Abstract
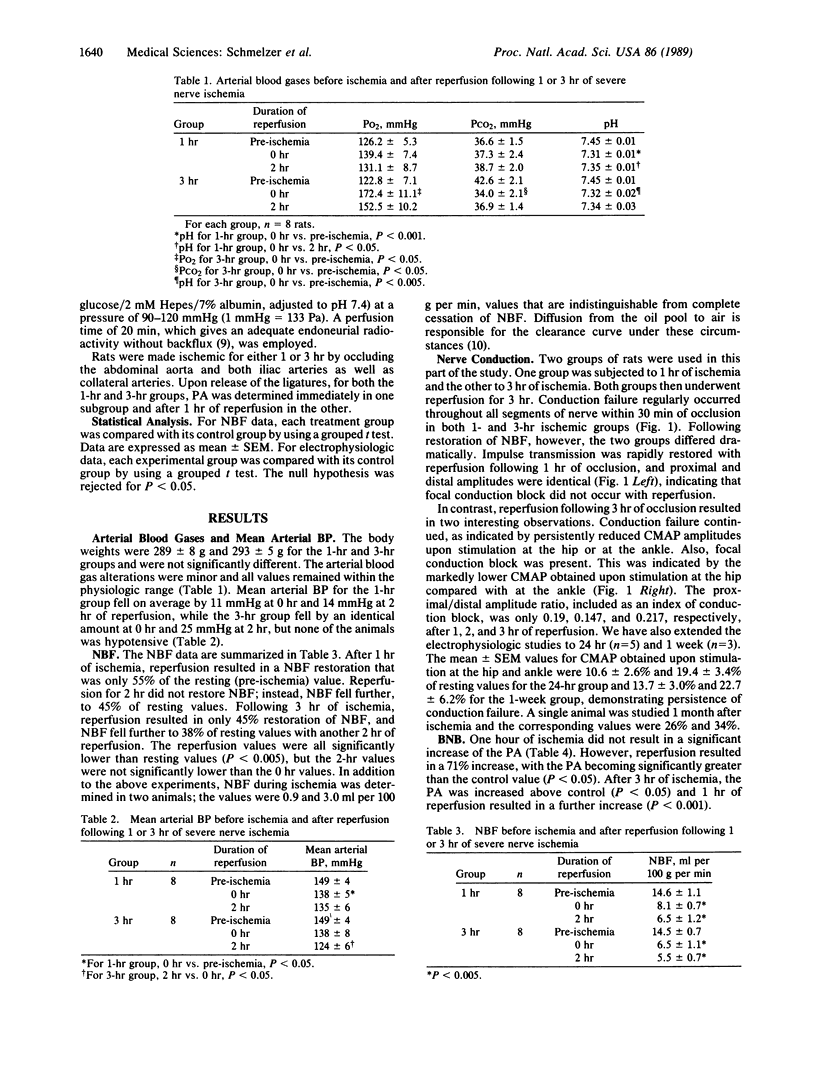
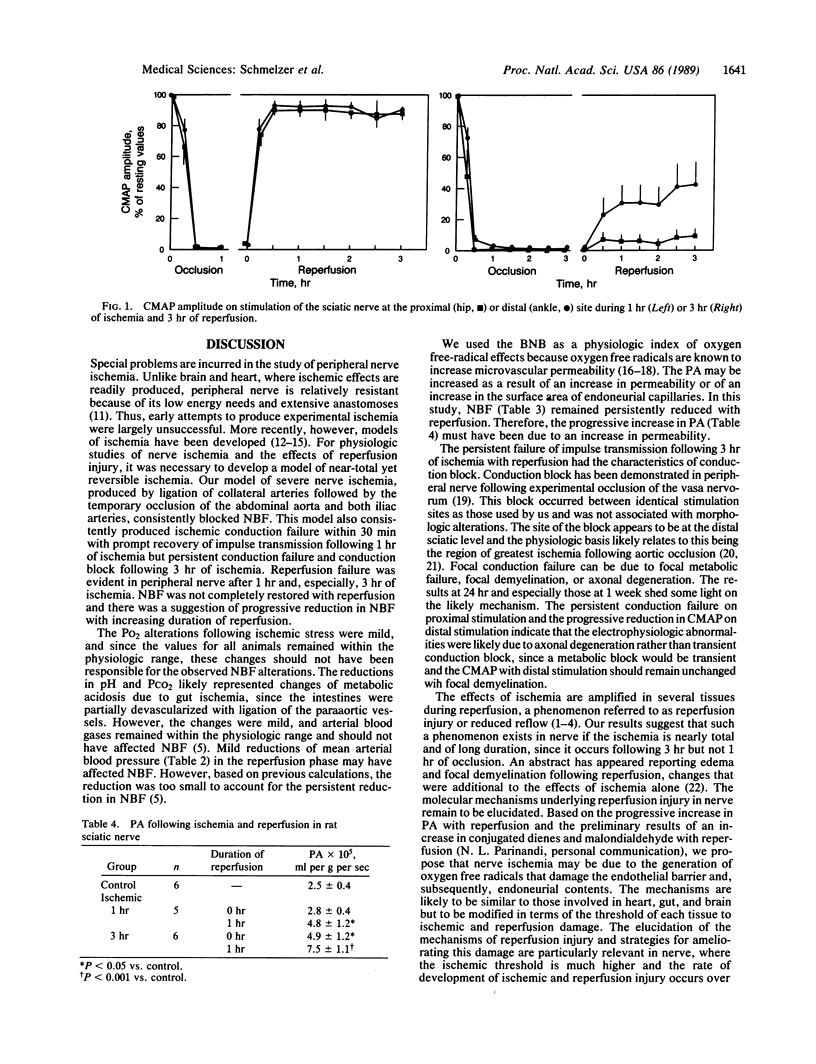
A rat model of severe nerve ischemia was used to study the effects of ischemia and reperfusion on nerve conduction, blood flow, and the integrity of the blood-nerve barrier. Conduction failure was consistently found in the sciatic-tibial nerve during 1- and 3-hr ischemic periods. Recovery of the compound muscle action potential was prompt and complete upon reperfusion following 1 hr of ischemia. However, after 3 hr of ischemia, recovery in the proximal portion of the sciatic nerve was less than 10%, and conduction block occurred in the distal portion of the nerve. Nerve blood flow was restored to only 55% and 45% of resting values following 1 and 3 hr, respectively, of ischemia and did not recover even after 2 hr of reperfusion. The blood-nerve barrier was not statistically impaired to the passage of [14C]sucrose following 1 hr of ischemia but was significantly impaired after 3 hr of ischemia. The permeability-surface area product was consistently greater following 1 hr of reperfusion than during the immediate reperfusion period. These data indicate that severe ischemia of peripheral nerve results in reperfusion injury, conduction block, and blood-nerve barrier disruption. Microvascular events, which may occur during reperfusion, may be important in amplifying the nerve fiber damage that began during ischemia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Del Maestro R. F., Björk J., Arfors K. E. Increase in microvascular permeability induced by enzymatically generated free radicals. II. Role of superoxide anion radical, hydrogen peroxide, and hydroxyl radical. Microvasc Res. 1981 Nov;22(3):255–270. doi: 10.1016/0026-2862(81)90096-0. [DOI] [PubMed] [Google Scholar]
- Demopoulos H. B., Flamm E. S., Pietronigro D. D., Seligman M. L. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl. 1980;492:91–119. [PubMed] [Google Scholar]
- Granger D. N., Höllwarth M. E., Parks D. A. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- Hearse D. J., Manning A. S., Downey J. M., Yellon D. M. Xanthine oxidase: a critical mediator of myocardial injury during ischemia and reperfusion? Acta Physiol Scand Suppl. 1986;548:65–78. [PubMed] [Google Scholar]
- Hess K., Eames R. A., Darveniza P., Gilliatt R. W. Acute ischaemic neuropathy in the rabbit. J Neurol Sci. 1979 Dec;44(1):19–43. doi: 10.1016/0022-510x(79)90220-x. [DOI] [PubMed] [Google Scholar]
- Korthals J. K., Wiśniewski H. M. Peripheral nerve ischemia. Part 1. Experimental model. J Neurol Sci. 1975 Jan;24(1):65–76. doi: 10.1016/0022-510x(75)90009-x. [DOI] [PubMed] [Google Scholar]
- Korthuis R. J., Granger D. N., Townsley M. I., Taylor A. E. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ Res. 1985 Oct;57(4):599–609. doi: 10.1161/01.res.57.4.599. [DOI] [PubMed] [Google Scholar]
- Low P. A., Schmelzer J. D. Peripheral nerve conduction studies in galactose-poisoned rats. Demonstration of increased resistance to ischemic conduction associated with endoneurial edema due to sugar alcohol accumulation. J Neurol Sci. 1983 Jun;59(3):415–421. doi: 10.1016/0022-510x(83)90026-6. [DOI] [PubMed] [Google Scholar]
- Low P. A., Tuck R. R. Effects of changes of blood pressure, respiratory acidosis and hypoxia on blood flow in the sciatic nerve of the rat. J Physiol. 1984 Feb;347:513–524. doi: 10.1113/jphysiol.1984.sp015079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg G. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. J Bone Joint Surg Am. 1975 Oct;57(7):938–948. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McManis P. G., Low P. A. Factors affecting the relative viability of centrifascicular and subperineurial axons in acute peripheral nerve ischemia. Exp Neurol. 1988 Jan;99(1):84–95. doi: 10.1016/0014-4886(88)90129-x. [DOI] [PubMed] [Google Scholar]
- Nukada H., Dyck P. J. Microsphere embolization of nerve capillaries and fiber degeneration. Am J Pathol. 1984 May;115(2):275–287. [PMC free article] [PubMed] [Google Scholar]
- Parry G. J., Brown M. J. Selective fiber vulnerability in acute ischemic neuropathy. Ann Neurol. 1982 Feb;11(2):147–154. doi: 10.1002/ana.410110207. [DOI] [PubMed] [Google Scholar]
- Parry G. J., Linn D. J. Transient focal conduction block following experimental occlusion of the vasa nervorum. Muscle Nerve. 1986 May;9(4):345–348. doi: 10.1002/mus.880090411. [DOI] [PubMed] [Google Scholar]
- Phillips S. C. Does ethanol damage the blood--brain barrier? J Neurol Sci. 1981 Apr;50(1):81–87. doi: 10.1016/0022-510x(81)90043-5. [DOI] [PubMed] [Google Scholar]
- Schmelzer J. D., Low P. A. The effect of hyperbaric oxygenation and hypoxia on the blood-nerve barrier. Brain Res. 1988 Nov 15;473(2):321–326. doi: 10.1016/0006-8993(88)90861-x. [DOI] [PubMed] [Google Scholar]
- Sladky J. T., Greenberg J. H., Brown M. J. Regional perfusion in normal and ischemic rat sciatic nerves. Ann Neurol. 1985 Feb;17(2):191–195. doi: 10.1002/ana.410170215. [DOI] [PubMed] [Google Scholar]
- Wei E. P., Christman C. W., Kontos H. A., Povlishock J. T. Effects of oxygen radicals on cerebral arterioles. Am J Physiol. 1985 Feb;248(2 Pt 2):H157–H162. doi: 10.1152/ajpheart.1985.248.2.H157. [DOI] [PubMed] [Google Scholar]


