Abstract
This study was designed to validate a high-resolution method to measure regional ventilation (V̇a) in small laboratory animals, and to compare regional V̇a and perfusion (Q̇) before and after methacholine-induced bronchoconstriction. A mixture of two different colors of 0.04-μm fluorescent microspheres (FMS) was aerosolized and administered to five anesthetized, mechanically ventilated rats. Those rats also received an intravenous injection of a mixture of two different colors of 15-μm FMS to measure regional blood flow (Q̇). Five additional rats were labeled with aerosol and intravenous FMS, injected with intravenous methacholine, and then relabeled with a second pair of aerosol and intravenous FMS colors. After death, the lungs were reinflated, frozen, and sequentially sliced in 16-μm intervals on an imaging cryomicrotome set to acquire signal for each of the FMS colors. The reconstructed lung images were sampled using randomly placed 3-mm radius spheres. V̇a within each sphere was estimated from the aerosol fluorescence signal, and Q̇ was estimated from the number of 15-μm FMS within each sphere. Method error ranged from 6 to 8% for Q̇ and 0.5 to 4.0% for V̇a. The mean coefficient of variation for Q̇ was 17%, and for V̇a was 34%. The administration of methacholine altered the distribution of both V̇a and Q̇ within lung regions, with a change in V̇a distribution nearly twice as large as that seen for Q̇. The methacholine-induced changes in V̇a were not associated with compensatory shifts in Q̇. Cryomicrotome images of FMS markers provide a high-resolution, anatomically specific means of measuring regional V̇a/Q̇ responses in the rat.
Keywords: cryomicrotome, fluorescent microspheres, methacholine
the spatial distribution of pulmonary ventilation (V̇a) and blood flow (Q̇) has been measured in larger laboratory animals using deposition of a fluorescent microsphere (FMS) aerosol and intravascular lodging of intravenously injected FMS (10). Relative regional V̇a or Q̇ is measured after death, utilizing fluorescence signals extracted from ∼2-cm3 pieces of dried lung. While this methodology is effective for larger laboratory animals, the lung piece size for fluorescence measurements is difficult to scale down for high-resolution measurements of V̇a and Q̇ that would permit investigation of V̇a/Q̇ relationships in small-animal models of injury and disease. High-resolution measurements of spatial Q̇ heterogeneity in rat lungs have been derived from analysis of cryomicrotome lung images of intravenously injected 15-μm FMS. Working from cryomicrotome images cut at 16-μm intervals, the spatial coordinates of 40,000 individual microspheres were identified, allowing for an accurate estimate of the heterogeneity of regional Q̇ in volumes of lung as small as an acinus (4). While this approach provides high-resolution measurements of regional Q̇ in rodents, the 1.0- to 0.04-μm FMS size used for aerosol deposition in the large-animal regional V̇a studies is a particle size well below the resolving power attainable with the cryomicrotome system. The fluorescence signal from deposited aerosol in a cryomicrotome image of lung represents both aerosol at the level of the slice and background signal from aerosol deeper within the frozen lung block, an effect that homogenizes the V̇a signal and hence underestimates the true extent of regional V̇a heterogeneity. In this study, we present an approach to obtain high-resolution measurements of regional V̇a in the rat, utilizing a corrected fluorescence signal derived from cryomicrotome images of FMS aerosol deposition. We compare the overall heterogeneity of V̇a measured using the cryomicrotome approach with six FMS aerosol-exposed rats, where the lungs were air-dried and cut into separate pieces for fluorescence measurement. We examine the cryomicrotome measurement error by analysis of signal differences of simultaneously administered FMS aerosols and describe the spatial heterogeneity of V̇a/Q̇ distribution in the rat lung at a resolution of 113 mm3, the volume of a 3-mm radius sphere. Finally, we present an application of this method by examining the effects of intravenous methacholine injection on the measurements of regional V̇a and Q̇.
METHODS
Animal preparation.
The University of Washington Animal Care Committee approved the experimental protocol. Sixteen ∼250-g male Sprague-Dawley rats were anesthetized by intraperitoneal injection of 140–160 mg/kg pentobarbital, sufficient to prevent withdrawal of paw after pinch. A tracheostomy was performed, and an internal jugular vein was cannulated. The animals were mechanically ventilated at a rate of 40 breaths/min with a tidal volume of 4 ml, utilizing a piston pump ventilator. The FMS aerosol was generated by an in-line ultrasonic aerosol-generating system (Microstat Ultrasonic Nebulizer, Mountain Medical Equipment, Littleton, CO) that passed the generated aerosol through a drying column before entry into the trachea. The FMS aerosol was generated from 2 ml of a 2.5% suspension of 0.04-μm FMS (Molecular Probes, Eugene, OR) in distilled water and was administered to the rats for 10 min. Regional Q̇ to rat lungs was marked by intravenous injection of 40,000 FMS (15-μm) of each color.
The first five rats (group 1) had two different 0.04-μm FMS aerosol labels (red and yellow) mixed for a single administration and two different 15-μm FMS (green and scarlet) mixed for intravenous injection. The administration of paired FMS markers for aerosol deposition and pulmonary Q̇ was used to explore the importance of measurement error in the FMS estimates of regional V̇a and Q̇. The second five rats (group 2) had single aerosol and single injected FMS colors administered at baseline, followed by a second set of aerosol and injected FMS colors administered 5 min after a 2- to 3-min intravenous injection of 30-μg of methacholine in 0.1 ml saline. (The tidal volume was reduced to 3 ml during the methacholine injection and then returned to 4 ml after 5 min.) Airway pressure, heart rate, carotid artery pressure, and oxygen saturation were measured continuously for the five methacholine rats, and four of those five rats had arterial blood-gas measurements. In this minimally invasive preparation, measurements of cardiac output and pulmonary artery pressure were not obtained. The final six anesthetized rats (group 3) received a 10-min administration of red and yellow 1.0-μm FMS aerosol.
Specimen preparation and imaging.
When the FMS label administration was completed, the animals were deeply anesthetized with 100 mg/kg ip pentobarbital and exsanguinated. The lungs were cut free from cardiac and mediastinal tissues. The lungs of the first 10 rats (groups 1 and 2) were prepared for cryomicrotome imaging. The lungs were filled via tracheal injection of 6 to 8 ml of 99% OCT (optimal cutting temperature media, Tissue-Tek OCT) and 1% India ink. The filled lung was frozen, suspended in a mixture of 90% OCT and 10% India ink, and frozen in the suspension OCT mixture. The frozen tissue block was fixed in a cryomicrotome (Imaging Cryomicrotome 6.0, Barlow Scientific, Olympia, WA) and shaved with 16-μm increments. Following every cryomicrotome cut, fluorescent emission images of the lung block were acquired for each of the four different fluorescent colors. The original cryomicrotome (4) has been upgraded with a new camera (Redlake MegaPlus II ES 3200, San Diego, CA) with a resolution of 2,184 × 1,472 pixels and a new control box (Redlake MegaPlus II Camera Controller Console).
The lungs of the final six rats receiving two FMS aerosols (group 3) were prepared for regional V̇a mapping by the same method used in the large-animal FMS studies. The lungs were inflated to 25-cmH2O airway pressure and air-dried. The dried lungs were cut into 28 approximately equal ∼300-mm3 volume pieces, excluding the external conducting airways. Each piece was weighed and then soaked for 48 h in 0.5 ml Cellosolve (Aldrich Chemical, Milwaukee, WI) to extract the fluorescence from the FMS labels. The fluorescence signals were then measured with a fluorescence spectrometer (Perkin-Elmer LS50B, Boston, MA). The fluorescence signals were corrected for spillover from adjacent colors in the emission spectrum using a matrix inversion program (13). The color signal for each piece was normalized by both mean signal and weight to express the measurements as V̇a per unit parenchyma, thereby establishing a mean piece V̇a value of 1.0 (10).
Image analysis of regional Q̇ in groups 1 and 2.
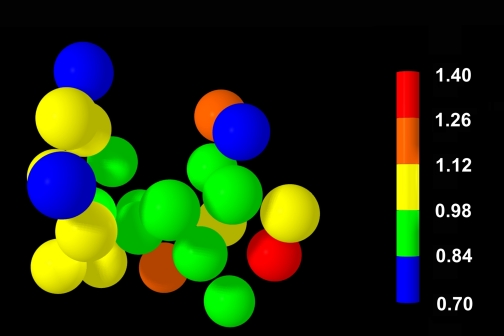
The 15-μm FMS count was obtained from the image set, as described by Glenny et al. (4). In brief, system software identifies the spatial coordinates of each deposited 15-μm FMS based on identification of any fixed spatial signal within the tissue block that persists through at least four 16-μm slices before vanishing. Regional Q̇ sampling in the lung specimen was based on 3-mm-radius spheres placed within the lung parenchyma. The spheres were centered on randomly selected points within the lung with the constraints that each sphere had to include at least 90% lung tissue and have no more than 10% radius overlap with any other sphere. The number of 3-mm radius ROI spheres placed in the group 1 and 2 rats ranged between 24 and 38. Figure 1 illustrates the placement of a typical set of 3-mm-radius spheres within the summed lung image. Regional Q̇ within each sphere was based on the sum of 15-μm FMS with spatial coordinates included within that sphere. The microsphere count in each sphere was normalized by the mean number of microspheres in all of the sampled spheres, so that the mean value representing Q̇ among spheres was 1.0.
Fig. 1.
Twenty-four 3-mm-radius spheres positioned within the lung viewed from a ventral aspect, with colors representing normalized blood flow (Q̇) relative to the number of 15-μm fluorescent microspheres (FMS) located within the sphere volume.
Image analysis of regional V̇a for groups 1 and 2.
Figure 2 illustrates the typical pattern 0.04-μm FMS aerosol deposition seen on a cryomicrotome section. The signal from the images of aerosol fluorescence was first corrected for differences in the incident light within a given slice by adjusting the voxels from each image in the stack by the normalized bright-field image. Next, a median filter covering pixels with the same x- and y-coordinates over five slices in the z-direction was applied to exclude portions of cryomicrotome images blocked by shavings from previous cryomicrotome cuts remaining on the tissue block at time of imaging. The processed lung images were then masked to include lung and exclude the largest airways and blood vessels. All pixels outside of the mask were excluded from the summation. Finally, each image was individually corrected for the background fluorescent glow originating from the cryomicrotome specimen block. The background estimate for each fluorescent image used a cumulative histogram of voxel gray-scale activity within the lung mask. The gray-scale value that included the lowest 5% of the pixels was taken as background, and that gray-scale value was subtracted from all of the remaining pixels in the image. The same 3-mm-radius sampling spheres chosen for the Q̇ measurements were then applied to the aerosol measurements. The sum of all corrected pixel signal for aerosol FMS contained within each sphere represented the regional V̇a signal for that sphere. The V̇a value for each sphere was normalized by the mean sphere V̇a signal, giving a mean relative V̇a value of 1.0.
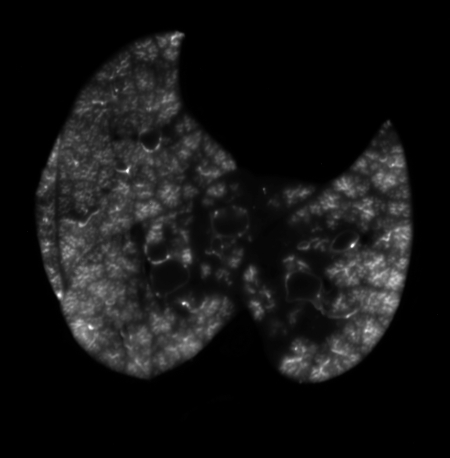
Fig. 2.
Typical cryomicrotome specimen image of deposition of 0.04-μm yellow FMS aerosol in caudal lobes, before image processing. The irregular linear markings within the lobes reflect the reduced aerosol deposition in the periphery of acini.
RESULTS
Estimate of measurement error.
Correlation coefficients for the measurements of the simultaneously administered paired FMS labels for V̇a and Q̇ in the group 1 rats are presented in Table 1. The between-label correlation coefficients for the 15-μm microspheres are lower, representing between 4 and 8% of the total variance of the Q̇ signal from the two colors, using a statistical model (5) to estimate the method noise. As the mean sphere 15-μm FMS count in each animal ranged between 220 and 390, Poisson noise alone accounts for the majority of that Q̇ method error. The uncorrected Pearson correlation coefficients for Q̇ presented in Table 1 can have the Poisson noise contribution removed, based on the average number of microspheres per region of interest (ROI) and the number of ROIs, as described by Polissar et al. (8). Utilizing that correction, the estimate of the correlation between paired colors for measurement of Q̇ was >0.99. The between-color variation is greater for the aerosol FMS, but, as the strength of the color signal for aerosol indicated that there were several thousand FMS per sphere volume, Poisson noise is negligible. The method noise for the V̇a measurements accounted for between 0.5 and 4% of the total variance among the spherical ROIs (5).
Table 1.
Simultaneously administered FMS
| Animal No. | Between-Color Correlation, V̇a | Between-Color Correlation, Q̇ |
|---|---|---|
| 1 | 0.99 | 0.89 |
| 2 | 0.99 | 0.82 |
| 3 | 0.92 | 0.95 |
| 4 | 0.97 | 0.84 |
| 5 | 0.94 | 0.93 |
Pearson correlation coefficients (r) for simultaneously administered pairs of fluorescent microsphere (FMS) aerosol colors [pulmonary ventilation (V̇a)] and FMS blood flow (Q̇) markers in the group 1 animals.
Heterogeneity of V̇a and Q̇.
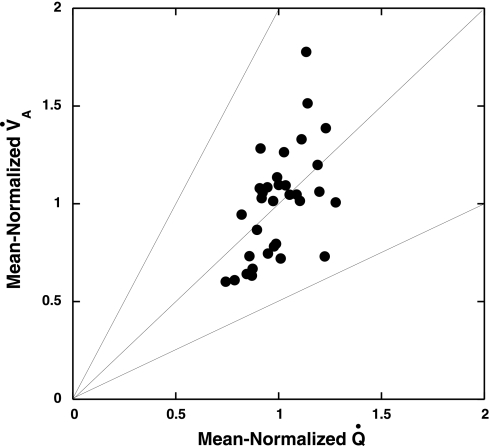
Table 2 presents coefficients of variation for V̇a and Q̇ for the five group 1 rats with two labels for each parameter (nos. 1–5) and for the five group 2 rats with the control measurements made before injection of methacholine (nos. 6–10). The coefficient of variation (CV) for Q̇ was fairly consistent among animals and also consistent with previous measurements made on rats at this level of scale (4). The CV for V̇a was consistently larger than that for Q̇. In addition, the individual Pearson correlations between V̇a and Q̇ among animals were weaker than those observed in FMS studies of larger animals (2, 7, 9, 10). Despite the relatively weaker correlations between V̇a and Q̇, nearly all of the ROIs remained within a V̇a/Q̇ range of 0.5 to 2.0 (Fig. 3).
Table 2.
Within-animal variability of V̇a and Q̇
| Animal No. | CV-V̇a,% | CV-Q̇, % | r |
|---|---|---|---|
| 1 | 28 | 12 | 0.70 |
| 2 | 74 | 11 | 0.38 |
| 3 | 34 | 19 | 0.23 |
| 4 | 27 | 13 | 0.56 |
| 5 | 30 | 16 | 0.61 |
| 6 | 26 | 16 | 0.41 |
| 7 | 16 | 19 | 0.00 |
| 8 | 29 | 11 | 0.54 |
| 9 | 34 | 17 | 0.64 |
| 10 | 52 | 32 | 0.81 |
| Mean ± SD | 35 ± 16 | 17 ± 6 | 0.49 ± 0.23 |
The percent coefficient of variation (CV) for V̇a and Q̇ among the 3-mm-radius spheres sampled within each lung for the 10 rats in groups 1 and 2. The r is shown between V̇a and Q̇ for those regions of interest within each rat lung.
Fig. 3.
Plot of pulmonary ventilation (V̇a) vs. Q̇ from a typical animal (no. 4), where the line of identity represents a V̇a/Q̇ of 1, and upper and lower lines represent V̇a/Q̇ values of 2 and 0.5, respectively.
Comparison with V̇a heterogeneity measurements from dried lungs (group 3 rats).
The CVs for aerosol distribution within the six air-dried lungs with individual piece fluorescence measurements (Table 3) show within-animal values very similar to the cryomicrotome measurements (Table 2). These dried lung measurements differ from the cryomicrotome spherical ROIs, because all alveolar parenchyma is included in these measurements, and the mean piece volume was roughly twice that of the 3-mm-radius spheres. The correlation between the simultaneously administered FMS aerosols delivered to the subsequently dried lungs was not different from that observed with the cryomicrotome images.
Table 3.
Individually measured pieces
| Animal No. | CV-V̇a,% | r |
|---|---|---|
| 11 | 25 | 0.96 |
| 12 | 37 | 0.98 |
| 13 | 44 | 0.85 |
| 14 | 38 | 0.99 |
| 15 | 28 | 0.98 |
| 16 | 25 | 0.97 |
| Mean ± SD | 33 ± 7 | 0.96 ± 0.05 |
CV of V̇a for individually measured cut pieces, and the Pearson correlation between the two simultaneously administered FMS colors for the group 3 animals.
Response to administration of methacholine (group 2 rats).
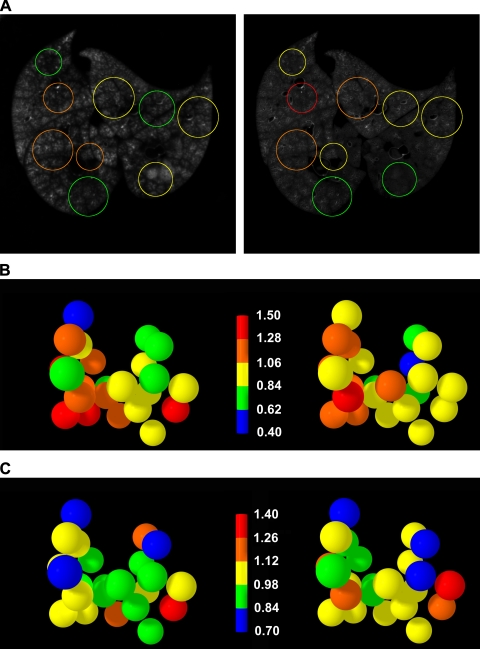
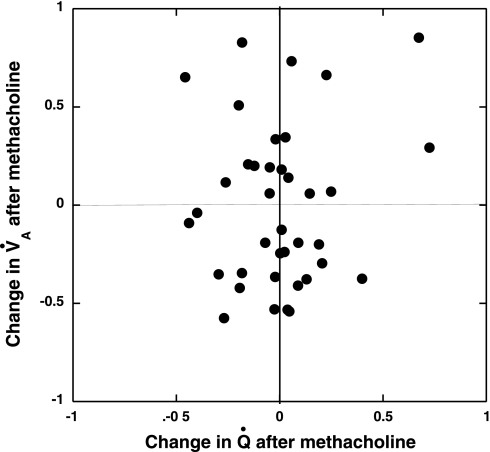
The injection of methacholine produced the expected changes in physiological measurements (Table 4), with significant increases in peak airway pressure and arterial Pco2 and associated decreases in O2 saturation and arterial Po2. Figure 4A shows the FMS aerosol deposition in a single cryomicrotome slice before (left) and after (right) administration of methacholine. Although the mean intensities of the two FMS colors are different, there are substantial differences in regional signal intensity among the regions demarcated by the colored circles. The colored circles represent sections through the randomly spaced sampling spheres. The magnitude of change in regional V̇a after methacholine injection in all sampled lung regions in this animal is illustrated in Fig. 4B. The majority of the regions demonstrate large increases or decreases in the allocation of regional V̇a. The shifts in regional Q̇ following the methacholine injection, illustrated using the same ROIs (Fig. 4C), reveal changes that are generally smaller than those shown for V̇a, shown in Fig. 4B. Table 5 shows the changes in CV for regional V̇a and Q̇, and the correlation between the two parameters before and after methacholine. The administration of methacholine did not change the CV for either V̇a or Q̇, but it produced substantial interregional shifts in both V̇a and Q̇, as illustrated by Fig. 4, B and C. The Pearson correlation between V̇a and Q̇ was not systematically changed by the methacholine. An illustration of the relative responses of V̇a and Q̇ to the methacholine stimulus (Fig. 5) plots the change in V̇a vs. the change in Q̇ for each sampled lung volume following the administration of methacholine.
Table 4.
Physiological response to MCh injection
| Paw, cmH2O | SAP, mmHg | O2 Sat, % | PaO2, Torr | PaCO2, Torr | |
|---|---|---|---|---|---|
| Control | 10.6 ± 0.6 | 91 ± 6 | 94.3 ± 1.9 | 80 ± 5 | 34 ± 1 |
| MCh | 13.1 ± 1.0* | 83 ± 3 | 80.9 ± 7.4* | 63 ± 10* | 42 ± 4* |
Values are means ± SD of changes induced by methacholine (MCh) for peak airway pressure (Paw), systolic arterial pressure (SAP), arterial oxygen saturation (O2 Sat), and arterial blood gases [arterial Po2 (PaO2), arterial Pco2 (PaCO2)].
Difference between control and post-MCh measurements, significant at P < 0.02 level.
Fig. 4.
A: single cryomicrotome slice images of FMS aerosol deposition labeled before (left) and after (right) methacholine administration. The colored circles correspond to the region of interest spheres shown in B. B: measurements of regional V̇a, comparing control (left) and postmethacholine (right) V̇a in the same 3-mm-radius spheres. Color scale represents V̇a normalized to the overall mean measurement. C: measurements of regional Q̇ before and after methacholine, with the same regions of interest shown in B.
Table 5.
Descriptive statistics before and after MCh injection
| CV V̇a,% |
CV Q̇,%, |
r |
||||
|---|---|---|---|---|---|---|
| Animal No. | Control | MCh | Control | MCh | Control | MCh |
| 6 | 26 | 22 | 16 | 16 | 0.41 | −0.03 |
| 7 | 16 | 36 | 19 | 19 | 0.00 | 0.64 |
| 8 | 29 | 25 | 12 | 16 | 0.54 | 0.19 |
| 9 | 34 | 26 | 17 | 23 | 0.64 | 0.63 |
| 10 | 52 | 33 | 32 | 42 | 0.81 | 0.74 |
| Mean ± SD | 31 ± 8 | 28 ± 5 | 19 ± 7 | 23 ± 10 | 0.48 ± 0.27 | 0.43 ± 0.32 |
Coefficient of variation for V̇a and Q̇ in the group 2 rats before and after MCh injection. The r control is the Pearson correlation coefficient between control V̇a and Q̇, and r MCh is the corresponding Pearson correlation after MCh.
Fig. 5.
Plot of the changes observed in regional V̇a and regional Q̇ following the injection of methacholine in a typical group 2 animal.
If the regional shifts in V̇a had been well compensated by shifts in Q̇, the data points of Fig. 5 would be aligned on a diagonal line running through the intercept, with a slope of 1. In fact, the data points fail to reveal any consistent pattern of Q̇ response to the methacholine-induced V̇a changes. Table 6 presents the standard deviations of those before-after differences for both V̇a and Q̇ in each animal, illustrating the magnitude of the shifts observed in V̇a and Q̇ among all of the group 2 animals and confirming the impression from Fig. 5 that the change in V̇a was nearly twice as large as the change in Q̇ following methacholine injection.
Table 6.
Reallocation of V̇a and Q̇ after MCh
| Animal No. | SD of Change in V̇a | SD of Change in Q̇ |
|---|---|---|
| 6 | 0.30 | 0.16 |
| 7 | 0.26 | 0.16 |
| 8 | 0.25 | 0.11 |
| 9 | 0.28 | 0.13 |
| 10 | 0.40 | 0.25 |
SD of the control-MCh differences for V̇a and Q̇ in all of the regions sampled in each animal from group 2.
DISCUSSION
Heterogeneity of Q̇.
This study demonstrates that regional V̇a and Q̇ can be simultaneously measured at high spatial resolution in small laboratory animals, and that changes in both can be quantified following an intervention. The spatial variability of Q̇ described here at the 113-mm3 scale, expressed as a CV, is consistent with previously reported measurements in rats (4), but is substantially smaller than that seen in large animals at the ∼2-cm3 level of scale. However, as the spatial variability of Q̇ is dependent on the number of vascular generations (6), the spatial CV needs to be compared by generation number and not absolute volume. By generation number, the 113-mm3 rat volume samples would be roughly equivalent to a sample size of ∼ 80-cm3 in a large animal, and that sample size corresponds to a CV in the 10–15% range seen in large laboratory animals (1). As we did not obtain measurements of cardiac output in this study, we do not have the ability to describe our findings in terms of Q̇ per unit tissue. This approach provides valid information concerning the heterogeneity of regional flow, but, without measurements of cardiac output, we are unable to predict measurement of respiratory gas exchange, as previously described in large-animal FMS studies (2).
Heterogeneity of V̇a.
The primary motivation for this study was to obtain accurate estimates of regional V̇a based on cryomicrotome images of fluorescent aerosol deposition. To determine whether the cryomicrotome measurement of V̇a measured in group 1 and 2 rats appropriately represented the regional deposition of aerosol, the six group 3 rats had paired FMS aerosol administered with the same aerosol system, but postdeath, the lungs were inflated, air-dried, and cut into the smallest piece size that still would permit extraction of an adequate fluorescence signal. The cut dried lung pieces had an average volume of ∼300 mm3, over twice the size of the 113-mm3 volume of the ROI spheres of the cryomicrotome measurements. The dried lung tissue measurements, which included the entire lung, demonstrated equally strong correlations between the simultaneously administered color markers for V̇a and comparable coefficients of variation (compare Tables 1 and Table 3). Thus an independent method of measuring aerosol deposition at a modestly larger level of scale gave similar coefficients of variation. In addition, this approach, including all of the lung parenchyma, provided assurance that the random sphere sampling gave appropriate estimates of the variability of regional V̇a and Q̇. These findings support the potential for the cryomicrotome aerosol distribution measurements to faithfully represent the regional deposition of aerosol in the lung.
Aerosol deposition measurements to represent regional V̇a.
FMS measurements of regional V̇a and Q̇ in pigs have accurately predicted measurements of both respiratory and inert-gas exchange in large animals (2), and those findings have served as a confirmation that the aerosol FMS measurements can appropriately represent regional V̇a in those studies. An unanticipated finding of this study was that, in contrast to the large-animal studies of regional V̇a and Q̇, the CV for V̇a was consistently larger than the CV for Q̇. While this possibly could reflect a characteristic of V̇a distribution in rodents or smaller animals in general, it also could be a problem related to mucous plugs in the lung. Examination of the individual V̇a images of rats 2 and 10 (see Table 2) showed very substantial distribution to a single lower lobe, suggesting that mucus could have blocked aerosol distribution to the remainder of the lung for part of the 10-min aerosol administration, leading to an increased CV artifact for the measurement of V̇a. However, excluding those two rats, the mean CV of V̇a for the remaining eight rats with lobes that appeared to be uniformly labeled was 28%, compared with the 17% mean CV of Q̇. Additional studies with small animals will be required to determine whether the observed higher V̇a heterogeneity represents a biological difference or undetected mucous plugs in the small airways. A second property of aerosol deposition that could account for a larger CV in the aerosol estimate of V̇a can be appreciated from examination of Figs. 2 and 4A. At the acinar level, the deposition of aerosol no longer appropriately reflects the distribution of alveolar gases, as the aerosol does not reach the periphery of the acini. While these FMS deposition images offer insight into acinar anatomy, using aerosol deposition to estimate regional V̇a at the acinar scale of measurement adds noise. The diffusive characteristics of gases become very important for gas delivery to the most peripheral alveoli, and, as even the smallest aerosols cannot penetrate to those regions, the aerosol estimate of regional V̇a will be more heterogeneous than an estimate done with gases. This artifact becomes relevant as the V̇a ROIs decrease towards the scale of a single acinus. However, given a rat acinar volume at total lung capacity of ∼3 mm3 (12), the uneven aerosol deposition effect would be too small to explain the difference between the CVs for V̇a and Q̇ noted with the 113-mm3 ROIs employed for this study.
While regional V̇a measured with CT images of xenon washout and FMS aerosol deposition have agreed acceptably in large-animal studies using ∼2-cm3 ROIs (11), the aerosol estimate will probably overestimate heterogeneity as resolution approaches the acinar level of scale. A method for very high-resolution measurement of regional V̇a utilizing gases has been reported with xenon washout synchrotron radiation computed tomography (3). Using anesthetized rabbits where the heterogeneity of regional V̇a could be measured using 3-mm3 ROIs, Bayat et al. (3) reported a CV of V̇a of 22%. Unfortunately, that method cannot provide comparable measurements of the CV of Q̇. Nevertheless, that reported extent of V̇a heterogeneity at the 3-mm3 level of scale matches well with the range of rat CV of Q̇ estimates made at a similar sample volume (4). The level of resolution where aerosol deposition begins to overestimate the extent of V̇a heterogeneity currently remains to be defined.
Assessment of V̇a/Q̇ heterogeneity in the rat.
The correlation noted between V̇a and Q̇ in the rats is weaker than that reported in larger animals (10). However, the lungs of larger animals have more branching generations of both airways and pulmonary arteries before alveolar surfaces are encountered. Because of the increasing heterogeneity of both V̇a and Q̇ produced by successive generations of branching, large animals will have a larger overall range of both V̇a and Q̇ at the acinar level, and this requirement alone will produce a higher Pearson correlation coefficient. Hence, for smaller animals, the correlation coefficient between V̇a and Q̇ is somewhat weaker, because the extent of heterogeneity attained at entry to acinar units is smaller. A lower correlation coefficient between V̇a and Q̇ for smaller animals does not necessarily indicate that the match between those parameters is worse, if the overall variance of both V̇a and Q̇ is correspondingly reduced. This is illustrated by Fig. 3, a typical plot of V̇a and Q̇ for this study, where the data points for both parameters are contained within a narrow V̇a/Q̇ range, despite a relatively low correlation coefficient. Wilson and Beck (15) demonstrated that, given multiple regional measurements of V̇a and Q̇, the overall extent of V̇a/Q̇ heterogeneity is dependent not only on the correlation between the two measurements, but also on the variance of each of those two parameters. The insight gained is that the correlation between regional V̇a and Q̇ measurements is dependent both on the size of the selected ROI and on the number of vascular or airway branchings that take place before the designated ROI is reached. Hence a Pearson correlation coefficient in isolation is not an adequate predictor of gas exchange properties, particularly in small animals, as they have fewer airway and vascular branches before the gas exchange surfaces are reached.
The changes in the regional allocation of V̇a and Q̇ among ROIs noted in the group 2 rats after the methacholine injection were relatively large (Table 6), but the administration of methacholine failed to produce an increase in the CV for V̇a (Table 5). The latter finding was not initially expected, but, given the unchanged minute ventilation, reduced V̇a to the regions with the most severe bronchospasm mandates increased V̇a in other regions of the lung. That reallocation of V̇a was not associated with compensatory changes in regional Q̇. Figure 5 illustrates the failure of Q̇ to adjust to the changes in V̇a distribution; if regional Q̇ had adjusted to maintain V̇a/Q̇ homogeneity, the data points on Fig. 5 would fall along a line, with a slope of 1 and an intercept at the origin. The measurements on Fig. 5 show substantial changes in both V̇a and Q̇ for all ROIs, with no suggestion of correlation, a finding confirmed by the hypoxemia and respiratory acidosis observed in all of the postmethacholine measurements.
Potential applications of cryomicrotome FMS measurements of V̇a and Q̇.
The advantage of this FMS methodology arises from the availability of multiple color labels, providing the opportunity to assess how the distribution of V̇a and Q̇ changes in response to interventions. These regional measurements of V̇a and Q̇ can define the size and location of the anatomic units responding to a stimulus. PET measurements of changes in V̇a distribution in asthma have provided valuable insights into disease mechanisms (14), but that approach lacks the resolution needed for application to small animals. The regional response of both V̇a and Q̇ to methacholine provides an example of insights that can be obtained with this technique in small-animal models of disease. In addition to the high level of resolution afforded by this approach, the same cryomicrotome images could also be used to define the conducting airway and pulmonary arterial tree structures. Those tree image sets, combined with the FMS deposition measurements, would provide the opportunity to divide the lung parenchyma into anatomically appropriate ROIs. Regional responses of the lung are likely propagated along the bronchovascular delivery trees, so the potential to identify anatomically appropriate ROIs for V̇a and Q̇ would represent a significant advance over the current approaches used to subdivide the lung. The availability of anatomically based ROIs would be especially important for applications to animal models of lung diseases.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL073598.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Altemeier WA, McKinney S, Glenny RW. Fractal nature of regional ventilation distribution. J Appl Physiol 88: 1551–1557, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Altemeier WA, Robertson HT, Glenny RW. Pulmonary gas-exchange analysis by using simultaneous deposition of aerosolized and injected microspheres. J Appl Physiol 85: 2344–2351, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Bayat S, Porra L, Suhonen H, Suortti P, Sovijarvi AR. Paradoxical conducting airway responses and heterogeneous regional ventilation after histamine inhalation in rabbit studied by synchrotron radiation CT. J Appl Physiol 106: 1949–1958, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Glenny RW, Bernard SL, Robertson HT. Pulmonary blood flow remains fractal down to the level of gas exchange. J Appl Physiol 89: 742–748, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Glenny RW, Polissar NL, McKinney S, Robertson HT. Temporal heterogeneity of regional pulmonary perfusion is spatially clustered. J Appl Physiol 79: 986–1001, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Glenny RW, Robertson HT. Fractal modeling of pulmonary blood flow heterogeneity. J Appl Physiol 70: 1024–1030, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Melsom MN, Kramer-Johansen J, Flatebo T, Muller C, Nicolaysen G. Distribution of pulmonary ventilation and perfusion measured simultaneously in awake goats. Acta Physiol Scand 159: 199–208, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Polissar NL, Stanford DC, Glenny RW. The 400 microsphere per piece “rule” does not apply to all blood flow studies. Am J Physiol Heart Circ Physiol 278: H16–H25, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Robertson HT, Glenny RW, Stanford D, McInnes LM, Luchtel DL, Covert D. High-resolution maps of regional ventilation utilizing inhaled fluorescent microspheres. J Appl Physiol 82: 943–953, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Robertson HT, Hlastala MP. Microsphere maps of regional blood flow and regional ventilation. J Appl Physiol 102: 1265–1272, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Robertson HT, Kreck TC, Krueger MA. The spatial and temporal heterogeneity of regional ventilation: comparison of measurements by two high-resolution methods. Respir Physiol Neurobiol 148: 85–95, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Rodriguez M, Bur S, Favre A, Weibel ER. Pulmonary acinus: geometry and morphometry of the peripheral airway system in rat and rabbit. Am J Anat 180: 143–155, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Schimmel C, Frazer D, Glenny RW. Extending fluorescent microsphere methods for regional organ blood flow to 13 simultaneous colors. Am J Physiol Heart Circ Physiol 280: H2496–H2506, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 434: 777–782, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Wilson TA, Beck KC. Contributions of ventilation and perfusion inhomogeneities to the V̇a/Q̇ distribution. J Appl Physiol 72: 2298–2304, 1992 [DOI] [PubMed] [Google Scholar]