Abstract
Intramuscular triglyceride (IMTG) has received considerable attention as a potential mechanism promoting insulin resistance. Endurance-trained athletes have high amounts of IMTG but are insulin sensitive, suggesting IMTG content alone does not change insulin action. Recent data suggest increased muscle lipid synthesis protects against fat-induced insulin resistance. We hypothesized that rates of IMTG synthesis at rest would be increased in athletes compared with controls. Eleven sedentary men and 11 endurance-trained male cyclists participated in this study. An intravenous glucose tolerance test was performed to assess insulin action. After 3 days of dietary control and an overnight fast, [13C16]palmitate was infused at 0.0174 μmol·kg−1·min−1 for 4 h, followed by a muscle biopsy to measure isotope incorporation into IMTG and diacylglycerol. Compared with controls, athletes were twice as insulin sensitive (P = 0.004) and had a significantly greater resting IMTG concentration (athletes: 20.4 ± 1.6 μg IMTG/mg dry wt, controls: 14.5 ± 1.8 μg IMTG/mg dry wt, P = 0.04) and IMTG fractional synthesis rate (athletes: 1.56 ± 0.37%/h, controls: 0.61 ± 0.15%/h, P = 0.03). Stearoyl-CoA desaturase 1 mRNA expression (P = 0.02) and protein content (P = 0.03) were also significantly greater in athletes. Diacylglycerol, but not IMTG, saturation was significantly less in athletes compared with controls (P = 0.002). These data indicate endurance-trained athletes have increased synthesis rates of skeletal muscle IMTG and decreased saturation of skeletal muscle diacylglycerol. Increased synthesis rates are not due to recovery from exercise and are likely adaptations to chronic endurance exercise training.
Keywords: intramuscular triglyceride, isotope, training, exercise, diacylglycerol
intramuscular triglyceride (IMTG) appears to be an important predictor of diabetes risk, since it is inversely related to insulin sensitivity in almost all populations studied (39). In contrast, endurance-trained athletes have a high concentration of IMTG but are very insulin sensitive, which has been termed the “athlete's paradox” (15). This observation has led to speculation that rates of intramuscular lipid synthesis, rather than concentration, may be related to improved insulin sensitivity (15, 46). Currently, there are no reports in the literature comparing IMTG synthesis in endurance-trained subjects.
There is growing evidence suggesting the ability to increase rates of IMTG synthesis and concentration is associated with insulin sensitivity (22, 31, 32, 49). Protection from fat-induced insulin resistance has been reported with increased IMTG synthesis in muscle cell culture (31), with transgenic mouse models increasing IMTG synthesis or inhibiting degradation (22, 32), and following a single exercise bout in humans (49). Increased synthesis rates of IMTG were associated with decreased ceramide and diacylglycerol (DAG) concentration (32, 49). These data suggest IMTG may act as a sink into which free fatty acids (FFA) are stored, protecting against insulin resistance during increased FFA uptake.
Skeletal muscle DAG is an intermediate of IMTG and phospholipid synthesis and degradation that is increased in insulin resistance (27, 35). Elevated DAG concentration can promote protein kinase C-ε and -θ activity and inhibit insulin signaling (36). The effect of endurance training on muscle DAG concentration is unclear, since both decreased (10) and unchanged (6) concentrations have been reported. Interestingly, there was a trend for decreased saturated lipid content of DAG after endurance training that increased insulin sensitivity in obese subjects (6). We previously reported that insulin-resistant chronic smokers had increased DAG saturation compared with nonsmokers (3). Therefore, data are accumulating suggesting intramuscular DAG saturation may relate to insulin sensitivity. We sought to evaluate whether DAG concentration and composition could explain changes in insulin sensitivity in highly trained endurance athletes. We performed this study to test the hypothesis that resting IMTG synthesis rates are greater and that IMTG and DAG saturations are lower in endurance-trained athletes compared with sedentary control subjects.
METHODS
Subjects.
Eleven healthy sedentary men and 11 endurance-trained male cyclists were recruited for this study (Table 1). Subjects gave informed consent and were excluded if they smoked, had diabetes, hyperlipidemia, or liver, kidney, thyroid, or lung disease, or were taking medications that affect glucose or lipid metabolism. Subjects were considered sedentary if they engaged in moderate to vigorous exercise <2 h/wk. The endurance-trained athletes in this study were professional (n = 6), category 1 (n = 1), or category 2 (n = 4) bicycle racers according to the United States Cycling Federation. Subjects were excluded if they had a body mass index <20 kg/m2 or >25 kg/m2. Subjects were weight stable in the 6 mo preceding participation in this research study. This study was approved by Institutional Review Boards at the University of Colorado Denver and Boulder.
Table 1.
Subject demographics
| Variable | Athletes | Controls |
|---|---|---|
| n | 11 | 11 |
| Age, yr | 23.3 ± 0.7 | 20.5 ± 0.7* |
| BMI, kg/m2 | 22.2 ± 0.3 | 22.7 ± 0.6 |
| Body fat, % | 8.3 ± 0.6 | 14.6 ± 0.9* |
| Fasting glucose, mg/dl | 83.6 ± 1.6 | 83.1 ± 2.8 |
| FFA, μmol/l | 535 ± 58 | 762 ± 105 |
| Triglycerides, mg/dl | 63 ± 4 | 78 ± 13 |
| Insulin, μU/ml | 3.2 ± 0.1 | 3.8 ± 0.2* |
| Si, mU·l−1·min−1 | 10.9 ± 1.5 | 5.3 ± 0.7* |
| Muscle DAG, μg/mg dry wt | 0.36 ± 0.02 | 0.36 ± 0.03 |
Values are means ± SE; n = no. of subjects.
P < 0.05, significant difference between athletes and controls.
Preliminary testing.
After a 12-h overnight fast, subjects reported to the General Clinical Research Center (GCRC), where they were given a health and physical exam and subjected to a fasting blood draw. Body composition was determined using dual-energy X-ray absorptiometry (DEXA) analysis (Lunar DPX-IQ; Lunar, Madison, WI). Insulin sensitivity was determined using an insulin-modified frequently sampled intravenous glucose tolerance test according to standard methods (4). The data were input into MINMOD (Millennium version; Los Angeles, CA) to derive the parameter of insulin action (Si). On a separate day, and following 48 h of recovery from the last exercise bout, endurance-trained subjects performed a maximal oxygen consumption (V̇o2max) test on an electronically braked cycle ergometer (Lode Excalibur; Quinton Instruments, Seattle, WA). Respiratory gases were measured via indirect calorimetry (respiratory mass spectrometer: Perkin Elmer MGA-1100, St Louis, MO; pneumotachograph: Hans Rudolph Series 3813, Kansas City, MO).
Diet and exercise control.
All subjects were given a prescribed diet for 3 days before admission to the GCRC. Daily caloric requirement was determined from the DEXA measurement of fat-free mass (FFM) using the equation daily energy intake = 1.4 kcal/day × [372 + (23.9 × FFM)] and from analysis of dietary records as previously published (16). The composition of this diet was 55% carbohydrate, 30% fat, and 15% protein. Energy intake and macronutrient composition were not significantly different during the 3-day diet control compared with habitual diet evaluated with a dietary record. The fat content of the diet was controlled with the composition of saturated, monounsaturated, and polyunsaturated fat in a 1:1:1 ratio. Subjects were asked to refrain from exercising for 48 h before the tracer infusion study.
Tracer infusion study.
After an overnight fast, subjects arrived at the GCRC at 7:00 AM. Blood samples were then taken for determination of background isotope enrichment using the heated hand technique (7). A continuous infusion of [13C16]palmitate (Isotec, Miamisburg, OH) was then initiated at 0.0174 μmol·kg−1·min−1 and continued throughout the study. Indirect calorimetry was performed to measure CO2 production (V̇co2) at 210 min into the infusion. Blood samples for hormones and substrates were obtained every 10 min from 210 to 240 min of the infusion. A percutaneous needle biopsy was performed following blood sampling after 240 min of isotope infusion for determination of IMTG and DAG concentration, composition, and synthesis (5). The muscle biopsies were taken from midway between the greater trochanter of the femur and the patella. The anatomic location and depth of the biopsy were as similar as possible between subjects to minimize variance in muscle fiber composition. Muscle was immediately flash frozen in liquid nitrogen and stored at −80°C until dissection and analysis.
Metabolite and hormone analyses.
Standard enzymatic assays were used to measure glucose and triglycerides (Olympus AU400e chemistry analyzer; Olympus America, Center Valley, PA), lactate (kit no. 826; Sigma, St. Louis, MO), and FFA (NEFA kit; Wako Chemicals, Richmond, VA). Insulin was measured using a radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX).
Muscle lipid analysis.
Skeletal muscle lipid extraction, isolation, and analysis were performed as previously described by our laboratory (3). Briefly, skeletal muscle samples were dissected free of extramuscular fat on ice, lyophilized, added to 1 ml of iced methanol along with internal standards of tripentadecanoic acid and dipentadecanoic acid, and homogenized (Omni TH; Omni International, Marietta, GA). Total lipids were extracted (45) and then added to solid-phase extraction columns (Supelclean LC-NH2, 3 ml; Supelco Analytical) to isolate FFAs, phospholipids, IMTG, and DAG. The FFA, phospholipid, IMTG, and DAG fractions were converted to fatty acid methyl esters (FAME), and the stable isotope ratios of 13C in FAMEs were measured using a gas chromatography-combustion isotope ratio mass spectrometer (GC/C-IRMS) system (Thermo Electron, Bremen, Germany). Concentration and composition analysis was performed on an HP 6890 GC with a 30-m DB-23 capillary column, connected to a HP 5973 MS. Peak identities were determined by retention time and mass spectra compared with standards of known composition.
Western blotting.
Frozen skeletal muscle samples were weighed and homogenized on ice using a Kontes glass homogenizer (Kimble/Kontes, Vineland, NJ) in buffer as previously described (3). Protein was extracted, concentration was measured (Calbiochem, San Diego, CA), and 40 μg of sample and internal standard were run on an SDS-PAGE 8% Bis-Tris gel (Invitrogen, Carlsbad, CA) using standard methods as previously described (3). The rabbit anti-human stearoyl-CoA desaturase 1 (anti-hSCD1) was purchased from Alpha Diagnostics (San Antonio, TX), peroxisome proliferator-activated receptor (PPAR)-α and -γ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and rabbit anti-β-actin was purchased from Cell Signaling (Danvers, MA). Secondary antibodies were obtained from Bio-Rad (Hercules, CA).
RT-PCR.
Total RNA was extracted from homogenized muscle biopsies using the RNeasy mini kit (Qiagen, Valencia, CA). RNA was analyzed and quantified using the Experion system (Bio-Rad). Reverse transcription was performed using 45 ng of total RNA with the iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR was performed using primer sets for genes of interest or two reference genes (all spanned exon-exon boundaries) and ABsolute Blue QPCR SYBR Green fluorescein mix (Thermo Fisher Scientific, Waltham, MA) following the manufacturer's protocol (Table 2). Reactions were run in duplicate on an iQ5 real-time PCR detection system (Bio-Rad) along with a no-template control per gene. RNA expression data were normalized to levels of ribosomal protein L13a and ubiquitin C using the comparative threshold cycle method.
Table 2.
RT-PCR primer sequences
| Forward Primer | Reverse Primer | |
|---|---|---|
| SCD1 | AACTGGTGATGTTCCAGAGGAGGT | CGCAAGAAAGTGGCAACGAACACA |
| DGAT1 | ATGTCTTTGCTGTGGCTGCATTCC | AAACACAGAATGGTGGCCAGGTTG |
| ATGL | ATCCCACTTCAACTCCAAGGACGA | GCAGGTTGTCTGAAATGCCACCAT |
| SREBP-1c | GGAGCCATGGATTGCACTTT | TCCCAGCATAGGGTGGGTCAAATA |
| PPAR-α | TGCACTGGAACTGGATGACAGTGA | ACGTTTAGAAGGCCAGGACGATCT |
| PPAR-γ | TCATCCTCTCAGGAAAGGCCAGTA | AGCGTCTTCTCAGCCATACACAGT |
| RPL13A | CCTGGAGGAGAAGAGGAAAGAGA | TTGAGGACCTCTGTGTATTTGTCAA |
| UBC | ATTTGGGTCGCGGTTCTTG | TGCCTTGACATTCTCGATGGT |
SCD1, stearoyl-CoA desaturase 1; DGAT1, diacylglycerol acyltransferase 1; ATGL, adipose triglyceride lipase; SREBP-1c, sterol regulatory element binding protein 1c; PPAR-α and -γ, peroxisome proliferator-activated receptor-α and -γ; RPL13A, ribosomal protein L13a; UBC, ubiquitin C.
Plasma palmitate analysis.
Methylation and extraction of plasma palmitate were performed as previously described (40). Samples were run on an HP 6890 GC with a 30-m DB-23 capillary column, connected to a HP 5973 MS. Enrichments were calculated based on a standard curve of known enrichments and corrected for variations in abundance (41). Peak identities were determined by retention time and mass spectra compared with standards of known composition.
Calculations.
IMTG fractional synthesis rate was calculated as previously described for use with stable isotopes in humans, using plasma palmitate as the precursor pool from which IMTG is synthesized (47): where EIMTG palm(t1) and Eplasma palm(t1) are enrichments of palmitate in IMTG and plasma after 4 h of infusion and EIMTG palm(t0) and Eplasma palm(t0) are enrichments of background palmitate in IMTG and plasma, respectively. Background enrichment of IMTG palmitate was determined using enrichment of stearate as previously described, eliminating the need for a baseline biopsy specifically for this purpose (17): where FFA represents the concentration of individual FFA species in IMTG and DAG after transmethylation.
Statistics.
Data are means ± SE. Differences in normally distributed data between controls and athletes were analyzed using one-way ANOVA (SPSS, Chicago, IL). Nonnormally distributed data were log-transformed before analysis using one-way ANOVA. Correlation analysis was performed using the Pearson product moment correlation. An α level of 0.05 was used throughout.
RESULTS
Subject characteristics and demographic information are shown in Table 1. All subjects were college-age men, although athletes were statistically older (P = 0.01). Both groups had a similar body mass index; however, athletes had significantly less body fat (P < 0.001) and a lower fasting insulin concentration (P = 0.03). There were no differences in fasting plasma glucose, FFA, or triglyceride concentrations. As expected, insulin sensitivity (Si) was significantly greater in athletes compared with controls (Table 1, P = 0.004). V̇o2max testing was not performed on sedentary controls, but they self-reported <2 h of planned physical activity per week. Athletes had been training 5.8 ± 1.6 yr and were training 14.8 ± 1.4 h/wk during this period of testing. They had V̇o2max (68.3 ± 2.1 ml·kg−1·min−1) and lactate threshold values (86.8 ± 0.9% V̇o2max) indicating a highly trained state (8).
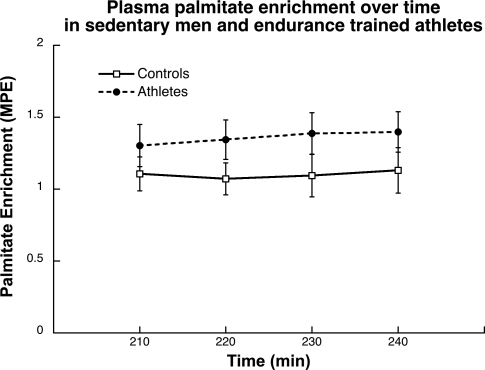
Plasma palmitate enrichment during the last 30 min of the 4-h infusion in both groups is shown in Fig. 1. The values were stable over time and were not significantly different (P = 0.18) between endurance-trained athletes and control subjects.
Fig. 1.
Plasma palmitate enrichment over time during the last 30 min of the 4-h infusion in endurance-trained athletes and control subjects. Values are means ± SE. MPE, mole percent excess.
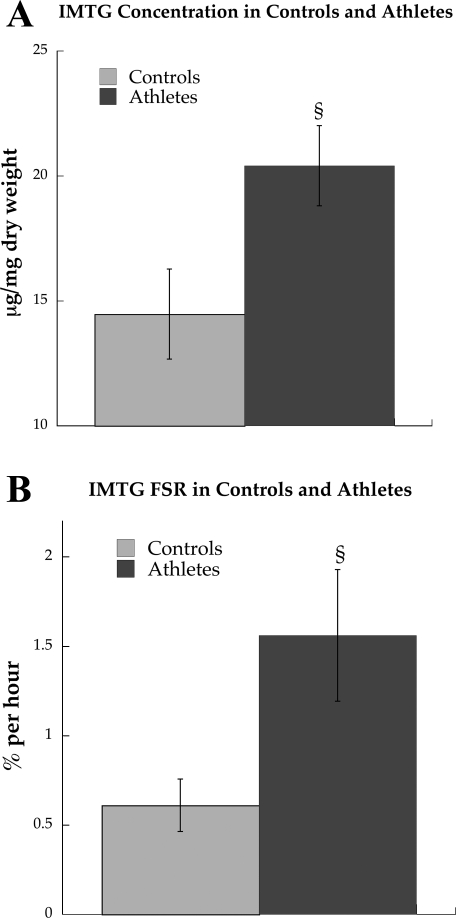
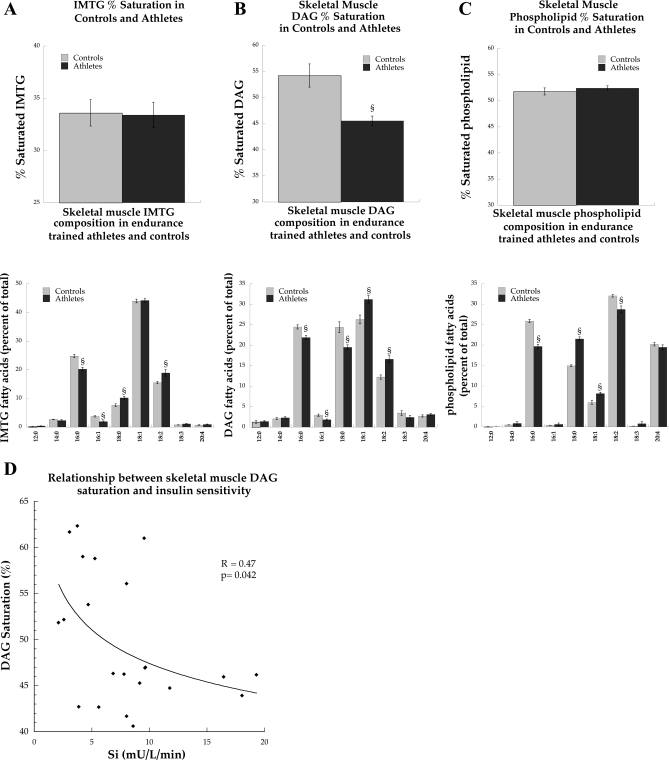
IMTG concentration was significantly greater in endurance-trained athletes compared with controls (Fig. 2A, P = 0.04). Increased IMTG concentration was paralleled by a significantly greater fractional synthesis rate (FSR) of the IMTG pool in athletes compared with controls (Fig. 2B, P = 0.03). The concentration of skeletal muscle DAG was not significantly different between groups (Table 1). Although there were differences in individual IMTG and phospholipid species, overall the percent saturations of IMTG and phospholipids were not significantly different between groups (Fig. 3, A and C). However, the overall percent saturation of DAG was significantly decreased in endurance-trained athletes compared with control subjects (Fig. 3B, P = 0.002). The correlation between Si and DAG percent saturation was statistically significant (R = 0.47, P = 0.04, Fig. 3D).
Fig. 2.
Intramuscular triglyceride concentration (IMTG; A) and fractional synthesis rate (FSR; B) in endurance-trained athletes and control subjects after an overnight fast at rest. Values are means ± SE. §P < 0.05, significantly different from control subjects.
Fig. 3.
IMTG (A), diacylglycerol (DAG; B), and phospholipid (C) %saturation and composition in endurance-trained athletes and control subjects after an overnight fast at rest. D: significant relationship between DAG %saturation and insulin sensitivity (Si). Values are means ± SE. §P < 0.05, significantly different from control subjects.
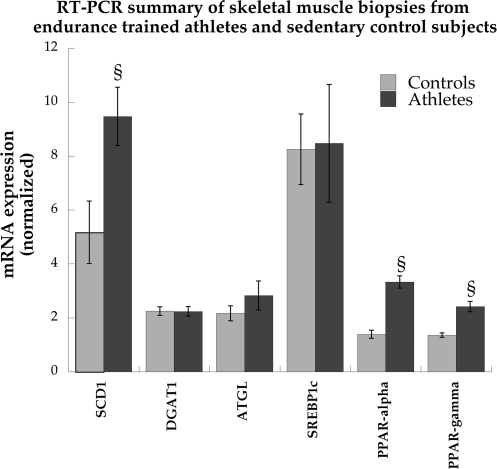
Data from RT-PCR analysis are shown in Fig. 4. We evaluated whether there were differences in the expression of genes involved in desaturation of long-chain acyl (LCA)-CoA (SCD1), IMTG synthesis (diacylglycerol acyltransferase 1; DGAT1), IMTG degradation (adipose triglyceride lipase; ATGL), nuclear transcription factors controlling expression of genes for mitochondrial fatty acid oxidation and β-oxidation (PPAR-α) and fatty acid synthesis (PPAR-γ), and a transcription factor regulating genes controlling lipogenesis (sterol regulatory element binding protein 1c; SREBP1c). The relative expression of SCD1 mRNA was significantly greater in athletes compared with control subjects (P = 0.01). There were no differences in relative mRNA expression of DGAT1, ATGL, and SREBP1c mRNA. PPAR-α (P < 0.0001) and PPAR-γ (P = 0.0002) mRNA expression were significantly greater in athletes compared with control subjects.
Fig. 4.
Skeletal muscle mRNA expression of stearoyl-CoA desaturase 1 (SCD1), diacylglycerol acyltransferase 1 (DGAT1), adipose triglyceride lipase (ATGL), sterol regulatory element binding protein 1c (SREBP1c), and peroxisome proliferator-activated receptor-α (PPAR-α) and -γ (PPAR-γ) in sedentary control subjects and endurance-trained athletes. RNA expression data were normalized to levels of ribosomal protein L13a and ubiquitin C using the comparative threshold cycle method. Values are means ± SE. §P < 0.05, significantly different from control subjects.
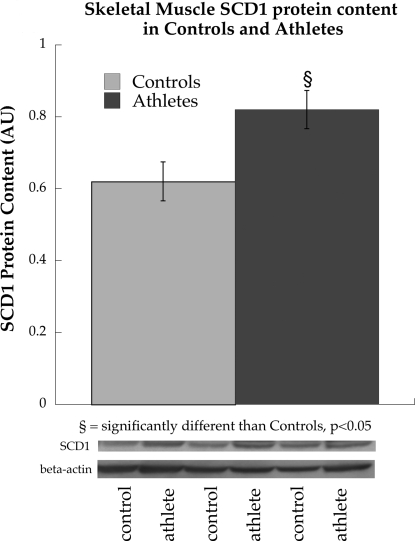
Similar to mRNA expression, protein content of SCD1 was also significantly increased in athletes compared with control subjects (P = 0.03, Fig. 5A). However, we found no differences in PPAR-γ [controls: 1.0 ± 0.07 arbitrary units (AU), athletes: 0.96 ± 0.04 AU, P = 0.68] or PPAR-α (controls: 1.0 ± 0.15 AU, athletes: 0.81 ± 0.16 AU, P = 0.47) protein content between groups.
Fig. 5.
Protein content of SCD1 in endurance-trained athletes and sedentary control subjects. Values are means ± SE. §P < 0.05, significantly different from control subjects. AU, arbitrary units.
The proportion of energy expenditure from whole body fat oxidation was determined using the respiratory exchange ratio and was not significantly different between groups (controls: 0.81 ± 0.02, athletes: 0.80 ± 0.02, P = 0.63). Correlations between Si and IMTG FSR (R2 = 0.07, P = 0.25) were not significant.
DISCUSSION
There is a growing body of literature suggesting increased rates of IMTG synthesis protect against insulin resistance during fat-induced insulin resistance in cell culture (31), rodents (22, 32), and humans (49). The historical view of IMTG negatively influencing insulin sensitivity is changing to a new view suggesting the ability to increase IMTG synthesis can prevent insulin resistance. These are the first published data comparing IMTG synthesis rates in endurance-trained athletes compared with those in sedentary subjects. There are two main findings from this study: 1) endurance-trained athletes have significantly increased synthesis rates of IMTG compared with sedentary control subjects, and 2) saturation of the intramuscular DAG pool is decreased in athletes compared with control subjects.
As expected, athletes in the current study were significantly more insulin sensitive and had greater IMTG content compared with controls. It has been hypothesized (15, 46) that increased flux through IMTG may be one mechanism by which athletes are “protected” from the negative relationship between IMTG content and insulin action observed in other populations (39). Our data corroborate this hypothesis and imply that IMTG synthesis, rather than concentration, may be an important link between intramuscular lipids and insulin action (15). After an exercise bout that reversed fatty acid-induced insulin resistance, Schenk and Horowitz (49) reported DAG concentration decreased and IMTG storage increased. These data suggested increased rates of IMTG synthesis prevented accumulation of skeletal muscle DAG. However, differences in insulin action in the current study cannot be explained by differences in DAG concentration, which was not different between groups. Our working hypothesis is that increased neutral lipid synthesis rates may enhance clearance of DAG into synthetic pathways (i.e., IMTG), decreasing interaction with protein kinase C (PKC) and insulin resistance (44). Interestingly, other investigators increased flux through IMTG synthesis in skeletal muscle, via either DGAT1 overexpression in mice (32) or a single bout of exercise (49), and reported protection against fat-induced insulin resistance. These data, combined with our data showing increased IMTG FSR in endurance-trained athletes, advance the idea that high rates of IMTG synthesis may be beneficial to insulin sensitivity. Therefore, increased rates of muscle lipid synthesis in endurance-trained athletes may be one mechanism that helps explain the “athlete's paradox.”
The current data showing increased IMTG FSR in athletes are different from reports in rodent literature where increased IMTG synthesis rates are associated with insulin resistance in skeletal muscle (21) and myocardium (38). It is possible that these differences are due to species effects in the regulation of IMTG. It also is possible that increased synthesis of IMTG occurs as a function of increased IMTG pool size. More data need to be published, specifically from insulin-resistant subjects, to determine the relationship between IMTG FSR and insulin sensitivity in humans. Our data suggest increased synthesis rates of the IMTG pool are associated with insulin sensitivity in humans.
We measured plasma insulin and FFA concentration, which inhibit IMTG FSR (11, 19, 20, 56), to determine whether regulation by these factors could explain differences in IMTG synthesis rates. There were no significant differences in the concentration of FFA between endurance-trained athletes and sedentary controls. We found a statistically lower fasting insulin concentration in athletes compared with controls; however, it is unlikely that such a small difference in concentration would alter IMTG flux considering the hyperinsulinemia required to decrease IMTG synthesis (20). Recovery from exercise also may influence IMTG use (29). Therefore, we asked subjects to refrain from exercise for 48 h before the study. These data suggest increased synthesis rates of IMTG are a result of chronic exercise training, and not an acute effect of recovery from exercise or alterations in known hormones or substrates that regulate IMTG synthesis.
In addition to intramuscular lipid content, composition of these lipids also may influence insulin sensitivity (30, 33). Alteration in hepatic lipid composition, with increased ratios of 16:1/16:0 and 18:1/18:0, without a change in steatosis, increased hepatic insulin action in a rodent model (33), highlighting the potential importance of lipid composition on insulin sensitivity. Furthermore, insulin-sensitizing lifestyle behaviors such as weight loss and exercise training have been reported to decrease saturation of LCA-CoA, ceramide, and DAG in obese humans (6, 24). Similar to previous reports (6), we did not find a significant difference in overall IMTG saturation between groups. However, overall DAG saturation was lower in athletes compared with controls. In addition, we found a significant inverse relationship between percent saturation of DAG and insulin sensitivity (P = 0.04). It is well established that diets rich in saturated fats decrease insulin sensitivity (52, 53). Therefore, it is possible that saturated lipid species may exert a negative influence on insulin sensitivity in skeletal muscle. The literature is unclear on the influence of DAG saturation on PKC activity, which is the leading mechanism explaining DAG-induced insulin resistance (27). PKC activation in vitro has been reported to be higher with unsaturated acyl chains on DAG (23, 48) or independent of saturation (28). However, palmitate administration in primary muscle cell culture increased palmitate incorporation into DAG and resulted in greater PKC activation (35). Our data suggest saturation of skeletal muscle DAG, even without changes in concentration, may be important in modulating insulin action in skeletal muscle after chronic endurance exercise training.
Lower intramuscular DAG saturation in athletes does not appear to be due to differences in dietary lipid composition. Measuring dietary fat intake is unreliable by dietary record (50), and therefore previous studies used the overall composition of muscle phospholipids as a surrogate measure of dietary lipid composition (1), which was not different between groups. Because there was no difference in IMTG saturation but a significant decrease in DAG saturation between groups, these data suggest that exercise training specifically changes the composition of intramuscular DAG in skeletal muscle.
Alterations in DAG composition in athletes may be due to increased enzymatic lipid desaturation. One such enzyme is SCD1, which converts saturated palmitoyl-CoA and stearoyl-CoA to monounsaturated palmitoleoyl-CoA and oleoyl-CoA, respectively. Studies reported SCD1 inhibition resulted in an obesity-resistant and insulin-sensitive phenotype in mice (37), and SCD1 content was elevated in obese subjects, which was related to decreased fat oxidation and increased fat storage (25). However, others reported SCD1 content was related to insulin sensitivity in humans (42), and increasing SCD1 content transiently in rat muscle cells protects against fat-induced insulin resistance (43). The definitive answer on the role of SCD1 content in insulin sensitivity is not clear. Based on our data and those of others (26), it appears chronic endurance exercise training increases SCD1 mRNA expression and protein content compared with controls. Increased SCD1 content in athletes may be one mechanism influencing decreased storage of saturated DAG in skeletal muscle.
Other intracellular lipids, including LCA-CoA (12) and ceramide in some (54, 55) but not all studies (51), have been implicated in muscle insulin resistance. Endurance training has been reported to decrease muscle ceramide concentration in rodents (9) and obese humans (6, 10), in addition to decreasing saturated ceramide species (6) in some, but not all, studies (51). The effect of endurance training on LCA-CoA is unclear. Therefore, although not measured in this study, it is possible that changes in LCA-CoA and ceramide content or composition also may influence insulin sensitivity in endurance-trained athletes.
There are several limitations to this study. We did not measure differences in muscle fiber type between subjects, so it is possible that muscle fiber type influenced our results. Palmitate was used as a tracer in this experiment, because this FFA has been previously reported to measure IMTG FSR (18, 47). It is possible that differences in IMTG FSR may be attained using an alternatively labeled FFA; however, previous studies have not found a difference between IMTG FSR measured with oleate and palmitate in rodent muscle (18). We used plasma palmitate as the precursor pool from which IMTG is synthesized in the calculation of IMTG FSR. Plasma FFA and intramuscular FFA enrichment have been used in previous studies; therefore the synthesis rates calculated are similar to some, but not all, published data (3, 17, 19, 21, 47). We used the enrichment of plasma palmitate during the final 30 min of isotope infusion as the enrichment of the precursor pool for IMTG synthesis. However, during a long infusion such as this, plasma triglyceride also becomes labeled with [13C16]palmitate, which increases with time (Table 3). Our calculations for IMTG synthesis do not include the contribution of labeled palmitate derived from the action of lipoprotein lipase on plasma triglyceride. We also cannot account for substrate cycling of labeled palmitate, where palmitate derived from IMTG degradation was reincorporated back into IMTG. Our calculations also assume linearity of triglyceride synthesis over 4 h and that no stearate was synthesized during the infusion by elongation of unlabeled palmitate with labeled acetyl-CoA and/or labeled palmitate with unlabeled or labeled acetyl-CoA. Our methods assume that there was no hydrolysis of muscle lipids during microdissection and no contamination of intramuscular triglyceride by extracellular triglyceride during sample processing. Finally, we used a whole body measure of insulin sensitivity, but it is likely that muscle lipid synthesis is more related to muscle insulin sensitivity, which may have decreased our ability to determine such a relationship.
Table 3.
Stable isotope enrichments for skeletal muscle and plasma lipid species
| Controls | Athletes | |
|---|---|---|
| IMTG stearate enrichment (AP) | 1.081 ± 0.0012 | 1.077 ± 0.0018 |
| DAG stearate enrichment (AP) | 1.086 ± 0.003 | 1.082 ± 0.004 |
| IMTG palmitate enrichment (AP) | 1.107 ± 0.005 | 1.147 ± 0.008* |
| DAG palmitate enrichment (AP) | 1.151 ± 0.013 | 1.190 ± 0.008* |
| Plasma TG-palmitate enrichment (MPE) | 0.24 ± 0.02 | 0.22 ± 0.01 |
| Plasma FFA-palmitate enrichment (MPE) | 1.10 ± 0.13 | 1.32 ± 0.14 |
Values are means ± SE.
P < 0.05, significant difference between controls and athletes. AP, atom percent from gas chromatography-combustion isotope ratio mass spectrometry (GC/C/IRMS) reflects the 13C enrichment of all carbons in the lipid fraction of interest; MPE, mole percent excess from GC/MS reflects enrichment above background of the M +16 isotopomer of palmitate in TG and FFA fractions.
To conclude, these are the first data in humans comparing rates of intramuscular triglyceride synthesis between endurance-trained and untrained individuals. These data are compelling but need to be verified in the future. Our data suggest endurance-trained athletes have significantly increased rates of IMTG synthesis, as well as concentration, compared with sedentary subjects. Furthermore, endurance-trained athletes have a similar DAG concentration to that of sedentary controls. Diacylglycerol saturation was decreased in athletes compared with sedentary subjects, which was significantly related to insulin sensitivity. These data indicate intramuscular lipid synthesis rates and/or DAG saturation may influence insulin sensitivity in endurance-trained athletes.
GRANTS
This work was partially supported by National Institutes of Health (NIH) General Clinical Research Center Grant RR-00036 and NIH Grants DK-064811 (to L. Perreault), DK-26356 (R. H. Eckel), and DK-059739 (B. C. Bergman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Andersson A, Nalsen C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr 76: 1222–1229, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. An evaluation of exercise and training on muscle lipid metabolism. Am J Physiol Endocrinol Metab 276: E106–E117, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes 58: 2220–2227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergman RN. Minimal model: perspective from 2005. Horm Res 64, Suppl 3: 8–15, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand 71: 140–150, 1967 [DOI] [PubMed] [Google Scholar]
- 6. Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJ, Dyck DJ. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 291: E99–E107, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Copeland KC, Kenney FA, Nair KS. Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am J Physiol Endocrinol Metab 263: E1010–E1014, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Coyle EF, Feltner ME, Kautz SA, Hamilton MT, Montain SJ, Baylor AM, Abraham LD, Petrek GW. Physiological and biomechanical factors associated with elite endurance cycling performance. Med Sci Sports Exerc 23: 93–107, 1991 [PubMed] [Google Scholar]
- 9. Dobrzyn A, Zendzian-Piotrowska M, Gorski J. Effect of endurance training on the sphingomyelin-signalling pathway activity in the skeletal muscles of the rat. J Physiol Pharmacol 55: 305–313, 2004 [PubMed] [Google Scholar]
- 10. Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dyck DJ, Miskovic D, Code L, Luiken JJ, Bonen A. Endurance training increases FFA oxidation and reduces triacylglycerol utilization in contracting rat soleus. Am J Physiol Endocrinol Metab 278: E778–E785, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Ellis BA, Poynten A, Lowy AJ, Furler SM, Chisholm DJ, Kraegen EW, Cooney GJ. Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol Endocrinol Metab 279: E554–E560, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Fink WJ, Costill DL, Pollock ML. Submaximal and maximal working capacity of elite distance runners. Part II. Muscle fiber composition and enzyme activities. Ann NY Acad Sci 301: 323–327, 1977 [DOI] [PubMed] [Google Scholar]
- 14. Friedlander AL, Jacobs KA, Fattor JA, Horning MA, Hagobian TA, Bauer TA, Wolfel EE, Brooks GA. Contributions of working muscle to whole body lipid metabolism are altered by exercise intensity and training. Am J Physiol Endocrinol Metab 292: E107–E116, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Grunwald GK, Melanson EL, Forster JE, Seagle HM, Sharp TA, Hill JO. Comparison of methods for achieving 24-hour energy balance in a whole-room indirect calorimeter. Obes Res 11: 752–759, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Guo Z, Jensen MD. Determination of skeletal muscle triglyceride synthesis using a single muscle biopsy. Metabolism 51: 1198–1205, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Guo Z, Jensen MD. Intramuscular fatty acid metabolism evaluated with stable isotopic tracers. J Appl Physiol 84: 1674–1679, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Guo Z, Zhou L. Fatty acids inhibit intramyocellular triglyceride synthesis and turnover acutely in high fat-fed obese rats. Horm Metab Res 38: 721–726, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Guo Z, Zhou L, Jensen MD. Acute hyperinsulinemia inhibits intramyocellular triglyceride synthesis in high-fat-fed obese rats. J Lipid Res 47: 2640–2646, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Guo ZK, Jensen MD. Accelerated intramyocellular triglyceride synthesis in skeletal muscle of high-fat-induced obese rats. Int J Obes Relat Metab Disord 27: 1014–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312: 734–737, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Hinderliter AK, Dibble AR, Biltonen RL, Sando JJ. Activation of protein kinase C by coexisting diacylglycerol-enriched and diacylglycerol-poor lipid domains. Biochemistry 36: 6141–6148, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Houmard JA, Tanner CJ, Yu C, Cunningham PG, Pories WJ, MacDonald KG, Shulman GI. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes 51: 2959–2963, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2: 251–261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ikeda S, Miyazaki H, Nakatani T, Kai Y, Kamei Y, Miura S, Tsuboyama-Kasaoka N, Ezaki O. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem Biophys Res Commun 296: 395–400, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Kerr DE, Kissinger LF, Gentry LE, Purchio AF, Shoyab M. Structural requirements of diacylglycerols for binding and activating phospholipid-dependent, Ca++-sensitive protein kinase. Biochem Biophys Res Commun 148: 776–782, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Kiens BK, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol Endocrinol Metab 275: E332–E337, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106: 2747–2757, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100: 3077–3082, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117: 1679–1689, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N, Iwasaki H, Iwasaki Y, Karasawa T, Kumadaki S, Matsui T, Sekiya M, Ohashi K, Hasty AH, Nakagawa Y, Takahashi A, Suzuki H, Yatoh S, Sone H, Toyoshima H, Osuga J, Yamada N. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med 13: 1193–1202, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Mittendorfer B, Sidossis LS, Walser E, Chinkes DL, Wolfe RR. Regional acetate kinetics and oxidation in human volunteers. Am J Physiol Endocrinol Metab 274: E978–E983, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Montell E, Turini M, Marotta M, Roberts M, Noe V, Ciudad CJ, Mace K, Gomez-Foix AM. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab 280: E229–E237, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55, Suppl 2: S9–S15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 99: 11482–11486, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am J Physiol Endocrinol Metab 290: E448–E455, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 40: 2118–2124, 1999 [PubMed] [Google Scholar]
- 41. Patterson BW, Zhao G, Klein S. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism 47: 706–712, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Peter A, Weigert C, Staiger H, Machicao F, Schick F, Machann J, Stefan N, Thamer C, Haring HU, Schleicher E. Individual stearoyl-CoA desaturase 1 expression modulates endoplasmic reticulum stress and inflammation in human myotubes and is associated with skeletal muscle lipid storage and insulin sensitivity in vivo. Diabetes 58: 1757–1765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinnamaneni SK, Southgate RJ, Febbraio MA, Watt MJ. Stearoyl CoA desaturase 1 is elevated in obesity but protects against fatty acid-induced skeletal muscle insulin resistance in vitro. Diabetologia 49: 3027–3037, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Qu X, Seale JP, Donnelly R. Tissue and isoform-selective activation of protein kinase C in insulin-resistant obese Zucker rats—effects of feeding. J Endocrinol 162: 207–214, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Rosendal J, Knudsen J. A fast and versatile method for extraction and quantitation of long-chain acyl-CoA esters from tissue: content of individual long-chain acyl-CoA esters in various tissues from fed rat. Anal Biochem 207: 63–67, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Russell AP. Lipotoxicity: the obese and endurance-trained paradox. Int J Obes Relat Metab Disord 28, Suppl 4: S66–S71, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Sacchetti M, Saltin B, Osada T, van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. J Physiol 540: 387–395, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sanchez-Pinera P, Micol V, Corbalan-Garcia S, Gomez-Fernandez JC. A comparative study of the activation of protein kinase C alpha by different diacylglycerol isomers. Biochem J 337: 387–395, 1999 [PMC free article] [PubMed] [Google Scholar]
- 49. Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schoeller DA, Bandini LG, Dietz WH. Inaccuracies in self-reported intake identified by comparison with the doubly labelled water method. Can J Physiol Pharmacol 68: 941–949, 1990 [DOI] [PubMed] [Google Scholar]
- 51. Skovbro M, Baranowski M, Skov-Jensen C, Flint A, Dela F, Gorski J, Helge JW. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 51: 1253–1260, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 40: 280–289, 1991 [DOI] [PubMed] [Google Scholar]
- 53. Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237: 885–888, 1987 [DOI] [PubMed] [Google Scholar]
- 54. Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol 18: 5457–5464, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang CN, O'Brien L, Brindley DN. Effects of cell-permeable ceramides and tumor necrosis factor-alpha on insulin signaling and glucose uptake in 3T3-L1 adipocytes. Diabetes 47: 24–31, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab 287: E120–E127, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Wolfe R. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992 [Google Scholar]