Abstract
An exaggerated exercise pressor reflex (EPR) occurs in the chronic heart failure (CHF) state, which contributes to exercise intolerance and excessive sympathoexcitation during exercise. Exercise training (ExT) improves abnormal cardiovascular reflexes in CHF. Whether ExT can normalize the exaggerated EPR function remains to be determined. This study was designed to investigate the effects of ExT on the EPR and on the mechanical or metabolic components of this reflex in sham-operated and CHF rats. The EPR was activated by static contraction induced by electrical stimulation of L4/L5 ventral roots. The afferent fibers associated with the mechanoreflex and metaboreflex were activated by passive stretch and hindlimb arterial injection of capsaicin (0.1 and 1 μg/kg, 0.2 ml), respectively. Heart rate, blood pressure, and sympathoexcitatory responses during the activation of these reflexes were compared in sham + sedentary (Sed), sham + ExT, CHF + Sed, and CHF + ExT rats. Compared with sham + Sed rats, CHF + Sed rats exhibited exaggerated heart rate and pressor and sympathoexcitatory responses to either static contraction or passive stretch, whereas the cardiovascular responses to injection of capsaicin were blunted. Eight to ten weeks of ExT normalized the exaggerated responses induced by static contraction or passive stretch and partially improved the blunted responses due to intra-arterial capsaicin in CHF rats. ExT had no significant effect on the EPR and mechanoreflex and metaboreflex functions in sham rats. These findings suggest a potential therapeutic role for ExT in minimizing arterial pressure and sympathetic outflow following activation of the EPR in the CHF state.
Keywords: muscle, sympathetic nerve activity, blood pressure, decerebration
a hallmark of patients suffering from chronic heart failure (CHF) is elevated sympathoexcitation and exercise intolerance (3, 7, 50, 53). Although the mechanisms responsible for the increase in sympathetic outflow and exercise intolerance in CHF are not known, it has recently been suggested that this may be due, in part, to an exaggerated exercise pressor reflex (EPR).
The EPR is a peripheral neural reflex originating in skeletal muscle that contributes significantly to the regulation of the cardiovascular system during exercise. The afferent arm of this reflex is composed of metabolically sensitive (predominantly group IV) and mechanically sensitive (predominantly group III) afferent fibers (4, 16, 17, 26). Evidence from animal studies has demonstrated that increases in heart rate (HR), arterial pressure (AP), and sympathetic nerve activity in response to activation of this reflex are enhanced in rats with CHF induced by myocardial infarction (22, 43, 44, 46). Furthermore, these studies also suggest that an enhanced mechanical component of this reflex (i.e., mechanoreflex) contributes to the exaggerated EPR in CHF, whereas the cardiovascular control of the metaboreflex is blunted (23, 47, 48). An exaggerated EPR can potentially increase cardiovascular risk and contribute to exercise intolerance during physical activity in CHF patients. A therapeutic strategy for preventing or slowing the progression of the exaggerated EPR may be beneficial in CHF patients.
Accumulating studies suggest that long-term exercise training (ExT), as a nonpharmacological treatment for CHF, increases exercise capacity, reduces sympathoexcitation, and improves cardiovascular function in CHF animals and patients (2, 9, 11, 19, 35). However, whether ExT also improves the exaggerated EPR function in the CHF state remains to be determined. In the present study, we investigated the effects of ExT on the exaggerated EPR in rats with CHF. Moreover, to determine the mechanism by which ExT affects EPR function, we further investigated the effects of ExT on the mechano- and metaboreflex in sham and CHF rats.
METHODS
Experiments were performed on male Sprague-Dawley rats weighing 420–510 g (n = 68). These experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and carried out under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Model of CHF
Heart failure was produced by coronary artery ligation, as previously described (8, 52). Briefly, the rat was ventilated at a rate of 60 breaths/min with 3% isoflurane during the surgical procedure. A left thoracotomy was performed through the fifth intercostal space, the pericardium was opened, the heart was exteriorized, and the left anterior descending coronary artery was ligated. Sham-operated rats were prepared in the same manner, but did not undergo coronary artery ligation. All of the rats (n = 27) survived from the sham surgery. However, ∼70% of rats (i.e., 28 of total 41 rats) survived from coronary artery ligation surgery.
In this study, the cardiac function in all experimental animals was measured by echocardiography (VEVO 770, Visual Sonics), as previously described (52). In addition, at the end of each acute experiment, a Millar catheter (SPR 524; size, 3.5-Fr; Millar Instruments, Houston, TX) was advanced through the carotid artery into the left ventricle (LV) to determine LV end-diastolic pressure and LV systolic pressure. The rats were then euthanized with an overdose of pentobarbital sodium. The hearts and lungs were removed, and the ratio of the infarct area to whole LV minus septum was measured.
Protocol for ExT
All animals were divided into four groups: sham + sedentary (Sed) (n = 13), sham + ExT (n = 14), CHF + Sed (n = 13), and CHF + ExT (n = 15). The rats (CHF, n = 2; sham, n = 1) that were unwilling to run steadily on the treadmill were excluded from the analysis. Rats were treadmill trained, as described previously by us and others (32, 37). Generally, ExT was started 2 wk after coronary ligation or sham operation. Rats ran at an initial speed of 10 m/min, 0% grade, 10–15 min/day during the 1st wk. The speed and grade of treadmill were gradually increased to 25 m/min and 10% grade, and the exercise duration was increased to 60 min/day during the 2nd and 3rd wk. Since the 4th wk, sham and CHF rats had the same average period of time and total workload (25 m/min, 10% grade, 60 min/day, 5 days/wk, 5–7 wk). Sed rats were used as the control. Echocardiography was performed on all rats at the completion of ExT. To assess the effectiveness of ExT, citrate synthase (CS) activity from soleus muscle homogenate was measured spectrophotometrically, as described previously (37). At the end of ExT, the soleus muscle and gastrocnemius muscle were taken, frozen on dry ice, and stored at −80°C until processed.
EPR Function Evaluation
After 8–10 wk of ExT (10–12 wk after the coronary ligation or sham operation), rats were used for acute experiments to evaluate the effects of ExT on the EPR and on mechanoreflex and metaboreflex functions. Sham-operated rats were used as control for CHF rats. Sed rats were used as control for ExT rats. EPR function was also evaluated in Sed rats 8–10 wk after sham or infarction procedures.
General surgical preparation.
Rats were anesthetized with isoflourane (Halocarbon Laboratories, River Edge, NY; 5% in O2). A jugular vein and the trachea were cannulated. After tracheal cannulation, the lungs were ventilated with an anesthetic mixture of 2–3% isoflourane and O2. The right carotid artery was catheterized for measurement of AP and mean AP (MAP). HR was derived from the AP pulse by using the cardiotachometer function of the PowerLab (ADInstruments, Colorado Springs, CO). Body temperature was maintained between 37 and 38°C by a heating pad.
Decerebration.
Previous studies have shown that the EPR is compromised by anesthesia, which is especially a concern in rats. However, after decerebration, the effects of anesthesia on the EPR were largely abolished (46). Therefore, a decerebrate rat model was used in the present studies. The decerebration procedure was performed as described by our laboratory and others (45, 51). Briefly, rats were placed in a stereotaxic apparatus (Stoelting, Chicago, IL) and customized spinal frame. The head and pelvis were stabilized. Before decerebration, the lungs were ventilated with the isoflourane-oxygen mixture. Dexamethasone (0.2 mg iv) was given to reduce brain edema and inflammatory responses from the decerebration. The remaining intact carotid artery was isolated and ligated to reduce bleeding during decerebration. Subsequently, a portion of bone superior to the central sagittal sinus was removed. The dura mater was breached and reflected. The cerebral cortex was gently aspirated to visualize the superior and inferior colliculi. With the use of a blunt instrument, the brain was perpendicularly sectioned precollicularly, and the transected forebrain aspirated. The cranial vault was filled with warm agar (37°C). After the decerebration had been completed, the lungs were ventilated with a mixture of room air and oxygen, instead of the anesthetic gas. A minimum recovery period of 1.25 h was employed postdecerebration before data collection began.
Activation of the EPR, mechanoreflex, and metaboreflex.
To activate both mechanically and metabolically sensitive skeletal muscle afferent fibers, static hindlimb contraction was induced by electrical stimulation of ventral roots. A laminectomy exposing the lower lumbar portions of the spinal cord (L2–L6) was performed. Electrically induced static muscle contraction of the triceps surae was performed by stimulating the peripheral end of L4/L5 ventral roots for 30 s. Constant-current stimulation was used at three times motor threshold (defined as the minimum current required to produce a muscle twitch) with a pulse duration of 0.1 ms at 40 Hz. All muscles of the hindlimb undergoing study were denervated, except for the triceps surae muscle.
Previous studies had reported that passive stretch, a pure mechanical stimulus, was used to selectively activate mechanically sensitive intramuscular afferents (group III) in decerebrate rats (45, 47). Therefore, in animals in which ventral root stimulation was performed, preferential activation of the mechanoreflex was achieved by passively stretching the triceps surae muscles using a calibrated rack and pinion system (Harvard Apparatus). Care was taken to match the peak tension developed in response to electrical stimulation during the passive stretch experiments.
It has been reported that the capsaicin-sensitive receptor (transient receptor potential vanilloid 1) is localized primarily to metabolically sensitive afferent fibers (group IV) in skeletal muscle (48). As a result, the exogenous chemical capsaicin can be used to preferentially activate these fibers. Therefore, in animals in which ventral root stimulation and passive stretch were performed, hindlimb arterial bolus injections of capsaicin (0.1 or 1.0 μg/kg, 0.2 ml) were used to preferentially activate the metaboreflex (48). Briefly, a catheter was placed in the right iliac artery with its tip advanced to the abdominal aortic bifurcation, ensuring that capsaicin was delivered to the left hindlimb through the left iliac artery. To limit drug delivery to the left hindlimb, the common iliac vein was reversibly ligated by a vascular occluder (Harvard). On injection of drug, the vein was occluded for 2 min to trap the injectate in the leg (48). In the present study, ventral root stimulation, passive stretch, and injection of capsaicin were performed in all rats (n = 13/each group, 4 groups). The order of the three maneuvers was randomized. The interval between two maneuvers was at least 15 min.
Recording of renal sympathetic nerve activity.
To evaluate EPR-evoked sympathetic activation, left renal sympathetic nerve activity (RSNA) was recorded in decerebrated rats (n = 6/each group). RSNA was recorded as previously described by our laboratory and others (22, 52). In brief, the left kidney was exposed retroperitoneally through a left flank incision. Sympathetic nerves running on or beside the renal artery were identified. About 3 mm of the nerve were carefully dissected free of connective tissue. A pair of flexible silver electrodes insulated by Teflon (786500, A-M system) was hooked on the nerve. The third electrode was placed subcutaneously, which served as a ground electrode. Subsequently, the exposed nerve and the electrode were embedded in Kwik-Sil gel (World Precision Instruments). Once the gel had hardened, the abdominal cavity was closed. RSNA was amplified and filtered (bandwidth: 100–3,000 Hz) using a Grass P55C preamplifier. The nerve signal was monitored on an oscilloscope. The signal from the oscilloscope was displayed on a computer, where it was rectified, integrated, sampled (1 kHz), and converted to a digital signal by the PowerLab data-acquisition system. At the end of the experiment, the rat was euthanized with an overdose of pentobarbital sodium. The maximum nerve activity (Max) occurred 1–2 min after the rat was euthanized. Background noise levels for sympathetic nerve activity were recorded 15–20 min after the rat was euthanized. Using the unit conversion of Powerlab Chart (AD Instruments) system, baseline nerve activity was taken as percent of Max after the noise level was subtracted.
Data Acquisition and Statistical Analysis
MAP, HR, RSNA, and muscle tension were acquired using PowerLab software. Baseline values were determined by analyzing at least 30 s of the data before muscle contraction. The peak response was determined in the period of the greatest change from baseline. RNSA is expressed as percentage of Max. The tension-time index (TTI) was calculated by integrating the area between the tension trace and the baseline level and is expressed in kilograms times seconds. Peak developed tension was calculated by subtracting the resting tension from the peak tension and is expressed in grams. All values are expressed as means ± SE. Differences between groups were determined by a two-way ANOVA followed by the Tukey post hoc test. P < 0.05 was considered statistically significant.
RESULTS
Evaluation of Body Weight, Organ Weight, and Baseline Hemodynamics
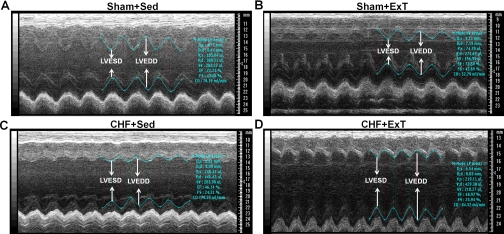
Echocardiographic and hemodynamic measurements of sham + Sed, sham + ExT, CHF + Sed, and CHF + ExT rats are summarized in Table 1. The heart weight and lung weight-to-body weight ratios were significantly higher in CHF rats than those in sham rats, suggesting cardiac hypertrophy and substantial pulmonary congestion in the CHF state. Moreover, in rats with CHF, a gross examination revealed a dense scar in the anterior ventricular wall. The mean infarct area was 42.5 ± 2.8% of the LV area. No infarcts were identified in sham rats. Pleural fluid and ascites were also found in the CHF rats, but none were found in the sham rats. There were no statistically significant differences in baseline MAP and HR between the sham and CHF rats. However, CHF rats exhibited elevated LV end-diastolic pressure and reduced LV systolic pressure compared with sham rats. Echocardiographic data show that LV end-systolic diameter, LV end-diastolic diameter, LV end-systolic volume, and LV end-dastolic volume were increased, whereas ejection fraction and fractional shortening were attenuated in CHF rats compared with sham rats (Fig. 1 and Table 1), indicating decreased cardiac function in CHF rats. After ExT, indexes of cardiac function were not significantly different between CHF + Sed and CHF + ExT rats, indicating that ExT did not improve cardiac function in CHF rats. ExT did not significantly affect MAP and HR in sham and CHF rats. However, CS activity, an important marker of skeletal muscle aerobic metabolism, increased by 54% in the soleus muscle of sham + ExT rats and by 49% in CHF + ExT rats compared with their respective controls (Table 1). Nonetheless, the CS activity in CHF + ExT rats was still lower than in sham + ExT rats (Table 1).
Table 1.
Hemodynamic and morphological data in sham and CHF rats following exercise training
| Sham + Sed | Sham + ExT | CHF + Sed | CHF + ExT | |
|---|---|---|---|---|
| n | 13 | 13 | 13 | 13 |
| Body weight, g | 470 ± 5 | 438 ± 6* | 464 ± 7‡ | 449 ± 6‡ |
| Heart weight, mg | 1514 ± 27 | 1533 ± 21 | 2521 ± 50§‡ | 2443 ± 48§‡ |
| Heart weight/body weight, mg/g | 3.2 ± 0.1 | 3.5 ± 0.1 | 5.4 ± 0.1§‡ | 5.5 ± 0.1§‡ |
| Wet lung weight/body weight, mg/g | 5.3 ± 0.6 | 5.7 ± 0.5 | 9.7 ± 0.8§‡ | 9.0 ± 0.6§‡ |
| MAP, mmHg | 98.1 ± 5.7 | 95.1 ± 4.7 | 89.8 ± 4.8 | 91.5 ± 4.9 |
| HR, beats/min | 368.2 ± 8.4 | 361.0 ± 10.1 | 382.2 ± 11.5 | 377.6 ± 10.1 |
| LVEDP, mmHg | 0.8 ± 0.6 | 1.4 ± 0.8 | 14.7 ± 2.3§‡ | 14.3 ± 2.6§‡ |
| LVSP, mmHg | 126.1 ± 6.5 | 120.5 ± 5.9 | 99.2 ± 5.5*‡ | 100.2 ± 5.6*‡ |
| LVESD, mm | 3.9 ± 0.2 | 3.6 ± 0.1 | 8.0 ± 0.3§‡ | 7.6 ± 0.3§‡ |
| LVEDD, mm | 7.2 ± 0.4 | 6.9 ± 0.4 | 10.7 ± 0.3§‡ | 10.5 ± 0.3§‡ |
| LVESV, μl | 74.8 ± 4.1 | 66.1 ± 3.4 | 365.5 ± 26.8§‡ | 354.4 ± 24.6§‡ |
| LVEDV, μl | 335.8 ± 26.2 | 329.5 ± 25.6 | 608.0 ± 27.1§‡ | 599.7 ± 28.2§‡ |
| EF, % | 75.7 ± 2.8 | 77.3 ± 3.2 | 40.2 ± 2.0§‡ | 41.3 ± 2.5§‡ |
| FS, % | 45.3 ± 1.9 | 46.8 ± 2.3 | 25.2 ± 2.4§‡ | 27.1 ± 2.1§‡ |
| Infarct size, % | 0 | 0 | 42.5 ± 2.8§‡ | 41.1 ± 2.4§‡ |
| CS, μmol·g−1·min−1 | 19.3 ± 2.3 | 29.8 ± 2.9* | 12.8 ± 1.2*‡ | 19.1 ± 1.4†‡ |
Values are means ± SE; n, no. of rats. Sed, sedentary; ExT, exercise training; CHF, chronic heart failure; MAP, mean arterial pressure; HR, heart rate; LVEDP, left ventricle end-diastolic pressure; LVSP, left ventricle systolic pressure; LVESD, left ventricle end-systolic diameter; LVEDD, left ventricle end-diastolic diameter; LVESV, left ventricle end-systolic volume; LVEDV, left ventricle end-diastolic volume; EF, ejection fraction; FS, fractional shortening; CS, citrate synthase activity.
P < 0.05 and
P < 0.01 vs. sham + Sed.
P < 0.05 vs. CHF + Sed.
P < 0.01 vs. sham + ExT.
Fig. 1.
M-mode echocardiographic images from sham + sedentary (Sed) (A), sham + exercise training (ExT) (B), chronic heart failure (CHF) + Sed (C), and CHF + ExT (D) hearts. Arrows demarcate left ventricular end-systolic diameter (LVESD) and end-diastolic diameter (LVEDD) dimensions. Notice that LVESD and LVEDD were increased in CHF + Sed and CHF + ExT rats compared with sham + Sed and sham + ExT rats.
Effects of ExT on HR, AP, and Sympathoexcitatory Responses to Static Contraction in Sham and CHF Rats
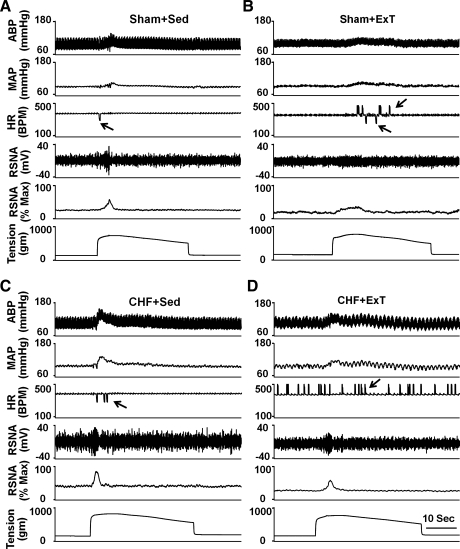
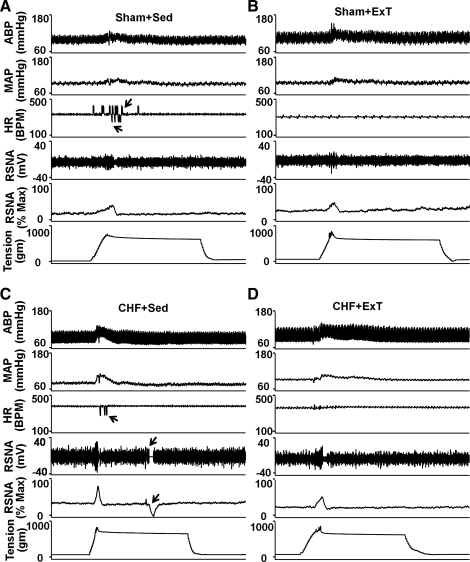
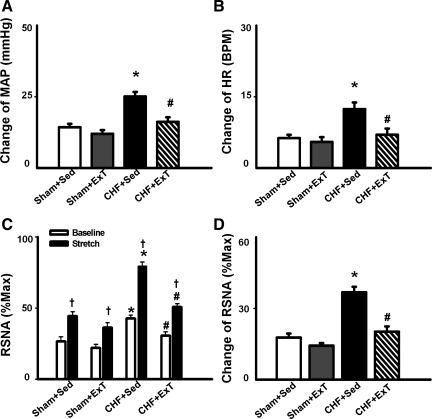
In the four groups of rats (n = 13/group), static contraction was induced by electrical stimulation of the L4/L5 ventral roots. Stimulation elicited increases in MAP and HR. Representative recordings from a rat in each of the four groups are shown in Fig. 2. Mean data are shown in Fig. 3. Compared with sham + Sed rats, CHF + Sed rats exhibited greater pressor and HR responses to a 30-s static contraction (Fig. 3, A and B). The exaggerated responses in CHF + Sed rats were attenuated in CHF + ExT rats. Although ExT trended to decrease MAP and HR responses to static contraction in sham rats, it did not reach statistical significance.
Fig. 2.
Original record showing the exercise pressor reflex (EPR) control of blood pressure, heart rate (HR), and renal sympathetic nerve activity (RSNA) in response to static contraction induced by electrical stimulation of L4/L5 ventral roots in sham + Sed (A), sham + ExT (B), CHF + Sed (C), and CHF + ExT (D) rats. In the HR panel, the arrows point to artifacts. ABP, arterial blood pressure; MAP, mean arterial pressure; Max, maximum RSNA; BPM, beats/min.
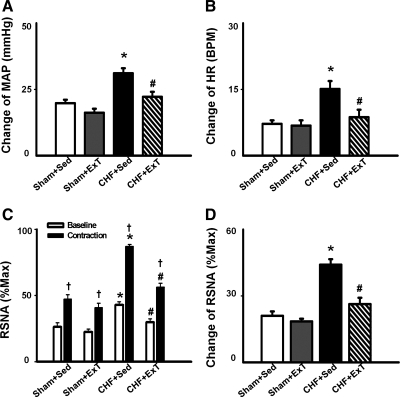
Fig. 3.
Mean data showing the effects of ExT on pressor (A), HR (B), and sympathoexcitatory (D) responses to a 30-s static contraction in sham and CHF rats. C: both baseline and contraction-induced RSNA were compared in sham + Sed, sham + ExT, CHF + Sed, and CHF + ExT rats. Values are means ± SE. *P < 0.05 vs. sham + Sed and sham + ExT. †P < 0.05 vs. baseline RSNA. #P < 0.05 vs. CHF + Sed.
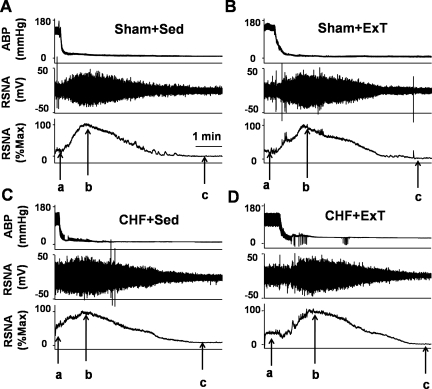
In 6 of 13 rats in each group, RSNA responses to 30-s static contraction were compared among the four groups to investigate the effect of ExT on EPR-evoked sympathoexcitation in sham and CHF rats. Figure 4 is a representative figure showing measurements of baseline RSNA, maximum RSNA (Max), and noise level in four groups. Baseline RSNA was expressed as percentage of Max after subtracting the noise level. As we expected, baseline RSNA in CHF + Sed was significantly increased compared with sham + Sed rats (42.8 ± 2.4 vs. 26.3 ± 3.1% of Max, P < 0.05). ExT in CHF significantly attenuated the basal RSNA (29.8 ± 2.7% of Max).
Fig. 4.
Original record showing blood pressure and RSNA responses to euthanasia with an overdose of pentobarbital sodium in sham + Sed (A), sham + ExT (B), CHF + Sed (C), and CHF + ExT (D) rats. In each rat, arrow a points to basal RSNA, arrow b points to maximum RSNA, and arrow c points to noise level. The Max occurred 1–2 min after the rat was euthanized. Baseline nerve activity was taken as percentage of Max after the noise level was subtracted. Notice that maximum RSNA is close in all 4 rats, whereas the CHF rat had a higher basal RSNA level than other rats.
During 30-s static contraction, RSNA was immediately increased at the onset of muscle contraction and then returned to baseline in 5–10 s (Fig. 2). Compared with sham + Sed and sham + ExT rats, CHF + Sed rats exhibited a larger RSNA response to static contraction (Fig. 3, C and D). However, the enhanced RSNA response to static contraction was significantly attenuated in CHF + ExT rats (Fig. 3D), indicating that ExT reduced the exaggerated sympathoexcitation during muscle stimulation in CHF rats. ExT also trended to decrease RSNA responses to static contraction in sham rats, but it did not reach statistic significance.
Effect of ExT on Mechanoreflex in Sham and CHF Rats
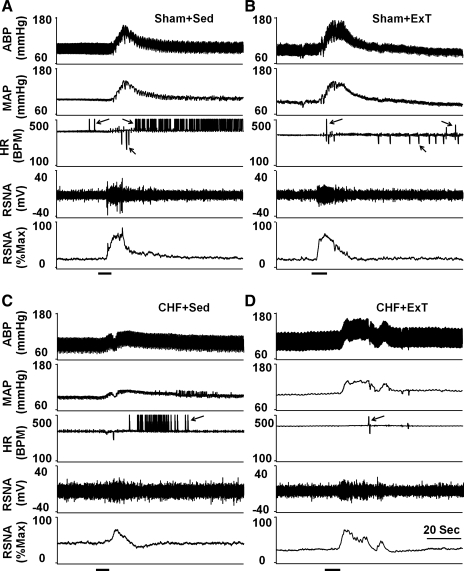
As shown in Figs. 5 and 6, passive stretch, a purely mechanical stimulus, elicited increases in MAP, HR, and RSNA in the four groups of rats. Compared with sham + Sed and sham + ExT rats, CHF + Sed rats exhibited greater pressor, HR, and RSNA responses to passive stretch, which were attenuated in CHF + ExT rats (Fig. 6). There was no significant difference in MAP, HR, and RSNA responses to passive stretch between sham + ExT and sham + Sed rats (Fig. 6).
Fig. 5.
Original record of the mechanoreflex control of blood pressure, HR, and RSNA in response to passive stretch in sham + Sed (A), sham + ExT (B), CHF + Sed (C), and CHF + ExT (D) rats. In the HR and RSNA panels, the arrows point to artifacts.
Fig. 6.
Mean data showing the effects of ExT on pressor (A), HR (B), and sympathoexcitatory (D) responses to passive stretch in sham and CHF rats. C: both baseline and stretch-induced RSNA were compared in sham + Sed, sham + ExT, CHF + Sed, and CHF + ExT rats. Values are means ± SE. *P < 0.05 vs. sham + Sed and sham + ExT. †P < 0.05 vs. baseline RSNA. #P < 0.05 vs. CHF + Sed.
Effect of ExT on Metaboreflex in Sham and CHF Rats
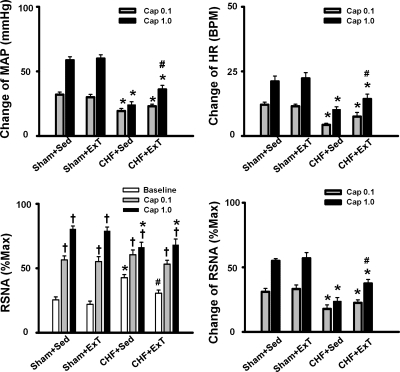
In the present study, the metaboreflex was activated by hindlimb arterial injection of capsaicin (0.1 or 1.0 μg/kg, 0.2 ml). As shown in Fig. 7, injection of capsaicin (1.0 μg/kg, 0.2 ml) increased MAP, HR, and RSNA in sham + Sed rats, whereas these responses to injection of capsaicin were significantly blunted in CHF + Sed rats (Figs. 7 and 8). ExT partially restored the blunted cardiovascular responses to injection of capsaicin (1.0 μg/kg, 0.2 ml; Fig. 7) in CHF rats. There was no significant difference in MAP, HR, and RSNA responses to injection of capsaicin between sham + ExT and sham + Sed rats (Fig. 8).
Fig. 7.
Original record of the metaboreflex control of blood pressure, HR, and RSNA in response to hindlimb arterial injection of capsaicin (1.0 μg/kg, 0.2 ml) in sham + Sed (A), sham + ExT (B), CHF + Sed (C), and CHF + ExT (D) rats. In the HR panel, the arrows point to artifacts. Capsaicin was injected during the horizontal bar.
Fig. 8.
Mean data showing the effects of ExT on pressor (A), HR (B), and sympathoexcitatory (D) responses to arterial injection of capsaicin (0.1 or 1.0 μg/kg, 0.2 ml) in sham and CHF rats. C: both baseline and capsaicin-induced RSNA were compared in sham + Sed, sham + ExT, CHF + Sed, and CHF + ExT rats. Values are means ± SE. *P < 0.05 vs. sham + Sed and sham + ExT. †P < 0.05 vs. baseline RSNA. #P < 0.05 vs. CHF + Sed.
Muscle Tension Produced by Static Contraction or Passive Stretch
In the present study, muscle peak developed tension induced by static contraction or passive stretch ranged from 650 to 800 g in all groups. There was no significant difference in TTI during either static contraction or passive stretch between groups (Table 2).
Table 2.
Tension-time indexes for either static contraction or passive stretch in sham + Sed, sham + ExT, CHF + Sed, and CHF + ExT rats
| Tension-Time Index, kg × s |
|||
|---|---|---|---|
| Group | n | Contraction | Stretch |
| Sham + Sed | 13 | 15.6 ± 2.4 | 15.3 ± 2.9 |
| Sham + ExT | 13 | 16.3 ± 2.8 | 15.9 ± 2.6 |
| CHF + Sed | 13 | 15.1 ± 2.2 | 15.7 ± 2.5 |
| CHF + ExT | 13 | 15.8 ± 2.7 | 15.5 ± 2.7 |
Values are means ± SE; n, no. of rats. There were no significant differences (P > 0.05) between means in any one group.
DISCUSSION
The primary findings of the present study demonstrated 1) that ExT attenuated the exaggerated EPR, as well as the excessive sympathoexcitation during static contraction in CHF rats; 2) that ExT normalized the enhanced mechanoreflex function in CHF rats; and 3) that ExT partially improved the blunted metaboreflex function in CHF rats.
Effect of ExT on the Exaggerated EPR in CHF State
Traditionally ExT was considered contraindicated for CHF patients. However, over the past decade, numerous clinical trials and small randomized studies have demonstrated that long-term regular exercise is safe in stable CHF patients and increases the quality of life as well as survival (1, 18, 31, 54). The beneficial effects of ExT include improved autonomic balance, reduced neurohumoral activation, an increase in exercise capacity, and ameliorated myopathy in CHF patients and animals (34, 41, 42). Furthermore, Piepoli et al. (38) first reported that 6-wk forearm training improved the abnormal exercise-evoked ventilation and cardiovascular responses in CHF patients, suggesting a beneficial effect of ExT on the abnormal muscle reflex function in CHF patients. However, due to intrinsic limitations of human research, the study of Piepoli et al. could not exclude the possibility that ExT plays a beneficial role by affecting central command, a mechanism whereby a volitional signal from the motor cortex or subcortical nuclei, responsible for recruiting motor units, activates cardiovascular activity during exercise (6, 10). However, in animal experiments, this limitation can be largely resolved by 1) using a decerebrate model to remove the cortical structures from which central command originates; and 2) electrical stimulation of the peripheral end of ventral roots to induce nonvolitional contraction. In the present study, by using a decerebrate rat model, we first observed that ExT improved the exaggerated EPR function, as well as excessive sympathoexcitation during static contraction in CHF rats. Interestingly, we noticed that, although ExT tended to attenuate EPR function in sham rats, the effect did not reach statistic significance.
The mechanism(s) by which ExT improves the exaggerated EPR function remains unclear. Both central and peripheral (muscle afferent) mechanisms may be involved in this process in the CHF state. In the present study, ExT also attenuated the exaggerated sympathoexcitation at rest in CHF rats, which was consistent with the findings of Gao et al. (9) and Liu et al. (24), who demonstrated that ExT normalizes the elevated RSNA in rabbits with pacing-induced CHF. Futhermore, Gao et al. reported that the beneficial effects of ExT on the excessive sympathoexcitation in CHF state is at least partially mediated by affecting central neural structures, such as the rostral ventrolateral medulla (9). Mueller and Hasser (33) also reported that alterations in neurotransmission at the level of the nucleus tractus solitarius contribute importantly to regulation of HR and sympathetic nerve activity in ExT rats. Based on these findings, it is reasonable to speculate that ExT may normalize the exaggerated EPR, in part, via a central mechanism.
Another possibility is that ExT may attenuate the exaggerated EPR by affecting muscle afferent pathways. Previous studies (5, 25) have shown that peripheral skeletal myopathy develops in CHF (e.g., muscle atrophy, decreased peripheral blood flow, fiber-type transformation, and reduced oxidative capacity), which can be reversed by ExT (11, 13). Iwamoto and Botterman (14) have reported that contraction of fast-twitch fiber (type II) evoked a larger pressor response to static contraction compared with slow-twitch fiber (type I) contraction, suggesting that type II fiber contraction may activate a larger number of muscle afferent receptors. In the CHF state, a muscle fiber-type shift from type I to type II could cause an exaggerated EPR. Since muscle fiber-type transformation in CHF can be reversed by ExT (11, 13), the improvement of abnormal fiber-type shift by ExT may subsequently affect muscle afferent function, and eventually ameliorate the exaggerated EPR function in the CHF state. However, the hypothesis above remains to be tested.
Effect of ExT on the Abnormal Mechano- and Metaboreflex Function in CHF Rats
Previous studies have established that there is an exaggerated EPR in CHF patients and animals (43, 46). Futhermore, studies by Mostoufi-Moab et al. (30) and Middlekauff et al. (27, 29) provide evidence that the mechanical component of the EPR (mechanoreflex) is enhanced in CHF patients. Using a decerebrate rat model, the studies of Smith et al. (47) and Li et al. (23). reported consistent findings that the mechanoreflex is elevated in CHF rats, which contributes to the exaggerated EPR function in CHF (23, 47). Based on the finding that ExT normalizes the exaggerated EPR function in the CHF state, it was important to determine whether the mechanoreflex is primarily affected by ExT. In the present study, we found that, compared with sham rats, CHF rats had an exaggerated mechanoreflex-evoked pressor and sympathoexcitatory response, which confirmed the findings of Smith et al. (47) and Li et al. (23). However, the exaggerated mechanoreflex function seen in CHF was prevented by 8–10 wk of ExT in the present study. In addition, the response to contraction and stretch was characterized by pressor and sympathetic responses that were brief at the onset of contraction or stretch and dissipated well before the end of stimulation, a classical characteristic of mechanoreflex activation. It is highly possible that a 30-s static contraction predominantly activates the mechanical component of EPR, as does passive stretch. This also may explain why ExT had similar effects between EPR and mechanoreflex induced by stretch in the present study. Therefore, the findings above suggest that ExT may partially alter EPR function by affecting the mechanoreflex in CHF rats.
The role of the metaboreflex in the exaggerated EPR function remains controversial. Based on measurements of ventilation, the studies of Piepoli et al. (39, 40) showed that CHF patients had an overactive metaboreflex compared with control subjects. However, based on measurements of blood pressure or sympathoexcitatory responses to postcontraction circulatory arrest (an isolated activator of the muscle metaboreflex), the studies of Sterns et al. (49), Negrão et al. (36), and Middlekauff et al. (28) showed that the metaboreflex control of blood pressure and sympathetic nerve activity was reduced in patients with CHF (New York Heart Association classes II to IV), indicating that metaboreflex function is blunted rather than exaggerated in CHF patients. In animal experiments, the studies of Smith et al. (47, 48) and Li et al. (23) reported that the pressor response to hindlimb injection of capsaicin (an exogenous metabolite to activate metaboreflex) was attenuated in CHF rats, supporting the fact that the metaboreflex control of cardiovascular function was blunted in CHF. However, by activating the muscle metaboreflex via a reduction of blood flow to the exercising muscles (hindlimbs) during dynamic exercise in conscious dogs, Hammond and colleagues (12) demonstrated that muscle metaboreflex activation in CHF induces augmented HR response, exaggerated peripheral vasoconstriction, and secretion of renin, vasopressin, and norepinephrine, indicating that the muscle metaboreflex-induced sympathoexcitation is augmented but not decreased in CHF. Therefore, the controversial conclusions that the metaboreflex is blunted or exaggerated in CHF may be partially due to different measurements of physiological parameters, such as ventilation, blood pressure, and sympathetic nerve activity. Different approaches used to activate the metaboreflex in these studies may also contribute to the dispute about the blunted or enhanced metaboreflex in CHF state. Here, following the studies of Smith et al. (48), we confirmed their findings that the pressor response to hindlimb administration of capsaicin was blunted in CHF rats. Furthermore, we also found that ExT slightly but significantly improved the blunted metaboreflex activated by capsaicin in CHF rats. However, compared with the effect of ExT on the exaggerated mechanoreflex in CHF rats, the effects of ExT on blunted metaboreflex control of blood pressure and sympathetic nerve activity were less. The underlying mechanisms by which ExT affect the mechanoreflex and metaboreflex functions in CHF state remain to be clarified.
Effect of ExT on Cardiac Function
In the present study, to evaluate the efficiency of ExT, we measured the CS activity in skeletal muscle in all groups. The data showed that ExT increased CS activity in skeletal muscle in both sham and CHF rats, indicating the efficiency of ExT in all groups. Although ExT modulated the exaggerated EPR as well as excessive sympathoexcitation at rest and during exercise in the CHF state, ExT did not improve the decreased cardiac function in CHF rats, indicating that the beneficial effect of ExT on the exaggerated EPR was not mediated by an improvement in cardiac function. In addition, previous studies have reported that ExT improves skeletal myopathy in the CHF state and increases exercise performance (2, 11, 15, 19). Interestingly, in this study, we found that TTI during static contraction was not increased by ExT in sham and CHF rats.
Limitations
There are several potential limitations in this study that should be discussed. First, to minimize the effects of anesthesia and central command on the EPR, a decerebrate rat model was used in the present study. However, decerebration removes higher levels of the brain, such as paraventricular nucleus of the hypothalamus, which may be involved in modulation of cardiovascular responses to ExT (21). Therefore, using a decerebrated animal model potentially enlarges the differences between studies in animals and humans and compromises the applicability of the present findings to conscious humans. Second, in the present study, stretch and capsaicin administration were used to stimulate the afferent fiber populations that are associated with the mechanoreflex and metaboreflex. However, it is likely that they do so in a manner that is different than during physiological contraction. For example, a study by Kaufman et al. (16) reported that, although capsaicin, an exogenous chemical, was used to activate the muscle metabosensitive fibers (group IV), ∼16% of group IV fibers are not responsive to capsaicin. In addition, it has been reported from Kaufman's group that blocking capsaicin receptors (transient receptor potential vanilloid 1) contributes little to the EPR induced by static contraction in cats (20). Finally, although we found that ExT prevents the exaggerated EPR in CHF rats, the present study did not determine what components of the reflex pathways, such as receptors, peripheral or central neural components, or end organs are likely to be engaged.
Summary
In conclusion, we have shown that ExT improves the exaggerated EPR, as well as the excessive sympathoexcitation during static contraction in CHF rats. The beneficial effect of ExT on the exaggerated EPR may be due to the normalization of the enhanced mechanical component of EPR in CHF. Finally, we also found that ExT partially improved the blunted metaboreflex control of AP and RSNA in CHF rats. These findings suggest that ExT, as a nonpharmacological therapeutic strategy, has potential clinical value in reducing the elevated sympathetic tone at rest and during exercise in the CHF state.
GRANTS
This work was supported by grant from National Heart, Lung, and Blood Institute (PO1 H62222). H.-J. Wang was supported by a postdoctoral fellowship from American Heart Association.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 99: 1173–1182, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 85: 2119–2131, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Cohn JN. Abnormalities of peripheral sympathetic nervous system control in congestive heart failure. Circulation 82: I59–I67, 1990 [PubMed] [Google Scholar]
- 4. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 85: 1751–1759, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59: 313–337, 1985 [DOI] [PubMed] [Google Scholar]
- 7. Francis GS. Neurohumoral mechanisms involved in congestive heart failure. Am J Cardiol 55: 15A–21A, 1985 [DOI] [PubMed] [Google Scholar]
- 8. Gao L, Schultz HD, Patel KP, Zucker IH, Wang W. Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension 45: 1173–1181, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115: 3095–3102, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hambrecht R, Fiehn E, Yu J, Niebauer J, Weigl C, Hilbrich L, Adams V, Riede U, Schuler G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol 29: 1067–1073, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflügers Arch 403: 369–376, 1985 [DOI] [PubMed] [Google Scholar]
- 14. Iwamoto GA, Botterman BR. Peripheral factors influencing expression of pressor reflex evoked by muscular contraction. J Appl Physiol 58: 1676–1682, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Jankowska EA, Wegrzynowska K, Superlak M, Nowakowska K, Lazorczyk M, Biel B, Kustrzycka-Kratochwil D, Piotrowska K, Banasiak W, Wozniewski M, Ponikowski P. The 12-wk progressive quadriceps resistance training improves muscle strength, exercise capacity and quality of life in patients with stable chronic heart failure. Int J Cardiol 130: 36–43, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 17. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 18. Khan MH, Sinoway LI. Muscle reflex control of sympathetic nerve activity in heart failure: the role of exercise conditioning. Heart Fail Rev 5: 87–100, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Kiilavuori K, Naveri H, Salmi T, Harkonen M. The effect of physical training on skeletal muscle in patients with chronic heart failure. Eur J Heart Fail 2: 53–63, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol 288: H1867–H1873, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koba S, Xing J, Sinoway LI, Li J. Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. Am J Physiol Heart Circ Physiol 294: H311–H321, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation 110: 3049–3054, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation 102: 1854–1862, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 85: 1364–1373, 1992 [DOI] [PubMed] [Google Scholar]
- 26. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol 287: H1937–H1943, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation 101: 784–789, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated muscle mechanoreflex control of reflex renal vasoconstriction in heart failure. J Appl Physiol 90: 1714–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Mostoufi-Moab S, Herr MD, Silber DH, Gray KS, Leuenberger UA, Sinoway LI. Limb congestion enhances the synchronization of sympathetic outflow with muscle contraction. Am J Physiol Regul Integr Comp Physiol 279: R478–R483, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol 34: 377–384, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Mueller PJ. Exercise training attenuates increases in lumbar sympathetic nerve activity produced by stimulation of the rostral ventrolateral medulla. J Appl Physiol 102: 803–813, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Mueller PJ, Hasser EM. Putative role of the NTS in alterations in neural control of the circulation following exercise training in rats. Am J Physiol Regul Integr Comp Physiol 290: R383–R392, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Negrao CE, Middlekauff HR. Adaptations in autonomic function during exercise training in heart failure. Heart Fail Rev 13: 51–60, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Negrao CE, Middlekauff HR. Exercise training in heart failure: reduction in angiotensin II, sympathetic nerve activity, and baroreflex control. J Appl Physiol 104: 577–578, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Negrao CE, Rondon MU, Tinucci T, Alves MJ, Roveda F, Braga AM, Reis SF, Nastari L, Barretto AC, Krieger EM, Middlekauff HR. Abnormal neurovascular control during exercise is linked to heart failure severity. Am J Physiol Heart Circ Physiol 280: H1286–H1292, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Pan YX, Gao L, Wang WZ, Zheng H, Liu D, Patel KP, Zucker IH, Wang W. Exercise training prevents arterial baroreflex dysfunction in rats treated with central angiotensin II. Hypertension 49: 519–527, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93: 940–952, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Piepoli MF, Coats AJ. Increased metaboreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol 102: 494–496, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Piepoli MF, Dimopoulos K, Concu A, Crisafulli A. Cardiovascular and ventilatory control during exercise in chronic heart failure: role of muscle reflexes. Int J Cardiol 130: 3–10, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Pliquett RU, Cornish KG, Patel KP, Schultz HD, Peuler JD, Zucker IH. Amelioration of depressed cardiopulmonary reflex control of sympathetic nerve activity by short-term exercise training in male rabbits with heart failure. J Appl Physiol 95: 1883–1888, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Rondon E, Brasileiro-Santos MS, Moreira ED, Rondon MU, Mattos KC, Coelho MA, Silva GJ, Brum PC, Fiorino P, Irigoyen MC, Krieger EM, Middlekauff HR, Negrao CE. Exercise training improves aortic depressor nerve sensitivity in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 291: H2801–H2806, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol 99: 5–22, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Smith SA, Mammen PP, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 108: 1126–1132, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111: 2056–2065, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation 84: 2034–2039, 1991 [DOI] [PubMed] [Google Scholar]
- 50. Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 81: 518–527, 1990 [DOI] [PubMed] [Google Scholar]
- 51. Wang HJ, Pan YX, Wang WZ, Zucker IH, Wang W. NADPH oxidase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J Appl Physiol 107: 450–459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am J Physiol Heart Circ Physiol 295: H1216–H1226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilson JR. Exercise intolerance in heart failure. Importance of skeletal muscle. Circulation 91: 559–561, 1995 [DOI] [PubMed] [Google Scholar]
- 54. Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094, 2007 [DOI] [PubMed] [Google Scholar]