Abstract
Pompe disease, a deficiency of lysosomal acid α-glucosidase, is a disorder of glycogen metabolism that can affect infants, children, or adults. In all forms of the disease, there is progressive muscle pathology leading to premature death. The pathology is characterized by accumulation of glycogen in lysosomes, autophagic buildup, and muscle atrophy. The purpose of the present investigation was to determine if myofibrillar dysfunction in Pompe disease contributes to muscle weakness beyond that attributed to atrophy. The study was performed on isolated myofibers dissected from severely affected fast glycolytic muscle in the α-glucosidase knockout mouse model. Psoas muscle fibers were first permeabilized, so that the contractile proteins could be directly relaxed or activated by control of the composition of the bathing solution. When normalized by cross-sectional area, single fibers from knockout mice produced 6.3 N/cm2 of maximum Ca2+-activated tension compared with 12.0 N/cm2 produced by wild-type fibers. The total protein concentration was slightly higher in the knockout mice, but concentrations of the contractile proteins myosin and actin remained unchanged. Structurally, X-ray diffraction showed that the actin and myosin filaments, normally arranged in hexagonal arrays, were disordered in the knockout muscle, and a lower fraction of myosin cross bridges was near the actin filaments in the relaxed muscle. The results are consistent with a disruption of actin and myosin interactions in the knockout muscles, demonstrating that impaired myofibrillar function contributes to weakness in the diseased muscle fibers.
Keywords: lysosomal glycogen storage disease, actin-myosin interaction
pompe disease (acid maltase deficiency, glycogen storage disease type II) is a lysosomal storage disorder caused by the deficiency of acid α-glucosidase (GAA) (9a, 27). Loss of the enzyme activity results in the accumulation of glycogen in various tissues, particularly in cardiac and skeletal muscles. Clinical manifestations depend largely on the levels of residual enzyme activity. The most severe infantile form is characterized by cardiomyopathy, skeletal muscle myopathy, and a rapid decline, leading to death due to cardiac failure in the 1st year of life (16). Progressive skeletal muscle weakness and wasting are the main clinical features in milder, late-onset forms of the disease. Even in its late-onset form, mortality due to respiratory insufficiency secondary to diaphragmatic weakness is high (28).
The diseased muscles exhibit atrophy, and enlarged lysosomes accumulate in the muscle cells (27). In a GAA knockout (GAA-KO) model of Pompe disease (22), we previously showed that accumulation of lysosomal glycogen in skeletal muscle leads to a profound disorder of autophagy (10, 11, 25), a lysosome-dependent intracellular pathway for degradation of long-lived proteins and damaged organelles (17).
The underlying mechanism of muscle weakness remains largely unknown. Hesselink et al. (13, 15) showed in another knockout model of Pompe disease that atrophy cannot fully account for the decline in muscle performance. In old knockout mice, only one-third of the muscle force loss could be attributed to a reduction in mass, whereas the remaining two-thirds is caused by what the authors refer to as a decrease in “muscle quality.” In addition, mathematical modeling of myofibrillar force transmission indicated that the effects of swollen lysosomes and debris cannot explain the severe loss of force (9). In the present study, single muscle fibers were used to directly assess myofibrillar function in the GAA-KO mouse model. Force levels, concentrations of contractile proteins, and structure of the filament assemblies within the single fibers were studied. Our results confirm that force generation in single muscle fibers is indeed impaired beyond what could be explained simply by atrophy. Furthermore, structural assessment by X-ray diffraction reveals profound disorder in the arrangement of the thick and thin filaments in the myofibrils. The data also indicate that the initial actin-myosin interactions leading to force generation are disrupted, and this may constitute a key factor in the decline of muscle strength in Pompe disease.
MATERIALS AND METHODS
Muscle fiber preparation.
Psoas muscles, which consist of >90% fast type II fibers, were dissected from 6-mo-old wild-type (WT) and GAA-KO mice. The membranes of the fibers were permeabilized with detergent so that the chemical environment of the myofilaments could be directly controlled through the bathing solution. Immediately after the animal's death, ∼0.5-mm-wide and ∼1-cm-long muscle strips were dissected and pinned at resting length to the bottom of a chamber covered with Sylgard (Dow Corning, Midland, MI). The muscle strips were incubated for 1 h at 5°C in skinning solution (see below) to which 1% Triton X-100 was added to permeabilize the cell membranes. The bathing solution was then replaced with skinning solution without detergent, and single ∼5-mm-long fibers were pulled from the bundle as previously described (30). The isolated single fibers were kept at resting length in skinning solution at 4–6°C for up to 3 days before use.
Solutions.
Skinning solution (pH 7.0) contained 5 mM KH2PO4, 5 mM MgAc, 5 mM EGTA, 3 mM Na2ATP, 50 mM creatine phosphate, 5 mM NaN3, 5 mM DTT, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO).
The relaxing (MgATP) solution contained 5 mM MgATP, 2 mM MgCl2, 2 mM EGTA, 5 mM DTT, 10 mM imidazole, and 20 mM creatine phosphate; ionic strength was adjusted to 200 mM with potassium propionate. An ATP-backup system, containing 109 U/ml of creatine kinase (Sigma-Aldrich), was included.
Activating solution contained relaxing solution and 3 mM CaCl2.
Lysis buffer contained 570 mM NaCl, 10 mM Tris·HCl (pH 8.0), 10% glycerol, 1% NP-40, 1 mM EDTA, protease inhibitor cocktail (1:100 dilution; Sigma-Aldrich), and phosphatase inhibitor cocktail set II (1:100 dilution; Calbiochem, San Diego, CA). Protease and phosphatase inhibitors were added just before use.
Mechanical measurements.
Single fibers were transferred to the experimental chamber for force measurement through a transfer boat. Both chambers were filled with relaxing solution so that the fibers would not be exposed to air during the transfer. In the experimental chamber, the two ends of the fiber were held by two tiny clips: one was connected to the force transducer, and the other was fixed to a rod. The sarcomere length was monitored by laser diffraction (8) and adjusted by moving either end of the fiber. The muscle chamber had one inlet and one outlet for exchanging the bathing solution, which was delivered by a syringe system under pressure. The time and rate of delivery were controlled electronically by LabView software (National Instruments, Austin, TX). Rapid exchange of solutions from relaxing to activating is essential to achieve a uniform activation level and mechanical stability. Generally, a volume equal to or greater than the specimen chamber (0.5 ml) was delivered to the chamber for each exchange. Two rounds of solution exchange were sufficient to achieve maximum force development or full relaxation. The rate of delivery was 1 ml/s.
The force transducer system was modified from an earlier design (12) consisting of an infrared light emitter, two infrared collectors, and a small hypodermic tube positioned in the gap between the emitter and the collectors. As force was generated by the muscle fiber, deflection of the tube attached to one end of the fiber would intercept various amounts of light reaching the collectors. The electrical signal converted from the collected light was processed by a Wheatstone bridge circuit. The sensitivity of the force transducer output was generally 0.0286 mg/mV. The response was linear between 0 and 100 mg of force and was calibrated after each experiment.
The temperature of the bathing solution in the chamber with the bundles was maintained at the preset temperatures ±1°C by two thermoelectric devices (Melcor, Trenton, NJ) controlled by a feedback circuit (Cambion, Cambridge, MA). Experiments were performed at 20°C.
After the muscle fiber was transferred to the experimental chamber, it was rinsed with relaxing solution two to three times, and the baseline of the force level was then determined. Activating solution was exchanged twice within a 5-s interval. Isometric force reached a steady level in ∼5 s and was stable for at least ∼30 s, decreasing <10% during this period. Activation was ended by replacement of the activating solution with relaxing solution. In general, three activation cycles were recorded.
Protein determination and gel electrophoresis.
The volumes of single fibers were calculated from measurements of fiber dimensions under relaxing conditions. The sarcomere length of each fiber was monitored by laser diffraction and adjusted to ∼2.4 μm. The diameter of each fiber was read through a calibrated eyepiece on a dissecting microscope at ×50 magnification at three or more points along the length of the fiber. Fiber length was determined at ×20 magnification. The shape of the fiber was assumed to be cylindrical, so that the volume was defined as follows: V = [(πD2)/4]L, where D is fiber diameter and L is fiber length.
To reduce random errors in determining protein concentrations, single fibers were combined into groups of seven to eight, and each group was placed into 30 μl of lysis buffer. Samples were lysed using an ultrasonic processor (model GE 50T) at 60 MHz for 60 s. Total protein concentrations were determined using the bicinchoninic acid assay (Pierce Chemical, Rockford, IL).
The lysed samples were diluted with SDS sample buffer, and 1 or 5 μg of total proteins were separated by electrophoresis on NuPAGE Bis-Tris gels (Invitrogen, Carlsbad, CA). Gels were stained with SYPRO Ruby total protein gel stain according to the manufacturer's protocol (Invitrogen). The gels were subsequently scanned using a Typhoon fluorescent scanner (GE Healthcare Life Sciences, Piscataway, NJ). Images were analyzed using ImageQuant (GE Healthcare Life Sciences). The relative concentrations of individual protein bands were calculated by multiplying the gel intensities by the corresponding total protein concentration.
X-ray diffraction.
Equatorial X-ray diffraction patterns were obtained from bundles of psoas muscle fibers consisting of ∼20 fibers from the WT and ∼40 fibers from the GAA-KO mice. The Beamline X27C at the National Synchrotron Light Source of the Brookhaven National Laboratory (Upton, NY) was used as the X-ray source. The experimental set-up has been described previously (29). The distance between the specimen and the electronic X-ray detector (Marresearch, Hamburg, Germany) was 1,500 mm. The exposure time to X-rays for each equatorial diffraction pattern was 60 s.
Animal care and experimental protocol (A009-08-06) were approved by the Animal Care and Use Committee of the National Institute of Arthritis and Musculoskeletal and Skin Diseases in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
RESULTS
Mechanical properties and myofibrillar protein content of skinned fibers.
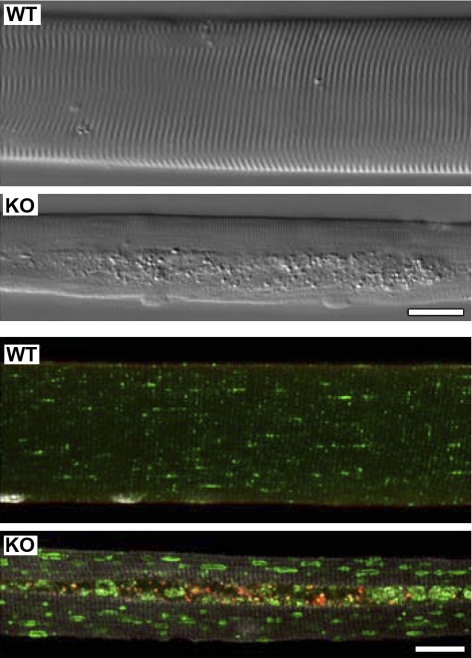
We verified that isolated psoas fibers from 6-mo-old GAA-KO mice exhibited the pathological features typical of Pompe disease. Muscular atrophy and autophagic buildup were clearly observed by interference microscopy (Fig. 1, top). In addition, lysosomal enlargement and accumulation of ubiquitinated proteins in the autophagic areas were detected by immunofluorescence (Fig. 1, bottom).
Fig. 1.
Pathological features of myofibers derived from fast (psoas) muscle of mice with Pompe disease. Top: differential interference contrast microscopy images of unstained single muscle fibers from a 6-mo-old wild-type (WT) and an acid α-glucosidase (GAA) knockout (KO) mouse. Centrally located autophagic accumulation is clearly visible throughout the length of the fiber from the GAA-KO mouse. Scale bar, 20 μm. Bottom: immunostaining of single fibers for mono- and polyubiquitinated conjugates (FK2, red; BIOMOL International, Philadelphia, PA) and for lysosomal-associated membrane protein 1 (green; BD Pharmingen, San Diego, CA). Procedure for isolation, fixation, and immunostaining of single fibers is described elsewhere (21). Centrally located area devoid of muscle striation corresponds to the region of autophagic accumulation in the GAA-KO fiber. Ubiquitin-positive structures are clearly seen in the GAA-KO fiber, and they are confined to the autophagic area. Lysosomal-associated membrane protein 1 staining is present throughout the fiber, in the autophagic area and on the enlarged lysosomes in the GAA-KO fiber. Lysosomes appear as small dots in the WT fiber, and there is no accumulation of the ubiquitin-positive structures. Scale bar, 20 μm.
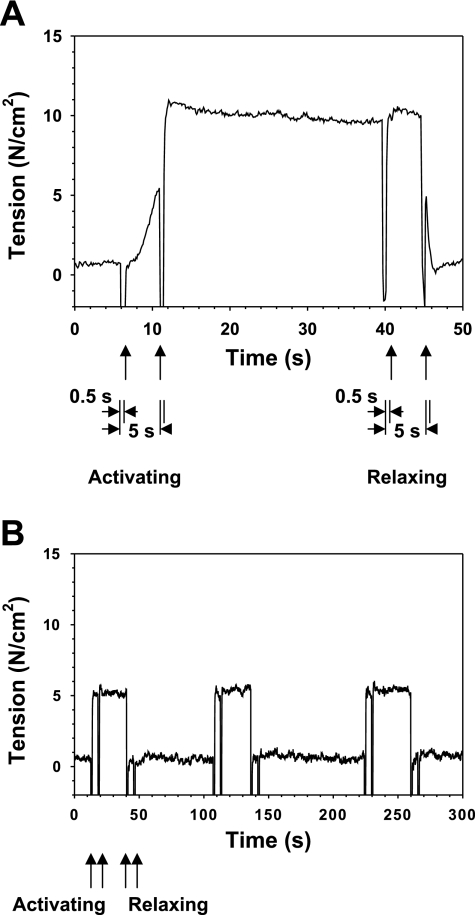
The GAA-KO fibers were much more difficult to isolate than single fibers from the WT mice, mainly because of excess connective tissue surrounding the fibers. However, undamaged fibers could be isolated and fully activated, and steady force levels could be maintained for several cycles of relaxation and activation (Fig. 2). In these experiments, chemical skinning was used to allow direct control of the solution bathing the myofibrils, eliminating membrane depolarization and intracellular Ca2+ release as factors that might affect the results.
Fig. 2.
Records of isometric tension generated by a single permeabilized psoas muscle fiber from a WT (A) and a GAA-KO (B) mouse. In A, muscle fiber was initially in the relaxed state at time 0. Activating solution was delivered to the muscle fiber at 5 s for 0.5 s; a second round of activating solution was delivered after a 5-s interval. After ∼30 s of steady tension, relaxing solution was delivered for 0.5 s, and another round of relaxing solution was delivered after a 5-s interval. In B, 3 relaxing-activating cycles demonstrate stability of the fiber. Similar cycles were observed for the WT mouse. Note different time scales in A and B. Mice were 6 mo of age, fiber diameters were 66 μm (A) and 59 μm (B), sarcomere length was 2.4 μm, and temperature was 20°C.
The maximum Ca2+-activated isometric forces and the force levels normalized by the fiber cross-sectional area generated by WT and GAA-KO single psoas fibers are shown in Table 1. The WT fibers generated considerably higher force than the GAA-KO fibers [40.8 ± 2.4 vs. 9.4 ± 0.7 (SE) mg]. When the forces were normalized by cross-sectional areas, the force generated by the GAA-KO fibers (6.3 ± 0.4 N/cm2) was 53% of that generated by the WT fibers (12.0 ± 0.8 N/cm2). The isometric force generated by WT psoas fibers is comparable to previously reported values for fast muscles from mice (26) and rabbits (5).
Table 1.
Isometric forces generated by single permeabilized psoas fibers from WT and GAA-KO mice
| Fiber Diameter, μm | Force, mg | Force/Area, N/cm2 | |
|---|---|---|---|
| WT | 66 ± 3 | 40.8 ± 2.4 | 12.0 ± 0.8 |
| GAA-KO | 44 ± 3 | 9.4 ± 0.7 | 6.3 ± 0.4 |
Values are means ± SE of 9 single fibers from 5 WT mice and 9 single fibers from 3 acid α-glucosidase (GAA) knockout (KO) mice. Isometric forces were generated by fully Ca2+-activated single permeabilized psoas muscle fibers; values were normalized by cross-sectional area. Sarcomere length = 2.4–2.5 μm, temperature = 20°C. Fiber diameter was measured in additional fibers from WT (n = 51) and GAA-KO (n = 53) mice: 76 ± 2 μm in WT and 47 ± 1 μm in GAA-KO. All GAA-KO values are significantly different from WT values (P < 0.001).
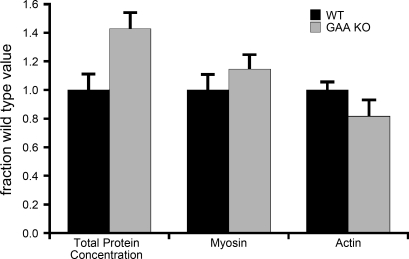
To determine if the impaired force generation in GAA-KO fibers could be explained by a decrease in contractile protein content, we performed biochemical protein assays and measured the amount of actin and myosin by gel electrophoresis. Although significant variability was observed between samples, the mean concentrations of myosin and actin determined by densitometry of gels stained with SYPRO Ruby were not significantly different between WT and GAA-KO fibers (Fig. 3). Similar results were obtained when the proteins were detected by silver stain of gels loaded with equal fiber volumes, instead of equivalent amounts of protein (not shown). The total protein concentration of GAA-KO fibers was increased relative to the WT controls (Fig. 3).
Fig. 3.
Concentrations of total protein, myosin heavy chain, and actin in WT and GAA-KO muscle fibers. Each parameter was normalized to the mean corresponding value in WT fibers.
Structural properties of skinned fibers.
In vertebrate skeletal muscle fibers, the myosin filaments are arranged in a crystal-like hexagonal filament lattice, with the actin filaments situated at the trigonal points. Equatorial X-ray diffraction patterns provide information on the distributions of mass within the filament lattice. The ratio of the intensities of the [1,1] and [1,0] reflections (I11/I10) is a measure of the fraction of myosin heads in the vicinity of and/or attached to actin, while a broadening of the reflections indicates disarray of filaments within the lattice. In addition, the lateral distances between filament backbones can be calculated from the diffraction patterns, yielding information regarding the overall compression or expansion of the myofibrils (19).
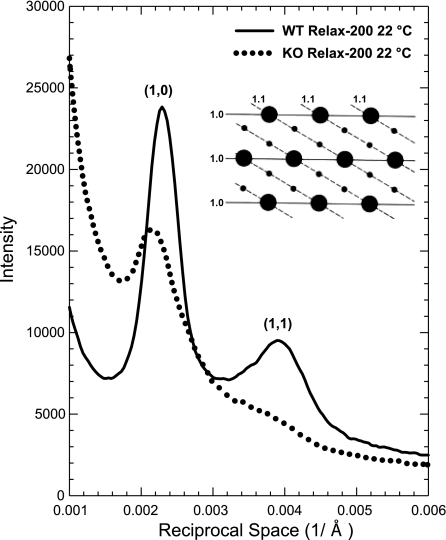
The equatorial diffraction patterns revealed several significant structural differences between the WT and the GAA-KO muscle fibers (Fig. 4, Table 2). The widths of the reflections were broader in the GAA-KO fibers, indicating disorder in the arrangement of the thick and thin filaments. I11/I10 in relaxed fibers was reduced from 0.30 in WT to 0.07 in GAA-KO fibers. The distance between the thick and the thin filaments was increased by 3.5%. It has been shown that, under constant ionic strength and temperature, such a change in the filament distance does not significantly affect force level (6).
Fig. 4.
X-ray intensity profiles of equatorial reflections obtained from psoas muscle from WT and GAA-KO mice. Reflections from the GAA-KO mouse are significantly broader, indicating disorder of the filament lattice in the muscle cells. Intensity ratio (I11/I10) of the reflections is greatly reduced, suggesting decreased interaction between myosin and actin. Inset: cross-sectional view of the hexagonal lattice formed by myosin and actin filaments that give rise to the equatorial reflections.
Table 2.
Structural parameters from X-ray diffraction
| I11/I10 | Normalized Half-Widths of [1,0] Reflection | Distance Between Thick and Thin Filaments, nm | |
|---|---|---|---|
| WT | 0.30 ± 0.02 | 1.00 | 29.0 ± 0.1 |
| GAA-KO | 0.07 ± 0.01 | 1.36 ± 0.07 | 30.1 ± 0.3 |
Values are means ± SE of 3 bundles from WT mice and 3 bundles from GAA-KO mice. X-ray diffraction parameters were obtained from fully relaxed permeabilized fiber bundles. Sarcomere length = 2.4–2.5 μm, temperature = 20°C. I11/I10, intensity ratio. All GAA-KO values are significantly different from WT values (P < 0.05).
DISCUSSION
In this study, we have shown that single permeabilized muscle fibers from GAA-KO mice produce ∼75% less force in response to Ca2+ activation than do WT fibers. Most of this decrease in force is evident even after normalization to fiber cross section, indicating that the severe reduction in force production cannot be attributed to atrophy alone. Several other factors that might contribute to muscle weakness in vivo can be ruled out in our experiments. Activation through the excitation-contraction coupling pathway was bypassed, and a high concentration of ATP was maintained, with an ATP-regenerating system present in the bathing solution. Reduced myofibrillar protein content is ruled out, since there were no gross changes in the concentration and pattern of the major proteins in the muscle fibers. In particular, myosin heavy chain and actin concentrations were unaltered in the GAA-KO muscle (Fig. 3).
Equatorial X-ray diffraction patterns obtained from GAA-KO fibers point to an extensive structural degradation in the organization of the myofilament lattice that may contribute to the impaired force generation. Normally, the thick myosin-containing filaments and the thin actin-containing filaments are distributed in an almost crystal-like hexagonal lattice. The broadening of the reflections (Fig. 4, Table 2) indicates that the filaments, including the myosin heads, are displaced from their normal positions. A likely cause of this is a reduction in the interaction between myosin and actin in the relaxed muscle. I11/I10 is a measure of the fraction of myosin heads (cross bridges) interacting with actin. The present result suggests that the myosin-actin interaction is greatly reduced in the GAA-KO mice.
It has been shown that myosin and actin interact weakly in a relaxed muscle fiber (3) and the weak interactions are precursors to force generation (7, 18). If the weak interaction is inhibited by, say, 50%, the force is reduced by a similar proportion. Under the conditions used in this study, there is a small but significant fraction (∼10% at 20°C) of myosin cross bridges that interacts with actin at all times in the WT mice (18). In the GAA-KO mice, on the basis of the X-ray diffraction data, the fraction is greatly reduced. Although an accurate estimate of the interacting actin-myosin complex requires precise knowledge of molecular conformations, the decrease in I11/I10 (Table 2) is consistent with a reduction of ∼50% or more in the actin-myosin interaction (19), leading to a reduction of force of a similar magnitude (18). Thus the present results demonstrate a site and potential mechanism of muscle weakness, but how this mechanism is linked to the lysosomal pathology of this illness remains uncertain.
A number of candidate molecules and structures might be considered possible links. Hesselink and colleagues (9, 14, 15), by studying the progression of glycogen buildup and muscle function over time, concluded that enlarged lysosomes could not fully explain the decreased mechanical performance per unit muscle mass, but they suggested that large clusters of noncontractile material and lipofuscin in the cytoplasm might hamper longitudinal force transmission and, hence, contractile function. We previously demonstrated in muscles from GAA-KO mice and patients with Pompe disease that the noncontractile material is autophagic debris that includes ubiquitinated proteins, lipofuscin, damaged autophagosomes, late endosomes/lysosomes, and other materials (21). Since this debris is mostly located in the core of the fiber, between and not within the fibrils (10, 20, 23, 24), simple impingement on the myofilaments is unlikely, and since the observed distance between myofilament backbones is, in fact, increased, lateral compression is unlikely to account for effects these structures may have on myofibrillar function.
Although direct mechanical forces from the larger accumulated structures do not appear to be the cause of altered actin-myosin interaction, other, smaller molecular structures deserve consideration. In addition to ubiquitinated proteins, desmin and cytoplasmic glycogen accumulation have been described in Pompe muscle fibers (13, 27). Any of these molecules or their breakdown products might have toxic effects that could disturb actin-myosin interaction. For example, toxic effects of misfolded ubiquitinated proteins have been demonstrated in several disorders of the central nervous system (1).
In summary, these experiments show that impaired myofibrillar function from disturbed actin-myosin interaction in the myofibrils contributes to skeletal muscle weakness in the GAA-KO mice beyond that accounted for by muscle atrophy or by direct mechanical effects of lysosomes and autophagic buildup. The chain of events leading to this effect remains to be charted.
GRANTS
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank the staff scientists at Beamline 37C, National Synchrotron Light Source, Brookhaven National Laboratory, for assistance in carrying out X-ray experiments, and Gustavo Gutierrez-Cruz for providing assistance to M. Galperin.
Present address of M. Galperin: Virginia Commonwealth University School of Medicine, Richmond, VA 23298.
REFERENCES
- 1. Bahr BA. Lysosomal modulatory drugs for a broad strategy against protein accumulation disorders. Curr Alzheimer Res 6: 438–445, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Bijvoet AG, Van de Kamp EH, Kroos MA, Ding JH, Yang BZ, Visser P, Bakker CE, Verbeet MP, Oostra BA, Reuser AJ, Van der Ploeg AT. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum Mol Genet 7: 53–62, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Brenner B, Schoenberg M, Chalovich JM, Greene LE, Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci USA 79: 7288–7291, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner B, Xu S, Chalovich JM, Yu LC. Radial equilibrium lengths of actomyosin cross-bridges in muscle. Biophys J 71: 2751–2758, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenner B, Yu LC. Equatorial X-ray diffraction from single skinned rabbit psoas fibers at various degrees of activation. Changes in intensities and lattice spacing. Biophys J 48: 829–834, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brenner B, Yu LC. Characterization of radial force and radial stiffness in Ca2+-activated skinned fibres of the rabbit psoas muscle. J Physiol 441: 703–718, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenner B, Yu LC, Chalovich JM. Parallel inhibition of active force and relaxed fiber stiffness in skeletal muscle by caldesmon: implications for the pathway to force generation. Proc Natl Acad Sci USA 88: 5739–5743, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brenner B, Yu LC, Podolsky RJ. X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys J 46: 299–306, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drost MR, Hesselink RP, Oomens CW, van der Vusse GJ. Effects of non-contractile inclusions on mechanical performance of skeletal muscle. J Biomech 38: 1035–1043, 2005 [DOI] [PubMed] [Google Scholar]
- 9a. Engel AG, Hirschhorn R, Huie ML. Acid maltase deficiency. In: Myology, edited by Engel AG, Franzini-Armstrong C. New York: McGraw-Hill, 2003, p. 1559–1586 [Google Scholar]
- 10. Fukuda T, Ewan L, Bauer M, Mattaliano RJ, Zaal K, Ralston E, Plotz PH, Raben N. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol 59: 700–708, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Fukuda T, Roberts A, Ahearn M, Zaal K, Ralston E, Plotz PH, Raben N. Autophagy and lysosomes in Pompe disease. Autophagy 2: 318–320, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Hellam DC, Podolsky RJ. Force measurements in skinned muscle fibres. J Physiol 200: 807–819, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hesselink RP, Gorselink M, Schaart G, Wagenmakers AJ, Kamphoven J, Reuser AJ, van der Vusse GJ, Drost MR. Impaired performance of skeletal muscle in α-glucosidase knockout mice. Muscle Nerve 25: 873–883, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Hesselink RP, Schaart G, Wagenmakers AJ, Drost MR, van der Vusse GJ. Age-related morphological changes in skeletal muscle cells of acid α-glucosidase knockout mice. Muscle Nerve 33: 505–513, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Hesselink RP, Van Kranenburg G, Wagenmakers AJ, van der Vusse GJ, Drost MR. Age-related decline in muscle strength and power output in acid 1–4 α-glucosidase knockout mice. Muscle Nerve 31: 374–381, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 148: 671–676, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8: 931–937, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kraft T, Chalovich JM, Yu LC, Brenner B. Parallel inhibition of active force and relaxed fiber stiffness by caldesmon fragments at physiological ionic strength and temperature conditions: additional evidence that weak cross-bridge binding to actin is an essential intermediate for force generation. Biophys J 68: 2404–2418, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malinchik S, Yu LC. Analysis of equatorial X-ray diffraction patterns from muscle fibers: factors that affect the intensities. Biophys J 68: 2023–2031, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raben N, Danon M, Gilbert AL, Dwivedi S, Collins B, Thurberg BL, Mattaliano RJ, Nagaraju K, Plotz PH. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab 80: 159–169, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Raben N, Hill V, Shea L, Takikita S, Baum R, Mizushima N, Ralston E, Plotz P. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum Mol Genet 17: 3897–3908, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L, LaMarca M, King C, Ward J, Sauer B, Plotz P. Targeted disruption of the acid α-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 273: 19086–19092, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Raben N, Takikita S, Pittis MG, Bembi B, Marie SKN, Roberts A, Page L, Kishnani PS, Schoser BGH, Chien YH, Ralston E, Nagaraju K, Plotz PH. Deconstructing Pompe disease by analyzing single muscle fibers. Autophagy 3: 546–552, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Ralston E, Swaim B, Czapiga M, Hwu WL, Chien YH, Pittis MG, Bembi B, Schwartz O, Plotz P, Raben N. Detection and imaging of non-contractile inclusions and sarcomeric anomalies in skeletal muscle by second harmonic generation combined with two-photon excited fluorescence. J Struct Biol 162: 500–508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schoser B, Hill V, Raben N. Therapeutic approaches in glycogen storage disease type II/Pompe disease. Neurotherapeutics 5: 569–578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seow CY, Ford LE. Shortening velocity and power output of skinned muscle fibers from mammals having a 25,000-fold range of body mass. J Gen Physiol 97: 541–560, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van der Ploeg AT, Reuser AJ. Pompe's disease. Lancet 372: 1342–1353, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Winkel LP, Hagemans ML, Van Doorn PA, Loonen MC, Hop WJ, Reuser AJ, Van der Ploeg AT. The natural course of non-classic Pompe's disease: a review of 225 published cases. J Neurol 252: 875–884, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Xu S, Gu J, Melvin G, Yu LC. Structural characterization of weakly attached cross-bridges in the A*M*ATP state in permeabilized rabbit psoas muscle. Biophys J 82: 2111–2122, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu LC, Brenner B. Structures of actomyosin cross-bridges in relaxed and rigor muscle fibers. Biophys J 55: 441–453, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]