Abstract
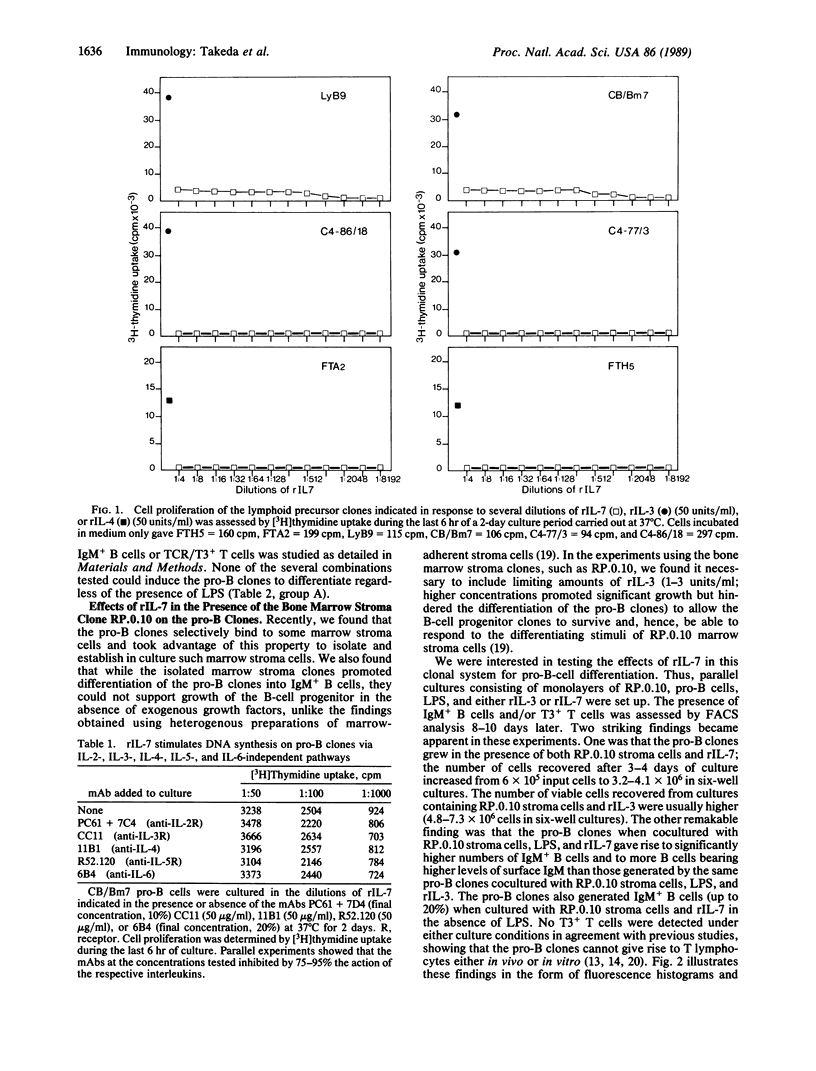
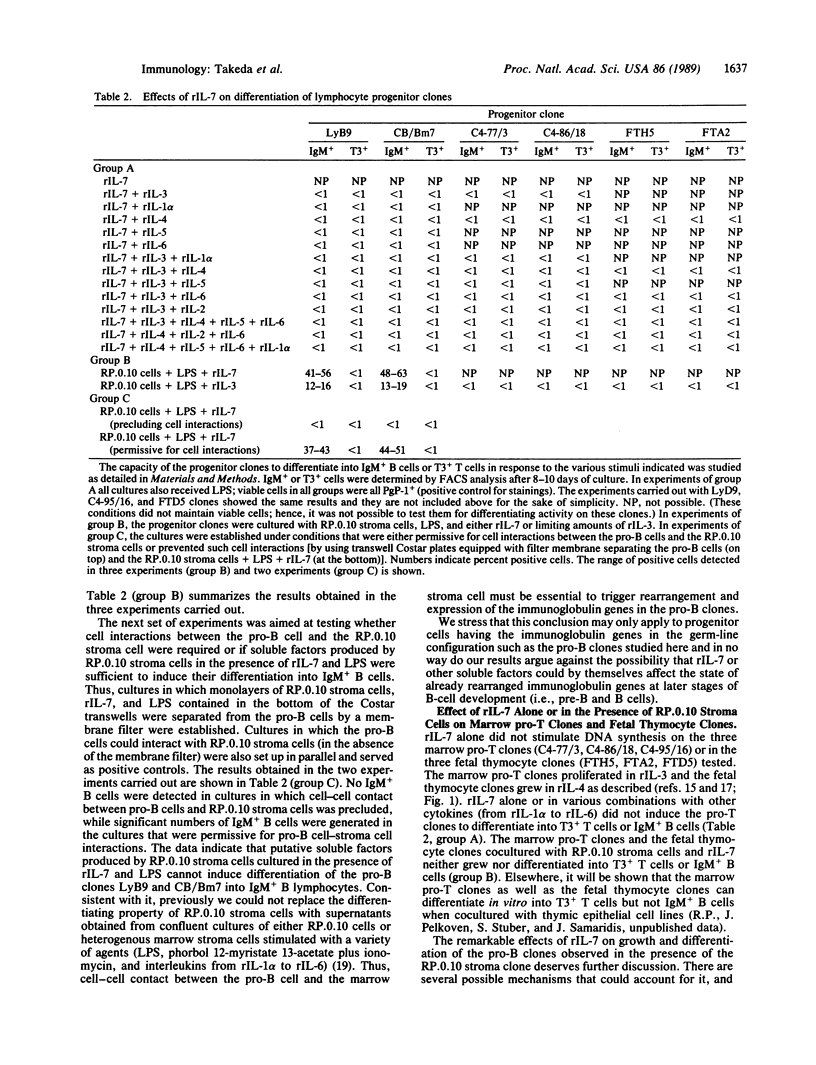
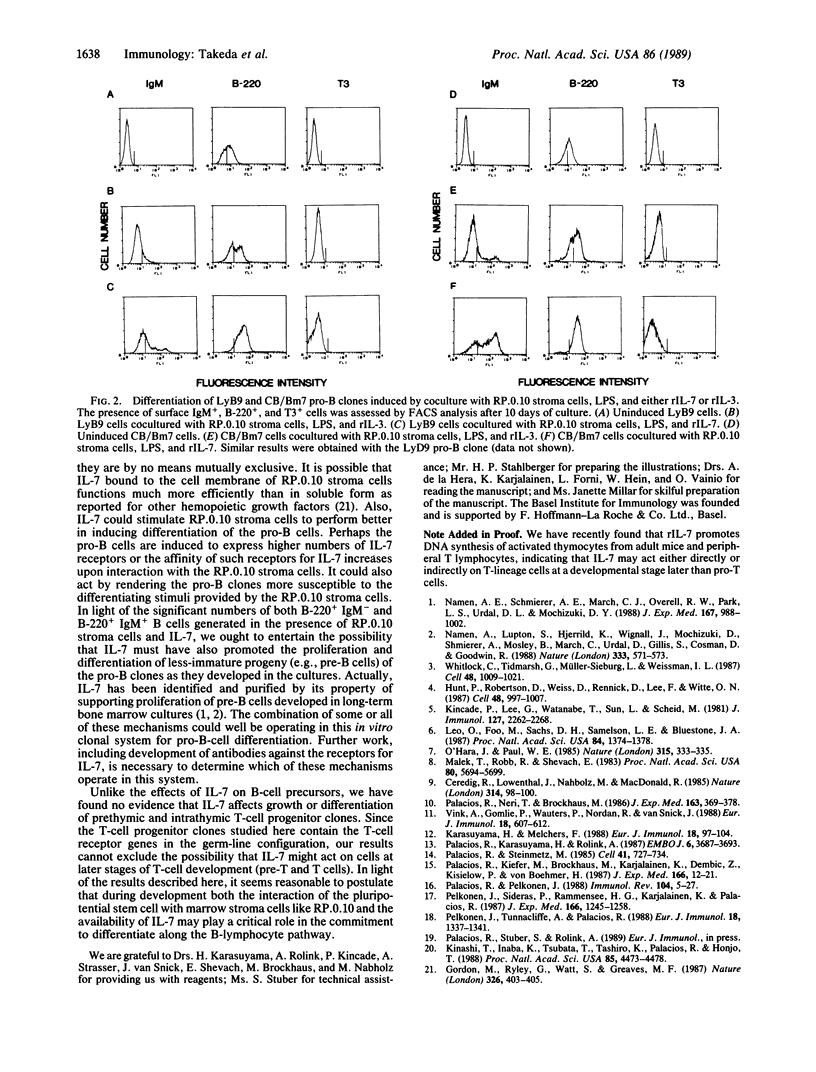
We have studied the effects of recombinant (r) interleukin 7 (IL-7) on growth and differentiation of marrow pro-B-lymphocyte clones (CB/Bm7, LyD9, LyB9), marrow pro-T-lymphocyte clones (C4-77/3, C4-86/18, C4-95/16), and fetal thymocyte clones (FTH5, FTA2, FTD5) in the presence or absence of the bone marrow stroma clone RP.0.10, which was selected for its ability to promote differentiation of the pro-B clones. rIL-7 alone stimulated some DNA synthesis (measured by [3H]thymidine uptake) but not actual growth (increase in cell number) of the pro-B clones. Antibodies against IL-4 and IL-6 or against receptors for IL-2, IL-3, and IL-5 did not inhibit this effect of rIL-7 on the pro-B clones. rIL-7 alone or in various combinations with other cytokines (from rIL-1 alpha to rIL-6) could not induce differentiation of the pro-B clones into IgM+ B cells regardless of the presence of lipopolysaccharide (LPS). The RP.0.10 marrow stroma cells by themselves do not support the growth of the pro-B clones. However, the pro-B clones grew when cultured with rIL-7 and monolayers of the RP.0.10 stroma cells. While the RP.0.10 stroma cells induced the pro-B clones to differentiate into IgM+ B cells but not T3+ T cells when cultured in the presence of LPS and rIL-3, the B-cell progenitor clones gave rise to significantly higher numbers of IgM+ B cells (up to 63%) and to many more B cells expressing higher levels of surface IgM when cocultured with rIL-7, LPS, and RP.0.10 stroma cells. The pro-B clones also generated IgM+ B cells (up to 20%) when cocultured with RP.0.10 stroma cells and rIL-7 in the absence of LPS. By using culture plates designed for testing requirements for cell-cell contact, we found that cell interactions between the pro-B cell and the marrow stroma cell are essential to induce rearrangement and expression of the immunoglobulin genes in the pro-B clones. Possible mechanisms to account for the remarkable effects of rIL-7 in the presence of RP.0.10 stroma cells on both growth and differentiation of the pro-B clones are discussed. Finally, rIL-7 alone or together with RP.0.10 stroma cells neither supported proliferation nor induced differentiation into T3+ T cells or IgM+ B cells of the marrow pro-T clones or the fetal thymocyte clones. In light of these findings, we postulate that the interaction of the pluripotential stem cell with marrow stroma cells like RP.0.10 and the availability of IL-7 could play a critical role in the commitment to develop along the B-lymphocyte pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Riley G. P., Watt S. M., Greaves M. F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. 1987 Mar 26-Apr 1Nature. 326(6111):403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kinashi T., Inaba K., Tsubata T., Tashiro K., Palacios R., Honjo T. Differentiation of an interleukin 3-dependent precursor B-cell clone into immunoglobulin-producing cells in vitro. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4473–4477. doi: 10.1073/pnas.85.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Schmierer A. E., March C. J., Overell R. W., Park L. S., Urdal D. L., Mochizuki D. Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988 Mar 1;167(3):988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Karasuyama H., Rolink A. Ly1+ PRO-B lymphocyte clones. Phenotype, growth requirements and differentiation in vitro and in vivo. EMBO J. 1987 Dec 1;6(12):3687–3693. doi: 10.1002/j.1460-2075.1987.tb02702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Kiefer M., Brockhaus M., Karjalainen K., Dembić Z., Kisielow P., von Boehmer H. Molecular, cellular, and functional properties of bone marrow T lymphocyte progenitor clones. J Exp Med. 1987 Jul 1;166(1):12–32. doi: 10.1084/jem.166.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Neri T., Brockhaus M. Monoclonal antibodies specific for interleukin 3-sensitive murine cells. J Exp Med. 1986 Feb 1;163(2):369–382. doi: 10.1084/jem.163.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Pelkonen J. Prethymic and intrathymic mouse T-cell progenitors. Growth requirements and analysis of the expression of genes encoding TCR/T3 components and other T-cell-specific molecules. Immunol Rev. 1988 Aug;104:5–27. doi: 10.1111/j.1600-065x.1988.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pelkonen J., Sideras P., Rammensee H. G., Karjalainen K., Palacios R. Thymocyte clones from 14-day mouse embryos. I. State of T cell receptor genes, surface markers, and growth requirements. J Exp Med. 1987 Nov 1;166(5):1245–1258. doi: 10.1084/jem.166.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen J., Tunnacliffe A., Palacios R. Thymocyte clones from 14-day mouse embryos. II. Transcription of T3 gamma gene may precede rearrangement of TcR delta and expression of T3 delta, T3 epsilon and T11 genes. Eur J Immunol. 1988 Sep;18(9):1337–1341. doi: 10.1002/eji.1830180906. [DOI] [PubMed] [Google Scholar]
- Vink A., Coulie P. G., Wauters P., Nordan R. P., Van Snick J. B cell growth and differentiation activity of interleukin-HP1 and related murine plasmacytoma growth factors. Synergy with interleukin 1. Eur J Immunol. 1988 Apr;18(4):607–612. doi: 10.1002/eji.1830180418. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Tidmarsh G. F., Muller-Sieburg C., Weissman I. L. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987 Mar 27;48(6):1009–1021. doi: 10.1016/0092-8674(87)90709-4. [DOI] [PubMed] [Google Scholar]



