Abstract
The functional roles of the medullary raphé, and specifically 5-HT neurons, are not well understood. It has previously been stated that the role of 5-HT has been so difficult to understand, because “it is implicated in virtually everything, but responsible for nothing”(Cowen PJ. Foreword. In: Serotonin and Sleep: Molecular, Functional and Clinical Aspects, edited by Monti JM, Prandi-Perumal SR, Jacobs BL, Nutt DJ. Switzerland: Birkhauser, 2008). Are 5-HT neurons important, and can we assign a general, or even specific, function to them given their diffuse projections? Recent data obtained from transgenic animals and other model systems indicate that the 5-HT system is not expendable, particularly during postnatal development, and likely plays specific roles in vital functions such as respiratory and thermoregulatory control. We recently provided a detailed and updated review of one specific function of 5-HT neurons, as central respiratory chemoreceptors contributing to the brain's ability to detect changes in pH/CO2 and stimulate adjustments to ventilation accordingly (9). Here, we turn our focus to recent data demonstrating that 5-HT neurons provide an essential excitatory drive to the respiratory network. We then further discuss their role in the CO2 chemoreflex, as well as other homeostatic functions that are closely related to ventilatory control. Last, we provide additional hypotheses/concepts that are worthy of further study, and how 5-HT neurons may be involved in human disease.
Keywords: temperature control, control of breathing
the contribution of the 5-HT system to central nervous system (CNS) function in general has been an enigma, and the contributions to mechanisms of homeostatic control (e.g., breathing, thermoregulation) are equally unclear. However, accumulating data over recent years have begun to clarify the roles of medullary 5-HT neurons and their transmitters, 5-HT, substance P (SP), and thyrotropin-releasing hormone (TRH) in the control of breathing. Our working hypothesis is that the major contributions of medullary raphé 5-HT neurons to ventilatory control are twofold: 1) they provide tonic, excitatory drive to multiple components of the respiratory network; and 2) they sense changes in tissue pH/CO2 via intrinsic membrane properties, and through changes in neurotransmitter release alter the level of tonic, excitatory drive to appropriately adjust ventilation (9; 50–53). This hypothesis is based on our interpretation of the existing body of literature, which was detailed in two recent previous reviews (9, 25) and further supported by recent experiments using transgenic mice (24, 26, 27). Here we discuss the data obtained from various knockout mouse models (see below) and other relevant data that provide support for the hypothesized roles of 5-HT neurons in the control of breathing, including eupneic ventilation and CO2 chemoreception, and discuss a conceptual view of the integration of the various functions of the 5-HT system.
RAPHÉ 5-HT NEURONS AND EUPNEIC VENTILATION
Studies aiming to elucidate the role of 5-HT in the control of breathing have resulted in mixed results, leading to conflicting conclusions that 5-HT is inhibitory, excitatory, or plays little or no role (reviewed in 25, 50). It is our view that it can be hard to interpret data from some of the complex experimental paradigms that have been used previously, especially those in an intact network. For example, attempts were made to block output of the 5-HT system using drugs that interfere with 5-HT synthesis such as para-chlorophenylalanine (PCPA; 44). This led to an increase in respiratory output and the conclusion that 5-HT neurons inhibit breathing. However, it is now known that depletion of brain 5-HT to 10% of control has no effect on the postsynaptic response to stimulation of 5-HT fibers (7), probably due to decreased inhibition of autoreceptors on 5-HT terminals and enhanced efficacy of 5-HT release. Thus, although PCPA markedly reduces total brain 5-HT levels, it appears that there can be a compensatory increase in synaptic vesicle release mechanisms to maintain a normal level of 5-HT release (6). Alternatively, or simultaneously, a reduction in cytosolic 5-HT levels in 5-HT neurons could lead to a decrease in somatodendritic 5-HT release (which may depend more on nonvesicular mechanisms). This would lead to a decrease in 5-HT1A receptor-dependent autoinhibition and greater release of SP and TRH, and both have powerful effects on respiratory output (18, 47, 68). Thus a decrease in 5-HT synthesis may actually cause a paradoxical increase in postsynaptic stimulation by 5-HT neurons.
Confusing results have also been obtained using focal stimulation experiments. Stimulation of some parts of the medullary raphé causes an increase in respiratory output, whereas stimulation in other parts causes inhibition of breathing (31). Although it is not clear that all these effects are due to stimulation of 5-HT neurons, these results have been interpreted as indicating that some 5-HT neurons have direct excitatory connections with the respiratory network whereas others have direct inhibitory connections. However, direct inhibitory connections have never been demonstrated. Instead, it is well known that there are recurrent collaterals between 5-HT neurons (63, 64), and increasing 5-HT suppresses 5-HT neuron activity (1), and as a result breathing could be inhibited when one group of 5-HT neurons is stimulated because they inhibit other 5-HT neurons that stimulate breathing. Thus data obtained during experiments manipulating the 5-HT system in vivo can sometimes be very difficult to interpret, because of these types of complexities in an intact system.
There are two bodies of work that led to the unequivocal conclusion that 5-HT neurons stimulate breathing, at least in neonates. First, experiments like those from Ptak et al. (49) using the in vitro neonatal brain slice demonstrate that there are direct excitatory connections from 5-HT neurons to many of the primary respiratory nuclei, and these are essential for rhythm generation (46, 49). Second, recent in vivo experiments show that genetic elimination of 5-HT neurons in knockout (KO) mice (as opposed to elimination of 5-HT itself) severely disrupts eupneic respiratory rhythm and leads to high postnatal mortality in neonates (27). Although deletion of 5-HT neurons does not decrease eupneic ventilation in adult mammals (26), we believe that much of the existing evidence is still consistent with the concept that 5-HT neurons play an exclusively excitatory role at all postnatal ages (see below). Although there are data that have been interpreted as disproving that possibility, we believe that there are alternative interpretations of those data.
MECHANISMS BY WHICH 5-HT NEURONS PROVIDE TONIC, EXCITATORY DRIVE TO THE RESPIRATORY NETWORK
There is expression of multiple receptors for 5-HT, SP, and TRH on respiratory neurons, and known projections from 5-HT neurons to multiple sites within the respiratory network (8, 55, 62). Recent functional data are consistent with a strong excitatory influence on the network and reveal some of the mechanisms by which 5-HT, SP, and TRH act to facilitate eupneic breathing. With the exception of 5-HT3 receptors, all 5-HT, SP [via neurokinin (NK)-1, -2, and -3] and TRH (1 and 2) receptors are G protein-coupled receptors (25). Activation of these receptors modifies the excitability of their target neurons through G protein-dependent second messengers, which alter the properties of ion channels to affect membrane excitability, in some cases without direct effects on membrane potential. This neuromodulation can increase or decrease membrane excitability, depending on the receptors being activated.
Recent in vitro experiments have specifically addressed how 5-HT neurons modulate components of the respiratory network. Ptak et al. (49) found that most raphé obscurus 5-HT neurons fire spontaneously in rhythmically active slice preparations, and these 5-HT neurons send axonal projections directly to both the pre-Bötzinger complex (pre-BötC) and hypoglossal motor nucleus, and they colocalize 5-HT and SP. In addition, many of these neurons also receive reciprocal excitatory connections from the respiratory network, with firing rate increasing during the inspiratory phase of the respiratory cycle (49). Moreover, cross-correlation analysis in vivo suggests there are synaptic connections from some raphé neurons (that were not identified as serotonergic) to respiratory neurons within the ventral and pontine respiratory groups (43). Thus these neurons are not peripheral to the respiratory network but are embedded within it.
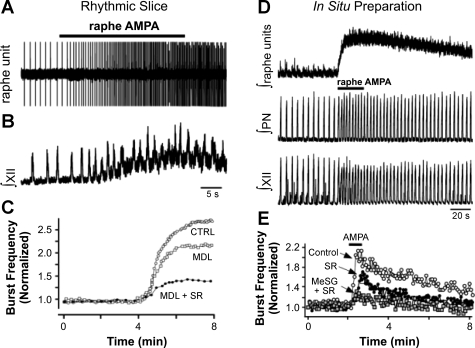
Not only are 5-HT neurons anatomically and functionally embedded within the respiratory network, but release of 5-HT and SP is required for generation of inspiratory motor output in rhythmically active slices (46, 47, 49, 59) and in the perfused brain stem-spinal cord (in situ) preparation (27, 49). Blockade of 5-HT receptors, alone or in combination with NK-1 receptors, eliminates respiratory motor output in neonatal slices and neonatal and juvenile perfused preparations (27, 49). In addition, an increase in firing rate of raphé 5-HT neurons induced by local α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor activation is followed by an increase in burst frequency in rhythmically active slices and in situ, and the augmented respiratory burst frequency can be blocked with antagonists of 5-HT (methysergide, ketanserin, or MDL-11,939) and/or NK-1 (SR 140333) receptors (Fig. 1; Ref. 49). Finally, local application of artificial cerebrospinal fluid with 0 mM K+ into the raphé obscurus eliminated both 5-HT neuron firing and network activity, consistent with the concept that respiratory motor output in the neonatal slice is dependent on tonic 5-HT neuron firing under baseline conditions (49).
Fig. 1.
Raphé-specific stimulation increases hypoglossal (XII) and phrenic motor outputs in vitro and in situ. Individual raphé unit activity (A) and integrated XII motor activity (B) both increase during midline raphé application of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) in rhythmic slices. C: the increase in XII motor output can be reduced with bath application of the 5-HT2A receptor antagonist MDL-11,939 (MDL), and further reduced with coapplication of the NK-1 receptor antagonist SR 140333 (SR). CTRL, control. D: similarly, focal application of AMPA into the raphé of perfused brain (in situ) preparations increases raphé unit activity, and phrenic (PN) and XII motor outputs. E: the stimulatory effect of raphé AMPA is blunted or nearly eliminated in situ with SR or methysergide (MeSG) + SR, respectively. [Adapted from Ptak et al. (49) with permission from the Society for Neuroscience.]
Data obtained by Toppin et al. (58) using the in situ perfused brain preparation suggests that blocking 5-HT receptors with ketanserin or methysergide has little effect on inspiratory motor output, defined as either “eupnea” or “gasping”. However, these data contrast with the findings of others using the same preparation. For example, Ptak et al. (49) showed that both hypoglossal and phrenic nerve motor outflows were completely dependent on endogenous activation of 5-HT and/or NK-1 receptors. The differences in the Ptak study from the Toppin study (58) included 1) the use of the highly selective antagonists MDL 11,939 (5-HT2A antagonist) and SR 140333 (NK-1 antagonist) instead of ketanserin and methysergide (less selective 5-HT antagonists); 2) the finding that combined block of 5-HT and NK1 receptors is more effective than either one alone; and 3) the Toppin study reported a large decrease in amplitude of phrenic output (>50%) with 30–40 μM methysergide, but used lower doses for the main experiments. It is not clear how effectively drugs have access to the brain interstitial space in the perfused juvenile rat brain, so higher doses may have been necessary for effective blockade.
The mechanisms of the effect of 5-HT neuron activity on respiratory output have begun to be elucidated. For example, hypoglossal motor neurons depolarize in response to activation of 5-HT2 (6, 15), NK-1, and TRH receptors, and this is due in part to inhibition of TWIK-related acid-sensitive K+ (TASK) channels (57). In intrinsic and nonintrinsic bursting pre-BötC neurons, 5-HT2A and NK1 receptor activation causes depolarization through modulation of cation leak conductances (49). Interestingly, these may include NALCN, a non-selective Na+ leak channel that is critical for generation of normal respiratory motor output (36). Neurotransmitters released by 5-HT neurons can also enhance rhythm generation by inducing pacemaker activity in respiratory neurons. For example, 5-HT induces intrinsic bursting in pre-BötC inspiratory neurons (49). Similarly, nanomolar concentrations of TRH can convert tonically firing neurons in the nucleus of the solitary tract (NTS) into intrinsic bursters by activating pacemaker currents (12). The in vivo significance of the ability of multiple transmitters released by 5-HT neurons to induce widespread bursting of respiratory neurons remains unclear. However, it is possible that 5-HT neurons allow neurons in multiple sites within the respiratory network to be able to generate the respiratory rhythm, and/or increase the stability of rhythmic network activity.
5-HT NEURONS PROVIDE TONIC, EXCITATORY DRIVE FOR EUPNEA IN VIVO
As discussed above, raphé 5-HT neurons are highly embedded within the respiratory network and are a source of necessary tonic, excitatory drive in rhythmic slices. However, it is not always clear whether data from in vitro preparations are relevant to the influence of 5-HT neurons in the intact respiratory network in vivo. We now turn our attention to transgenic mouse models to provide further insights and potentially validate the hypothesis that 5-HT neurons provide tonic, excitatory drive to breathe in vivo. The strategy of these mouse models is to create dysfunction in either a component of 5-HT neuron function (transporter, receptor, key synthetic enzyme), or by varying degrees of 5-HT neuron loss, and determine the consequences on physiological control mechanisms. While these transgenic lines carry drawbacks, such as compensation due to loss of function from early in development, they have also provided important insight into the function of the 5-HT system.
One such model is derived from the genetic deletion of the “E-twenty six” (ETS) transcription factor Pet-1, which is uniquely expressed in 5-HT neurons. Pet-1 null mice retain only 20–30% of the normal complement of central 5-HT neurons, and demonstrate anxiety-like behavior (19), poor maternal care (33), and aggression (19), and an unstable breathing pattern as neonates (14). Pet-1 null mice display a disrupted breathing pattern at postnatal day 4.5 (P4.5) (including many apneas), which resolves around P9 to give rise to a stable, eupneic breathing pattern (14). The younger mice also have altered autoresuscitation responses to acute anoxia, with delays in onset of gasping and reestablishment of eupnea. Surprisingly, the number and “quality” of gasps is unaffected (54), despite the evidence that gasping is dependent on 5-HT signaling (59). Thus Pet-1 KO mice show disrupted eupneic breathing patterns and autoresuscitory responses as neonates, which improve with age.
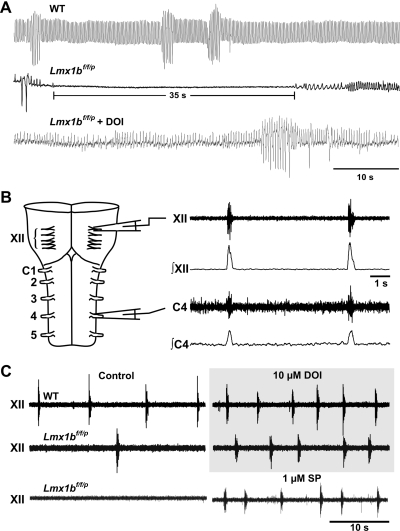
To generate mice with more complete loss of 5-HT neurons, an alternative conditional knockout strategy was employed. LoxP sites were inserted such that they flank exons 4–6 of LIM homeobox transcription factor 1β (Lmx1b) gene. These “floxed” Lmx1b (Lmx1bf/f) animals were then mated to mice in which the gene for cre-recombinase was inserted downstream of the enhancer region for Pet-1 (69), giving rise to Lmx1bf/f/p mice. Thus Lmx1b would only be deleted in 5-HT neurons of Pet-1 cre-expressing mice. These Lmx1bf/f/p mice have complete (>99%) and specific loss of central 5-HT neurons and severely reduced levels of CNS 5-HT (<50 pg/mg in KO mice compared with ∼500 mg/pg wet tissue weight in the WT mice, and what was detected was likely due to contamination by peripheral blood 5-HT) and undetectable levels of 5-hydroxyindolacetic acid (5-HIAA) in the KO mice compared with ∼350 pg/mg wet tissue weight in the WT mice (69). This occurs without any major anatomic malformations of the brain, and without any detectable change in other monoamine systems. Lmx1bf/f/p mice can live to adulthood, and as adults have normal breathing at rest, with the exception of reduced ventilatory frequency measured when ambient temperature is near the thermoneutral zone (26). Measurements of minute ventilation (V̇e), frequency, and the ratio of ventilation to oxygen consumption (V̇e/V̇o2; an index of the ability of ventilation to keep up with metabolic demand) are decreased when ambient temperature is ∼25°C, in part due to a decreased core temperature (24). Thus baseline ventilation is relatively normal in adult Lmx1bf/f/p mice, suggesting that 5-HT neurons are not essential for breathing (although see below). In contrast, during the postnatal period there is severe hypoventilation and frequent apnea in Lmx1bf/f/p mice (27). For the first 2 wk of life, Lmx1bf/f/p mice have severe and frequent apnea, with some apneas lasting 35–55 s (Fig. 2A). When apnea was defined as a respiratory pause > 1 s in duration, 2-day-old (P2) Lmx1bf/f/p mice have ∼7 apneas/min. When apneas were defined as longer than 5 s, they occur >50 times/h and are present in 100% of animals studied. By summing apnea durations and dividing by the length of time each animal was studied, it was found that P2 Lmx1bf/f/p mice typically spend 30–40% of the time apneic.
Fig. 2.
Mice lacking 5-HT neurons display severe apnea in early development. A: raw traces of baseline ventilation in wild-type (WT; top trace) and Lmx1bf/f/p mice (middle trace) at 4 days of age. Note the long apnea in the Lmx1bf/f/p mouse. Apneas are eliminated after intraperitoneal injection of the 5-HT2A agonist 2,5-dimethoxy-iodoamphetamine (DOI; bottom trace). B: schematic of the isolated brain stem-spinal cord (en bloc) preparation, showing raw and integrated inspiratory motor output from the XII and cervical (C) nerve roots. C: XII motor output is reduced in en bloc preparations from postnatal day 2 (P2) Lmx1bf/f/p mice under control conditions (left) but is restored to the level of WT preparations by bath application of DOI or substance P (SP). [Adapted from Hodges et al. (27) with permission from the Society for Neuroscience.]
Lmx1bf/f/p mice have slower growth rate and higher estimated mortality (∼23%) than wild-type (WT) mice during early neonatal life (27). In addition, growth rate in Lmx1bf/f/p mice increases and exceeds that of WT mice at the age at which apneas resolve. Since hypoxia can cause growth retardation (39), it is likely that the low growth rate in Lmx1bf/f/p mice is due to the severe disruption of eupneic ventilation, as the augmented growth rate after the second postnatal week coincides with loss of apnea. However, ventilation and the V̇e/V̇o2 ratio remained decreased beyond P28, indicating that improvement in eupneic ventilation continues well beyond the age at which apneas disappear.
5-HT plays a role as a trophic factor and is a key contributor to cortical network development (61). Thus the abnormal breathing in neonatal Lmx1bf/f/p mice could be due to disrupted network formation during development. This possibility was tested by measurements of respiratory motor output from hypoglossal and cervical spinal nerve roots in isolated brain stem-spinal cord (en bloc) preparations from P2 mice (27). WT preparations displayed regular and frequent bursts simultaneously in the hypoglossal and cervical nerve roots under control conditions (Fig. 2). Under these same conditions, Lmx1bf/f/p preparations had severely depressed burst generation, in many cases failing to burst for minutes. However, regular and frequent bursting was elicited by the 5-HT2A receptor-specific agonist 2,5-dimethoxy-iodoamphetamine (DOI), SP (alone or in combination with DOI), or 5-HT. Since respiratory output could be normalized by simply replacing the missing neuromodulators, the problem is not due to altered network formation during development (i.e., not due to a loss of trophic influences). It also suggests that although 5-HT might cross from the periphery to the CNS due to a “leaky” blood-brain barrier during embryonic development (13), baseline ventilation is not being supported by 5-HT receptor stimulation from peripheral 5-HT in early postnatal life. Thus respiratory output is likely abnormal in vivo due to a lack of neuromodulatory drive—a conclusion that is supported by the finding that systemic treatment of P2 Lmx1bf/f/p pups with DOI stimulates breathing rate and volume and decreases apnea (Fig. 2).
These in vivo and in vitro data demonstrate that 5-HT neurons provide the neonatal respiratory network with tonic drive that is essential for generation of respiratory output. The normalization of breathing with age can be interpreted in two ways. One possibility is that breathing is only dependent on input from 5-HT neurons in immature animals. Another possibility is that breathing is dependent on input from 5-HT neurons at all ages, but adult Lmx1bf/f/p mice have compensated for the loss of 5-HT neurons using some other mechanism. Existing data do not distinguish between these possibilities. However, specific antagonists of 5-HT2A (MDL 11,939) and NK-1 (SR 140333) receptors consistently eliminate inspiratory motor output in in situ preparations from young (P6–P8) and older (P35) rats (27, 49), suggesting that activation of these receptors is required for driving inspiratory motor output even at older ages.
THE IMPORTANCE OF 5-HT NEURONS IN THE HYPERCAPNIC VENTILATORY RESPONSE
5-HT neurons are among a limited number of neurons that are thought to be central respiratory chemoreceptors (9, 50, 51). We have previously defined a respiratory chemoreceptor as a cell that possesses an intrinsic ability to respond to physiologically relevant changes in Pco2 (or pH), and consequently drives the appropriate adjustment of ventilation (51). As summarized in previous reviews (9, 25, 50, 51), the evidence in support of the hypothesis that 5-HT neurons fit this definition is substantial and continues to build. The evidence that 5-HT neurons are central respiratory chemoreceptors includes 1) their anatomic location near penetrating arteries would allow them to faithfully monitor blood Pco2; 2) those on the ventral surface of the medulla overlap the location of the classically described rostral and caudal “chemosensitive zones” (5); 3) their large and intrinsic pH/CO2 sensitivity in primary cell culture and brain stem slices in vitro (52, 53, 65, 67); 4) their increased activity during hypercapnia in vivo (17, 32, 48, 60); 5) the decreased ventilatory response to hypercapnia in vivo after nonselective lesions of the raphé (23) or 5-HT neuron-specific neurotoxins (40, 42) or genetic deletion of 5-HT neurons (20, 24, 26); 6) injection of the carbonic anhydrase inhibitor acetazolamide into the raphé creates a local acidosis, and stimulates ventilation in anesthetized rats (4); 7) reverse microdialysis of high-CO2 solutions within the medullary raphé at single sites (21, 41) or at multiple sites (22) increases ventilation in unanesthetized rats and goats in vivo; and 8) postnatal development of pH/CO2 sensitivity of 5-HT neurons occurs in parallel with the hypercapnic ventilatory response in vivo (11, 52, 56, 66, 67).
Male Pet-1 KO mice (which lack 70% of 5-HT neurons) have a decreased ventilatory response to hypercapnia as adults, while females do not (20). Neither male nor female Pet-1 KO mice display abnormalities in resting ventilation, or the hypoxic ventilatory response as adults. Likewise, knockout of the 5-HT transporter (SERT) also blunts the hypercapnic ventilatory response (34). SERT KO males have a 68% reduction of the hypercapnic ventilatory response, while females have a 22% reduction (34). Interestingly, SERT KO mice are characterized by increased extracellular 5-HT concentrations in the brain, which has been shown to decrease both 5-HT neuron activity (via autoinhibition) and to decrease 5-HT1A receptor binding (16, 37). It is not clear how this would influence the response of 5-HT neurons to hypercapnia, but the increase in autoinhibition has the potential to blunt the response of these neurons to a pH stimulus. Together these findings further support a role for 5-HT neurons in the CO2 chemoreflex.
In Lmx1bf/f/p mice the hypoxic ventilatory response is normal in the thermoneutral temperature range and only modestly reduced in cooler conditions (24, 26). Importantly, the hypercapnic ventilatory response is reduced by 50% under both conditions, irrespective of ambient or core temperature, and both male and female Lmx1bf/f/p mice are equally affected. Intracerebroventricular (ICV) infusion of 5-HT in Lmx1bf/f/p mice augments both baseline ventilation and the hypercapnic ventilatory response, essentially restoring the latter to near control levels (26). This effect on the hypercapnic ventilatory response could occur via enhancement of network excitability in response to chemoreceptor input, such as by potentiating the response to input from peripheral chemoreceptors (43). Alternatively, the increased extracellular 5-HT could enhance chemosensitivity of nonserotonergic chemoreceptors, such as those proposed to exist in the retrotrapezoid nucleus or the NTS. The decrease in the CO2 chemoreflex in these knockout models is consistent with the hypothesis that 5-HT neurons are central respiratory chemoreceptors, but the restoration of the CO2 chemoreflex by exogenous 5-HT indicates that there are mechanisms beyond intrinsic chemosensitivity by which 5-HT neurons enhance chemoreception. Thus 5-HT neurons may act both as sensors of pH themselves and as facilitators of chemoreception by other groups of neurons. Data from a variety of approaches support the proposed role of 5-HT neurons as central chemoreceptors, and the consistent and specific deficit in the hypercapnic ventilatory response in transgenic models reinforces the concept that 5-HT neurons play a major role in the CO2 chemoreflex in vivo.
THERMOREGULATION AND TRANSGENIC MODELS OF 5-HT SYSTEM DYSFUNCTION
Interestingly, in addition to altered CO2 chemoreception, each of the genetic mouse models discussed above also displays differing degrees of thermoregulatory dysfunction, consistent with the hypothesized role of raphé (5-HT) neurons as a critical relay point for descending hypothalamic drive to heat generation mechanisms (38). When these deficits are considered in combination, they provide a striking parallel with the deficiencies thought to contribute to sudden infant death syndrome (SIDS) (30, 45), which is itself characterized by multiple abnormalities in the 5-HT system. Thus it is relevant to discuss the observations of altered thermoregulation along with the known effects of 5-HT system dysfunction on ventilatory control.
Pet-1 KO, SERT KO, tryptophan hydroxylase 2 (TPH2) KO, and Lmx1bf/f/p mice all maintain appropriate core temperatures under normal conditions, but not in response to a thermal stress. Even the mild thermal stress of a convective air current at a temperature of 24–25°C is sufficient to decrease core temperature in Lmx1bf/f/p mice by ∼1.5°C (24). Decreasing the environmental temperature to 4°C typically decreases core temperature in WT mice by 0.5–1.5°C, and they can maintain this temperature for days (29). In contrast, Pet-1 KO mice decrease core temperature on average by 3.5°C over 4 h in response to the same challenge (unpublished observations), similar to the temperature drop reported in SERT KO mice under the same conditions (35). In response to the same challenge, core temperature in Lmx1bf/f/p mice precipitously drops more than 6°C in 1 h, and they have to be physically removed from the cold environment and actively heated or body temperature will continue to drop to below 26°C (26). Further analysis of the dysfunction in Lmx1bf/f/p mice clearly indicates attenuation of heat generation mechanisms, including reduced shivering and a failure to sustain nonshivering (brown adipose tissue) thermogenesis during cold exposure. However, there is a normal peripheral vasoconstrictor response to cold, in addition to retained ability to distinguish and select a preferred surface temperature. Thus Lmx1bf/f/p mice display normal heat conservation mechanisms and thermosensory perception but cannot appropriately generate heat by shivering or nonshivering thermogenesis during cold exposure.
Audero et al. (3) reported another mouse model in which the 5-HT1A receptor is overexpressed specifically in 5-HT neurons. These mice have spontaneous “events” characterized by bradycardia and hypothermia that lead to death in some cases. In the same report, it was confirmed that 5-HT1A receptor overexpression led to decreased 5-HT neuron activity in vitro. It remains unclear if the hypothermia associated with the events is the cause, a contributor to, or unrelated to spontaneous death. However, the hypothermia is consistent with that seen in other models of 5-HT dysfunction.
TPH2 KO mice have selective and near complete loss of central 5-HT, without loss of 5-HT neurons (2). Paralleling the observations in Lmx1bf/f/p mice (69), there is not a change in norepinephrine or dopamine levels (2). Likewise, TPH2 KO mice show high mortality (50%) in early life, decreased growth rates, and altered thermoregulation. However, in this case the mortality was attributed primarily to poor maternal care (2), as seen in Pet-1 KO mice (33). Exposure to 4°C over a period of 4 h led to decreases in core body temperature to ∼32.5°C in TPH2 knockouts, which is similar to that observed in Pet-1 KO mice, but far less severe than that seen in Lmx1bf/f/p mice. This may be due to the fact that many 5-HT neurons remain in Pet-1 KO mice, and although 5-HT neurons lack 5-HT in TPH2 KO mice they would still release SP and TRH.
In summary, the observations of altered thermoregulation in models of 5-HT system dysfunction are consistent with the hypothesized role of raphé 5-HT neurons as a critical “relay” point in transmitting (excitatory) information to thermoeffector neurons within the heat generation pathways emanating from the hypothalamic centers, particularly those important to cold defense mechanisms.
CAN WE ASSIGN A GENERAL FUNCTION TO 5-HT NEURONS: ARE THEY IMPORTANT?
Mice lacking 5-HT neurons show high neonatal mortality and deficits in eupneic ventilation in the form of severe and frequent apnea. In addition, the hypercapnic ventilatory responses, but not hypoxic ventilatory responses, are deficient in these and other transgenic models of 5-HT system dysfunction during adulthood. Moreover, core body temperature is normal when thermal “stress” is minimal (thermoneutral), but environmental cooling exposes a marked inability to maintain core temperature, consistent with a major role for 5-HT neurons in thermoregulatory defense. These observations suggest that in addition to providing tonic, excitatory drive to breathe, 5-HT neurons provide an error detection and/or correction mechanism that aims to minimize deviations in body temperature and or pH/CO2. These functions may be shared by a common pool of 5-HT neurons, or there may be independent, distinctly dedicated subpopulations of 5-HT neurons. Based on the observation that many 5-HT neurons in the midbrain are also CO2 sensitive, one might envision that these neurons provide an arousal stimulus in response to hypercapnia (due to hypoventilation or apnea) during sleep. A key component of this concept is further characterizing the targets and biophysical mechanisms by which 5-HT neurons can modify postsynaptic excitability at various sites. Based on the data collected to date we continue to favor an integrative role of 5-HT neurons in homeostatic control mechanisms, through intrinsic properties and/or synaptic interactions that contribute to regulation of ventilation relative to metabolic and thermoregulatory demands.
SIDS has long been associated with breathing and thermoregulatory dysfunction and is thought to include defects in arousal (28, 30). In addition, multiple defects in the 5-HT system have now been characterized in postmortem brain stem tissues from SIDS cases, including decreased 5-HT1A receptor binding and SERT density, and increased numbers of morphologically distinct (possibly immature) 5-HT neurons (45). Although they do not “model” SIDS, the transgenic mouse models studied to date provide important insight into how a defect in the 5-HT system could lead to death. They point strongly to specific roles for 5-HT neurons in respiratory and thermoregulatory control, with a large contribution to CO2 chemoreception. Further studies are required to increase our understanding of the fundamental roles of 5-HT in homeostatic regulatory systems, and how these defects could precipitate death in SIDS.
GRANTS
This study was supported by the National Institutes of Health, the Bumpus Foundation, and the Department of Veterans Affairs Medical Center (West Haven, CT).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Aghajanian GK, Graham AW, Sheard MH. Serotonin-containing neurons in brain: depression of firing by monoamine oxidase inhibitors. Science 169: 1100–1102, 1970 [DOI] [PubMed] [Google Scholar]
- 2. Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA 106: 10332–10337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Banchaabouchi MA, Corradetti R, Gross C. Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science 321: 130–133, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. J Appl Physiol 80: 108–115, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci 5: 401–402, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Brandes IF, Zuperku EJ, Stucke AG, Jakovcevic D, Hopp FA, Stuth EA. Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs. J Neurophysiol 95: 3449–3459, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaput Y, Lesieur P, de MC. Effects of short-term serotonin depletion on the efficacy of serotonin neurotransmission: electrophysiological studies in the rat central nervous system. Synapse 6: 328–337, 1990 [DOI] [PubMed] [Google Scholar]
- 8. Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett 105: 34–40, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168: 49–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cowen PJ. Foreword. In: Serotonin and Sleep: Molecular, Functional and Clinical Aspects, edited by Monti JM, Prandi-Perumal SR, Jacobs BL, Nutt DJ. Switzerland: Birkhauser, 2008 [Google Scholar]
- 11. Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol 101: 1097–1103, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science 229: 67–69, 1985 [DOI] [PubMed] [Google Scholar]
- 13. Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res 314: 119–129, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5-HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol 159: 85–101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med 167: 563–569, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Gobbi G, Murphy DL, Lesch K, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther 296: 987–995, 2001 [PubMed] [Google Scholar]
- 17. Haxhiu MA, Tolentino-Silva F, Pete G, Kc P, Mack SO. Monoaminergic neurons, chemosensation and arousal. Respir Physiol 129: 191–209, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Hedner J, Hedner T, Wessberg P, Lundberg D, Jonason J. Effects of TRH and TRH analogues on the central regulation of breathing in the rat. Acta Physiol Scand 117: 427–437, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37: 233–247, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Hodges MR, Best S, Deneris ES, Richerson GB. Adult Pet-1 knockout mice exhibit an attenuated hypercapnic ventilatory response. Program no. 352. 4 2005 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2005 [Google Scholar]
- 21. Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol 96: 1815–1824, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol 97: 2303–2309, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV. Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphe area of awake goats. J Appl Physiol 97: 2236–2247, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol 164: 350–357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol 164: 222–232, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hodges MR, Tattersall G, Harris MB, McEvoy S, Richerson D, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29: 10341–10349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hunt CE, Brouillette RT. Sudden infant death syndrome: 1987 perspective. J Pediatr 110: 669–678, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Jimenez M, Leger B, Canola K, Lehr L, Arboit P, Seydoux J, Russell AP, Giacobino JP, Muzzin P, Preitner F. Beta(1)/beta(2)/beta(3)-Adrenoceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Lett 530: 37–40, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. Serotonin and the brainstem in the sudden infant death syndrome: a review. Annu Rev Pathol 4: 517–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lalley PM. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol 380: 373–385, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larnicol N, Wallois F, Berquin P, Gros F, Rose D. c-fos-like immunoreactivity in the cat's neuraxis following moderate hypoxia or hypercapnia. J Physiol (Paris) 88: 81–88, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci 11: 1001–1003, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol 586: 2321–2329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li A, Nattie EE. SERT knock-out mice have altered control of breathing and thermoregulation. FASEB J 21: 2007 [Google Scholar]
- 36. Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell 129: 371–383, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods 140: 169–181, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci 19: 67–74, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Mortola JP, Xu LJ, Lauzon AM. Body growth, lung and heart weight, and DNA content in newborn rats exposed to different levels of chronic hypoxia. Can J Physiol Pharmacol 68: 1590–1594, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Mueller RA, Towle AC, Breese GR. Supersensitivity to the respiratory stimulatory effect of TRH in 5,7-dihydroxytryptamine-treated rats. Brain Res 298: 370–373, 1984 [DOI] [PubMed] [Google Scholar]
- 41. Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol 90: 1247–1257, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol 556: 235–253, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nuding SC, Segers LS, Shannon R, O'Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphe-pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci 364: 2501–2516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olson EB, Jr, Dempsey JA, McCrimmon DR. Serotonin and the control of ventilation in awake rats. J Clin Invest 64: 689–693, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci 24: 7549–7556, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pete G, Mack SO, Haxhiu MA, Walbaum S, Gauda EB. CO2-induced c-Fos expression in brainstem preprotachykinin mRNA containing neurons. Respir Physiol Neurobiol 130: 265–274, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Richerson GB, Wang W, Hodges MR, Dohle CI, ez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol 90: 259–266, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol 129: 175–189, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci 6: 1139–1140, 2003 [DOI] [PubMed] [Google Scholar]
- 54. St-John WM, Li A, Leiter JC. Genesis of gasping is independent of levels of serotonin in the Pet-1 knockout mouse. J Appl Physiol 107: 679–685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6: 557–618, 1981 [DOI] [PubMed] [Google Scholar]
- 56. Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol 127: 135–155, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25: 399–410, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol 103: 220–227, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci 26: 2623–2634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. Eur J Neurosci 26: 331–344, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Voss MD, De CD, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol 295: 208–218, 1990 [DOI] [PubMed] [Google Scholar]
- 63. Wang RY, Aghajanian GK. Collateral inhibition of serotonergic neurones in the rat dorsal raphe nucleus: pharmacological evidence. Neuropharmacology 17: 819–825, 1978 [DOI] [PubMed] [Google Scholar]
- 64. Wang RY, Aghajanian GK. Antidromically identified serotonergic neurons in the rat midbrain raphe: evidence for collateral inhibition. Brain Res 132: 186–193, 1977 [DOI] [PubMed] [Google Scholar]
- 65. Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511: 433–450, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90: 1001–1011, 1999 [DOI] [PubMed] [Google Scholar]
- 67. Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85: 2224–2235, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Yamamoto Y, Lagercrantz H, von Euler C. Effects of substance P and TRH on ventilation and pattern of breathing in newborn rabbits. Acta Physiol Scand 113: 541–543, 1981 [DOI] [PubMed] [Google Scholar]
- 69. Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci 26: 12781–12788, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]