Abstract
The loss of skeletal muscle mass during aging, sarcopenia, increases the risk for falls and dependence. Resistance exercise (RE) is an effective rehabilitation technique that can improve muscle mass and strength; however, older individuals are resistant to the stimulation of muscle protein synthesis (MPS) with traditional high-intensity RE. Recently, a novel rehabilitation exercise method, low-intensity RE, combined with blood flow restriction (BFR), has been shown to stimulate mammalian target of rapamycin complex 1 (mTORC1) signaling and MPS in young men. We hypothesized that low-intensity RE with BFR would be able to activate mTORC1 signaling and stimulate MPS in older men. We measured MPS and mTORC1-associated signaling proteins in seven older men (age 70 ± 2 yr) before and after exercise. Subjects were studied identically on two occasions: during BFR exercise [bilateral leg extension exercise at 20% of 1-repetition maximum (1-RM) with pressure cuff placed proximally on both thighs and inflated at 200 mmHg] and during exercise without the pressure cuff (Ctrl). MPS and phosphorylation of signaling proteins were determined on successive muscle biopsies by stable isotopic techniques and immunoblotting, respectively. MPS increased 56% from baseline after BFR exercise (P < 0.05), while no change was observed in the Ctrl group (P > 0.05). Downstream of mTORC1, ribosomal S6 kinase 1 (S6K1) phosphorylation and ribosomal protein S6 (rpS6) phosphorylation increased only in the BFR group after exercise (P < 0.05). We conclude that low-intensity RE in combination with BFR enhances mTORC1 signaling and MPS in older men. BFR exercise is a novel intervention that may enhance muscle rehabilitation to counteract sarcopenia.
Keywords: occlusion, S6 kinase 1, resistance exercise, aging, sarcopenia, skeletal muscle
as humans age, there is a gradual loss of skeletal muscle mass, strength, and fiber size, termed sarcopenia (2, 40). This loss of muscle and increased frailty may begin as early as middle age, but it is more pronounced in individuals over 65 years of age (20, 27). It has been estimated that between one-quarter and one-half of all individuals over age 65 are sarcopenic (6, 28), which increases the risk of disability and loss of functional capacity in the elderly (42). Given the rapidly aging population, research that pursues potential interventions to attenuate or prevent the age-related loss of skeletal muscle is increasingly important.
Resistance exercise training has been shown to be a beneficial intervention to protect against the effects of sarcopenia, with training studies showing increases in muscle protein synthesis and mass in both the old and young (24, 33, 62, 63). However, the training studies often show a more robust muscle protein synthetic and strength response in the young than in the elderly (33, 59). This may be due to an inability of older individuals to lift an amount of weight sufficient to induce hypertrophy or an inability of aging muscle to respond to resistance exercise (34, 48). The mechanisms responsible for how resistance exercise induces muscle hypertrophy are not completely understood; however, it does appear that activation of a key cell growth pathway, the mammalian target of rapamycin (mTOR) complex 1 (mTORC1), is an important regulatory mechanism of muscle hypertrophy (7, 41). More recently, we have shown (14) that mTORC1 activation is necessary for the resistance exercise-induced stimulation of muscle protein synthesis since the administration of rapamycin (a specific mTOR inhibitor) to humans before exercise prevented the contraction-induced increase in muscle protein synthesis.
On the other hand, a few studies have now shown that low-intensity [20–50% 1-repetition maximum (1-RM)] resistance exercise in combination with blood flow restriction (BFR) to the working muscles produces increases in muscle size and strength similar to those of traditional, high-intensity resistance exercise (1, 49–52). A recent study from our lab (21) reported an increase in muscle protein synthesis and the phosphorylation of p70 ribosomal S6 kinase 1 (S6K1), a downstream target of mTORC1, after a single bout of low-intensity resistance exercise with reduced muscle blood flow. With recent studies demonstrating that traditional high-intensity resistance exercise produces a smaller response or is incapable of stimulating muscle protein synthesis in older humans (34, 37, 48), the use of a novel muscle rehabilitation intervention, such as low-intensity resistance in combination with BFR, may prove beneficial in stimulating mTORC1 signaling and muscle protein synthesis and eventually in improving or maintaining muscle mass in older individuals.
Therefore, the primary aim of the present study was to determine the effect of an acute bout of low-intensity resistance exercise with BFR on mTORC1 signaling and muscle protein synthesis in older men. We hypothesized that a single bout of low-intensity resistance exercise with reduced muscle blood flow would enhance mTORC1 signaling and stimulate muscle protein synthesis in older men. However, our secondary aim was to assess a number of different regulators of translation initiation and muscle protein synthesis (e.g., MAPK signaling pathway and several stress/hypoxia-associated proteins) in an effort to better elucidate the cellular mechanisms underlying muscle growth following low-intensity resistance exercise combined with BFR.
EXPERIMENTAL PROCEDURES
Subjects
We studied seven older male subjects on two separate occasions. All subjects were healthy and physically active but were not currently engaged in an exercise training program. All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch. Screening of subjects was performed with clinical history, physical examination, stress test, and laboratory tests, including complete blood, count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test (OGTT), hepatitis B and C screening, human immunodeficiency virus (HIV) test, thyroid-stimulating hormone, lipid profile, urinalysis, drug screening, and ECG. On two separate occasions (>5 days apart) and >5 days before the study was conducted, each subject was tested for muscle strength by measuring his 1-RM on a leg extension machine (Cybex-VR2, Medway, MA) located within the Institute for Translational Sciences-Clinical Research Center (ITS-CRC) Exercise Laboratory. The higher of the two 1-RM values obtained was used to determine the weight (20% of 1-RM) for the resistance exercise portion of the study. The subjects had a mean age of 70 ± 2 yr, weight of 77 ± 4 kg, height of 1.70 ± 0.03 m, body mass index of 26.5 ± 0.6 kg/m2, body fat of 23.6 ± 1.2%, and a 1-RM of 79 ± 4 kg.
Study Design
The subjects were initially randomized to an infusion study in which they performed resistance exercise during BFR (BFR group) or a control group in which the subjects performed resistance exercise with no restriction of blood flow (Ctrl group). After a minimum of three weeks following the first visit, subjects assigned to the BFR group during infusion study 1 repeated the protocol without BFR (Ctrl). Subjects assigned to the Ctrl group during infusion study 1 then completed the blood flow-restricted exercise protocol (BFR).
Infusion study 1.
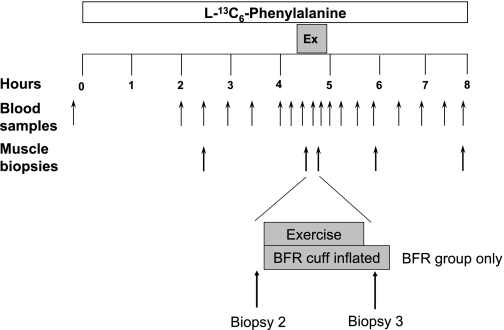
Each subject was admitted to the ITS-CRC of the University of Texas Medical Branch the day before the exercise study, and a dual-energy X-ray absorptiometry (DEXA) scan (Hologic QDR 4500W, Bedford, MA) was performed to measure body composition and lean mass. The subjects were then fed a standard dinner, and a snack was given at 2200. The subjects were studied after an overnight fast under basal conditions and refrained from exercise for 48 h before study participation. All subjects were studied during the same time of day (0600–1500). The morning of the infusion study, at 0600 an 18-gauge polyethylene catheter was inserted into an antecubital vein for tracer infusion. Another 18-gauge polyethylene catheter was inserted retrogradely in a hand vein of the opposite arm, which was kept in a heated pad for arterialized blood sampling. After a background blood sample was drawn, a primed continuous infusion of l-[ring-13C6]phenylalanine (Isotec, Sigma-Aldrich, Miamisburg, OH) was begun and maintained at a constant rate until the end of the experiment (Fig. 1). The priming dose for the labeled phenylalanine was 2 μmol/kg, and the infusion rate was 0.05 μmol·kg−1·min−1. Two and a half hours after the initiation of the tracer infusion, the first muscle biopsy was obtained from the lateral portion of the vastus lateralis of the leg, with the biopsy site between 15 and 25 cm from the midpatella. The biopsy was performed with a 5-mm Bergström biopsy needle by sterile procedure under local anesthesia (1% lidocaine). Muscle tissue was immediately blotted, frozen in liquid nitrogen, and stored at −80°C until analysis. Two hours after the first biopsy, a second biopsy was taken from the same incision. The biopsy needle was inclined at a different angle so that the second biopsy was taken ∼5 cm apart from the first. This method has been used previously by us (13, 21) and others (34). Leg circumference was measured at a point 20 cm from the midpatella before exercise, immediately after exercise, and at half-hour increments after exercise for 2 h to track changes in leg circumference. A pulse oximeter was placed on the toe of each subject so that oxygen saturation could be monitored before, during, and immediately after exercise. During this baseline period, cell signaling data were obtained from the first biopsy and the rate of muscle protein synthesis was calculated as the rate of incorporation of the tracer between the two baseline biopsies (see Muscle Fractional Synthetic Rate below).
Fig. 1.
Study design. Blood and muscle samples were taken at the times indicated by the arrows. Ex, exercise. Exercise was performed immediately after biopsy 2. Biopsy 3 was taken immediately after exercise with the blood flow restriction (BFR) cuff still inflated.
BFR group.
After the second biopsy was obtained, a lower extremity pressure cuff (Kaatsu-Master Mini, Sato Sports Plaza, Tokyo, Japan) was placed around the most proximal portion of each leg. While the subject was seated on a chair, the pressure cuff was increased to 120 mmHg for 30 s, and the air pressure was released. The pressure cuff was then inflated four more times, with each period being increased by 20 mmHg. Each period lasted 30 s, and then the cuff was released for 10 s between periods until a final pressure of 200 mmHg was reached. With the pressure maintained at 200 mmHg, the subjects then performed a set of 30 repetitions of bilateral leg extension exercise at 20% of 1-RM, followed by a 30-s rest period. Subjects subsequently performed three more sets of 15 repetitions with 30-s rest intervals for a total of four sets and 75 repetitions. Immediately after the fourth set, a third muscle biopsy was taken, and then the pressure was released from the cuff. Total time for the exercise period was ∼4–5 min. Blood was obtained at selected intervals over the next 3 h, and muscle biopsies were obtained at 1 and 3 h after exercise.
Resistance exercise without blood flow restriction (Ctrl group).
The subjects in the Ctrl group performed an exercise protocol identical to that in the BFR group except that the cuff was not inflated and no pressure was applied to the legs. Blood and muscle biopsy postexercise collection times were identical to those described for the BFR group.
Infusion study 2.
Three weeks after the first visit, subjects assigned to the BFR group during infusion study 1 repeated the protocol without BFR (Ctrl). Subjects assigned to the Ctrl group during infusion study 1 then completed the blood flow-restricted exercise protocol (BFR). Subjects were initially randomized to either the Ctrl or the BFR group, and therefore seven subjects were studied for both groups.
D-Dimer Blood Test
Blood was also sampled before exercise and at 15 min after exercise to measure D-dimer, a fibrin degradation product. D-dimer concentration can be used to diagnose thrombosis. A negative result can almost rule out thrombosis, and its use in this study was to exclude thrombosis in the present protocol after release of the blood flow restriction cuff. D-dimer concentrations in the blood were analyzed with a latex agglutination assay according to the manufacturer's instructions (Fisher Diagnostics, Middletown, VA).
SDS PAGE and Western Blot Analysis
Details of the immunoblotting procedures have been published previously (12). Briefly, ∼30–50 mg of frozen tissue was homogenized (1:9 wt/vol) and centrifuged for 10 min at 4°C, followed by the removal of the supernatant. Biopsy 1 was used as the baseline measure for all subjects. Total protein concentrations were determined by using the Bradford assay (Bio-Rad, Hercules, CA, Smartspec Plus spectrophotometer). The supernatant was diluted (1:1) in a sample buffer mixture containing 125 mM Tris (pH 6.8), 25% glycerol, 2.5% SDS, 2.5% β-mercaptoethanol, and 0.002% bromphenol blue and then boiled for 3 min at 100°C. Fifty micrograms of total protein was loaded into each lane, and the samples were separated by electrophoresis (150 V for 60 min) on a 7.5% or 15% polyacrylamide gel (Bio-Rad, Criterion). A molecular weight ladder (Bio-Rad, Precision Plus protein standard) was also included on each gel, as was a normalization control. After electrophoresis, protein was transferred to a polyvinylidene difluoride membrane (Bio-Rad) at 50 V for 60 min. Blots were incubated in a single primary antibody overnight at 4°C (antibody concentrations are described below). The next morning, blots were incubated in secondary antibody for 1 h at room temperature. Chemiluminescent solution (ECL plus, Amersham BioSciences, Piscataway, NJ) was applied to each blot. After a 5-min incubation, optical density measurements were obtained with a phosphoimager (Bio-Rad) and densitometric analysis was performed with Quantity One software (Bio-Rad, version 4.5.2). Data are expressed as the fold change from baseline in phosphorylation divided by total protein content (in arbitrary units) normalized to a rodent standard in Figs. 4 and 5. Biopsy 1 serves as the baseline value for all proteins measured. In Tables 2 and 3, data are expressed as either phosphorylation divided by total protein content or total protein content (in arbitrary units) normalized to a rodent standard, as indicated.
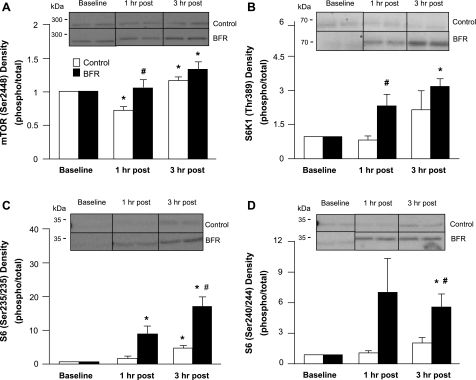
Fig. 4.
Mammalian target of rapamycin (mTOR), S6 kinase 1 (S6K1), and S6. Data represent phosphorylation/total fold change of mTOR at Ser2448 (A), S6K1 at Thr389 (B), S6 at Ser235/236 (C), and S6 at Ser240/244 (D) at baseline and 1 and 3 h after exercise. Representative immunoblot images are shown. *Significantly different from baseline (P < 0.05); #significantly different from Ctrl subjects at corresponding time point (P < 0.05).
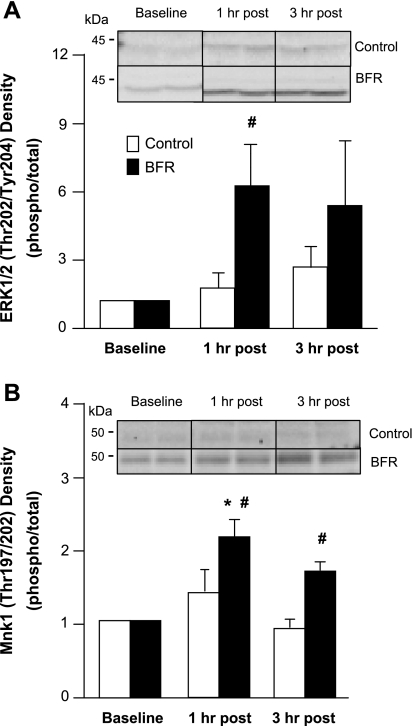
Fig. 5.
Extracellular signal-regulated kinase 1/2 (ERK1/2) and mitogen-activated protein kinase-interacting kinase 1 (Mnk1). Data represent phosphorylation/total fold change of ERK1/2 at Thr202/Tyr204 (A) and Mnk1 at Thr197/202 (B) at baseline and 1 and 3 h after exercise. Representative immunoblot images are shown. *Significantly different from baseline (P < 0.05); #significantly different from Ctrl subjects at corresponding time point (P < 0.05).
Table 2.
Protein expression and phosphorylation immediately after exercise and before release of pressure cuff
| mTOR (Ser2448) | S6K1 (Thr389) | 4E-BP1 (Thr37/46) | eEF2 (Thr56) | rpS6 (Ser235/236) | rpS6 (Ser240/244) | AMPKα (Thr172) | eIF2Bε (Ser539) | FAK (Tyr576/577) | ERK1/2 (Thr202/Tyr204) | Mnk1 (Thr197/202) | HIF-1α | REDD1 | HSP70 | IL-6 | Akt (Thr308) | FOXO3a (Ser253) | IGF-I | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl | 0.84 ± 0.16 | 0.49 ± 0.21 | 0.47 ± 0.04* | 1.94 ± 0.44 | 0.44 ± 0.13* | 0.64 ± 0.35 | 0.45 ± 0.16 | 0.12 ± 0.06 | 0.97 ± 0.09 | 9.36 ± 3.87 | 0.12 ± 0.02* | 1.30 ± 0.30 | 4.43 ± 0.44 | 0.60 ± 0.08 | 1.04 ± 0.22 | 2.57 ± 0.89 | 0.53 ± 0.07 | 0.91 ± 0.10 |
| BFR | 0.52 ± 0.09 | 0.31 ± 0.10* | 0.63 ± 0.07 | 1.22 ± 0.08 | 0.89 ± 0.76 | 0.53 ± 0.27 | 0.36 ± 0.05 | 0.08 ± 0.02 | 1.27 ± 0.18 | 4.49 ± 1.41 | 0.06 ± 0.01† | 1.19 ± 0.28 | 3.27 ± 0.36 | 0.78 ± 0.08 | 0.75 ± 0.07 | 2.43 ± 0.42 | 0.55 ± 0.06 | 0.92 ± 0.08 |
Values are means ± SE expressed in arbitrary units. mTOR, mammalian target of rapamycin; S6K1, S6 kinase 1; 4E-BP1, eukaryotic initiation factor 4E binding protein 1; eEF2, eukaryotic elongation factor 2; rpS6, ribosomal protein S6; AMPKα, AMP-activated protein kinase α; eIF2Bε, eukaryotic initiation factor 2Bε; FAK, focal adhesion kinase; ERK1/2, extracellular signal-regulated kinase 1/2; Mnk1, mitogen-activated protein kinase-interacting kinase 1; HIF-1α, hypoxia-inducible factor 1α; REDD1, regulated in development and DNA damage responses 1; HSP70, heat shock protein 70; IL-6, interleukin-6; FOXO3a, Forkhead Box O3a; IGF-I, insulin-like growth factor I. Proteins with an indicated phosphorylation site are measured as a phospho-to-total ratio. Proteins without a regulatory phosphorylation site are measured as total protein alone.
Significantly different from baseline (P < 0.05);
significantly different from Ctrl at same time point (P < 0.05).
Table 3.
Protein expression and phosphorylation during postexercise recovery
| Baseline | 1 h Post | 3 h Post | |
|---|---|---|---|
| Translation initiation/elongation | |||
| eEF2 (Thr56) | |||
| Ctrl | 1.89 ± 0.48 | 1.59 ± 0.17 | 1.78 ± 0.09 |
| BFR | 1.36 ± 0.14 | 1.27 ± 0.11 | 1.43 ± 0.16 |
| 4E-BP1 (Thr37/46) | |||
| Ctrl | 0.59 ± 0.06 | 0.45 ± 0.02 | 0.46 ± 0.05 |
| BFR | 0.61 ± 0.08 | 0.53 ± 0.04 | 0.54 ± 0.04 |
| eIF2Bε (Ser539) | |||
| Ctrl | 0.08 ± 0.05 | 0.05 ± 0.02 | 0.07 ± 0.02 |
| BFR | 0.08 ± 0.02 | 0.08 ± 0.01 | 0.07 ± 0.01 |
| Upstream mTOR regulators | |||
| AMPKα (Thr172) | |||
| Ctrl | 0.36 ± 0.09 | 0.31 ± 0.08 | 0.30 ± 0.06 |
| BFR | 0.30 ± 0.02 | 0.31 ± 0.04 | 0.37 ± 0.07 |
| HIF-1α | |||
| Ctrl | 1.39 ± 0.30 | 1.69 ± 0.38 | 1.37 ± 0.37 |
| BFR | 1.41 ± 0.33 | 1.30 ± 0.27 | 1.37 ± 0.37 |
| REDD1 | |||
| Ctrl | 2.92 ± 0.72 | 3.47 ± 0.85 | 3.21 ± 1.06 |
| BFR | 3.12 ± 0.44 | 4.26 ± 0.89 | 3.74 ± 0.87 |
| Stress proteins | |||
| HSP70 | |||
| Ctrl | 0.78 ± 0.13 | 1.02 ± 0.06 | 0.82 ± 0.11 |
| BFR | 0.85 ± 0.12 | 0.87 ± 0.09 | 0.78 ± 0.07 |
| IL-6 | |||
| Ctrl | 0.98 ± 0.17 | 1.14 ± 0.07 | 0.87 ± 0.10 |
| BFR | 0.80 ± 0.14 | 0.87 ± 0.12 | 0.66 ± 0.06 |
| FAK (Tyr576/577) | |||
| Ctrl | 0.89 ± 0.07 | 0.95 ± 0.09 | 1.06 ± 0.12 |
| BFR | 1.30 ± 0.23 | 1.23 ± 0.23 | 1.17 ± 0.17 |
| Insulin signaling | |||
| Akt (Thr308) | |||
| Ctrl | 1.94 ± 0.29 | 2.14 ± 0.36 | 2.61 ± 0.42 |
| BFR | 1.93 ± 0.33 | 2.75 ± 0.57 | 3.32 ± 0.87* |
| FOXO3a (Ser253) | |||
| Ctrl | 0.54 ± 0.04 | 0.61 ± 0.08 | 0.53 ± 0.07 |
| BFR | 0.61 ± 0.04 | 0.59 ± 0.08 | 0.52 ± 0.06 |
| IGF-I | |||
| Ctrl | 1.00 ± 0.09 | 0.89 ± 0.09 | 0.87 ± 0.08* |
| BFR | 0.90 ± 0.07 | 0.86 ± 0.08 | 0.85 ± 0.07 |
Values are means ± SE expressed in arbitrary units. Proteins with an indicated phosphorylation site are reported as a phospho-to-total ratio. Proteins without a regulatory phosphorylation site are reported as total protein content.
Significantly different from baseline (P < 0.05).
Antibodies
The following primary antibodies used were purchased from Cell Signaling (Beverly, MA): phospho-mTOR (Ser2448), phospho-p70 S6K1 (Thr389), phospho-ribosomal protein S6 (rpS6) (Ser235/236), phospho-rpS6 (Ser240/244), phospho-eukaryotic initiation factor 4E binding protein 1 (4E-BP1) (Thr37/46), phospho-AMP-activated protein kinase α (AMPKα) (Thr172), phospho-mitogen-activated protein kinase-interacting kinase 1 (Mnk1) (Thr197/202), phospho-extracellular signal-regulated kinase 1/2 (ERK1/2) (Thr202/Tyr204), phospho-eukaryotic elongation factor 2 (eEF2) (Thr56), phospho-focal adhesion kinase (FAK) (Tyr576/577), phospho-Akt (protein kinase B) (Thr308), phospho-Forkhead Box O3a (FOXO3a) (Ser253), total mTOR, total p70 S6K1, total eEF2, total rpS6, total 4E-BP1, total AMPKα, total Mnk1, total ERK1/2, total hypoxia-inducible factor α (HIF-1α), total eukaryotic initiation factor 2Bε (eIF2Bε), total interleukin (IL)-6, total heat shock protein 70 (HSP70), total Akt, total FOXO3a, and total FAK. Total insulin-like growth factor I (IGF-I) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), phospho-eIF2Bε (Ser539) was purchased from GeneTex (San Antonio, TX), and total regulated in development and DNA damage responses 1 (REDD1) protein was purchased from ProteinTech Group (Chicago, IL). All antibodies were used in a dilution of 1:1,000 except for phospho-S6K1 (1:500). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (1:2,000) was purchased from Amersham BioSciences.
Gene Expression
Details of RNA isolation, cDNA synthesis and real-time quantitative PCR (qPCR) have been described elsewhere (16). Briefly, RNA concentration and integrity were determined with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The 28S-to-18S ratio was 1.24 ± 0.02, and the RNA integrity number (RIN) was 8.05 ± 0.09, which we considered acceptable (1–10 scale: 10 best). Human REDD1, REDD2, and GAPDH primer sets have been published previously (16). The mean cycle threshold (Ct) from each sample (run in duplicate) was normalized to the internal control, GAPDH, after which relative fold changes were determined as described by Livak and Schmittgen (36). The data are expressed as percent change from baseline.
Hormones and Glucose/Lactate
Serum concentrations of growth hormone (GH) and cortisol were determined (Calbiotech, Spring Valley, CA) via ELISA at select time points according to the manufacturer's instructions. Plasma glucose and lactate concentrations were measured with an automated glucose and lactate analyzer (YSI, Yellow Springs, OH). Four blood draws were taken before exercise, and these samples were averaged and reported as the baseline values. All subsequent blood draws were analyzed and reported individually.
Muscle Fractional Synthetic Rate
Muscle intracellular free amino acids and muscle proteins were extracted as previously described (57, 58). Muscle intracellular free concentration and enrichment of phenylalanine was determined by gas chromatography-mass spectrometry (GCMS; 6890 Plus GC, 5973N MSD, 7683 autosampler, Agilent Technologies, Palo Alto, CA) using an appropriate internal standard (l-[15N]phenylalanine) (57, 58). Mixed muscle protein-bound phenylalanine enrichment was analyzed by GCMS after protein hydrolysis and amino acid extraction (57, 58) with the external standard curve approach (11). We calculated the fractional synthetic rate of mixed muscle proteins (FSR) by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEp/t) and using the precursor-product model to calculate the synthesis rate: FSR = (ΔEp/t)/[(EM1 + EM2)/2]·60·100, where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, and EM1 and EM2 are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies; data are expressed as percentage per hour. Baseline FSR is the increment in protein-bound phenylalanine enrichment from biopsy 1 to biopsy 2, while the postexercise FSR is the increment in protein-bound phenylalanine enrichment from biopsy 3 to biopsy 5, encompassing the 3-h postexercise period.
Statistical Analysis
All values are expressed as means ± SE. Comparisons were performed by ANOVA with repeated measures, the effects being group (BFR, Ctrl) and time. Post hoc testing was performed with Bonferroni when appropriate. Significance was set at P ≤ 0.05. All analyses were done with SigmaStat 11.0 (Systat Software, San Jose, CA).
RESULTS
Serum Hormones and Metabolites
There were no significant differences in plasma glucose or lactate between groups at baseline (Table 1). Plasma lactate increased significantly (P < 0.05, Table 1) in both groups during the exercise bout (P < 0.05), remained elevated in the BFR group for 45 min after exercise, and was also higher compared with Ctrl for 30 min after exercise. Plasma lactate values returned to baseline in the Ctrl group after exercise. Plasma glucose increased significantly after exercise for 45 min in the BFR group compared with baseline and was higher than the Ctrl group (P < 0.05; Table 1), which did not change after exercise.
Table 1.
Plasma lactate and glucose measurements before and after resistance exercise
| Postexercise, Minutes |
||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Exercise | 15 | 30 | 45 | 60 | 120 | 180 | |
| Lactate, mmol/l | ||||||||
| BFR | 1.2 ± 0.1 | 2.7 ± 0.6* | 3.3 ± 0.4*† | 2.4 ± 0.4*† | 1.8 ± 0.3* | 1.7 ± 0.2 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| Ctrl | 1.3 ± 0.2 | 2.9 ± 0.6* | 1.7 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Glucose, mmol/l | ||||||||
| BFR | 5.3 ± 3.2 | 5.3 ± 3.2 | 6.0 ± 3.6† | 5.7 ± 3.9† | 5.6 ± 3.7† | 5.5 ± 3.2 | 5.2 ± 2.3 | 5.2 ± 2.4 |
| Ctrl | 5.3 ± 3.8 | 5.3 ± 3.0 | 4.9 ± 5.5 | 4.8 ± 5.9 | 5.1 ± 2.2 | 5.0 ± 2.2 | 5.0 ± 2.2 | 4.9 ± 1.9 |
Values are means ± SE. BFR, resistance exercise with blood flow restriction; Ctrl, resistance exercise without blood flow restriction.
Significantly different from baseline (P < 0.05);
significantly different from Ctrl at same time point (P < 0.05).
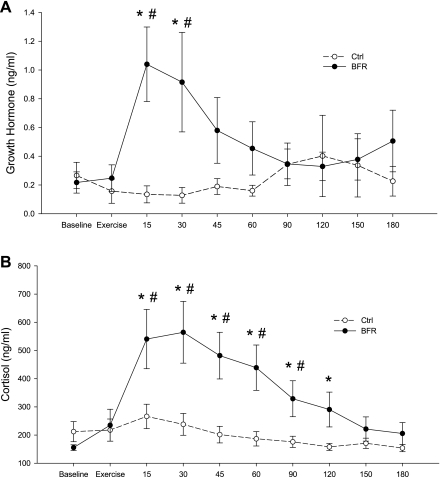
Cortisol concentrations were elevated only in the BFR group for 2 h after exercise from baseline values (P < 0.05; Fig. 2) and then returned to near-resting values (P > 0.05). After exercise, cortisol values were significantly higher than those in the Ctrl group during the first 90 min after exercise in the BFR group (P < 0.05). Serum cortisol concentrations did not change during or after exercise in the Ctrl group (P > 0.05).
Fig. 2.
Peripheral vein serum hormone concentrations. Samples were obtained from BFR and control (Ctrl; no BFR) subjects before and during exercise and for 3 h after exercise. A: serum growth hormone (ng/ml). B: serum cortisol (ng/ml). *Significantly different from baseline (P < 0.05); #significantly different from Ctrl subjects at corresponding time point (P < 0.05).
Serum GH concentrations increased significantly 15 min after exercise and remained elevated for 30 min after exercise in the BFR group compared with baseline (P < 0.05; Fig. 2). Serum GH concentration in the BFR group was also higher than the Ctrl group for 30 min after exercise (P < 0.05; Fig. 2). GH concentration did not change after exercise in the Ctrl group (P > 0.05).
D-Dimer Test for Thrombosis
Blood samples were taken from subjects during the basal period and immediately after exercise to test for D-dimer, a fibrin degradation product, to test for potential thrombosis in subjects due to the application of the pressure cuff during exercise in the BFR group. Subjects in the BFR group had a mean D-dimer value of 0.37 ± 0.09 μg/ml before exercise and a value of 0.39 ± 0.09 μg/ml immediately after exercise. There was no significant difference between values obtained before exercise and those taken after exercise (P > 0.05).
Leg Circumference
Leg circumference increased by an average of 2.5 ± 0.6 cm immediately after exercise and remained elevated for 30 min after exercise in the BFR group (P < 0.05). Similarly, leg circumference increased 1.3 ± 0.3 cm immediately after exercise in the Ctrl group but was significantly lower than that in the BFR group (P < 0.05).
Oxygen Saturation
Oxygen saturation, as measured by a pulse oximeter on the large toe of each subject, was 98.1 ± 0.9% in the Ctrl group and 96.6 ± 1.8% in the BFR group at baseline, with no difference between groups (P > 0.05). During exercise, oxygen saturation significantly dropped to 91.1 ± 1.0% in the Ctrl group (P < 0.05). Oxygen saturation also decreased to 74.5 ± 4.8% during exercise in the BFR group, which was significantly decreased from baseline and lower than the Ctrl group (P < 0.05). Immediately after the completion of exercise, the oxygen saturation levels in both groups returned to baseline values.
Muscle Protein Synthesis
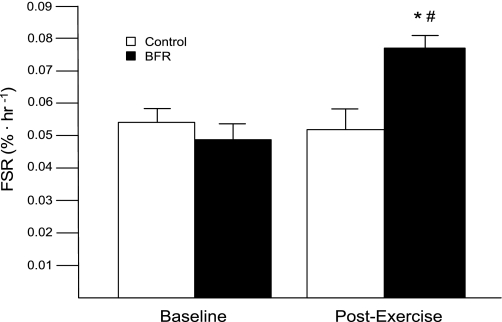
Mixed muscle protein FSR was significantly increased at 3 h after exercise in the BFR group compared with baseline and was higher than that in the Ctrl group (P < 0.05; Fig. 3). There was no increase in FSR after exercise in the Ctrl group.
Fig. 3.
Mixed muscle fractional synthetic rate (FSR): muscle protein synthesis as expressed by the mixed muscle FSR in BFR and Ctrl subjects before exercise and during 3 h of postexercise recovery. *Significantly different from baseline (P < 0.05); #significantly different from Ctrl (P < 0.05).
Protein Expression Immediately After Exercise
The muscle biopsy taken immediately after exercise was obtained before release of the pressure cuff in order to assess what effect the occlusion of blood flow during exercise may be having on selected cellular growth and stress proteins. Therefore, as shown in Table 2, we compared protein expression/phosphorylation between groups at this individual time point. There was no difference in the phosphorylation of mTOR (Ser2448), Akt (Thr308), FOXO3a (Ser253), eEF2 (Thr56), AMPKα (Thr172), ERK1/2 (Thr202/Tyr204), rpS6 (Ser240/244), eIF2Bε (Ser539), or FAK (Tyr576/577) between groups or from baseline (P > 0.05; Table 2) immediately after exercise. There also was no difference in the total protein content of IGF-I, REDD1, HIF-1α, HSP70, or IL-6 (P > 0.05; Table 2). Phosphorylation of 4E-BP1 (Thr37/46) decreased from baseline in the Ctrl group (P < 0.05). Phosphorylation of S6K1 (Thr389) increased from baseline in the BFR group (P < 0.05; Table 2). The phosphorylation of Mnk1 (Thr197/202) and rpS6 (Ser235/236) was increased from baseline in the Ctrl group (P < 0.05; Table 2), and the phosphorylation of Mnk1 was also significantly higher than in the BFR group (P < 0.05; Table 2).
mTORC1 Signaling During Postexercise Recovery
Phosphorylation of mTOR at Ser2448 was higher at 1 h after exercise in the BFR group compared with the Ctrl group (P < 0.05; Fig. 4A). Phosphorylation remained significantly elevated from baseline at 3 h after exercise in the BFR group (P < 0.05; Fig. 4A). The Ctrl group showed an increase in mTOR phosphorylation at 3 h after exercise (P < 0.05; Fig. 4A).
Phosphorylation of S6K1 at Thr389 was significantly increased at 1 and 3 h after exercise in the BFR group (P < 0.05; Fig. 4B). S6K1 phosphorylation status was unchanged after exercise in the Ctrl group.
Phosphorylation of rpS6 at Ser235/236 increased ∼10- and 18-fold from baseline at 1 and 3 h after exercise, respectively, in the BFR group (P < 0.05; Fig. 4C). S6K1 phosphorylation at Ser235/236 was increased from baseline at 3 h after exercise in the Ctrl group but was significantly lower than the BFR group (P < 0.05; Fig. 4C). Phosphorylation of rpS6 at Ser240/244 increased significantly from baseline at 3 h after exercise in the BFR group (P < 0.05; Fig. 4D) and was also higher than the Ctrl group (P < 0.05; Fig. 4D). There were no statistical differences in S6 phosphorylation at Ser240/244 at any time point in the Ctrl group (P > 0.05; Fig. 4D).
There was no difference in the phosphorylation of 4E-BP1 at Thr37/46 or eEF2 at Thr56 in either group at any time point (P > 0.05; Table 3). The phosphorylation of eIF2Bε at Ser539 also did not change in either group (P > 0.05; Table 3).
Upstream Regulators of mTORC1 During Postexercise Recovery
REDD1 gene expression decreased 46% from baseline in the Ctrl group and 41% from baseline in the BFR group at 3 h after exercise (P < 0.05), while REDD2 gene expression decreased 34% from baseline in the Ctrl group and 42% from baseline in the BFR group 3 h after exercise (P < 0.05). However, there was no difference in REDD1 total protein content at baseline or after exercise in either the BFR or Ctrl group, nor were there any group differences (P > 0.05; Table 3). HIF-1α total protein did not change with time or between groups (P > 0.05; Table 3). The phosphorylation of AMPKα at Thr172 (a measure of both α1 and α2 catalytic subunit phosphorylation status) was not different at any time point in either group (P > 0.05; Table 3).
Stress-Associated Proteins During Postexercise Recovery
There was no difference in HSP70 total protein content at baseline or after exercise in either the BFR or Ctrl group (P > 0.05; Table 3). IL-6 total protein did not change with time or between groups (P > 0.05; Table 3). The phosphorylation of FAK at Tyr576/577 was not different at exercise or 1 or 3 h after exercise in either group (P > 0.05; Table 3).
Insulin Signaling During Postexercise Recovery
There was no difference over time in the phosphorylation of Akt at Thr308 in the Ctrl group after exercise (P > 0.05; Table 3), but at 3 h after exercise there was an increase in Akt phosphorylation in the BFR group compared with baseline (P < 0.05; Table 3). There was no difference in the phosphorylation of FOXO3a (Ser253) at baseline or after exercise in either the BFR or the Ctrl group (P > 0.05; Table 3). IGF-I total protein did not change with time in the BFR group (P > 0.05; Table 3), but at 3 h after exercise there was a decrease in total protein content of IGF-I in the Ctrl group only (P < 0.05; Table 3).
MAPK Signaling During Postexercise Recovery
ERK1/2 phosphorylation at Thr202/Tyr204 increased fourfold at 1 h after exercise in the BFR group and was statistically higher than the Ctrl group at this point (P < 0.05; Fig. 5A). There was no difference in ERK1/2 phosphorylation at any time point in the Ctrl group (P > 0.05; Fig. 5A). Mnk1 phosphorylation at Thr197/202 was increased at 1 h after exercise in the BFR group compared with baseline and was higher than that in the Ctrl group (P < 0.05; Fig. 5B). Mnk1 was also elevated at 3 h after exercise in the BFR group compared with the Ctrl group (P < 0.05; Fig. 5B). The phosphorylation of Mnk1 did not change in the Ctrl group (P > 0.05; Fig. 5B).
DISCUSSION
The primary and novel finding from our study was that skeletal muscle protein synthesis was stimulated after an acute bout of low-intensity (20% 1-RM) resistance exercise in combination with BFR in older men. Specifically, muscle protein synthesis increased by 56% (P < 0.05) in the BFR group 3 h after performance of a bout of low-intensity resistance exercise with BFR, while muscle protein synthesis did not increase in the Ctrl group, who exercised without BFR. In the BFR group, there was also a significant increase in the phosphorylation of S6K1 and rpS6, suggesting enhanced mTORC1 signaling following exercise with BFR. The enhanced mTORC1 signaling is indicative of improved translation initiation, which likely explains the increase in muscle protein synthesis with BFR. Our recent report (14) that the contraction-induced increase in muscle protein synthesis is dependent on the activation of mTORC1 in human muscle would support the findings from the present study that mTORC1 activation with BFR exercise is playing a key role in stimulating muscle protein synthesis and likely muscle hypertrophy over time. The activation of protein synthesis may also have been due to other regulators of translation initiation, as we report concurrent activation of mTORC1 and MAPK signaling pathways with BFR exercise.
It is well established that high-intensity resistance exercise is a potent stimulus for muscle protein synthesis and hypertrophy (44, 48, 62, 63), and we have shown (12) that a single bout of resistance exercise at an intensity of 70% 1-RM increased muscle protein synthesis in addition to increasing phosphorylation of mTOR and S6K1 during postexercise recovery. An acute bout of leg resistance exercise at 20% of 1-RM does not stimulate muscle protein synthesis in young healthy men (21), and it is clear that high-intensity (rather than low intensity) resistance exercise is effective at increasing muscle hypertrophy with training, as confirmed by a direct correlation between the phosphorylation of S6K1 in the first few hours following an acute bout of high-intensity resistance exercise and the percent change in muscle mass following several weeks of high-intensity resistance exercise training in rodents (5) and humans (53). Several studies have shown that the gradual activation of mTORC1 and its downstream effector S6K1 in the recovery phase following high-intensity resistance exercise (9, 12, 32) is associated with increases in protein synthesis (5, 7, 26, 46).
Interestingly, a few studies have now shown that low-intensity (20–50% 1-RM) resistance exercise in combination with a restriction in blood flow to the working muscles produces increases in muscle size and strength similar to those from traditional, high-intensity resistance exercise (1, 49–52). We recently demonstrated (21) that S6K1 phosphorylation and muscle protein synthesis were increased in young men after a bout of low-intensity (20% 1-RM) resistance exercise with BFR. The present study used an identical study design and found a similar increase in S6K1 phosphorylation and muscle protein synthesis in the older men compared with the younger men in our previous study (21). Therefore, the increase in mTORC1 signaling (e.g., increased phosphorylation of S6K1 and its downstream target S6) following BFR exercise strongly suggests that improved translation initiation and ribosomal biogenesis is responsible for the increase in the rate of muscle protein synthesis we observed.
Although mTORC1 signaling appears to be an important regulator of translation initiation, other pathways are likely involved (e.g., MAPK pathway). For example, ERK1/2 can phosphorylate Mnk1, which in turn can activate the eIF4E translation initiation factor (19, 60, 61). Two recent studies have shown age-related differences at baseline and after exercise in the MAPK-associated proteins (60, 61). In agreement with those studies, we previously showed (13) that the phosphorylation of ERK1/2 and Mnk1 is blunted in the elderly after a bout of high-intensity resistance exercise and essential amino acid ingestion. In the present study, ERK1/2 and Mnk1 phosphorylation increased in the BFR group, which indicates that a concurrent activation of both the mTORC1 and MAPK signaling pathways is likely needed to induce a maximal muscle protein synthetic response after resistance exercise and that BFR exercise is capable of activating both pathways in older human skeletal muscle.
It has been suggested that increases in anabolic hormones such as GH following resistance exercise are necessary for muscle growth (22). In response to an acute bout of high-intensity resistance exercise and/or low-intensity resistance exercise with BFR, circulating GH concentration increases (13, 21, 52). In our study, peak GH concentrations after exercise increased ninefold in the BFR group compared with the Ctrl group; however, we cannot determine from our study design whether the GH response following BFR exercise played a role in activating mTORC1 signaling. We suspect that this is not the case, since muscle hypertrophy following resistance exercise can occur in the absence of large changes in anabolic hormones (59a). On the other hand, the increase in cortisol in the present study likely indicates that BFR exercise produces a similar stress response (compared with traditional high-intensity exercise) in the contracting muscle.
Other mechanisms such as energetic and/or hypoxic stress may also play a role in regulating the muscle growth response following BFR exercise. This was based on data showing that AMPK is activated during energetic stress and can inhibit mTOR via phosphorylation, and activation, of tuberous sclerosis complex 2 (TSC2) (8, 29). For instance, our lab (12) recently showed that “during” a bout of high-intensity resistance exercise, AMPK activity increased and muscle protein synthesis decreased, and AMPK may play a role in regulating the different muscle protein synthesis response between young and old rodents following overload-induced hypertrophy (55). Furthermore, mTOR is regulated by upstream factors HIF-1α and REDD1 (3, 35). Increased expression of HIF-1α and REDD1 during hypoxia inhibits the mTOR pathway (30, 31). However, we found no changes in selected markers of energetic (AMPK) or hypoxic (HIF-1α, REDD1/2) stress, which suggests that energetic or hypoxic stress was not occurring to a significant extent during BFR exercise.
Muscle protein accretion with chronic BFR exercise may also be influenced by changes in protein breakdown. Akt, in its active form, can phosphorylate members of the FOXO transcription factors, which then modulates their cellular localization to the cytosol, where they cannot act to alter gene expression (10, 47). FOXO3a can alter the expression of genes controlling numerous cellular processes, including the ubiquitin proteasome pathway via Atrogin-1 (10, 47). In the present study, we observed no influence of BFR exercise on the phosphorylation of Akt or FOXO3a, which is consistent with our previous study in young subjects showing no change in Atrogin-1 gene expression after BFR exercise (15). Therefore, it does not appear that BFR exercise alters the Akt-FOXO3a-ubiquitin proteasome pathway and that an increase in translation initiation may be the primary target of BFR exercise (rather than modulating proteolysis) during early postexercise recovery.
Resistance exercise has also been shown to increase the expression of several heat shock proteins, including HSP70 (54) which may protect against the damage induced by a second bout of exercise (38). Heat shock protein response to exercise is also attenuated in older individuals (56). The anti-inflammatory effect of exercise is also modulated through the release of IL-6 by the muscle (25, 43). The infusion of IL-6 into human skeletal muscle results in a rapid and sustained increase in heat shock protein mRNA (18). In our present study, we did not observe any increase in either HSP70 or IL-6 protein expression after BFR exercise. This may be due to the low intensity of the weight lifted, the relatively short duration of the exercise protocol (∼5 min), or the fact that both the heat shock protein and anti-inflammatory responses to exercise are blunted with aging (23, 56).
Another potential cellular mechanism that may explain the increase in muscle protein synthesis following BFR exercise is cell swelling-induced activation of mTORC1 signaling. In our study we found that leg circumference was increased to a much larger extent in the BFR group. Obviously, leg circumference is not a direct measure of cell swelling; however, it should be noted that cell swelling is an anabolic proliferative signal in which proteins associated with osmosensing are activated (i.e., FAK and ERK1/2) (4, 39). We did find a greater phosphorylation of ERK1/2 in the BFR subjects after exercise but found no difference in FAK phosphorylation, which may have increased before the time at which we collected the biopsy. However, the present study design prevented us from verifying this or verifying to what extent cell swelling may influence the postexercise response. Finally, another possible mechanism for mTORC1 activation may have been an increase in reactive hyperemia and hence nutritive flow to the muscle after the release of the pressure cuff. Future studies need to explore this possibility, because an increase in blood flow and amino acid availability can directly stimulate mTORC1 and muscle protein synthesis (45).
The BFR exercise in our study was well tolerated by all participants. Subjects completed the BFR exercise with verbal encouragement and were asked to report their rate of perceived exertion (RPE) on a 0–10 scale. Most reported a score of 7–8 during the end of the exercise protocol, and the discomfort observed was comparable to that experienced during high-intensity resistance exercise. Subjects were monitored for risk of potential deep vein thrombosis with D-dimer tests, none of which returned with a positive result. Subjects did not report any side effects immediately after exercise or 1 wk after the infusion protocol.
In summary, we have shown that an acute bout of low-intensity resistance exercise combined with BFR stimulates muscle protein synthesis and enhances the phosphorylation of proteins in both the mTORC1 and MAPK signaling pathways. Neither signaling pathway or muscle protein synthesis changed in the low-intensity exercise group without occlusion of blood flow. Consequently, we conclude that the concurrent activation of the mTORC1 and MAPK signaling pathways appears to be an important cellular mechanism responsible for the enhanced muscle protein synthesis during low-intensity resistance exercise with BFR. Therefore, BFR exercise is a well-tolerated and novel intervention that may enhance muscle rehabilitation to counteract sarcopenia.
GRANTS
This study was supported by National Institutes of Health Grants AR-049877 (to B. B. Rasmussen), P30-AG-024832, and T32-HD-07539. This study was conducted at the Institute for Translational Sciences Clinical Research Center (ITS-CRC) at the University of Texas Medical Branch and supported in part by Grant 1UL1RR029876-01 from the National Center for Research Resources, National Institutes of Health. This study was also partially supported by the University of Texas Medical Branch Center for Rehabilitation Sciences and Sato Sports Plaza Co., Ltd.
DISCLOSURES
Our study was partially supported by Sato Sports Plaza Co., Ltd., the maker of the blood flow restriction device we used during this study. T. Abe has received research support from this company, and this support allowed S. Fujita to fly to Galveston to help perform the occlusion experiments. However, please note that this support was very minor and Sato Sports had no involvement in the study design, data analysis, or interpretation of the results. This was completely under the control of B. B. Rasmussen and his lab.
ACKNOWLEDGMENTS
We thank the nurses and staff at the ITS-CRC for their assistance in screening, admitting, and assisting with the subjects during data collection and Ming Zheng and Shelley Medina for technical assistance.
REFERENCES
- 1. Abe T, Sato Y, Inoue K, Midorikawa T, Yasuda T, Kearns CF, Koizumi K, Ishii N. Muscle size and IGF-1 increased after two weeks of low-intensity “Kaatsu” resistance training. Med Sci Sports Exerc 36: S353–S353, 2004 [Google Scholar]
- 2. Aniansson A, Grimby G, Rundgren A. Isometric and isokinetic quadriceps muscle strength in 70-year-old men and women. Scand J Rehabil Med 12: 161–168, 1980 [PubMed] [Google Scholar]
- 3. Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem 278: 29655–29660, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Atherton PJ, Szewczyk NJ, Selby A, Rankin D, Hillier K, Smith K, Rennie MJ, Loughna PT. Cyclic stretch reduces myofibrillar protein synthesis despite increases in FAK and anabolic signalling in L6 cells. J Physiol 587: 3719–3727, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baar K, Esser K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147: 755–763, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277: 23977–23980, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553: 213–220, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–009 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 6: 421–424, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 104: 1452–1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drummond MJ, Fujita S, Takash A, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 40: 691–698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol 106: 1403–1411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Febbraio MA, Steensberg A, Fischer CP, Keller C, Hiscock N, Pedersen BK. IL-6 activates HSP72 gene expression in human skeletal muscle. Biochem Biophys Res Commun 296: 1264–1266, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Fluckey JD, Knox M, Smith L, Dupont-Versteegden EE, Gaddy D, Tesch PA, Peterson CA. Insulin-facilitated increase of muscle protein synthesis after resistance exercise involves a MAP kinase pathway. Am J Physiol Endocrinol Metab 290: E1205–E1211, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88: 1321–1326, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 103: 903–910, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Hakkinen K, Pakarinen A, Alen M, Kauhanen H, Komi PV. Neuromuscular and hormonal adaptations in athletes to strength training in two years. J Appl Physiol 65: 2406–2412, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Hamada K, Vannier E, Sacheck JM, Witsell AL, Roubenoff R. Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J 18: 264–266, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Hartman JW, Moore DR, Phillips SM. Resistance training reduces whole-body protein turnover and improves net protein retention in untrained young males. Appl Physiol Nutr Metab 31: 557–564, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Hiscock N, Chan MHS, Bisucci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J 18: 992–994, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Hornberger TA, Mateja RD, Chin ER, Andrews JL, Esser KA. Aging does not alter the mechanosensitivity of the p38, p70S6k, and JNK2 signaling pathways in skeletal muscle. J Appl Physiol 98: 1562–1566, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56: B209–B217, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 57: M772–M777, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Inoki K, Zhu TQ, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 271: 17771–17778, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol Cell Physiol 271: C1172–C1180, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Koopman R, Zorenc AHG, Gransier RJJ, Cameron-Smith D, van Loon LJC. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab 290: E1245–E1252, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101: 531–544, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell 21: 521–531, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−DeltaDeltaCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Mayhew D, Kim J, Cross J, Ferrando A, Bamman M. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol 107: 1655–1662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McArdle A, van der Meulen JH, Catapano M, Symons MCR, Faulkner JA, Jackson MJ. Free radical activity following contraction-induced injury to the extensor digitorum longus muscles of rats. Free Radic Biol Med 26: 1085–1091, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol 135: 1633–1642, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nair KS. Aging muscle. Am J Clin Nutr 81: 953–963, 2005 [DOI] [PubMed] [Google Scholar]
- 41. O'Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol 587: 3691–3701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 12: 86–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 98: 1154–1162, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 20: 768–769, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reynolds TH, Bodine SC, Lawrence JC., Jr Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem 277: 17657–17662, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sheffield-Moore M, Paddon-Jones D, Sanford AP, Rosenblatt JI, Matlock AG, Cree MG, Wolfe RR. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab 288: E922–E929, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Shinohara M, Kouzaki M, Yoshihisa T, Fukunaga T. Efficacy of tourniquet ischemia for strength training with low resistance. Eur J Appl Physiol Occup Physiol 77: 189–191, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 86: 308–314, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88: 2097–2106, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Takarada Y, Tsuruta T, Ishii N. Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Jpn J Physiol 54: 585–592, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102: 145–152, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Thompson HS, Scordilis SP, Clarkson PM, Lohrer WA. A single bout of eccentric exercise increases HSP27 and HSC/HSP70 in human skeletal muscle. Acta Physiol Scand 171: 187–193, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Thomson DM, Gordon SE. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J Appl Physiol 98: 557–564, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Vasilaki A, McArdle F, Iwanejko LM, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev 127: 830–839, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101: 2000–2007, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78: 250–258, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol Endocrinol Metab 268: E422–E427, 1995 [DOI] [PubMed] [Google Scholar]
- 59a. West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 587: 5239–5247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol 547: 977–987, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Williamson DL, Kubica N, Kimball SR, Jefferson LS. Exercise-induced alterations in extracellular signal-regulated kinase 1/2 and mammalian target of rapamycin (mTOR) signalling to regulatory mechanisms of mRNA translation in mouse muscle. J Physiol 573: 497–510, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men ≥76 yr old. Am J Physiol Endocrinol Metab 277: E118–E125, 1999 [DOI] [PubMed] [Google Scholar]
- 63. Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol Endocrinol Metab 265: E210–E214, 1993 [DOI] [PubMed] [Google Scholar]