Abstract
We have demonstrated that stimulation of somatic afferents during electroacupuncture (EA) inhibits sympathoexcitatory cardiovascular rostral ventrolateral medulla (rVLM) neurons and reflex responses. Furthermore, EA at P5-P6 acupoints over the median nerve on the forelimb activate serotonin (5-HT)-containing neurons in the nucleus raphe pallidus (NRP). The present study, therefore, examined the role of the NRP and its synaptic input to neurons in the rVLM during the modulatory influence of EA. Since serotonergic neurons in the NRP project to the rVLM, we hypothesized that the NRP facilitates EA inhibition of the cardiovascular sympathoexcitatory reflex response through activation of 5-HT1A receptors in the rVLM. Animals were anesthetized and ventilated, and heart rate and blood pressure were monitored. We then inserted microinjection and recording electrodes in the rVLM and NRP. Application of bradykinin (10 μg/ml) on the gallbladder every 10 min induced consistent excitatory cardiovascular reflex responses. Stimulation with EA at P5-P6 acupoints reduced the increase in blood pressure from 41 ± 4 to 22 ± 4 mmHg for more than 70 min. Inactivation of NRP with 50 nl of kainic acid (1 mM) reversed the EA-related inhibition of the cardiovascular reflex response. Similarly, blockade of 5-HT1A receptors with the antagonist WAY-100635 (1 mM, 75 nl) microinjected into the rVLM reversed the EA-evoked inhibition. In the absence of EA, NRP microinjection of dl-homocysteic acid (4 nM, 50 nl), to mimic EA, reduced the cardiovascular and rVLM neuronal excitatory reflex response during stimulation of the gallbladder and splanchnic nerve, respectively. Blockade of 5-HT1A receptors in the rVLM reversed the NRP dl-homocysteic acid inhibition of the cardiovascular and neuronal reflex responses. Thus activation of the NRP, through a mechanism involving serotonergic neurons and 5-HT1A receptors in the rVLM during somatic stimulation with EA, attenuates sympathoexcitatory cardiovascular reflexes.
Keywords: presympathetic neurons, visceral and somatic afferents, long-loop pathway
for many years, acupuncture and, more recently, electroacupuncture (EA) have been used to treat a number of diseases, including myocardial ischemia, arrhythmias, and hypertension (24, 32, 34, 43). Acupuncture or EA applied at Neiguan-Jianshi acupoints (P5-P6) of the pericardial meridian, through stimulation of the median nerve in the wrist, significantly modulate cardiovascular function (7, 8, 18). More specifically, EA stimulation at P5-P6 acupoints with low current and low frequency (2–4 mA, 2 Hz, 0.5 ms) reduces the extent of myocardial ischemia in response to an imbalance between oxygen supply and demand during reflex autonomic stimulation (18).
The rostral ventrolateral medulla (rVLM) is an important medullary region that participates in the modulation of sympathoexcitatory cardiovascular reflex responses by EA (19, 36, 37). Opioid μ- and δ-receptors, as well as nociceptin and γ-amino-butyric acid (GABAA) in rVLM, play an important role during the immediate EA-inhibitory influence on the sympathoexcitatory cardiovascular reflex response (10, 19, 35).
In addition to its immediate effects, acupuncture has the ability to cause prolonged modulation of cardiovascular excitatory reflexes for as long as 10–12 h in unanesthetized animals (42). In this regard, in a series of investigations, we have examined a long-loop pathway and the neurochemicals involved in the underlying mechanisms of the EA-related inhibitory influences on the cardiovascular responses, lasting for 60–90 min in anesthetized animals. We have identified that the hypothalamic arcuate nucleus, mesencephalic ventrolateral periaqueductal gray (vlPAG), and rVLM are important regions involved in the neuronal circuitry (20, 22, 23, 38). GABA and opioids, but not nociceptin, are involved in the prolonged cardiovascular effects of EA (35). The current study explores an additional medullary region, the nucleus raphe pallidus (NRP) and contributing neurotransmitters.
The medullary midline or raphe nuclei, through their influence on neurons in caudal and rostral VLM, modulate sympathetic outflow and cardiovascular responses (9, 40). The raphe, specifically the NRP, the most ventral subdivision of the raphe, contains serotonergic neurons that project to the rVLM (3). Involvement of raphe nuclei and their projections to the rVLM in EA-mediated modulation of the cardiovascular reflex responses have not been evaluated. Our previous anatomical data have shown that serotonin-containing neurons in the NRP may contribute to the EA cardiovascular response (14). Serotonin1A or 5-hydroxytryptamine (5-HT1A) receptors in the rVLM contribute to sympathoinhibition of cardiovascular responses to severe hemorrhage and inhibit somatosympathetic cardiovascular reflexes (12, 27). We, therefore, hypothesized that a serotonin projection from the NRP to the rVLM through a 5-HT1A mechanism participates in the EA inhibition of cardiovascular sympathoexcitatory reflex responses. These data have been published in preliminary form (28).
MATERIALS AND METHODS
Surgical Procedures
The animal use and care committee at the University of California, Irvine, approved all surgical and experimental protocols of this study. All procedures were carried out in accordance with the US Society for Neuroscience and the National Institutes of Health guidelines. The minimal possible number of cats was used to obtain reproducible and statistically significant results. Cats of both sexes were anesthetized initially with an injection of ketamine (40 mg/kg im). The femoral vein and artery were cannulated for administration of drugs and fluids and measurement of arterial blood pressure (Statham P 23 ID, Oxnard, CA), respectively. An intravenous injection of α-chloralose (50 mg/kg iv) was administered. Supplemental α-chloralose (5–10 mg/kg iv) was given, if the animals exhibited a corneal reflex, withdrew a limb in response to a noxious stimulus during the experiment, or displayed an unstable respiratory pattern or blood pressure. Heart rate (HR) was derived from the arterial pressure pulse by a biotech (Gould Instrument, Cleveland, OH). Intubation of the trachea facilitated artificial respiration (Harvard pump, model 662, Ealing, South Natick, MA). Arterial blood gases were examined frequently (Radiometer, Model ABL-3, Westlake, OH) and were maintained within the normal physiological range (Po2, 100–150 Torr; Pco2, 28–35 Torr; pH 7.35–7.45) by intravenous administration of 8% sodium bicarbonate or by adjusting the ventilator. Body temperature was kept between 36 and 38°C, using a water-perfused heating pad and an external heat lamp, as needed.
A right lateral laparotomy was performed to expose the surface of the gallbladder and to isolate the splanchnic nerve. This allowed direct placement of a bradykinin (BK) presoaked pledget on the serosal surface of the gallbladder. To quantify neuronal activity in the rVLM, we placed a bipolar flexible platinum stimulating electrode around the splanchnic nerve for stimulation of the cardiovascular sympathetic afferent reflex pathway. The stimulating electrode was connected to an isolation unit and a stimulator (Grass, model S88) and was held in place with hypoxy dental glue (Pentron, Wallington, CT). The abdominal wall was closed to prevent desiccation and heat loss and was reopened only for BK application on the gallbladder. A craniotomy was performed after the animal was stabilized with a Kopf stereotaxic head frame to expose the dorsal surface of the medulla to access the NRP and rVLM. The animal's neural axis was stabilized with a spinal holder (Kopf Instrument, Tujunga) before laminectomy to expose the spinal cord at T1-T4. To correlate renal sympathetic nerve (RSN) activity with rVLM discharge frequency, an incision was made in the right flank region to expose the retroperitoneal RSN, while the left splanchnic nerve was isolated to evoke rVLM neuronal activity. A dissecting microscope (Zeiss) was used to isolate a branch of the renal nerve, which was covered with warm mineral oil and placed across one pole of the recording electrode. The other pole of the recording electrode was grounded with a saline presoaked cotton thread to the animal.
Microinjection electrodes consisting of a guide tube with an outer diameter of 0.75 mm and an injection cannula with an inner diameter of 0.4 mm were inserted into the NRP and/or the rVLM to examine the cardiovascular responses. A three-barrel glass pipette electrode was used to evaluate rVLM neuronal activity. One barrel of the pipette electrode was filled with saline or the 5-HT1A receptor antagonist (WAY-100635). The other two barrels contained a platinum recording electrode with 0.5 M sodium acetate containing 2% Chicago sky blue (Sigma Chemical, St. Louis, MO) and 3 M NaCl to balance the current. A microinjection electrode was positioned perpendicularly to the dorsal surface of the medulla, 3.5 mm rostral to obex, and advanced ventrally 4.8 mm to reach the NRP. Alternatively, in experiments in which a closely located rVLM electrode was used (see below), the NRP electrode was positioned at the obex and advanced rostro-ventrally ∼6 mm at a 53° angle to the dorsal surface of the medulla. We positioned the rVLM electrode or a three-barrel pipette perpendicularly to the dorsal surface of the medulla using visual approximation, 3–3.5 mm laterally and 3–3.5 mm rostrally relative to the obex, and it was advanced 5 mm ventrally. At the end of the experiment, the recording and microinjection sites were marked with Chicago blue dye for later histological confirmation following administration of drugs into the NRP and rVLM. Acupuncture needles were inserted to a depth of ∼4 mm, bilaterally, at the Neiguan-Jianshi acupoints (P5-P6). Needles at these acupoints were located 2–3 cm proximal to the flexor crease on the wrist and were separated by 5–7 mm. They were connected to an isolation unit and stimulator (Grass, model S88) to deliver bipolar stimuli.
Methods of Blockade
The role of serotonin in the rVLM during EA was evaluated by microinjection of the 5-HT1A receptor antagonist WAY-100635 (1 mM, 50–75 nl, Research Biochemical International) (2) 20 min after stimulation was terminated. Saline served as the control.
The importance of the NRP in the acupuncture response was determined by microinjection of kainic acid (KA; 1 mM, 50 nl) (38) into the midline medullary region. Thus, either 50 nl of KA or its vehicle control, 0.9% saline, was injected into the NRP after termination of stimulation at a time when the cardiovascular effects of EA were still present.
To demonstrate an inhibitory role of the serotonergic projection from the NRP to the rVLM during sympathoexcitatory cardiovascular response, the NRP was activated by microinjection of dl-homocysteic acid (DLH, 4 nM, 50 nl), to mimic EA, while the 5-HT1A receptors in the rVLM were blocked with WAY-100635. In addition, to confirm the NRP-rVLM projection, we recorded splanchnic nerve-evoked rVLM extracellular neuronal activity in response to microinjection of DLH in the NRP before and after iontophoresis of the 5-HT1A receptor antagonist in the rVLM. Iontophoresis using a Neuro Phore BH-2 system (Medical System, Greenvale, NY) with saline vehicle or WAY-100635 before a third DLH-NRP microinjection lasted for 2 min. A current of 120–130 nA was used for iontophoresis.
Stimulating and Recording Methods
Repeated stimulation every 10 min of the gallbladder with BK (10 μg/ml) or splanchnic nerve (2 Hz, 0.4–0.6 mA, 0.5 ms) induced consistent increases in blood pressure or neuronal rVLM activity (35). The median nerves beneath the acupoints P5-P6 were stimulated bilaterally with EA at 2–4 Hz, 2–4 mA, using a 0.5-ms pulse (38). Previous study has shown that these stimulation parameters applied at P5-P6 activate both group III and IV afferents in the median nerve to decrease sympathoexcitatory cardiovascular responses (18, 39). We applied 30 min of EA to simulate clinical use of this procedure. Separately, the intermediolateral (IML) column at T1-T4 was stimulated electrically with 0.1–0.4 mA, 2 Hz, and 0.5 ms to induce antidromic stimulation and to test for collision (20, 35–37). The location of the IML was determined preliminarily during the experiment with electrical stimulation (10–40 μA), which evoked a small, reproducible excitatory response of 5–10 mmHg and confirmed anatomically after the experiment.
RSN recording.
A subgroup of rVLM neurons was characterized as presympathetic by demonstrating a relationship between rVLM discharge and renal sympathetic activity. To record renal nerve activity, a recording electrode placed around the renal nerve was attached to a high-impedance probe (model HIP511), and the signal was amplified (Grass P511), monitored with an oscilloscope (model 2201, Tektronix, Beaverton, OR), and processed with a Pentium IV computer for offline analysis through an analog-to-digital converter CED micro1401 MK II interface system. A window discriminator was set with a threshold above the noise level to assess renal nerve discharge activity (20).
Extracellular rVLM recordings.
Single-unit activity of rVLM neurons was recorded with a platinum electrode inserted in a three-barrel pipette positioned in the rVLM. Action potentials were amplified with a preamplifier (Grass P511) attached to a high-impedance probe (Grass H1P5) and then filtered (0.3–10 kHz) and monitored with an oscilloscope (Tektronix 2201). Renal nerve activity, rVLM action potentials, blood pressure, and HR were digitized with a data-acquisition CED micro1401 MK II interface system. Data were analyzed offline with a Pentium IV computer and CED Spike 2 Windows software. Rectified and nonrectified action potentials were analyzed both visually and with the Spike 2 program using wave shape recognition algorithms to allow detection of similar wave shapes, heights, and latencies of response. Peristimulus time histograms were constructed for each neuron to assess evoked responses to stimulation of splanchnic or median nerves. Relationships between rVLM neuronal activity and blood pressure or renal nerve activity were assessed by both time and frequency domain analyses using arterial pulse and spike-triggered averaging and coherence analysis (20, 35). Examination for baroreceptor afferent input with either nitroglycerin (2.5 mg/ml) or phenylephrine (2 mg/ml) provided additional characterization of rVLM neurons.
Retrograde Tracing and c-Fos Staining: Microinjection of a Retrograde Tracer into Rat rVLM
To anatomically examine for a direct projection between the NRP and rVLM that might be involved in EA-mediated inhibition, using stereotaxic positioning to guide placement of the tip of the injection pipette in the medulla in the region of the rVLM, we microinjected a retrograde tracer in rats (350–500 g), as we have described previously (20). A mixture of ketamine-xylazine (80:12 mg/ml, Sigma) was used to induce (0.3–0.4 ml im) and maintain (0.1–0.2 ml im) anesthesia. Body temperature was monitored with a rectal probe and was maintained at 37°C. HR and oxygen saturation were monitored using a pulse oximeter (Nonin Medical, Plymouth, MN). The rat was placed on a stereotaxic apparatus (David Kopf Instruments). A 2-cm, 1-in. incision was made to expose the skull. A burr hole (4-mm diameter) was made in the occipital bone, according to the following coordinates: 12.0–12.5 mm caudal from the bregma; 2.0–2.5 mm from the midline, 8.5 mm deep from the dural surface. One hundred nanoliters of a retrogradely transported tracer that is retrogradely transported, rhodamine-labeled fluorescent microspheres in suspension (0.04 μm, Molecular Probes, Eugene, OR) were unilaterally injected into the rVLM through a glass micropipette. The wound was sutured shut. The microspheres were transported during the 10- to 12-day recovery and maintenance period.
Terminal procedures occurred 10–12 days after administration of the retrograde tracer. Rats were re-anesthetized with the ketamine-xylazine, as described above. After tracheostomy and intubation, cannulation and monitoring for vital signs were similar to the procedures described earlier above. Animals were stabilized for 4 h. Then EA or sham-operated controls for EA were conducted over a 30-min period (as described, see below). As described in our previous studies (20), 90 min following termination of EA or the control procedure, rats were deeply anesthetized with a large dose of the ketamine-xylazine (0.5–0.7 ml im). Transcardial perfusion was performed using 500 ml of 0.9% saline solution followed by 500 ml of 4% paraformaldehyde. The medulla oblongata was harvested and sliced into coronal sections (30 μm) with a cryostat microtome (Leica CM1850 Heidelberger Strasse, Nussloch, Germany). The sections were scanned to identify the sites of microinjection of the microsphere tracer. If the tracer injections of microsphere tracers were located in the right region of the rVLM, identified according to their best matched standard stereotaxic plane, as shown in Paxinos and Watson's atlas for the rat (30), the brain sections were immunohistochemically stained for c-Fos protein, as described below.
Immunohistochemistry: Immunohistochemical Fluorescent Labeling for c-Fos
The staining procedures were similar to those described in our previous studies (20). In phosphate-buffered saline containing 3% Triton X-100 and 1% normal donkey serum, brain tissues were incubated with a primary mouse monoclonal anti-c-Fos antibody (1:2,000 dilution; Santa Cruz Biotechnology, CA) for 48 h at 4°C. Sections then were incubated with fluorescein-conjugated donkey anti-mouse antibody (1:200; Jackson Immunoresearch Laboratories) at 4°C for 24 h. The sections were mounted on slides and coverslipped with mounting medium (Vector Laboratories). No stain was detected when the primary or secondary antibody was omitted in the immunohistochemical control studies.
Experimental Protocols
Role of NRP.
To increase blood pressure, visceral chemosensitive afferents on the gallbladder were stimulated with a BK-soaked 1-cm2 pledget of filter paper every 10 min. At the maximal blood pressure increase, the filter paper was removed, and the gallbladder was rinsed three to four times with normal saline to remove excess BK. The increase in blood pressure, serving as an index of the visceral afferent reflex, was evaluated as the difference between mean arterial blood pressure (MAP) before application of BK and at peak of the reflex response. We first examined repeatability of the blood pressure response to nine sequential visceral reflex stimulations induced by application of BK to the gallbladder in a group of five animals. Then, in another group of six animals, the reflex responses were induced twice, followed by EA for 30 min, during which time the gallbladder was stimulated every 10 min. The NRP was inactivated with KA (1 mM, 50 nl) after 18 min of EA. Saline served as the vehicle control in five other animals.
Role of rVLM 5-HT1A receptors.
Reflex increases in blood pressure were evoked every 10 min. As in the previous protocol, we observed two consistent increases in blood pressure, followed by 30 min of EA applied bilaterally at the P5-P6 acupoints, beginning 5 min before the third application of BK. EA was terminated 5 min after the fifth gallbladder stimulation. Recovery of the inhibitory EA effect was examined for up to 70 min. We evaluated the role of serotonin in the rVLM during EA's action on the cardiovascular responses by microinjecting WAY-100635 or saline vehicle control in the rVLM (five animals in each group). We also evaluated the influence of 5-HT1A receptor blockade on the primary (gallbladder) reflex response in five additional animals.
Serotonergic input from NRP to rVLM.
To examine for the presence of inhibitory projections between the NRP and the rVLM, microinjection and recording electrodes were positioned in both nuclei in five animals. During repeated stimulation of the gallbladder, the NRP was stimulated by four consecutive microinjections of DLH 2 min before the second through fifth application of BK. The existence of a serotonergic projection from the NRP was determined by microinjecting the 5-HT1A receptor antagonist into the rVLM before the third NRP activation.
In addition to cardiovascular reflex responses, we examined the neuronal discharge of rVLM neurons during NRP-rVLM serotonergic inhibition. We initially identified rVLM neurons that received convergence of splanchnic and median nerves, NRP, and baroreceptor afferents. Then we examined for cardiac rhythmicity and either direct projection to IML using antidromic stimulation (n = 5) or the relationship of rVLM activity with renal sympathetic discharge (n = 10). Due to the complexity of the preparation, we found that we could not reliably insert an electrode into the IML to identify sympathetic premotoneurons. However, demonstration of a relationship between rVLM and renal sympathetic discharge allowed classification of the remainder of rVLM neurons as presympathetic.
We used a number of techniques to categorize rVLM neurons. First, we identified cells that receive visceral and somatic afferent convergent input. Convergence of splanchnic and median nerve input evoked activity of 30 stimuli at 2 Hz over a 15-s period was recorded to allow construction of peristimulus time histograms. Evoked discharge was established as the difference between peak response and prestimulation activity. Peristimulus histograms were used to evaluate rVLM neuronal responses to stimulation of the NRP substrate and blockade of serotonin rVLM receptors. Second, in some cases, we antidromically evoked activity in rVLM neurons from the IML, which was stimulated at a frequency of 2 Hz and a duration of 0.5 ms, to locate premotor sympathetic cells. Neurons that responded to antidromic stimulation were examined further for constant latency, stable threshold of the evoked all-or-none response, and a faithful response to high rates of stimulation (100–200 Hz). Regular responses to high-frequency stimulation helped establish an absence of variable synaptic delay. Then the neurons were evaluated for collision of IML-evoked antidromic action potentials and splanchnic or median nerve-evoked orthodromic activity. The refractory period also was measured to determine the critical time interval (latency plus refractory period) during which the ortho- and antidromic spikes collide. The conduction velocity of premotoneurons was determined from the distance between the recording and stimulating electrodes and the antidromic latency. Third, time and frequency domain analyses on blood pressure and rVLM signals were used to identify cardiovascular sympathoexcitatory rVLM neurons (20). Fourth, cardiovascular rhythmicity over a period of 5 min was determined by evaluating the relationship between blood pressure or RSN activity and rVLM neuronal discharge rate using pulse or spike-triggered averaging, as well as coherence (35, 38). Fifth, neurons were examined for their responsiveness to cardiovascular stimulation by altering baroreceptor input with administration of nitroglycerin or phenylephrine.
Once the cells were characterized, we examined their evoked responses to repeated splanchnic nerve stimulation every 10 min. Consistency of responses of five neurons was evaluated during repeated stimulation. NRP serotonergic input to 13 rVLM neurons was examined with four consecutive microinjections of DLH into the NRP and iontophoresis of WAY-100635 (1 mM, 120 nA, 2 min) or saline into the rVLM just before the third injection of DLH. DLH was microinjected into the NRP 2 min before splanchnic nerve evoked rVLM activity was recorded.
Histology
At the end of each experiment, animals were euthanized under deep α-chloralose anesthesia, followed by saturated KCl. Recording and/or microinjection sites were marked by either iontophoresis and/or injection of 2% Chicago blue dye. Thereafter, the brain was removed and fixed in 10% paraformaldehyde for at least 2 days. Brain stems were sliced with a microtome cryostat in 60-μm coronal sections. Recording and microinjection sites were reconstructed from the dye spots with the aid of a microscope (Nikon) and software (Corel presentation). The sites were plotted on coronal sections separated by 1.1- and 1.4-mm intervals with respect to the obex (5).
C-Fos Expression Induced by EA
Previously, we have shown a significant increase in c-Fos labeling in the NRP of cats following EA stimulation at the P5-P6 acupoints, compared with sham-operated controls (14). In the present study, we sought to identify c-Fos expression induced by EA in neurons containing the retrograde tracer. Low-frequency EA (0.5 ms pulses, 2 Hz, 1–4 mA) at the P5-P6 acupoints was applied in rats subjected to EA. EA was maintained for 30 min, as described in the above physiological protocols. The stimulation intensity was sufficient to produce mild, repetitive paw flexion in each forelimb. Insertion of acupuncture needles into the P5-P6 acupoints without electrical stimulation served as sham controls for EA. Animals were included for data analysis if the site for microinjection was found to be in the rVLM, as determined by subsequent histological examination mentioned above.
Data Analysis
Evoked activity was measured as the increase in number of spikes above baseline and presented as means ± SE. The assumption of normal data distribution was analyzed with the Kolmogorov-Smirnov test. Blood pressure responses to BK were analyzed with a one-way repeated-measures pairwise multiple-comparison analysis of variance, followed post hoc with the Student-Newman-Keuls test. SigmaStat and SigmaPlot software (Jandel Scientific, San Rafael, CA) were used for statistical analysis and graphing. The level of statistical significance was chosen as P < 0.05.
We also evaluated time and frequency relationships between rVLM neuronal activity and arterial blood pressure or sympathetic activity using pulse- or spike-triggered averaging, as well as coherence analysis. Thus time domain analysis involved either arterial pulse-triggered or spike-triggered averaging. A threshold was set at the systolic phase of the arterial pulse for the former method, while the latter used spike height discrimination and waveform recognition to sort action potentials during the evaluation period of 300 s. Averages of the arterial pulse and histograms of sympathetic discharge and rVLM neuronal activity were constructed as in our laboratory's previous studies (20, 22, 36).
Frequency domain analysis involved assessment of the coherence between rVLM activity and arterial blood pressure or RSN activity using a fast Fourier transform algorithm. We recorded data using a sampling rate of 10,000 Hz. Reconstructed data utilized every 10th sample, including assessment of the mean and peak amplitudes and the maximum and minimum slopes of the original spike to preserve the action potentials. The spikes were sorted and identified with a window discriminator to construct histograms before coherence analysis. The number of data sections (15–20, each lasting for 12.8 s) was chosen to determine the average histogram. Autospectra of rVLM discharge and arterial blood pressure or RSN activity were generated with fast Fourier transform. Thus coherence was generated with seven overlapping windows, each with a length of 12.8 s, consisting of 256 bins, with bin widths of 50 ms. The autospectral analysis was adopted from Shin et al. in 1995 (33) using contiguous segments of 256 beats with 50% overlap between the segments. The frequency resolution was 1/12 s or 0.08 Hz. The coherence function (normalized cross-spectrum) provided a measure of the strength of linear correlation of rVLM neuronal activity and blood pressure or RSN activity at each frequency. Coherence values of ≥0.5 were chosen to reflect a statistically significant relationship between rVLM spikes and arterial blood pressure (35).
Brain sections were scanned and examined with a standard fluorescent microscope (Nikon, E400, Melville, NY). Two epifluorescence filters (B-2A and G-2A) equipped in a fluorescent microscope were used to identify single stains appearing as green (fluorescein) or red (rhodamine) in each brain section. Sections containing the NRP were identified according to their best matched standard stereotaxic plane, as shown in Paxinos and Watson's atlas for the rat (30).
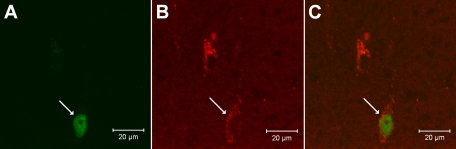
Selected sections containing the NRP were evaluated with a laser scanning confocal microscope (Zeiss LSM 510, Meta System, Thornwood, NY) after examination with a fluorescent microscope to confirm colocalization of microsphere tracer with c-Fos. This apparatus was equipped with argon and HeNe lasers and allowed operation of multiple channels. Lasers of 488- and 543-nm wavelengths were used to excite fluorescein (green) and rhodamine (red), respectively. Digital fluorescent images were captured and analyzed with software (Zeiss LSM) provided with the confocal microscope. Each confocal section analyzed was limited to 0.5-μm thickness in the Z-plane. Images containing two colors in the same plane were merged to reveal the relationship between two labels (see Fig. 7). Single- and double-labeled neurons were evaluated.
Fig. 7.
Confocal microscopic images showing an NRP neuron double-labeled with c-Fos (A) and the retrograde microsphere tracer (B) injected into the rVLM of a rat (bregma −12.24 mm). C: merged image from A and B. Arrows in A, B, and C, respectively, indicate neurons containing c-Fos (green) and the retrograde tracer (red) and colocalization of c-Fos with the microsphere tracer. Scale bars in A–C represent 20 μm.
The numbers of single- and double-labeled cells in similar sections were counted in each animal. Comparisons between the two groups were analyzed with the Student's t-test. Values were considered to be significantly different when P < 0.05. Data are expressed as means ± SE.
RESULTS
Role of NRP in Acupuncture Effect
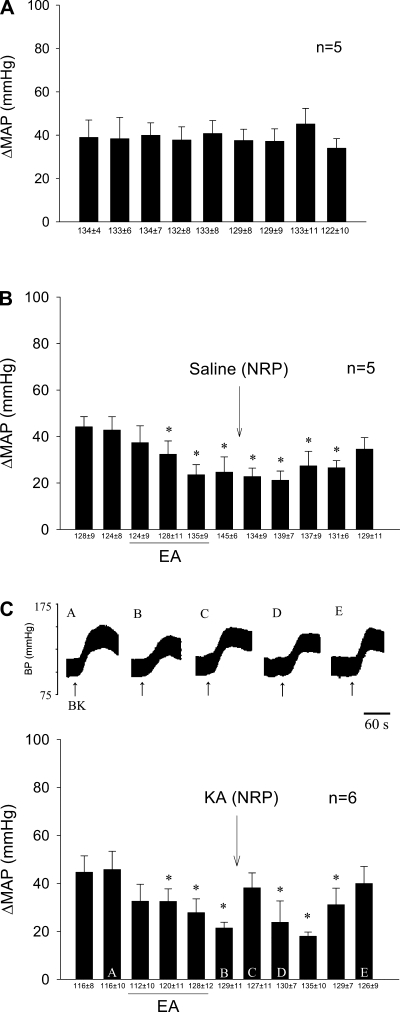
The cardiovascular sympathoexcitatory responses to repeated stimulation of the gallbladder with BK every 10 min were consistent. HR and MAP before the onset of each reflex response were consistent throughout this protocol (Fig. 1A). Sympathoexcitatory cardiovascular responses were reduced by EA and remained inhibited after microinjection of saline into the NRP. However, depolarization blockade of neurons in the NRP with KA reversed the EA-related inhibition of the cardiovascular sympathetic reflex (Fig. 1, B and C). Baseline blood pressure did not change following microinjection of KA.
Fig. 1.
Role of nucleus raphe pallidus (NRP) in electroacupuncture (EA) modulation of cardiovascular sympathoexcitatory reflex response. A and B: 30-min stimulation of P5-P6 overlying the median nerve caused prolonged attenuation of the increase in blood pressure (BP). B and C: depolarization blockade of the NRP with kainic acid (KA) reversed the inhibitory effects of EA. C: letters A–E above each BP tracing (top) represent bars (bottom). Bars represent increases (Δ) in mean arterial BP (MAP) induced by gallbladder simulation. BK, bradykinin. Means ± SE below histogram bars represent baseline MAP. *Significantly different compared with control MAP, P < 0.05.
Role of 5-HT1A Receptors in the rVLM During Acupuncture
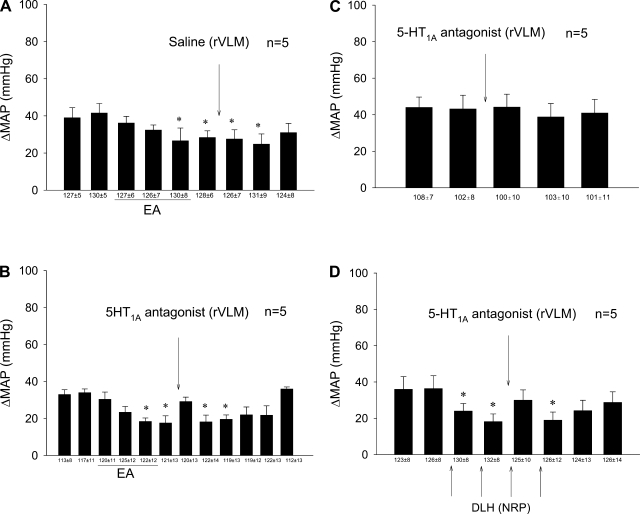
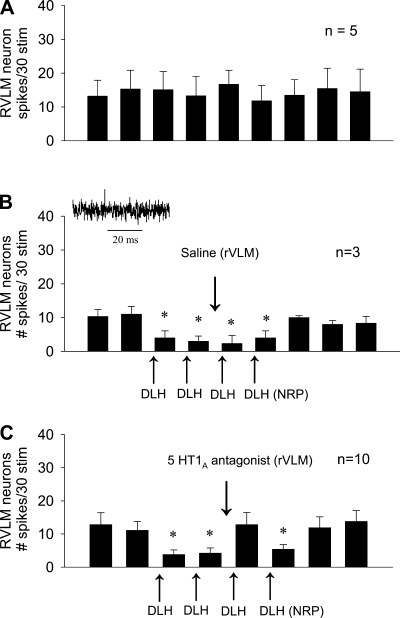
Stimulation by 30 min of EA reduced the cardiovascular reflex responses during and for over 40 min after activation of the median nerves. Microinjection of saline into the rVLM did not affect the EA-related inhibition (Fig. 2A). Blockade of 5-HT1A receptors in the rVLM reversed the inhibitory effects of EA from 18 ± 4 to 29 ± 2 mmHg, but did not influence the primary cardiovascular reflex responses (Fig. 2, B and C).
Fig. 2.
Role of serotonin1A or 5-hydroxytryptamine (5-HT1A) receptors in rostral ventrolateral medulla (rVLM) during EA-cardiovascular modulation. A: 30-min stimulation of P5-P6 led to prolonged attenuation of the increase in BP in the presence of saline vehicle microinjection into the rVLM. B: the serotonin antagonist (WAY-100635), however, reversed the effects of EA. C: in contrast, the antagonist did not influence the cardiovascular responses in the absence of EA. D: microinjection of dl-homocysteic acid (DLH) into the NRP, like EA, caused sympathoinhibition, a response that was reversed by administration of the serotonin antagonist into the rVLM. Bars represent increases in MAP induced by gallbladder simulation. Means ± SE below histogram bars represent baseline MAP. *Significantly different compared with control MAP, P < 0.05.
Serotonergic Input From NRP to rVLM
Neurons in the NRP were activated sequentially with DLH four times, to mimic EA, during repeated gallbladder stimulation every 10 min. The sympathoexcitatory cardiovascular responses were reduced after each DLH microinjection. Baseline arterial blood pressure was decreased transiently by 5–10 mmHg with each NRP-DLH microinjection. Serotonergic projections to the rVLM were identified by noting the significant reversal of the DLH inhibition of the cardiovascular reflex response following administration of WAY-100635 in the rVLM (Fig. 2D).
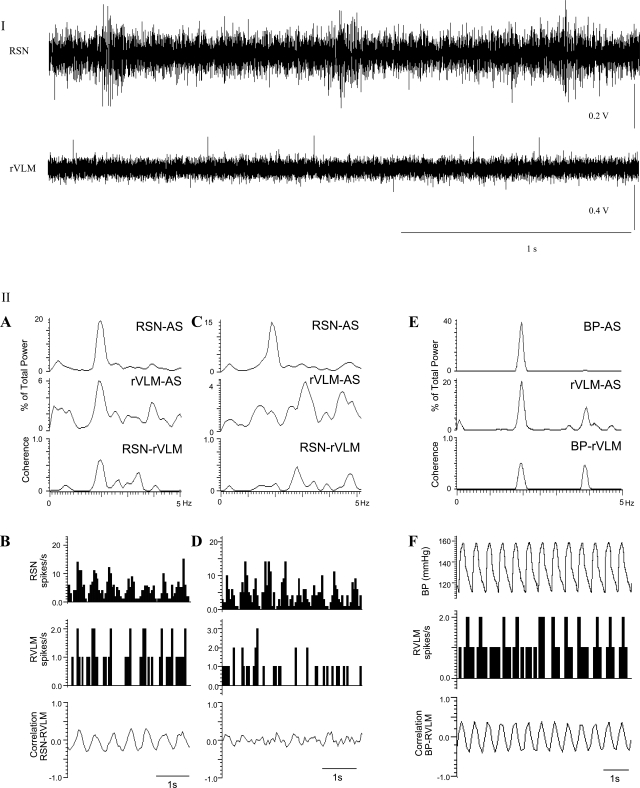
In addition to reflex cardiovascular responses, we evaluated rVLM neuronal activity to confirm the role of the NRP-rVLM serotonergic connection in the EA response. We studied only neurons that received convergent somatic and visceral input and were responsive to baroreceptor afferent stimulation and thus could be classified as cardiovascular excitatory cells. In this group of neurons, the change in MAP of 50 ± 3 mmHg with nitroglycerin increased rVLM activity from 2.6 ± 0.93 to 3.5 ± 1.0 spikes/s. Conversely, phenylephrine decreased the firing rate from 2.3 ± 0.63 to 1.8 ± 0.58 spikes/s in response to an increase in blood pressure of 108 ± 34 mmHg. We recorded activity of four out of five cells that were identified as premotoneurons using the collision technique. Another five cardiovascular rVLM neurons in which we did not examine antidromic input or record together with the renal multiunit sympathetic nerve activity received convergent input from the NRP, splanchnic, baroreceptor, and median nerves. The remaining eight rVLM neurons were recorded simultaneously with RSN activity. All eight rVLM neurons studied were found to be correlated with RSN activity, while four others, as noted above, were identified as premotor using collision from the IML. Thirteen of fourteen rVLM neurons examined received input from the NRP. Frequency domain analysis of RSN and rVLM autospectra generated a coherence of 0.64 ± 0.02 at a frequency of 2.6 ± 0.1 Hz (equivalent to a HR of 156 beats/min). Baseline activity of this group of rVLM neurons averaged 2.9 ± 0.75 spikes/s. Coherence values and baseline activity were not different between the two groups of rVLM neurons, i.e., four premotor and 14 presympathetic rVLM neurons. An example of an rVLM neuron whose activity was correlated with the RSN and arterial blood pressure and that demonstrated significant cardiac rhythmicity using coherence analysis is shown in Fig. 3. Another rVLM cell shown in Fig. 4 was identified as premotoneuron that projected to the IML, as shown by collision of an antidromically evoked spike with a splanchnic nerve-induced orthodromic action potential.
Fig. 3.
Characterization of a cardiovascular sympathoexcitatory rVLM neuron that received convergent input from splanchnic nerve, median nerve, NRP, and baroreceptors. I (top): the activities of the renal sympathetic nerve (RSN) and the rVLM neuron are shown. II (bottom), A: the rVLM neuron displayed similar rhythmicity with renal sympathetic activity documented as a coherence of 0.6 at a frequency at 1.9 Hz and autospectra (AS). B: the neuron also demonstrated a close correlation in time, as measured with spike-triggered averaging. C and D: another rVLM cell (control neuron) and its correlation with renal sympathetic activity. Note lack of coherence and correlation. E: the same rVLM neuron discharged at an identical frequency of 1.9 Hz and demonstrated a coherence of 0.52 when its activity was gated with BP. F: the pulse-triggered averaging of BP and rVLM discharge frequency of this neuron. AS, ?.
Fig. 4.
Antidromic collision of a premotor rVLM neuron. The three panels display tracings that, from left to right, represent the splanchnic nerve-induced evoked spike, intermediolateral column (IML) stimulus, and antidromic-evoked action potential, respectively. The neuron had a conduction velocity of 11.5 m/s, a latency of 10 ms, and a refractory period of 7.6 ms. The middle tracing displays collision between the splanchnic nerve-induced orthodromic and the IML-evoked antidromic spikes. ↓, Time of stimulation of the splanchnic nerve; ↓, time of stimulation of the IML.
Sympathoexcitatory cardiovascular rVLM neurons that received median nerve and NRP input demonstrated consistent splanchnic nerve evoked responses when stimulated every 10 min (Fig. 5A). The discharge rate of rVLM neurons was decreased after each of four DLH NRP microinjections (Fig. 5B). Blockade of rVLM 5HT1A receptors reversed the inhibitory action of NRP activation (Fig. 5C).
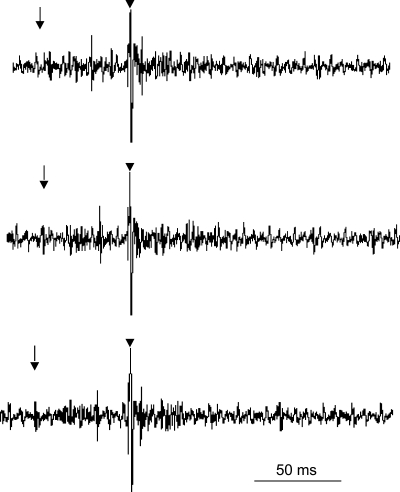
Fig. 5.
Role of 5-HT1A receptors in NRP-induced inhibition of rVLM neuronal activity. A: repeatable responses of premotor rVLM neurons following splanchnic nerve stimulation. B: DLH activation of the NRP neurons decreased discharge of sympathoexcitatory cardiovascular rVLM neurons. Inset: example of a rVLM spike. C: the serotonin antagonist (WAY-100635) reversed the effects of DLH-related inhibition of these rVLM neurons. *P < 0.05.
Anatomical Location of Recording and Stimulating Sites
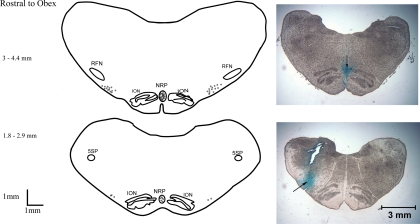
All rVLM recording and microinjection sites were confined to an area 1.8–4.4 mm rostral to the obex. The sites were located 2.87–3.75 mm lateral to the midline and 0.1–0.8 mm from the ventrolateral surface in the region of the rVLM, which is lateral to the inferior olivary nucleus and ventral and medial to the facial and retrofacial nuclei, as described by several authors, including ourselves (6, 15, 37) (Fig. 6). Microinjection sites in the NRP were located in the midline between the inferior olivary nucleus and the pyramidal tracts. The majority of the injection sites in the raphe pallidus were located 3–4 mm rostral to the obex. One rVLM neuron that did not receive input from the NRP was located in the NRP 2.1 mm rostral to the obex. All sites of stimulation of the spinal cord between T2 and T5 were identified to be in the IML, located in the central lateral gray area of the spinal cord, as described by Morrison and Gebber (29).
Fig. 6.
Composite map displaying sections of the medulla with sites of microinjections and recordings. Original pictures illustrate the sites (↓) of microinjection in the NRP (top) and recording in the rVLM (bottom). RFN, retrofacial nucleus; ION, inferior olivary nucleus; 5SP, alaminar spinal trigeminal nucleus.
NRP Neurons Colabeled With Retrograde Tracer and c-Fos in the NRP
In both EA-treated (n = 5) and control rats (n = 4), we observed that perikarya labeled with the retrograde microsphere tracer were distributed rostrocaudally throughout the NRP, following microinjection into the rVLM. Like our findings in cats (14), we noted more neurons with Fos immunoreactivity in EA-treated rats than in controls (19 ± 3 vs. 7 ± 2; P < 0.05) in the NRP. Importantly, we also found Fos nuclei colocalized with neurons that were labeled with the retrograde tracer from the rVLM. These double-labeled neurons also were observed significantly more frequently in EA-treated rats than in controls (9 ± 1 vs. 3 ± 1; P < 0.05), while there were similar numbers of neurons labeled with the retrograde tracer in NRP of both groups (24 ± 3 vs. 21 ± 4; EA treated vs. controls; P > 0.05). Photomicrographs in Fig. 7 show examples of confocal images displaying a neuron double-labeled with c-Fos (following median nerve stimulation) and microspheres in the NRP of an EA-treated rat.
DISCUSSION
Neurons in NRP activated by stimulation of acupoints P5-P6 overlying the median nerves contain serotonin, among other neurotransmitters (14). The medullary raphe nuclei are known to modulate autonomic reflexes (4). In this respect, activation of neurons in the raphe pallidus that project to sympathetic premotoneurons in the rVLM decreases baseline blood pressure through sympathoinhibition (3, 9, 25, 40). Similarly, EA modulates rVLM neurons to inhibit cardiovascular autonomic responses (35, 38). The present study provides several novel observations: 1) the NRP is involved in cardiovascular reflex modulation by EA; 2) EA modulates cardiovascular responses through 5-HT1A receptors in the rVLM; 3) a direct projection from EA-activated NRP neurons to rVLM exist; and 4) serotonergic projections from the NRP to the rVLM contribute to the EA-cardiovascular responses.
Serotonin is an important neurotransmitter in the medullary raphe. The caudal raphe, where the majority of EA-activated neurons exist (14), contains ∼15% of all serotonergic neurons in the brain (17). Serotonin plays a role in many conditions, including mood disorders, nociception, movement, sleep cycles, and autonomic cardiovascular regulation (17, 31). The raphe obscurus, for example, inhibits cardiovascular reflexes through a serotonergic mechanism (41). We recently have shown significant activation of neurons in the medullary raphe pallidus following stimulation at P5-P6. EA-induces activation of c-Fos containing neurons that often contain serotonin in a region 3.5 mm rostral to the obex (14), which coincides with the site of microinjections in the present study. Chemical and EA-related electrical activation of NRP neurons in this region demonstrate serotonergic input to the rVLM, which, in turn, decreases cardiovascular reflex sympathoactivation, thus underscoring the importance of serotonin in the cardiovascular modulation by EA.
Caudal medullary raphe neurons related to the cardiac cycle and sympathetic nerve discharge are frequently sympathoinhibitory (26). In this respect, microinjection of glutamate into the raphe pallidus decreases MAP and RSN activity and the discharge rate of sympathoexcitatory rVLM neurons (9, 40). In the present study, splanchnic nerve-evoked activation of sympathoexcitatory presympathetic cardiovascular rVLM neurons, as well as visceral sympathoexcitatory reflexes, was inhibited by DLH-induced activation of the NRP, as a surrogate for EA. The NRP-rVLM projections that participate in the EA modulatory response depend, at least in part, on activation of 5-HT1A receptors.
There are two potential limitations in the present study. First, we used rats rather than cats to demonstrate the presence of cells with direct projections from the NRP to the rVLM that were activated by EA. However, our laboratory has previously shown that the anatomic circuitry participating in the EA-cardiovascular inhibitory response in rats and cats is virtually identical (1, 20). We, therefore, believe that our data obtained in rats with respect to this projection likely also apply to cats.
Second, we found that only about one-fourth of the rVLM neurons examined could be identified as premotor rVLM neurons. On the other hand, we were able to demonstrate that the remainder of the rVLM neurons were presympathetic in nature, in that they showed a strong relationship to sympathetic outflow. As such, the rVLM neurons evaluated in the present study all appeared to play a role in regulating sympathetic nerve activity.
We have identified a long-loop pathway involved in EA modulation of excitatory cardiovascular reflexes. More specifically, neurons in the hypothalamic arcuate nucleus, vlPAG, and rVLM process information during somatic afferent stimulation that ultimately leads to prolonged (often >90 min) inhibition of sympathetic outflow following a single 30-min application of acupuncture (20, 22, 36–38). Reciprocal projections between the arcuate nucleus and the vlPAG likely contribute, in part, to the long-lasting EA inhibition of sympathoexcitatory and cardiovascular responses (21, 35). Serotonergic projections from the NRP to the rVLM may form an important part of this long-loop pathway.
The medullary raphe nuclei receive input from midbrain regions, including the vlPAG (11). Activation of neurons in the vlPAG inhibits activity of rVLM premotor sympathetic neurons (38) and is essential for the EA-cardiovascular sympathoinhibition in the rVLM (20). Direct projections from the vlPAG to the rVLM exist (13). However, it is unclear if EA relies on a direct or indirect pathway from the vlPAG to the rVLM, with the latter likely processed through the NRP. Additional studies will need to be conducted to determine the importance of direct vs. indirect connections between the vlPAG and the rVLM in the EA-cardiovascular response. However, our observation that 5-HT1A receptor activation is required for the full expression of the EA-related sympathoinhibition suggests that the NRP is a required component, since this region is so rich in serotonin (17).
Helke et al. (16) have demonstrated direct projections from the NRP to the IML of the spinal cord. These projections are serotonergic and are thus inhibitory in nature. Although we have shown an important connection from the NRP that is processed through the rVLM, future studies will need to evaluate possible direct connections from the raphe to sympathetic motoneurons in the spinal cord IML. We do believe, however, that the rVLM connections are of paramount importance, since blockade of neurons in this region largely eliminates the EA-cardiovascular response (10, 19, 35–37).
Conclusion
The present study shows that, during EA, serotonergic neurons in the medullary raphe, in particular, the raphe pallidus, project to sympathoexcitatory cardiovascular neurons in the rVLM and through a 5-HT1A receptor mechanism participate in EA-related sympathoinhibition.
GRANTS
National Heart, Lung, and Blood Institute, Bethesda, MD, Grants HL-72125 and HL-63313 and the Larry K. Dodge and Susan Samueli Endowed Chairs (J. C. Longhurst), supported this work.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge Dr. Peng Li, Kin K. Hang, and R. Cabatbat for technical assistance, and Y. Cao (Department of Physiology, Shanghai Medical College, Fu Dan University, China) for development of the software.
REFERENCES
- 1. Adams DB. The Behavioral and Brain Sciences. Brain Mechanisms for Offense, Defense and Submission. Cambridge, UK: Cambridge University Press, 1979, vol. 2 [Google Scholar]
- 2. Bago M, Dean C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by 5-HT1A receptors in the RVLM. Am J Physiol Regul Integr Comp Physiol 280: R976–R984, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bago M, Marson L, Dean C. Serotonergic projections to the rostroventrolateral medulla from midbrain and raphe nuclei. Brain Res 945: 249–258, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Barman SM, Gebber GL. Subgroups of rostral ventrolateral medullary and caudal medullary raphe neurons based on patterns of relationship to sympathetic nerve discharge and axonal projections. J Neurophysiol 77: 65–75, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Berman AL. The Brainstem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison, WI: University of Wisconsin Press, 1968 [Google Scholar]
- 6. Campos RR, McAllen RM. Cardiac sympathetic premotor neurons. Am J Physiol Regul Integr Comp Physiol 272: R615–R620, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Chao DM, Shen LL, Tjen-A-Looi S, Pitsillides KF, Li P, Longhurst JC. Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am J Physiol Heart Circ Physiol 276: H2127–H2134, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Cheung L, Li P, Wong C. Mechanism of Acupuncture Therapy and Clinical Case Studies. New York: Taylor and Francis, 2001 [Google Scholar]
- 9. Coleman M, Dampney RAL. Powerful depressor and sympathoinhibitory effects evoked from neurons in the caudal raphe pallidus and obscurus. Am J Physiol Regul Integr Comp Physiol 268: R1295–R1302, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Crisostomo M, Li P, Tjen-A-Looi SC, Longhurst JC. Nociceptin in rVLM mediates electroacupuncture inhibition of cardiovascular reflex excitatory response in rats. J Appl Physiol 98: 2056–2063, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Dean C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by the caudal midline medulla. Am J Physiol Regul Integr Comp Physiol 289: R1477–R1481, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Dean C, Bago M. Renal sympathoinhibition mediated by 5-HT1A receptors in the RVLM during severe hemorrhage in rats. Am J Physiol Regul Integr Comp Physiol 282: R122–R130, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Farkas E, Jansen ASP, Loewy AD. Periaqueductal gray matter input to cardiac-related sympathetic premotor neurons. Brain Res 792: 179–192, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Guo ZL, Moazzami A, Tjen-A-Looi S, Longhurst J. Responses of opioid and serotonin containig medullary raphe neurons to electroacupuncture. Brain Res 1229: 125–136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guyenet PG, Haselton JR, Sun MK. Sympathoexcitatory neurons of the rostroventrolateral medulla and the origin of the sympathetic vasomotor tone. Prog Brain Res 81: 105–116, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Helke CJ, Capuano S, Tran N, Zhou H. Immunocytochemical studies of the 5-HT1A receptor in ventral medullary neurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactiviy. J Comp Neurol 397: 261–270, 1997 [PubMed] [Google Scholar]
- 17. Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat 26: 331–343, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Li P, Pitsillides K, Rendig S, Pan HL, Longhurst J. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation 97: 1186–1194, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Li P, Tjen-A-Looi S, Longhurst J. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Auton Neurosci 89: 38–47, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Li P, Tjen-A-Looi S, Guo Z, Fu LW, Longhurst JC. Long-loop pathways in cardiovascular electroacupuncture responses. J Appl Physiol 106: 620–630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li P, Tjen-A-Looi S, Guo Z, Longhurst JC. Reciprocal neuronal projections between arcuate and ventrolateral periaqueductal gray participate in long-lasting EA inhibition of reflex blood pressure elevation. FASEB J 22: 737.–9., 2008 [Google Scholar]
- 22. Li P, Tjen-A-Looi S, Longhurst J. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am J Physiol Heart Circ Physiol 290: H2535–H2542, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Li P, Tjen-A-Looi S, Longhurst J. Role of arcuate nucleus and ventrolateral periaqueductal gray in electroacupuncture inhibition of sympathoexcitatory cardiovascular reflex response (Abstract). FASEB J 20: A734, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Lin MC, Nahin R, Gershwin ME, Longhurst JC, Wu KK. State of complementary and alternative medicine in cardiovascular, lung and blood. Circulation 103: 2038–2041, 2001 [DOI] [PubMed] [Google Scholar]
- 25. McCall RB. GABA-mediated inhibition of sympathoexitatory neurons by midline medullary stimulation. Am J Physiol Regul Integr Comp Physiol 255: R605–R615, 1988 [DOI] [PubMed] [Google Scholar]
- 26. McCall RB, Clement ME. Identification of serotonergic and sympathetic neurons in medullary raphe nuclei. Brain Res 477: 172–182, 1989 [DOI] [PubMed] [Google Scholar]
- 27. Miyawaki T, Goodchild AK, Pilowsky PM. Rostral ventral medulla 5-HT1A receptors selectively inhibit the somatosympathetic reflex. Am J Physiol Regul Integr Comp Physiol 280: R1261–R1268, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Moazzami A, Tjen-A-Looi SC, Guo ZL, Longhurst JC. Serotonergic projections from nucleus raphe pallidus to rostral ventrolateral medulla participate in acupuncture modulation of cardiovascular excitatory reflexes (Abstract). FASEB J 582: 23, 2007 [Google Scholar]
- 29. Morrison S, Gebber G. Axonal branching patterns and funicular trajectories of raphespinal sympathoinhibitory neurons. J Neurophysiol 53: 759–772, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Pérgola P, Alper R. Vaospressin and autonomic mechanisms mediate cardiovascular actions of central serotonin. Am J Physiol Regul Integr Comp Physiol 260: R1188–R1193, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Richter A, Herlitz J, Hjalmarson A. Effect of acupuncture in patients with angina pectoris. Eur Heart J 12: 175–178, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. Assessment of training-induced autonomic adaptations in athletes with spectral analysis of cardiovascular variability signals. Jpn J Physiol 45: 1053–1069, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Tam KC, Yiu HH. The effect of acupuncture on essential hypertension. Am J Chin Med 3: 369–375, 1975 [DOI] [PubMed] [Google Scholar]
- 35. Tjen-A-Looi SC, Li P, Longhurst JC. Role of medullary GABA, opioids, and nociceptin in prolonged inhibition of cardiovascular sympathoexcitatory reflexes during electroacupuncture in cats. Am J Physiol Heart Circ Physiol 293: H3627–H3635, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Tjen-A-Looi SC, Li P, Longhurst JC. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neuron by electroacupuncture in cats. Auton Neurosci 106: 119–131, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Tjen-A-Looi SC, Li P, Longhurst JC. Medullary substrate and differential cardiovascular response during stimulation of specific acupoints. Am J Physiol Regul Integr Comp Physiol 287: R852–R862, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Tjen-A-Looi SC, Li P, Longhurst JC. Midbrain vIPAG inhibits rVLM cardiovascular sympathoexcitatory responses during acupuncture. Am J Physiol Heart Circ Physiol 290: H2543–H2553, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Tjen-A-Looi S, Fu LW, Zhou W, Longhurst JC. Role of unmyelinated fibers in electroacupuncture cardiovascular responses. Auton Neurosci 118: 43–50, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Verberne AJM, Sartor DM, Berke A. Midline medullary depressor responses are mediated by inhibition of RVLM sympathoexcitatory neurons in rats. Am J Physiol Regul Integr Comp Physiol 276: R1054–R1062, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Wang WH, Li P. Influence of defence area stimulation on neurons in nucleus raphe obscurus. Chin J Physiol Sci 7: 184–189, 1991 [Google Scholar]
- 42. Yao T, Andersson S, Thoren P. Long-lasting cardiovascular depressor response following sciatic stimulation in spontaneously hypertensive rats. Evidence for the involvement of central endorphin and serotonin systems. Brain Res 244: 295–303, 1982 [DOI] [PubMed] [Google Scholar]
- 43. Yin S, Cao Y, Zhang J. Treatment of primary hypotension by electroacupuncture at Neiguan and Gongsun–a report of 100 cases. J Tradit Chin Med 24: 193, 2004 [PubMed] [Google Scholar]