brain Res 852: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 15. El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med 41: 217–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fidone S, Dinger BG, Gonzalez C. Mechanisms of carotid body chemoreception. In: The Lung Biology in Health Disease, vol. X, The Regulation of Breathing, edited by Dempsey JA, Pack AI. New York: Dekker, 1995, p. 391–471 [Google Scholar]
- 17. Fidone SJ, Gonzalez C, Obeso A, Gomez-Nino A, Dinger B. Biogenic amine and neuropeptide transmitters in carotid body chemotransmission: experimental findings and perspectives. In: Hypoxia: The Adaptations, edited by Sutton JR, Coates G, Remmers JE. Toronto: Decker, 1990, p. 116–126 [Google Scholar]
- 18. Fu XW, Wang D, Nurse CA, Dinauer MC, Cutz E. NADPH oxidase is an O2 sensor in airway chemoreceptors: evidence from K+ current modulation in wild-type and oxidase-deficient mice. Proc Natl Acad Sci USA 97: 4374–4379, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994 [DOI] [PubMed] [Google Scholar]
- 20. He L, Dinger B, Gonzalez C, Obeso A, Fidone S. Function of NADPH oxidase and signaling by reactive oxygen species in rat carotid body type I cells. Adv Exp Med Biol 580: 155–160, 2006 [DOI] [PubMed] [Google Scholar]
- 21. He L, Dinger B, Sanders K, Hoidal J, Obeso A, Stensaas L, Fidone S, Gonzalez C. Effect of p47phox gene deletion on ROS production and oxygen sensing in mouse carotid body chemoreceptor cells. Am J Physiol Lung Cell Mol Physiol 289: L916–L924, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kummer W, Acker H. Immunohistochemical demonstration of four subunits of neutrophil NAD(P)H oxidase in type I cells of carotid body. J Appl Physiol 78: 1904–1909, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, Park JW. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal 18: 499–507, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Liu X, He L, Stensaas L, Dinger B, Fidone S. Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body. Am J Physiol Lung Cell Mol Physiol 296: L158–L166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science 241: 580–582, 1988 [DOI] [PubMed] [Google Scholar]
- 27. McDonald DM. Peripheral chemoreceptors: structure-function relationships of the carotid body. In: Regulation of Breathing, part I, edited by Hornbein TF. New York: Dekker, 1981, p. 105–319 [Google Scholar]
- 28. Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci 120: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 29. O'Kelly I, Lewis A, Peers C, Kemp PJ. O2 sensing by airway chemoreceptor-derived cells: protein kinase C activation reveals functional evidence for involvement of NADPH oxidase. J Biol Chem 275: 7684–7692, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2+-activated K+ current. Neurosci Lett 119: 253–256, 1990 [DOI] [PubMed] [Google Scholar]
- 32. Peers C, Kemp PJ. Acute oxygen sensing: diverse but convergent mechanisms in airway and arterial chemoreceptors. Respir Res 2: 145–149, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29: 4903–4910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol 94: 2342–2349, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Pequignot JM, Hellstrom S. Intact and sympathectomized carotid bodies of long-term hypoxic rats. Virchows Arch 400: 235–243, 1983 [DOI] [PubMed] [Google Scholar]
- 37. Pérez-Garcia MT, López-López JR, Riesco AM, Hoppe UC, Marbán E, González C, Johns DC. Viral gene transfer of dominant-negative Kv4 construct suppresses an O2-sensitive K+ current in chemoreceptor cells. J Neurosci 20: 5689–5695, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol 88: 2287–2295, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol 90: 1986–1994, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol 91: 17–23, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Prabhakar NR, Fields RD, Baker T, Fletcher EC. Intermittent hypoxia: cell to system. Am J Physiol Lung Cell Mol Physiol 281: L524–L528, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Sallmann S, Juttler E, Prinz S, Petersen N, Knopf U, Weiser T, Schwaninger M. Induction of interleukin-6 by depolarization of neurons. J Neurosci 20: 8637–8642, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanders KA, Sundar KM, He L, Dinger B, Fidone S, Hoidal JR. Role of components of the phagocytic NADPH oxidase in oxygen sensing. J Appl Physiol 93: 1357–1364, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Wang D, Youngson C, Wong V, Yeger H, Dinauer MC, Vega-Saenz De Miera E, Rudy B, Cutz E. NADPH-oxidase and a hydrogen peroxide-sensitive K+ channel may function as an oxygen sensor complex in airway chemoreceptors and small cell lung carcinoma cell lines. Proc Natl Acad Sci USA 93: 13182–13187, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang ZY, Bisgard GE. Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microsc Res Tech 59: 168–177, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Wijayanti N, Kietzmann T, Immenschuh S. Heme oxygenase-1 gene activation by the NAD(P)H oxidase inhibitor 4-(2-aminoethyl) benzenesulfonyl fluoride via a protein kinase B, p38-dependent signaling pathway in monocytes. J Biol Chem 280: 21820–21829, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Wyatt CN, Peers C. Ca2+-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol 483.3: 559–565, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]