Abstract
Systemic maternal inflammation contributes to preterm birth and is associated with development of bronchopulmonary dysplasia (BPD). Infants with BPD exhibit decreased alveolarization, diffuse interstitial fibrosis with thickened alveolar septa, and impaired pulmonary function. We tested the hypothesis that systemic prenatal LPS administration to pregnant mice followed by postnatal hyperoxia exposure is associated with prolonged alterations in pulmonary structure and function after return to room air (RA) that are more severe than hyperoxia exposure alone. Timed-pregnant C3H/HeN mice were dosed with LPS (80 μg/kg) or saline on gestation day 16. Newborn pups were exposed to RA or 85% O2 for 14 days and then to RA for an additional 14 days. Data were collected and analyzed on postnatal days 14 and 28. The combination of prenatal LPS and postnatal hyperoxia exposure generated a phenotype with more inflammation (measured as no. of macrophages per high-power field) than either insult alone at day 28. The combined exposures were associated with a diffuse fibrotic response [measured as hydroxyproline content (μg)] but did not induce a more severe developmental arrest than hyperoxia alone. Pulmonary function tests indicated that hyperoxia, independent of maternal exposure, induced compliance decreases on day 14 that did not persist after RA recovery. Either treatment alone or combined induced an increase in resistance on day 14, but the increase persisted on day 28 only in pups receiving the combined treatment. In conclusion, the combination of systemic maternal inflammation and neonatal hyperoxia induced a prolonged phenotype of arrested alveolarization, diffuse fibrosis, and impaired lung mechanics that mimics human BPD. This new model should be useful in designing studies of specific mechanisms and interventions that could ultimately be utilized to define therapies to prevent BPD in premature infants.
Keywords: bronchopulmonary dysplasia, preterm birth, neonatal lung injury, perinatal stress
preterm birth, defined as delivery before 37 wk of gestation, has increased over the past decade in developing countries (2). Maternal infections and inflammatory states are commonly associated with preterm birth and include extrauterine sources of inflammation, such as periodontal (38), urinary tract (8), and respiratory (9) infections or chronic stresses, such as cigarette smoke (5). Often these infections are subclinical and even unnoticed but can be substantial contributors to the inflammatory responses that initiate preterm birth (37). Although the inflammation is maternal, the fetus is exposed to the results of maternal responses, such as increased expression of cytokines, chemokines, or lipid mediators, through the circulation (21, 30). These early exposures could prime the infants, making them more susceptible to insults after delivery than infants not exposed to maternal inflammation (4). The causes and timing of preterm delivery associated with maternal inflammation are not understood and can range from extremely preterm (23–28 wk of gestation) to late preterm (>32 wk of gestation). Although any premature infant is at increased risk, extremely premature infants (<1,000 g body wt) are the most vulnerable and often require extensive supportive care that can include mechanical ventilation and exposure to elevated levels of O2. These common therapeutic interventions could provide a “second hit” to primed newborns.
Bronchopulmonary dysplasia (BPD) is a multifactorial disease with an incidence of 30–75% in <1,000-g-birth-weight infants, thereby often afflicting the smallest and most vulnerable of the preterm population (7, 35). BPD is clinically defined as the need for respiratory support at 36 wk corrected gestational age (16, 24) and has been associated with long-term abnormalities in lung mechanics or function.
Chorioamnionitis, an acute infection of the fetal and maternal membranes, is strongly associated with the development of BPD (15), most likely through inflammatory effects on pulmonary development (19). Nevertheless, the link between chronic or systemic maternal infection or inflammation and the development of BPD has been well established (10–12).
With recent advances in therapies that increase survival rates in extremely premature infants, the etiology of BPD has changed from a disease that was secondary to supportive care in sick preterm infants to one in which an extremely immature lung exposed to more subtle insults develops arrested alveolarization and diffuse fibrosis (14, 16, 24). Newborn rodents are at a stage in lung development similar to extremely preterm infants and offer a reasonable model for studying lung development. Exposure of newborn rodents to hyperoxia has been useful in the study of the effects of oxidant-induced inflammatory responses on arrested alveolarization. However, rodents are born surfactant-sufficient and capable of oxygenation in room air, while extremely preterm infants are surfactant-deficient and often unable to sufficiently oxygenate at birth. Consequently, the results of O2 alone in a surfactant-sufficient rodent lung do not truly represent the pathophysiology often observed in preterm infants (22). Perinatal inflammation and neonatal O2 supplementation are often present in preterm infants and contribute to the complications of prematurity, including BPD. The addition of maternal inflammation to previously established rodent models of hyperoxia exposure provides an opportunity to investigate a potentially biologically relevant interaction (1, 16, 31).
Currently, lamb and rat models are used to investigate the complementary effects of fetal inflammation induced by intra-amniotic lipopolysaccharide (LPS) injection and subsequent neonatal hyperoxia (20, 34). These established models directly expose the fetus to agents that mimic the clinical characteristics consistent with chorioamnionitis. The responses observed in the offspring are fewer and larger alveoli, similar to those in infants with BPD. Alternatively, systemic administration of LPS to pregnant animals mimics systemic maternal stress or inflammatory responses that are also closely associated with preterm birth in humans. Previous studies have reported that systemic LPS exposure alone in pregnant mice leads to delayed alveolarization and prolonged pulmonary inflammation in pups (29). However, the effects of systemic maternal LPS exposure and subsequent neonatal hyperoxia have not been explored and are likely to be additive. The present study tests the hypothesis that administration of LPS to pregnant mice followed by exposure of the newborn pups to hyperoxia is associated with alterations in pulmonary structure and function. Furthermore, we hypothesize that these alterations persist after return to room air and are more severe than hyperoxia exposure alone.
METHODS
Animal models.
Animal study protocols were approved by the Institutional Animal Care and Use Committee at the Research Institute at Nationwide Children's Hospital. C3H/HeN mice (6–8 wk old) were purchased from Harlan Sprague Dawley (Indianapolis, IN). The C3H/HeN strain was chosen because of our previous experience with these mice in models of hyperoxic lung injury (25, 27, 28). Males and females were paired, and the presence of a vaginal plug was designated embryonic (E) day 1 (E1). On E16, dams were injected intraperitoneally with LPS (serotype 0111:B4, catalog no. 437627, Calbiochem) or an equal volume of saline (3). The highest LPS dose (80 μg/kg) that produced a consistently viable litter was chosen. Newborn mice from saline- or LPS-injected dams were pooled and redistributed randomly to the two dams in separate cages within 12 h of birth. One litter of pups was exposed to 85% O2 for 14 days followed by room air exposure for another 14 days. The other litter of pups that received the same treatment on E16 was maintained in room air for 4 wk. To avoid O2 toxicity in the dams and to eliminate maternal effects between the groups, the nursing dams were rotated between their hyperoxic and room air litters every 24 h. Furthermore, only dams with the same E16 treatment were matched. Body weights were recorded at birth and on days 1, 3, 7, 14, and 28.
Tissue preparation.
Mice were euthanized on day 14 or 28 by injection of pentobarbital sodium (200 mg/kg ip). After pentobarbital administration, a thoracotomy was done, the right bronchus was ligated, and the right lungs were removed, weighed, and snap frozen. The tracheas were cannulated, and the left lungs were inflation fixed with 10% buffered formalin at a pressure of 25 cmH2O for ≥15 min. After equilibration, left lungs were removed and fixed in buffered formalin overnight. Subsequently, lung tissues were washed five times in PBS and embedded in paraffin following serial dehydrations in increasing concentrations of ethanol. Embedded lungs were sliced perpendicular to the lung base (apical-basal axis) into 4-μm sections, with portions of the upper, middle, and base of the lung attained for analysis, and stained with hematoxylin and eosin or Sirius red as previously described (25, 28).
Lung morphometrics.
Tissue sections from 14- or 28-day-old mice were stained with hematoxylin and eosin for morphometric analyses. To assess uniform and proportional samples for each lung, five nonoverlapping photomicrographs in different sections, representing an area of 362,818 μm2, were captured at ×100 magnification with a microscope (model BX-40, Olympus Optical) and a digital camera (Diagnostic Instruments) under identical lighting conditions and optical settings by an investigator blinded to group. Five images per animal were analyzed and averaged using research-based digital image analysis software (Image-Pro Plus 6.3, Media Cybernetics, Silver Spring, MD) and a custom macro written for automated assessments of alveolar morphometry as described by Park et al. (25). Air spaces within the image were selected by color segmentation, and incomplete air spaces (those touching the image edge) were excluded from analysis. The macro was used to determine the number of complete air spaces in the image, the average air space size (area, in μm2), and the total perimeter per high-power field (HPF, in μm). In addition, the air space septal wall thickness was measured randomly in five individually intact alveoli by manual identification of the septal wall edges and measurement of the width. At least five measurements were made per HPF and averaged. Intra-animal and interobserver variability in these measurements were routinely <10% (25).
Picro-Sirius red quantification.
Collagen in the tissue from 14- and 28-day-old mice was assessed utilizing Picro-Sirius red staining. After deparaffinization and rehydration through xylenes and graded alcohols, slides were transferred to 0.2% phosphomolybdic acid for 2 min. After they were washed with distilled water, the slides were incubated in 0.1% Sirius red-saturated picric acid for 90 min (18). To assess uniform and proportional samples for each lung, five nonoverlapping photomicrographs in different sections were captured from four to five mice per group at ×100 magnification by brightfield microscopy using a microscope (model BX-40, Olympus) and a digital camera (Diagnostic Instruments) under identical lighting conditions and optical settings by an investigator blinded to group assignments. Then a picture was taken with polarized light from the same area. Picro-Sirius red stains collagen red on a pale yellow background in brightfield microscopy, whereas under polarized light, collagen appears bright orange-red and/or bright green (6). Images were analyzed using research-based digital image analysis software (Image-Pro Plus 6.3), and a macro written for automated quantification of number of spots and total area stained positive for Picro-Sirius red was used.
Hydroxyproline assay.
Frozen right lungs from 14- or 28-day-old mice were homogenized in 1 ml of deionized water and incubated with 65 μl of 50% trichloroacetic acid for 20 min on ice. After centrifugation at 15,000 g for 20 min at 4°C, the pellet was suspended in 1 ml of 12 N hydrochloric acid and incubated for 16 h at 110°C. Samples were resuspended in 2 ml of deionized water. Hydroxyproline content from five to seven mice per group was determined by a colorimetric assay as described previously (32). A hydroxyproline standard dilution series (catalog no. H54409, Sigma, St. Louis, MO) was used to calculate micrograms of hydroxyproline per right lung.
Lung macrophage and neutrophil counts.
Macrophage and neutrophil counts were performed on lung tissue sections from 14- or 28-day-old mice. The primary antibody for neutrophils was rat anti-mouse (Serotec, Kingston, UK), and the sections were exposed to a dilution of 1:500. The secondary antibody was rabbit anti-rat (catalog BA-4001, Vector), which was utilized at a dilution of 1:200. For macrophage staining, rat anti-mouse Mac3 monoclonal antibody (catalog no. 550292, BD Pharmingen, San Diego, CA) was used at a dilution of 1:500 as the primary antibody and rabbit anti-rat (catalog no. BA-4001, Vector) at a dilution of 1:200 as the secondary antibody.
Pulmonary function test.
Mice were weighed on day 14 or 28 and anesthetized by an intraperitoneal injection of ketamine (200 mg/kg) and xylazine (20 mg/kg). Two-thirds of the dose was given initially to induce anesthesia. After an adequate level of anesthesia was established, mice were tracheostomized, and the trachea was cannulated. The remaining anesthetics were given before the cannula was connected to the FlexiVent system (SCIREQ, Montreal, PQ, Canada) for forced oscillation measurements. Mice were ventilated with a tidal volume of 10 ml/kg at a frequency of 350 breaths/min and a positive end-expiratory pressure of 2 cmH2O. The maximal vital capacity perturbation (named TLC by SCIREQ) was used to determine the mean displaced volume (relative to weight). Furthermore, the “snapshot perturbation” maneuver was conducted to measure resistance and compliance. Then the ventilator produced a broadband frequency (0.5–19.75 Hz) for 8 s. This forced oscillation perturbation, primewave-8 (named by SCIREQ), was applied to measure central airway resistance (13). Finally, pressure-volume (PV) loops were generated to obtain static compliance. A coefficient of determination of 0.95 was used as the lower limit for measurements. For each parameter, two measurements were assessed and averaged (36).
Statistics.
Statistical analyses were performed by two-way ANOVA with exposure at E16 as one variable and O2 exposure as another. All data are presented as means ± SE, and the results of the two-way ANOVA are indicated. When there were differences noted by two-way ANOVA, individual differences were detected using modified t-test post hoc with P < 0.05 as significant. All analyses were performed with SPSS Windows version 15.0 (SPSS, Chicago, IL).
RESULTS
Approximately 20% of the pregnant dams injected with LPS did not give birth. However, the LPS-treated dams that delivered had similar numbers of pups per litter, and weights of these live born pups were similar to weights of the pups delivered by the saline-treated dams. No pups died during the hyperoxia or room air exposures, no signs of distress were observed in any group, and there were no differences in weights over the 28 days of observation (see supplemental data in the online version of this article).
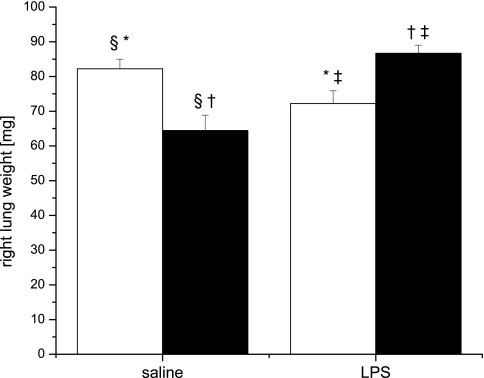
Right lung weight and lung morphometry were evaluated on days 14 and 28. On day 14, there were no differences in right lung weight between groups (data not shown). Mice exposed to prenatal saline and postnatal hyperoxia (saline/O2) or prenatal LPS and postnatal room air (LPS/RA) had lower right lung weights at day 28 than mice exposed to prenatal saline and postnatal room air (saline/RA; Fig. 1). Interestingly, there were no differences in the right lung weights between saline/RA- and LPS/O2-exposed mice. Statistical analyses indicated a significant two-way interaction between systemic maternal LPS injection and neonatal hyperoxia exposure on right lung weight at day 28. This interaction persisted even when right lung weights were normalized to body weights (see supplemental data).
Fig. 1.
Pup right lung weight at day 28. Pups were exposed to prenatal saline or LPS and postnatal room air (open bars) or 85% O2 for 14 days (solid bars) and then returned to room air for 14 days. Data were analyzed by 2-way ANOVA and modified t-test post hoc (n = 9). An interaction between prenatal LPS and postnatal hyperoxia exposure was observed. Values are means ± SE. Identical symbols above bars indicate statistical significance between groups: *P ≤ 0.05; §,†,‡P ≤ 0.01.
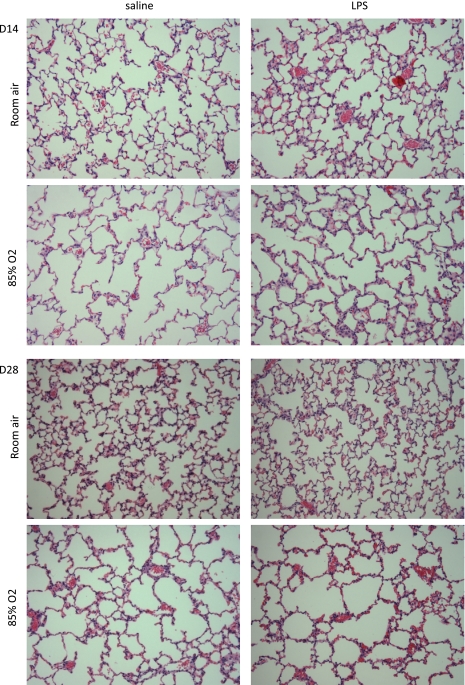
Alveolarization was assessed by histological analyses on lung sections obtained at days 14 and 28 (Fig. 2). Hematoxylin-and-eosin-stained lung sections from day 14 pups exposed to hyperoxia demonstrated decreased alveolar number and increased size, similar to previous reports, regardless of maternal exposure (Table 1). Number of air spaces per HPF, mean air space area, and total air space perimeter were affected by hyperoxia or LPS treatment independently. Furthermore, there was a statistical interaction between prenatal LPS and postnatal hyperoxia exposure on number of air spaces, air space area, and total air space perimeter per HPF. Distinct differences among treatment groups were observed in septal wall thickness, and there was an additive effect of the combined exposures at day 14.
Fig. 2.
Histological lung sections from pups exposed to prenatal saline or LPS and postnatal room air or 85% O2. Top: histological sections from pups euthanized at day 14 (D14). Bottom: histological sections from pups euthanized at day 28 (14 days in O2 followed by return to room air for 14 days; D28). Inflation-fixed lung sections were stained with hematoxylin and eosin. Photomicrographs are representative of 6 mice per group. Magnification ×100.
Table 1.
Morphometric measurements
| Treatment | No. of Air Spaces | Mean Area, μm2 | Mean Perimeter, μm | Total Perimeter, μm | Wall Thickness, μm |
|---|---|---|---|---|---|
| Day 14 | |||||
| Saline/RA | 142.8 ± 6 | 8,848 ± 387 | 515 ± 27 | 74,335 ± 4,701 | 10 ± 0.18 |
| Saline/85% O2 | 49.9 ± 1† | 26,848 ± 956† | 858 ± 34† | 42,808 ± 1,968† | 10 ± .025 |
| LPS/RA | 109.1 ± 1† | 12,078 ± 189* | 558 ± 10 | 66,836 ± 1,497 | 13 ± 0.4* |
| LPS/85% O2 | 55.4 ± 3† | 23,587 ± 1,557† | 820 ± 26† | 49,723 ± 1,663† | 23 ± 1.75† |
| Day 28 | |||||
| Saline/RA | 117.1 ± 7 | 7,562 ± 331 | 391 ± 17 | 45,082 ± 2,834 | 12 ± 0.32 |
| Saline/85% O2 | 75.1 ± 7† | 10,969 ± 888† | 481 ± 25* | 35,230 ± 2,478† | 14 ± 0.7† |
| LPS/RA | 143 ± 7* | 6,019 ± 400† | 358 ± 14 | 5,0451 ± 1,905 | 15 ± 0.3† |
| LPS/85% O2 | 51.2 ± 5† | 14,680 ± 1,926† | 587 ± 65† | 28,703 ± 2,390† | 21 ± 0.33† |
Values are means ± SE. RA, room air. Data were analyzed by 2-way ANOVA with modified t-test post hoc (n = 6 per group):
P ≤ 0.05 and
P ≤ 0.01 vs. saline/RA.
Pups that were exposed to hyperoxia for 14 days and recovered in room air for 14 days still showed altered pulmonary histology compared with pups that were exposed to room air for the entire time course, independent of prenatal exposure (Fig. 2). Morphometric analyses of histological lung sections demonstrated persistent deficits in lung development at day 28 as a function of exposure to hyperoxia; these deficits were exacerbated by prenatal LPS exposure as assessed by the number of air spaces, mean air space area, and total air space perimeter per HPF (Table 1). In each of these assessments, there was a significant statistical interaction between prenatal LPS and postnatal hyperoxia exposure. Also, saline/O2 exposure for 14 days induced an increase in septal wall thickness that persisted at day 28 (Table 1). Mice exposed to LPS/RA demonstrated an even greater increase in septal wall thickness than those exposed to saline/O2 on day 14. Furthermore, LPS/O2 exposure induced an impressive increase in septal wall thickness that was higher than the other three groups and persisted even after day 28. Statistical analyses of septal wall thickness indicated independent effects of prenatal LPS and postnatal hyperoxia exposure for 14 days as well as a two-way interaction, indicating that the combination of prenatal LPS and postnatal hyperoxia exposure was acting synergistically to produce a persistent phenotype even after 14 days of room air recovery.
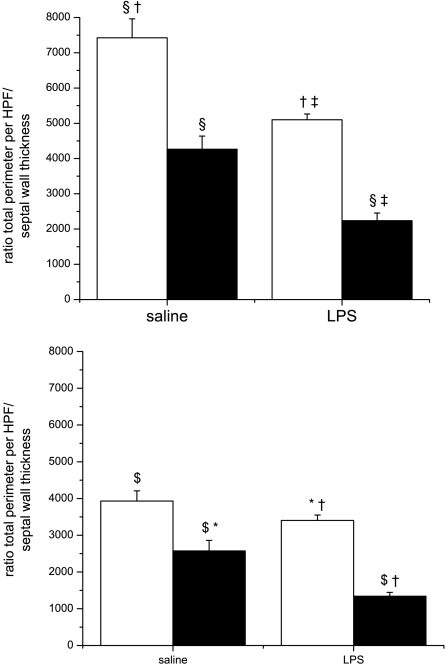
The consequences of increased septal wall thickness and impaired alveolarization for gas exchange are illustrated as a ratio of diffusion surface to distance (total alveolar perimeter per HPF divided by mean septal thickness; Fig. 3) at day 14 or 28. Either single exposure decreased the ratio of total air space perimeter per HPF to septal wall thickness compared with control mice that were exposed to saline/RA. The combination of both exposures (LPS/O2) further decreased the ratio compared with either single treatment. This finding would translate to even greater impairment of gas exchange. Statistical analyses indicated independent effects of prenatal LPS and postnatal hyperoxia exposure, as well as a two-way interaction for both time points, suggesting that the combination of prenatal LPS injection and postnatal hyperoxia exposure was again acting synergistically, even after room air recovery.
Fig. 3.
Ratios of total perimeter per high-power field (HPF) to septal wall thickness. Top: pups were exposed to prenatal saline or LPS and postnatal room air (open bars) or 85% O2 (solid bars) for 14 days, and ratio was measured at day 14. Bottom: pups were exposed to prenatal saline or LPS and postnatal room air (open bars) or 85% O2 (solid bars) for 14 days, followed by 14 days recovery, and ratio was measured at day 28. Data were analyzed by 2-way ANOVA with modified t-test post hoc (n = 6 per group). Effects of prenatal LPS, postnatal hyperoxia exposure, and an interaction between prenatal LPS and postnatal hyperoxia exposure were observed. Values are means ± SE. Identical symbols above bars indicate statistical significance between groups: *P ≤ 0.05; $,§,†,‡P ≤ 0.01.
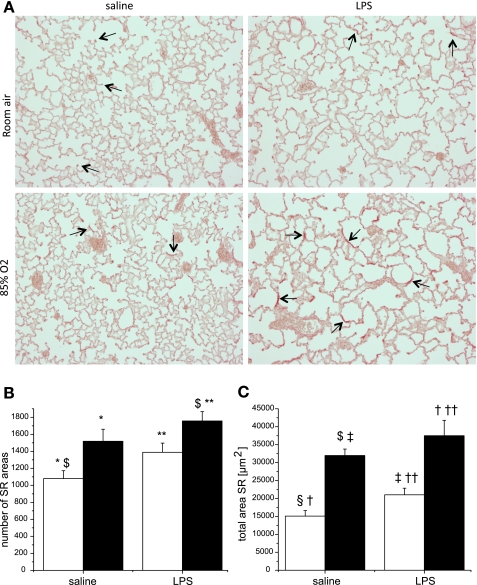
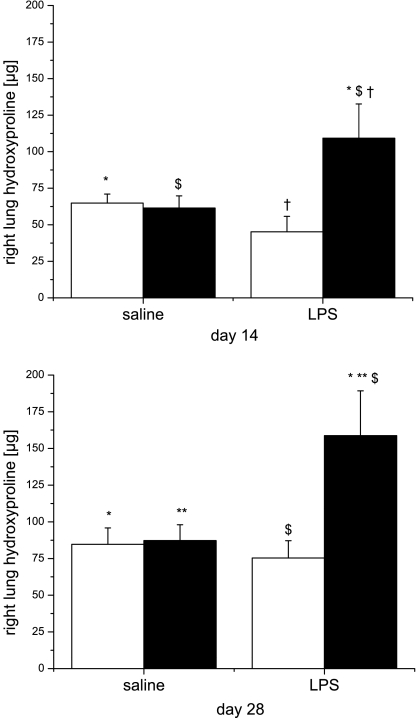
One explanation for increased septal thickness would be increased collagen deposition and fibrosis. To test this hypothesis, we stained lung sections with Picro-Sirius red (23) and measured hydroxyproline content in lung homogenates. Qualitatively, the dominant Picro-Sirius red staining was at the tips of the alveolar septa in mice that were exposed to saline/RA at days 14 and 28 (arrows in Fig. 4). However, the staining redistributed from the tips of the septa toward the alveolar walls (arrows in Fig. 4) in animals that were exposed to prenatal LPS or postnatal hyperoxia. The combination of prenatal LPS and postnatal hyperoxia exposure further accentuated the redistribution of staining, which was persistent at day 28, after 14 days of room air recovery (Fig. 4). Quantification of the Picro-Sirius red-stained area in lung tissues indicated a dominant effect of prenatal LPS exposure (Fig. 4). Measurement of hydroxyproline content revealed that neither saline/O2 nor LPS/RA exposure was related to higher hydroxyproline content at day 14 or 28 compared with pups exposed to saline/RA. However, there was a marked increase in hydroxyproline content in the lungs of pups that were exposed to LPS/O2 at day 14, which was persistent even after 14 days of room air recovery (day 28). Statistical analyses indicated an independent effect of hyperoxia exposure on day 14 and a two-way interaction between prenatal LPS and postnatal hyperoxia exposure on hydroxyproline content for both time points (Fig. 5).
Fig. 4.
Collagen deposition in lung tissues. A: histological lung sections from pups exposed as described in methods were stained with Picro-Sirius red and assessed at day 28. B and C: positive-stained areas from lungs exposed to prenatal saline or LPS and postnatal room air (open bars) or 85% O2 were quantified for number and total area. Five fields for each slide were averaged, and data were analyzed by 2-way ANOVA and modified t-test post hoc (n = 6 per group). Effects of prenatal LPS and postnatal hyperoxia exposure were observed in the numbers of Sirius red (SR) spots and total Sirius red area. Values are means ± SE. Identical symbols above bars indicate statistical significance between groups: *,**P ≤ 0.05; $,§,†,‡,††P ≤ 0.01.
Fig. 5.
Hydroxyproline contents in pup right lungs. Pups were exposed as described in methods, and hydroxyproline content was assessed at day 14 (top) and day 28 (bottom). Data were analyzed by 2-way ANOVA and modified t-test post hoc (n = 5–7 mice). An effect of hyperoxia and an interaction between LPS and hyperoxia on hydroxyproline contents were identified. Values are means ± SE. Identical symbols above bars indicate statistical significance between groups: *,**P ≤ 0.05; $,§,†P ≤ 0.01.
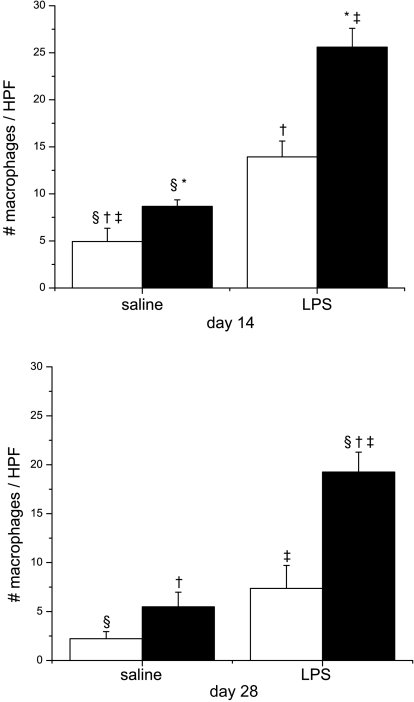
An influx of inflammatory infiltrates into the alveolar space could contribute to the development of fibrosis. To test this hypothesis, we stained histological lung sections obtained on days 14 and 28 with anti-neutrophil and anti-macrophage antibodies. No increase in neutrophil count was observed due to LPS or hyperoxia exposure alone, but a substantial effect of combined prenatal LPS and postnatal hyperoxia exposure was observed on day 14 (see supplemental data). However, the increases in neutrophil counts were not significant at day 28, indicating a recovery after return to room air (data not shown). Statistical assessment of lung neutrophil accumulation indicated an independent effect of systemic maternal LPS exposure and an interaction between prenatal maternal LPS and postnatal hyperoxia exposure on day 14 only. Macrophage accumulation in the lung on day 14 was greater in the hyperoxia-exposed pups than in the room air controls; however, prenatal LPS alone increased the macrophage accumulation, and this effect was compounded in the pups that were exposed to LPS/O2 (Fig. 6). Statistical analyses indicated independent effects of prenatal LPS and postnatal hyperoxia exposure and an interaction between both factors. Persistence of inflammation after room air recovery was also assessed on lung tissue sections at day 28. Lung macrophage accumulation was evident, as there were still higher lung macrophage counts at day 28 in mice exposed to LPS/O2 than in any other group (Fig. 6). Statistically, there were independent effects of prenatal LPS and postnatal hyperoxia exposure, as well as a two-way interaction.
Fig. 6.
Macrophage accumulation in lung tissues. Immunohistochemical assessment of macrophage numbers was performed on tissue sections obtained from pups exposed to prenatal saline or LPS and postnatal room air (open bars) or 85% O2 (solid bars) for 14 days (top) or 28 days (bottom). [See photomicrographs in supplemental data.] Inflation-fixed lung sections were stained with Mac3 antibodies, and number of macrophages was quantified by digital analyses of the sections. Macrophages were counted in 5 fields for each slide and averaged. Data were analyzed by 2-way ANOVA and modified t-test post hoc (n = 4–5 mice per group and time point). On day 14, there was an effect of prenatal LPS, an effect of postnatal hyperoxia, and an interaction between prenatal LPS and postnatal hyperoxia. On day 28, statistical analyses indicated persistent effects of prenatal LPS and postnatal hyperoxia exposure and an interaction between prenatal LPS and postnatal hyperoxia macrophage numbers. Values are means ± SE. Identical symbols above bars indicate statistical significance between groups: *P ≤ 0.05; §,†,‡P ≤ 0.01.
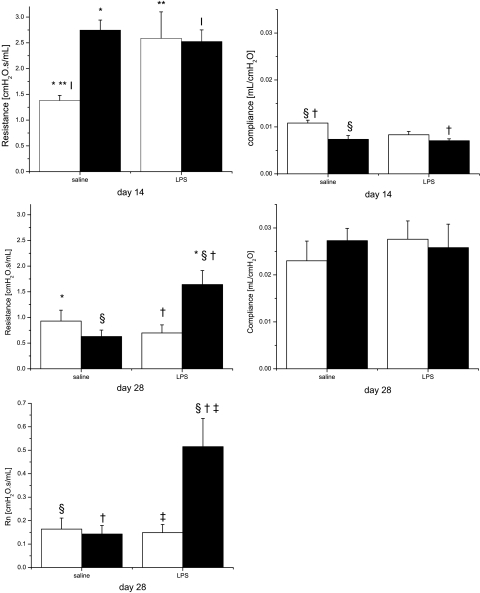
The functional impact of structural changes and diffuse fibrosis on lung mechanics was assessed by pulmonary function tests. We measured total airway resistance and compliance at both time points and, additionally, central airway resistance at day 28. In mice that were exposed to postnatal hyperoxia independent of their maternal exposures, resistance on day 14 was higher than in saline/RA-exposed pups (Fig. 7). Interestingly, pups that were exposed to LPS/RA for 14 days also exhibited a higher resistance than pups exposed to saline/RA. Statistical analyses indicated a significant two-way interaction of prenatal LPS and postnatal hyperoxia exposure. Compliance was also affected by hyperoxia, in that it was lower in mice exposed to hyperoxia at day 14, regardless of prenatal treatment (Fig. 7). Statistical analyses indicated independent effects of prenatal LPS injection and postnatal hyperoxia exposure. To evaluate whether the changes observed at day 14 were persistent, pulmonary function tests were performed at day 28, after 2 wk of room air recovery. There was no difference in lung compliance between groups, but total and central airway resistances were higher in pups that were exposed to LPS/O2 than all other groups. Only the combination of prenatal LPS and postnatal hyperoxia exposure induced prolonged alterations in lung mechanics. Statistical analysis indicated an effect of prenatal LPS exposure and further significant two-way interaction between prenatal LPS and postnatal hyperoxia exposure in total airway resistance. Furthermore, we found independent effects of prenatal LPS injection and postnatal hyperoxia exposure and an additional interaction between both treatments on the central airway resistance. These results indicate that the combination of both insults synergistically affected pulmonary function and that the change in resistance persisted after recovery. These statistical differences were unchanged after correction for displaced volumes.
Fig. 7.
Pulmonary function tests. Pulmonary resistance and compliance were measured in pups exposed to prenatal saline or LPS and postnatal room air (open bars) or 85% O2 (solid bars). Top: measurements on day 14; middle: measurements on day 28 following room air recovery; bottom: central airway resistance (Rn) on day 28. Data were analyzed by 2-way ANOVA and modified t-test post hoc (n = 5–6 mice per treatment group). Effects of prenatal LPS and an interaction between prenatal LPS and postnatal hyperoxia on total airway resistance were observed. Effects of prenatal LPS and postnatal O2 and an interaction of prenatal LPS and postnatal O2 on central airway resistance were also observed. Values are means ± SE. Identical symbols above bars indicate statistical significance between groups: *,**,|P ≤ 0.05; §,†,‡P ≤ 0.01.
DISCUSSION
In the modern era of neonatology, infants with BPD exhibit decreases in lung alveolarization but, importantly, also exhibit variable and diffuse interstitial fibrosis, thickened alveolar septa, and impaired lung function that persist into adulthood (14). Many rodent models have mimicked the decrease in alveolarization and/or increase in septal wall thickness due to hyperoxia exposure or fetal inflammation independently. However, the combination of systemic prenatal LPS and postnatal hyperoxia exposure creates a phenotype that closely resembles human infants with BPD, including the changes in lung structure and function. In light of the reported associations between maternal inflammation, preterm birth, and BPD, the “double-hit” model described in these studies is highly relevant in a investigation of the mechanism of maternal effects on the development of diseases in human infants.
Increases in septal wall thickness due to hyperoxia exposure have been previously reported (28, 33) and may reflect important biological processes. The ratio of total perimeter per HPF to septal wall thickness was calculated as a measure of surface area available for gas exchange. Either insult alone was able to decrease this ratio at day 14, but the more exaggerated, additive response in the double-hit animals is notable (Fig. 3, top). This deficiency recovered in the prenatal LPS-exposed pups, but not in the hyperoxia-exposed pups, and remained exaggerated in the LPS- and hyperoxia-exposed pups at day 28 (Fig. 3, bottom). These findings indicate that the pups exposed to both insults had structural defects that could impair gas exchange that were more severe than those in mice exposed to a single insult and that the more severe deficit persisted after return to room air.
To investigate the mechanisms associated with increased septal wall thickness, we stained lung tissue sections with Pico-Sirius red. The staining was located at the tips of the alveolar septae in pups from dams exposed to saline and neonatal exposure to room air, which we interpret as normal development at alveolar crests. Either single insult alone was associated with a qualitative change in the staining location away from the tips of the alveolar septa and more into the interstitium (Fig. 4). This change in staining pattern was further exaggerated in the tissues from pups having received the combined exposures. The intensity of the interstitial staining was moderately increased from day 14 to day 28 in the pups having received both exposures, while the pattern of the staining appeared to be recovered in either of the single-insult groups by day 28. A similar pattern of fibrosis has been observed recently in BPD patients that is more diffuse and less severe than in old BPD (14). Consistent with the Picro-Sirius red histological findings, hydroxyproline content increased in the lung tissues of pups that were exposed to both perinatal LPS and postnatal hyperoxia (Fig. 5). Only the combination of prenatal LPS and postnatal hyperoxia exposure led to quantitative increases in the amount of hydroxyproline, and this increase persisted, suggesting that the differences in Picro-Sirius red staining noted in the other groups (saline/O2 and LPS/RA) were primarily qualitative.
Other investigations have described substantial mortality in models of neonatal hyperoxia exposure, but no animal mortality was observed in the present studies, and body weights of the pups were not affected by prenatal LPS exposure and/or postnatal hyperoxia exposure. Similar growth rates in room air and hyperoxia are contrary to our previous report, and the reasons for differences are unknown (28). In the earlier report, pups exposed to 85% O2, as in these studies, did not exhibit deficits in growth as significant as those observed in pups exposed to 95% O2, and minor differences in exposure environment or the stress associated with injecting the dam could have potentiated our current results. However, the data are reassuring, in that differences in the lung phenotype are unlikely to be related to nutritional differences between groups (17). Either single exposure resulted in decreases in lung weight compared with saline/RA-exposed mice. In contrast, lung weight was higher in the LPS/O2-exposed mice than in the saline/O2- or LPS/RA-exposed mice. These differences in lung weights are not likely to be due to pulmonary edema, as these mice had recovered in room air for 14 days (Fig. 1), and increased pulmonary fibrosis was the more logical explanation. Interestingly, while there was a profound decrease of alveolarization in the prenatal LPS-exposed, postnatal hyperoxia-exposed group, there was no difference in lung weight compared with mice exposed to prenatal saline and postnatal room air. These differences indicate that the lungs are heavier, and this additional weight could be attributable to the increased fibrosis that persists in these animals on day 28 (Fig. 1).
Macrophage accumulation is associated with increased collagen deposition, and in other models of lung inflammation, macrophage accumulation is strongly associated with profibrotic responses (26). There was an independent effect of prenatal LPS exposure on lung macrophage numbers. The marked differences in macrophage accumulation in neonatal mice were not dependent on hyperoxia exposure, suggesting that LPS administration to the dams induces a fetal inflammatory response that persists without obvious exposures to other inflammatory agents through day 14 of life (Fig. 6, top). However, the macrophage responses were even greater in the pups receiving the combined insults than in those exposed to prenatal LPS alone, and this enhanced response persisted up to day 28, even after return to room air (Fig. 6, bottom). This finding suggests that maternal inflammatory insults can have effects on the offspring that are quite persistent. Furthermore, the additional increase in macrophages in the lungs of pups that were exposed to LPS/O2 indicates that hyperoxia augments the neonatal inflammatory responses caused by maternal LPS exposure. These data suggest that macrophage accumulation is likely to be important mechanistically in the development of the fibrosis observed in this model.
The effects of hyperoxia exposure on pulmonary alveolar development are similar to those previously reported (25, 28). Interestingly, we also observed an independent effect of maternal LPS exposure on the number of air spaces. Consistent with other reports that provide evidence of an association between prenatal LPS exposure, fetal pulmonary inflammation, and decreased surfactant protein expression, our data indicate that maternal inflammation leads to defects in alveolar development independent of postnatal hyperoxia exposure at day 14 but recovers to near saline/RA levels by day 28. Similarly, the combination of maternal LPS and postnatal hyperoxia exposure is not different from hyperoxia exposure alone at day 14, suggesting that the effects of 85% O2 may overwhelm the independent effect of LPS administration to the dam (29). The more significant finding, however, is that after 14 days of room air recovery, the LPS/O2-exposed pups still exhibited severe alveolarization deficiencies at day 28.
The functional significance of macrophage accumulation and diffuse fibrosis was assessed in measurements of lung function. Consistent with other studies, lung compliance was decreased and resistance was increased with hyperoxia alone. However, these parameters were not affected or only minimally affected by LPS alone. Furthermore, there were no or only minor additional effects of LPS and hyperoxia on lung mechanics compared with hyperoxia alone (Fig. 7, top). Once the groups were returned to room air for 14 days, the resistance decreased in the hyperoxia-exposed group, indicating a recovery of lung function. More important than temporary effects on pulmonary compliance is that, instead of recovering, the combined-exposure group demonstrated even more severe increases in total resistance (Fig. 7, middle) and substantial increases in central airway resistance (Fig. 7, bottom).
In conclusion, we have developed a rodent model of systemic maternal inflammation followed by neonatal oxidant stress. Our studies support the hypothesis that the combined effects of neonatal hyperoxia exposure and systemic maternal inflammation are responsible for a phenotype that shares many histological and pathophysiological characteristics of BPD, which include acute and prolonged alterations in lung structure and function. These findings imply that the combination of perinatal and postnatal insults may result in a worse neonatal outcome than either single event alone. Furthermore, neonatal hyperoxia, in addition to systemic maternal inflammation, induces a fibrotic response and a BPD-like phenotype that closely mimics human disease. Additional studies to elucidate the specific molecular mechanisms responsible for the development of the lung phenotype observed in these studies may be helpful in designing interventions in this model and, perhaps, in premature infants at risk for the development of BPD.
GRANTS
We gratefully acknowledge the support of National Heart, Lung, and Blood Institute Grant HL-068948.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1. Aly H. Is there a strategy for preventing bronchopulmonary dysplasia? Absence of evidence is not evidence of absence. Pediatrics 119: 818–820, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Behrman RE, Stith Butler A. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press, 2007 [PubMed] [Google Scholar]
- 3. Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol 188: 203–208, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Cao L, Wang J, Tseu I, Luo D, Post M. Maternal exposure to endotoxin delays alveolarization during postnatal rat lung development. Am J Physiol Lung Cell Mol Physiol 296: L726–L737, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res 6 Suppl 2: S125–S140, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Constantine VS, Mowry RW. Selective staining of human dermal collagen. II. The use of picrosirius red F3BA with polarization microscopy. J Invest Dermatol 50: 419–423, 1968 [DOI] [PubMed] [Google Scholar]
- 7. Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med 358: 1700–1711, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Freak-Poli R, Chan A, Tucker G, Street J. Previous abortion and risk of pre-term birth: a population study. J Matern Fetal Neonatal Med 22: 1–7, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Getahun D, Ananth CV, Oyelese Y, Peltier MR, Smulian JC, Vintzileos AM. Acute and chronic respiratory diseases in pregnancy: associations with spontaneous premature rupture of membranes. J Matern Fetal Neonatal Med 20: 669–675, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 371: 75–84, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groneck P, Goetze-Speer B, Speer CP. Inflammatory bronchopulmonary response of preterm infants with microbial colonisation of the airways at birth. Arch Dis Child 74: F51–F55, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Groneck P, Schmale J, Soditt V, Stutzer H, Gotze-Speer B, Speer CP. Bronchoalveolar inflammation following airway infection in preterm infants with chronic lung disease. Pediatr Pulmonol 31: 331–338, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 29: 710–717, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res 2: 27–32, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Johnston CJ, Stripp BR, Piedbeouf B, Wright TW, Mango GW, Reed CK, Finkelstein JN. Inflammatory and epithelial responses in mouse strains that differ in sensitivity to hyperoxic injury. Exp Lung Res 24: 189–202, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Junqueira LC, Cossermelli W, Brentani R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn 41: 267–274, 1978 [DOI] [PubMed] [Google Scholar]
- 19. Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child 91: F132–F135, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med 14: 2–7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Le Monnier A, Autret N, Join-Lambert OF, Jaubert F, Charbit A, Berche P, Kayal S. ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes. Infect Immun 75: 950–957, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montes GS, Krisztan RM, Junqueira LC. Preservation of elastic system fibers and of collagen molecular arrangement and stainability in an Egyptian mummy. Histochemistry 83: 117–119, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 276: 357–368, 1967 [DOI] [PubMed] [Google Scholar]
- 25. Park MS, Rieger-Fackeldey E, Schanbacher BL, Cook AC, Bauer JA, Rogers LK, Hansen TN, Welty SE, Smith CV. Altered expressions of fibroblast growth factor receptors and alveolarization in neonatal mice exposed to 85% oxygen. Pediatr Res 62: 652–657, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Richerson HB, Seidenfeld JJ, Ratajczak HV, Richards D, Butler JE, Swanson P. Models of pulmonary fibrosis: misadventures and ramifications. Chest 75: 267–269, 1979 [PubMed] [Google Scholar]
- 27. Rogers LK, Tipple TE, Britt RD, Welty SE. Hyperoxia exposure alters hepatic eicosanoid metabolism in newborn mice. Pediatr Res 67: 144–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rogers LK, Tipple TE, Nelin LD, Welty SE. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr Res 63: 33–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salminen A, Paananen R, Vuolteenaho R, Metsola J, Ojaniemi M, Autio-Harmainen H, Hallman M. Maternal endotoxin-induced preterm birth in mice: fetal responses in toll-like receptors, collectins, and cytokines. Pediatr Res 63: 280–286, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci 23: 299–305, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Speer CP. Pulmonary inflammation and bronchopulmonary dysplasia. J Perinatol 26 Suppl 1: S57–S63, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta 18: 267–273, 1967 [DOI] [PubMed] [Google Scholar]
- 33. Torday JS, Rehan VK. Mechanotransduction determines the structure and function of lung and bone: a theoretical model for the pathophysiology of chronic disease. Cell Biochem Biophys 37: 235–246, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Ueda K, Cho K, Matsuda T, Okajima S, Uchida M, Kobayashi Y, Minakami H, Kobayashi K. A rat model for arrest of alveolarization induced by antenatal endotoxin administration. Pediatr Res 59: 396–400, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med 14: 358–366, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, Janssens W. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol 42: 96–104, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Vidaeff AC, Ramin SM. From concept to practice: the recent history of preterm delivery prevention. II. Subclinical infection and hormonal effects. Am J Perinatol 23: 75–84, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG 113: 135–143, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.