Abstract
As chronic stress and depression have become recognized as significant risk factors for peripheral vascular disease in patients with no prior history of vasculopathy, we interrogated this relationship utilizing an established mouse model of chronic stress/depressive symptoms from behavioral research. Male mice were exposed to 8 wk of unpredictable chronic mild stress (UCMS; e.g., wet bedding, predator sound/smell, random disruption of light/dark cycle), with indexes of depressive behavior (coat status, grooming, and mobility) becoming exacerbated vs. controls. In vascular rings, constrictor (phenylephrine) and endothelium-independent dilator (sodium nitroprusside) responses were not different between groups, although endothelium-dependent dilation (methacholine) was attenuated with UCMS. Nitric oxide synthase (NOS) inhibition was without effect in UCMS but nearly abolished reactivity in controls, while cyclooxygenase inhibition blunted dilation in both. Combined blockade abolished reactivity in controls, although a significant dilation remained in UCMS that was abolished by catalase. Arterial NO production was attenuated by UCMS, although H2O2 production was increased. UCMS mice demonstrated an increased, although variable, insulin resistance and inflammation. However, while UCMS-induced vascular impairments were consistent, the predictive power of aggregate plasma levels of insulin, TNF-α, IL-1β, and C-reactive peptide were limited. However, when separated into tertiles with regard to vascular outcomes, insulin resistance and hypertension were predictive of the most severe vascular impairments. Taken together, these data suggest that aggregate insulin resistance, inflammation, and hypertension in UCMS mice are not robust predictors of vascular dysfunction, suggesting that unidentified mechanisms may be superior predictors of poor vascular outcomes in this model.
Keywords: vasodilation, endothelium-derived factors, peripheral vascular disease, nitric oxide, models of chronic stress, clinical depression
clinical diagnoses of depression and depressive symptoms are becoming increasingly common among patients with coronary artery and peripheral vascular disease, with ∼20% of patients displaying angiographic evidence of vascular disease suffering from major depressive disorders (21). In addition, previous studies have provided evidence that clinical depression or depressive symptoms of sufficient severity can serve as independent risk factors for ischemic heart disease (20). However, in recent clinical trials, antidepressant therapy failed to improve cardiac outcomes among patients with major depression (3, 4), suggesting that a distinct pathophysiological mechanism may be responsible for the compelling associations between clinical depression and the evolution of vascular disease. Patient-based studies have suggested that physiological differences are present between patients suffering from depression/depressive symptoms and control patients (4, 5), with one study suggesting that platelet nitric oxide synthase (NOS) activity and plasma nitric oxide (NO) levels were reduced in patients with depressive disorders (8). However, the validity of these reports has been criticized as suffering from a poor level of control, and heterogeneity in data acquisition procedures. To further interrogate the characteristics linking clinical depression with vascular disease, the contributing mechanisms, functional outcomes, and potential for interventional success, it is necessary to appropriately integrate an animal model for depression/depressive symptoms with data collected using human cohorts.
Previously, investigators developed the unpredictable chronic mild stress (UCMS) model for developing depression/depression-like behaviors in rodents (16, 22, 26, 28), with the underlying assumption that a common source for developing depression is an environment of continual stressors resulting from uncontrollable exogenous factors, and the genetic component of depressive behavior being a function of heritable differences in susceptibility to imposed stressors (16, 19). The UCMS model is considered to be the most appropriate rodent model for human clinical depression, based on its ability to reproduce the development of many human depressive symptoms, including anhedonia and learned helplessness (16, 19, 25).
Although the UCMS model of depression in mice has been well established in the behavioral sciences, the application of this model to interrogate the association between depressive disorders and peripheral vascular disease has received little attention. The purpose of this study was to determine the relationships between chronic stress/depressive symptoms and vascular function in a mouse strain that is highly susceptible to the development of depression/depressive symptoms as a result of the UCMS protocol, the BALB/cJ (16, 27). This study evaluated the hypothesis that the UCMS protocol, leading to depressive behaviors in mice, would cause significant impairments to vascular reactivity that are consistent with the development of peripheral vascular disease, including increased constrictor reactivity and blunted dilator responses. Results from this study suggest that chronic depressive symptoms in mice strongly associated with poor vascular endothelial function and that this may have significant implications for the regulation of vascular tone, thrombosis, atherogenesis, and tissue/organ perfusion.
MATERIALS AND METHODS
Animals.
Male BALB/cJ mice (Jackson) were fed standard chow and drinking water ad libitum and were housed in the animal care facility at the West Virginia University Health Science Center (WVU HSC). All protocols received prior institutional animal care and use committee (IACUC) approval. At 9 wk of age, mice were divided into two groups, control and UCMS (below). After 8 wk duration under either condition, mice were anesthetized with injections of pentobarbital sodium (50 mg/kg ip), and a carotid artery was cannulated for determination of arterial pressure. Venous blood aliquots were collected for biochemical evaluation of plasma markers of dysfunction (below).
UCMS protocol.
All mice were singly housed, with the control group in a separate, nearby quiet room. Alternatively, in the UCMS group, mice were randomly exposed to the following stressors on multiple occasions throughout each 24-h period: 1) damp bedding: 10 oz of water was added to each standard cage for the next 3 h; 2) water: all bedding was removed and ∼0.5 in. of water was added to empty cage for the next 3 h; 3) each cage was tilted to 45° with or without bedding for 3 h; 4) social stress: each mouse was switched into a cage of a neighboring mouse for 3 h; 5) no bedding lasting for 3 h or, on two occasions each week, overnight; 6) succession of light/dark cycles, lasting 30 min throughout a 24-h period; and 7) exposure to predator smells (e.g., cat fur) and/or sounds (e.g., cat growling).
After 8 wk, all mice were subjected to a series of behavioral tests that were aimed at evaluating the outcomes of the UCMS procedures.
Coat status.
This evaluation was done throughout the duration of the UCMS protocol. The total cumulative score was computed by giving an individual score of 0 (clean) or 1 (dirty) to eight body parts (head, neck, dorsal coat, ventral coat, tail, forelimb, hindlimb, and genital region).
Splash test.
This test was used to evaluate acute grooming behavior, defined as cleaning of the fur by licking or scratching. A 10% sucrose solution was sprayed on the dorsal coat of each mouse, and grooming activity was recorded for 5 min. The viscosity of the sucrose solution will dirty the coat and induce grooming behavior, with depressive symptoms characterized by an increased latency (idle time between spray and initiation of grooming) and decreased frequency (number of times grooming a particular body part).
Tail suspension test.
Mice subjected to the short term, inescapable stress of tail suspension will develop an immobile posture, with longer periods of immobility in mice exhibiting a depressed behavior. Mice were suspended by the tail on a horizontal bar 35 cm from the base platform using adhesive tape. The latency to the first bout of immobility and the duration of total immobility were recorded for 6 min.
Measurements of vascular reactivity.
In each mouse, the thoracic aorta was removed, rinsed in physiological salt solution, cleared of surrounding tissue, and cut in 2- to 3-mm segments. Each ring was mounted in a myobath chamber between a fixed point and a force transducer (World Precision Instruments) and set to 0.5-g tension for 45 min to equilibrate. The organ baths contained physiological salt solution at 37°C and were aerated with 95% O2-5% CO2. Rings were preconditioned by treatment with 10−7 M phenylephrine for 5 min, at which time 10−5 M methacholine was added to the bath to assess endothelial integrity. Any ring that failed to demonstrate both a brisk constrictor response to phenylephrine and viable endothelial function was discarded. Subsequently, rings were treated with increasing concentrations of phenylephrine (10−10 M–10−3 M) to assess constrictor reactivity. For the assessment of dilator reactivity, rings were pretreated with 10−6 M phenylephrine and exposed to increasing concentrations of methacholine (10−10 M–10−3 M) and sodium nitroprusside (10−10 M–10−3 M). To assess the roles of nitric oxide, cyclooxygenase and hydrogen peroxide in modulating vascular responses to the agonist treatments, concentration-response curves were also conducted following treatment of the rings with Nω-nitro-l-arginine methyl ester (l-NAME) (10−4 M), indomethacin (10−5 M), and catalase (10−4 M), respectively.
Measurement of vascular nitric oxide and hydrogen peroxide bioavailability.
From a cohort of mice within each group, the aorta was used to assess vascular NO and H2O2 production using amperometric sensors (World Precision Instruments). Briefly, aortas were isolated, cleaned, and placed within a sealed chamber filled with physiological salt solution equilibrated with 95% O2-5% CO2. Within this chamber, a nitric oxide sensor (ISO-NOPF 100) and a hydrogen peroxide sensor (ISO-HPO-100) were inserted and a baseline current was obtained. Subsequently, methacholine (10−6 M) was added to the chamber and the changes in current were determined. To verify that responses represented NO and H2O2 release, these procedures were repeated following addition of l-NAME (10−4 M) or catalase (10−4 M) to the chamber.
Analyses of endothelial NOS expression.
For determination of endothelial NOS (eNOS) expression, aortas were homogenized and proteins were separated under denaturing conditions on 10% SDS-polyacrylamide gel, which were then transferred to a PVDF membrane and blocked. Subsequently, blots were incubated with mouse anti-eNOS/NOS Type III mAb (BD Transduction Laboratories), washed, and incubated with appropriate horseradish peroxidase-conjugated secondary antibody. GE Healthsciences ECL advance kits were used to visualize proteins, and all eNOS bands were normalized to expression of actin.
Biochemical analyses.
In all samples, blood glucose was determined using a commercially available glucometer (Freestyle). Lipid panels for cholesterol and triglycerides were performed using commercially available kits (Wako). Additionally, oxidant stress was assessed through determination of plasma nitrotyrosine (Cayman). Finally, using a multiplexed procedure, plasma insulin and markers of inflammation were assessed using commercially available kits (Millipore).
Data and statistical analyses.
Mechanical responses following challenge with methacholine or phenylephrine were fit with the three-parameter logistic equation:
where y represents the isometric tension; min and max represent the lower and upper bounds, respectively, of the change in tone with agonist concentration; x is the logarithm of the agonist concentration; and logED50 represents the logarithm of the agonist concentration (x) where the response (y) is halfway between the bounds. The use of the three-parameter logistic equation is appropriate for the analysis of sigmoidal concentration-response relationships, as it simultaneously provides estimates of the curve maximum (upper bound), minimum (lower bound) and the dose at which the dependent variable reaches 50% of maximum (ED50).
All data are presented as means ± SE. Significant differences between groups were determined using ANOVA. In all cases, Student-Newman-Keuls post hoc test was used when appropriate and P < 0.05 was taken to reflect statistical significance.
RESULTS
Table 1 presents data describing the characteristics of mouse groups in the present study. At the time of use, plasma insulin, triglyceride concentrations, and plasma levels of nitrotyrosine were significantly greater in UCMS mice than in controls. No differences were determined between body mass, mean arterial pressure, plasma glucose, and cholesterol levels.
Table 1.
Baseline characteristics between mouse groups in the present study
| Control | UCMS | |
|---|---|---|
| Mass, g | 31 ± 2 | 29 ± 4 |
| MAP, mmHg | 84 ± 5 | 93 ± 4 |
| Insulinplasma, ng/ml | 1.3 ± 0.2 | 3.9 ± 0.6* |
| Glucoseblood, mg/dl | 102 ± 8 | 104 ± 7 |
| Cholesterolplasma, mg/dl | 75 ± 8 | 81 ± 8 |
| Triglyceridesplasma, mg/dl | 102 ± 8 | 128 ± 10* |
| Nitrotyrosineplasma, ng/ml | 17 ± 2 | 27 ± 4* |
Values are means ± SE. All mice are male, aged 17–18 wk.
UCMS, unpredictable chronic mild stress; MAP, mean arterial pressure.
P < 0.05 vs. control.
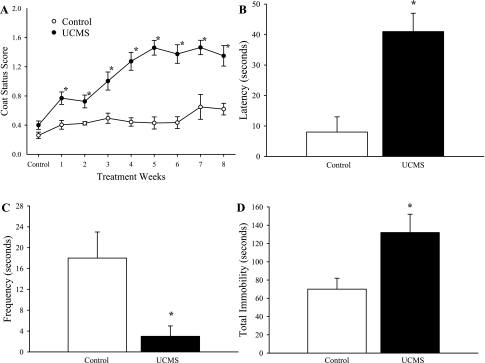
The impact of the UCMS protocol on depressive behaviors in mice is summarized in Fig. 1. Throughout the duration of the UCMS period, coat status in mice undergoing the stress protocol was consistently poorer compared with that in controls (Fig. 1A). In response to the sucrose spray, control mice demonstrated both a more rapid (Fig. 1B) and a more frequent (Fig. 1C) grooming response compared with that exhibited by mice following the UCMS protocol. In response to tail suspension, control mice demonstrated a significantly shorter period of immobility compared with that in UCMS mice (Fig. 1D).
Fig. 1.
Depressive symptoms following 8 wk of the unpredictable chronic mild stress (UCMS) protocol in BALB/cJ mice. Data are presented for coat status (A), the latency (B) and frequency (C) of facial grooming following a 10% sucrose solution spray, and the total period of immobility during the tail suspension test (D) for control and UCMS mice. *P < 0.05 vs. control; n = 25 for control; n = 26 for UCMS.
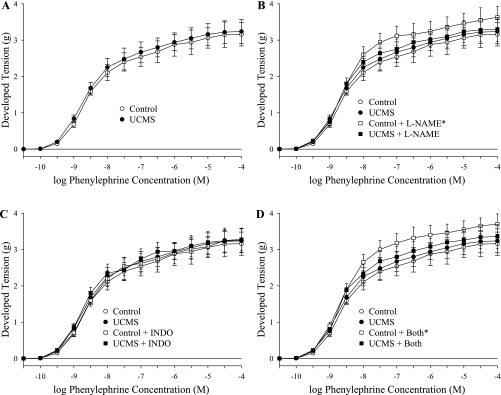
Figure 2 summarizes the constrictor responses of aortic rings from the two mouse groups in response to increasing concentrations of phenylephrine. As shown in Fig. 2A, there was no evidence of an altered constrictor reactivity of rings from UCMS mice in response to phenylephrine. However, when treated with the NOS inhibitor l-NAME (Fig. 2B), the constrictor responses of rings from control mice increased significantly, a response not present in UCMS. Treatment of vascular rings from either group with indomethacin had no impact on the constrictor response to phenylephrine (Fig. 2C), and combined treatment with both l-NAME and indomethacin was not different from that for l-NAME alone (Fig. 2D).
Fig. 2.
Constrictor responses of aortic rings to increasing concentrations of phenylephrine from mice under control conditions (open symbols: ○/☐) and after 8 wk of UCMS (filled symbols: ●/■). Data are presented under control conditions (A; control, upper bound = 3.2 ± 0.2 g; UCMS, upper bound = 3.3 ± 0.3 g), in response to NOS inhibition with Nω-nitro-l-arginine methyl ester (l-NAME) (B; control + l-NAME, upper bound = 3.8 ± 0.2 g; UCMS + l-NAME, upper bound = 3.5 ± 0.2 g), cyclooxygenase inhibition with indomethacin (INDO) (C; control + INDO upper bound = 3.3 ± 0.3 g; UCMS + INDO, upper bound = 3.4 ± 0.3 g), or both (D; control + both, upper bound = 3.9 ± 0.3 g; UCMS + both, upper bound = 3.5 ± 0.3 g). *P < 0.05 vs. responses in untreated vascular rings from control mice; n = 10–11 for control; n = 10–12 for UCMS.
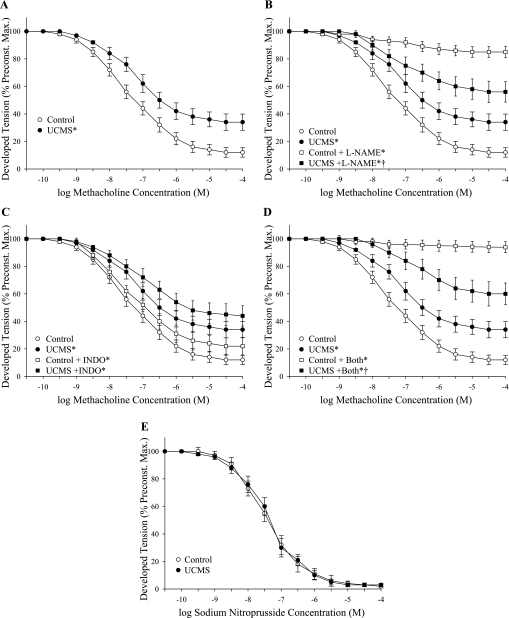
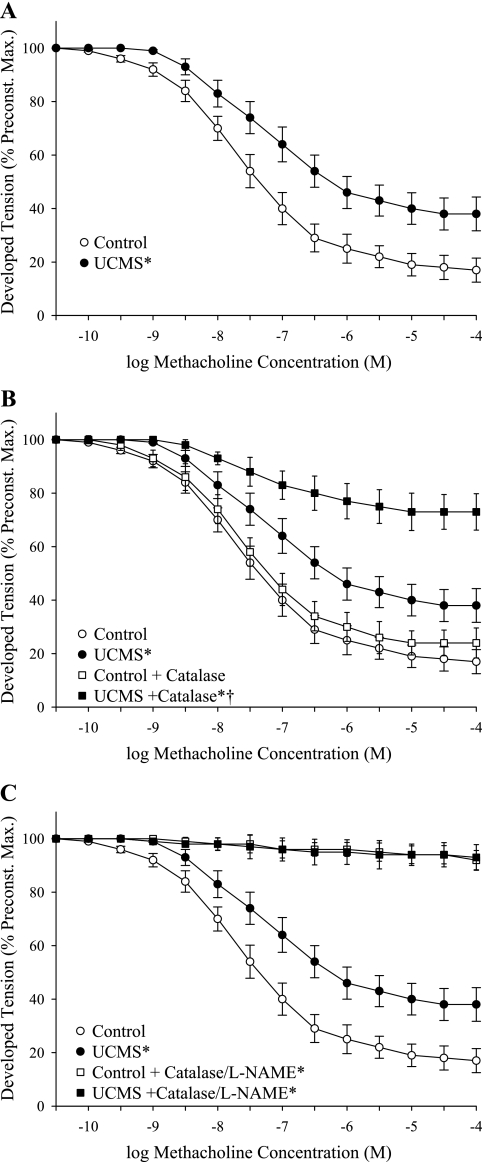
The dilator responses of vascular rings from control and UCMS mice are summarized in Fig. 3. Rings from UCMS mice demonstrated a significant reduction in methacholine-induced dilation compared with responses in rings from control mice (Fig. 3A). While pretreatment of rings with l-NAME nearly abolished methacholine-induced reactivity in control mice, NOS blockade had only a minor impact on dilation in rings from UCMS mice (Fig. 3B). Further, while methacholine-induced dilation was only mildly impacted by treatment with indomethacin in both groups (Fig. 3C), treatment with l-NAME and indomethacin produced results that were comparable to that for l-NAME alone (Fig. 3D). The dilation of aortic rings to increasing concentrations of the NO donor sodium nitroprusside did not differ between control and UCMS mice (Fig. 3E).
Fig. 3.
Dilator responses of aortic rings to increasing concentrations of methacholine from mice under control conditions (open symbols: ○/☐) and following 8 wk of UCMS (filled symbols: ●/■). Data are presented under control conditions (A; control, lower bound = 10.3 ± 2.4%; UCMS, lower bound = 33.6 ± 4.4%), in response to NOS inhibition with l-NAME (B; control + l-NAME, lower bound = 84.5 ± 3.2%; UCMS + l-NAME, lower bound = 54.6 ± 5.4%), cyclooxygenase inhibition with INDO (C; control + INDO, lower bound = 21.2 ± 4.1%; UCMS + INDO, lower bound = 43.4 ± 5.2%), or both (D; control + both, lower bound = 93.4 ± 2.4%; UCMS + both, lower bound = 59.2 ± 5.6%). Data are also presented for vascular responses to increasing concentrations of sodium nitroprusside (E; control, lower bound = 2.5 ± 1.1%; UCMS, lower bound = 2.9 ± 1.3%). *P < 0.05 vs. responses in untreated vascular rings from control mice; †P < 0.05 vs. responses in untreated vascular rings from UCMS mice; n = 8–9 for control; n = 7–9 for UCMS.
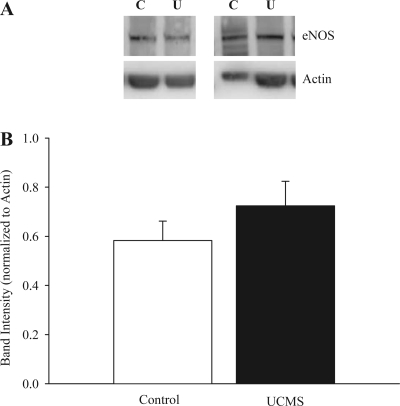
The arterial expression of eNOS in control and UCMS mice was not altered by the UCMS protocol (Fig. 4). These results suggest that any change in NO bioavailability and the impaired NO-dependent component of methacholine-induced dilation in UCMS mice does not reflect a change in vascular eNOS expression.
Fig. 4.
Representative blots describing expression of endothelial nitric oxide synthase (eNOS) from aortas in control and UCMS mice (A). B presents densitometric analysis of band intensity between control and UCMS mice; n = 5 for control and for UCMS.
Figure 5 presents data describing the impact of treatment of aortic rings from both mouse groups with catalase on methacholine-induced dilation. Treatment of rings with catalase, while having minimal impact of dilator reactivity in control mice, significantly reduced dilator responses in UCMS mice (Fig. 5, A and B). Further, combined treatment of rings with l-NAME and catalase nearly abolished responses of vascular rings to methacholine in both control and UCMS groups (Fig. 5C).
Fig. 5.
Dilator responses of aortic rings to increasing concentrations of methacholine from mice under control conditions (open symbols: ○/☐) and following 8 wk of UCMS (filled symbols: ●/■). Data are presented under control conditions (A; control, lower bound = 16.6 ± 3.9%; UCMS, lower bound = 37.4 ± 4.6%), following pretreatment of rings with the H2O2 scavenger catalase (B; control + catalase, lower bound = 23.8 ± 4.4%; UCMS + catalase, lower bound = 72.3 ± 6.1%), or following combined treatment with catalase and l-NAME (C; control + catalase/l-NAME, lower bound = 91.4 ± 2.8%; UCMS + catalase/l-NAME, lower bound = 92.6 ± 3.1%). *P < 0.05 vs. responses in untreated vascular rings from control mice; †P < 0.05 vs. responses in untreated vascular rings from UCMS mice; n = 8 for control; n = 8–9 for UCMS.
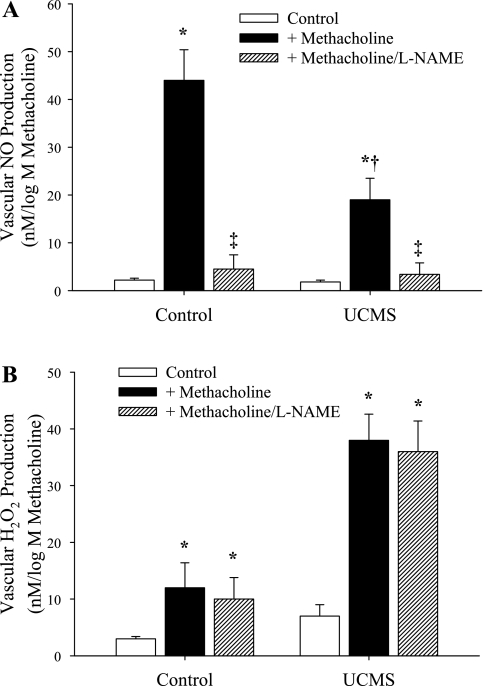
The results of biochemical assays for methacholine-induced NO and H2O2 production are summarized in Fig. 6, A and B, respectively. Aortas from control mice demonstrated a robust NO production in response to challenge with methacholine compared with levels in unstimulated vessels, while that from UCMS mice was significantly attenuated (Fig. 6A), paralleling the mechanical responses presented in Fig. 3. In contrast, methacholine challenge resulted in a limited production of H2O2 in aortas from control animals, yet caused a strong increase in H2O2 release in vessels of UCMS (Fig. 6B), providing further support for the data presented in Fig. 5. The production of H2O2 in response to application of methacholine in either group was unaffected by pretreatment with l-NAME.
Fig. 6.
Aortic production of nitric oxide (A) and H2O2 (B) following methacholine challenge in mice under control conditions and following 8 wk of UCMS. Data are presented for arteries under control (unstimulated) conditions, following challenge with methacholine and following methacholine challenge in the presence of NOS inhibition with l-NAME. *P < 0.05 vs. responses in unstimulated vessels in that group; †P < 0.05 vs. responses in vessels from control animals following challenge with methacholine; ‡P < 0.05 vs. responses in vessels from that group following challenge with methacholine; n = 5 for control; n = 6 for UCMS.
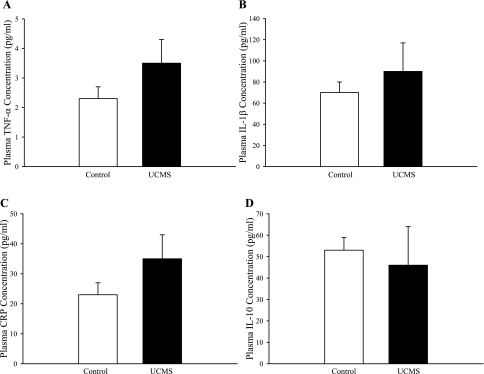
Plasma levels of inflammatory cytokines in control and UCMS mice are summarized in Fig. 7. Although mean values were elevated for TNF-α (Fig. 7A), IL-1β (Fig. 7B), and C-reactive peptide (CRP; Fig. 7C) in UCMS mice compared with control, a high variability in these measurements between animals within the UCMS group prevented differences from reaching statistical significance. Plasma levels of IL-10 (Fig. 7D) were not different between the two groups of mice.
Fig. 7.
Plasma levels of markers of inflammation in control mice and in mice following 8 wk of UCMS. Data are shown for TNF-α (A), IL-1β (B), C-reactive peptide (CRP) (C), and IL-10 (D); n = 24 for control and for UCMS.
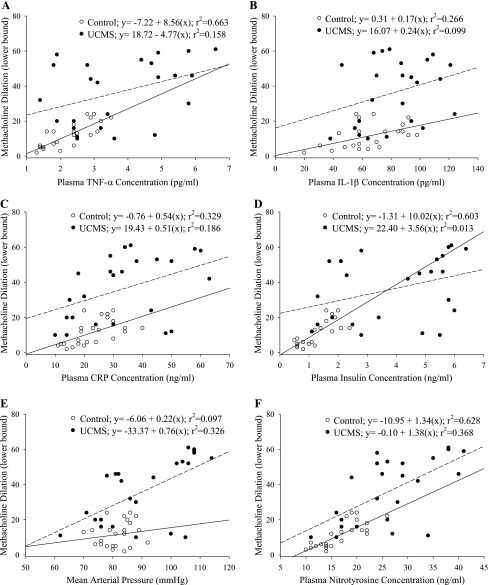
Given the variability in markers of peripheral vascular disease risk in the UCMS mice, it is important to determine the extent to which these substances were predictive of poor vascular outcomes in this model of chronic stress/depressive symptoms. As presented in Fig. 8, plasma levels of TNF-α (Fig. 8A), insulin concentration (Fig. 8D), and nitrotyrosine (Fig. 8F) were well correlated with vascular reactivity in control mice (with r2 > 0.6 for each), with other risk factors [IL-1β (Fig. 8B), CRP (Fig. 8C), and arterial pressure (Fig. 8E)] being less predictive. However, none of the measured risk factors were strongly predictive of the vascular outcomes in the UCMS mice where r2 values ranged between 0.013 for insulin and ∼0.3 for both arterial pressure and nitrotyrosine.
Fig. 8.
Correlations between vascular reactivity and individual vascular disease risk factors in control (open symbols, solid line) and UCMS mice (filled symbols, dashed line). Data are presented as the lower bound in the methacholine concentration-response curve for an individual mouse vs. plasma concentrations of TNF-α (A), IL-1β (B), CRP (C), insulin (D), mean arterial pressure (E), and plasma levels of nitrotyrosine (F). Also presented are lines of best fit through the data for each group, with the resulting equations and r2 values presented in the legend.
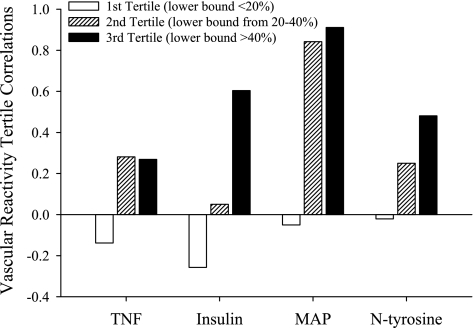
However, if relationships in Fig. 8 are separated into tertiles with regard to vascular outcome, these data are more informative. In Fig. 9, vascular dysfunction is separated as such, with the first tertile representing mild vascular dysfunction (lower bound of the methacholine-induced dilation <20% of initial tension), the second tertile representing moderate vascular dysfunction (lower bound between 20 and 40% of initial tension), and the third tertile representing severe vascular dysfunction (lower bound >40% of initial tension). When presented in this fashion where the correlation between specific risk factors and the graded severity of vascular dysfunction in UCMS mice is presented, it is apparent that mild levels of vascular dysfunction in response to UCMS are not well predicted by any of the risk factors determined. In mice where a moderate reduction in dilator reactivity was present (second tertile), this degree of vascular impairment was well predicted where a significant elevation in arterial pressure was the primary outcome of the UCMS protocol. The strongest levels of vascular dysfunction were observed in mice where the UCMS protocol was able to create a more insulin-resistant, hypertensive state.
Fig. 9.
Correlations between vascular reactivity and individual cardiovascular disease risk factors in control and UCMS mice following separation of the lower bound of the methacholine concentration-response curves into tertiles based on the severity of impairment in dilator responses. Data are presented as the correlation between a given tertile of vascular impairment severity and a specific vascular disease risk factor. Please see text for details.
DISCUSSION
With a growing body of clinical and epidemiological evidence suggesting a link between chronic stress/depression and the progression of vascular disease, it is vital to utilize an appropriate animal model such that experiments can be designed that will interrogate these processes at a high level of resolution, for mechanistic contributors, functional implications, and interventional strategies. The UCMS model for the establishment of depressive symptoms in rodents has been repeatedly validated within behavioral research and has demonstrated a remarkably broad utility. Not only is this model used for general studies involving chronic stress and depression (19, 25), it has also been employed as an excellent means for evaluating interventional and potentially ameliorative therapies (12, 19, 25), as the depressive state in rodents manifests behaviors that mimic those in human subjects afflicted with clinical depression (25, 27). One of the main underlying assumptions of clinical depression and depressive symptoms is that the presence of chronic exogenous stressors will initiate depression/depressive symptoms in all afflicted subjects, but that underlying genetic, heritable traits are directly relevant to the severity of these symptoms rather than basic susceptibility per se (25, 27). The veracity of this fundamental assumption has been evaluated previously in multiple mouse strains in response to the imposition of the UCMS protocol. Interestingly, across numerous mouse strains, the BALB/cJ was consistently demonstrated as being among the most susceptible to the UCMS protocol, with the most severe development of depressive symptoms as a result of the chronic stressors (10, 15). Given that previous studies have identified the BALB/cJ strain to be somewhat less susceptible to vascular dysfunction (11, 17) underscores the potential importance of the relationships between chronic stress/depression and negative vascular outcomes under investigation in the present study.
Building on the results from previous studies of impaired vascular function in human subjects with increasing depressive symptom severity (3), the UCMS mouse model was used to further interrogate these relationships. With the success of the UCMS protocol in producing depressive behaviors in mice (Fig. 1), the present results demonstrated that this was associated with significant impairments and alterations to vascular reactivity. Net reactivity of aortic rings to phenylephrine was not significantly different between control and UCMS mice, a response that is in contrast to the recent work from Neves et al. (18), although that study utilized rats and a much shorter duration of UCMS exposure. However, in the present study application of l-NAME resulted in an increased constrictor response in control mice only, suggesting a level of NO production that buffers phenylephrine-induced constriction, an observation that was completely absent in UCMS mice (Fig. 2). This lack of an impact of l-NAME on adrenergic reactivity suggested that one of the effects of UCMS was a reduction in vascular NO bioavailability, although the responses of vascular smooth muscle to exogenous NO appear to be intact as responses to sodium nitroprusside were unaffected.
This concept of impaired endothelial function was further explored through interrogation of dilator responses to the endothelium-dependent agonist methacholine. While methacholine-induced vasodilation in rings from control animals was overwhelmingly a function of NO production, this pattern was altered substantially in UCMS mice (Fig. 3). Following the UCMS protocol, methacholine-induced dilation, while blunted vs. responses from control mice demonstrated a reduced dependence on, and role for, NO bioavailability. However, as combined treatment with l-NAME revealed a residual dilator response to methacholine, these results suggest that a compensatory dilator mechanism develops in UCMS mice where NO bioavailability is compromised. Based on the data presented in Fig. 4, it seems unlikely that this reduction in vascular NO bioavailability reflects a reduction in NOS expression within the aortic endothelium, as expression was not altered between control and UCMS mice.
As summarized in Fig. 5, treatment of aortic rings from UCMS mice with the hydrogen peroxide scavenger catalase reduced methacholine-induced dilation, while having minimal impact on responses in rings from control mice. Further, combined treatment of vascular rings with l-NAME and catalase nearly abolished vascular responses to methacholine in both control and UCMS mice. These results strongly suggest that the vascular production of H2O2 in response to challenge with methacholine may represent a compensatory mechanism which helps to maintain normal vascular mechanical responses to vasoactive stimuli. This conceptual paradigm received additional support through the measurements of NO and H2O2 production in aortas of control and UCMS mice following challenge with methacholine. Presented in Fig. 6, the reduction in agonist-induced NO bioavailability in UCMS mice was associated with an increased H2O2 production vs. responses in aortas from control mice.
One of the more intriguing elements to the present study was assessing major contributing mechanisms that could underlie the demonstrated changes in vascular reactivity with chronic UCMS in mice. As evidenced by the data summarized in Fig. 8, vascular reactivity in control mice demonstrated a predictable correlation with markers of inflammation (Fig. 8, A–C), an index of insulin resistance (Fig. 8D), and a plasma marker of oxidant stress (Fig. 8F). However, in UCMS mice, these correlations were much less robust as neither these markers, nor arterial pressure (Fig. 8E), were able to effectively predict the reduced dilator responses in UCMS-mice, with only arterial pressure and plasma nitrotyrosine demonstrating r2 values in excess of 0.3. This is a particularly interesting outcome in that UCMS mice developed insulin resistance that, while not severe, could certainly contribute to alterations in endothelium-dependent vascular reactivity through multiple mechanism associated with NO bioavailability, oxidant stress, and chronic inflammation (7, 14, 24). Given this, we elected to analyze the data after dividing the vascular outcome into tertiles based on the severity of the impaired dilator reactivity (Fig. 9). The results from these analyses were particularly intriguing, as mild vascular impairments as a result of the UCMS protocol occurred largely in the absence of alterations to insulin sensitivity, chronic oxidant stress, inflammation, or elevated arterial pressure. However, moderate impairments in vascular reactivity in UCMS mice were associated with the development of increased arterial pressure only, were weakly predicted by oxidant stress and inflammation, and were independent of insulin sensitivity. It was only at the highest levels of vascular dysfunction as a result of UCMS where both hypertension and insulin resistance (with the associated elevation in oxidant stress) became robust predictors of the negative outcomes.
Taken together, these data provide for multiple compelling avenues for future investigation. First, a greater understanding of reduced NO bioavailability with UCMS warrants attention, via determination eNOS activity and its regulatory processes. Additionally, future studies should be organized to distinguish chronic stress/depression from a general lack of physical activity (and the possible role of insulin resistance in this process; Ref. 23) as a result of the UCMS protocol, as the mice experiencing the stress procedures did manifest a greater degree of behavior (delayed/reduced motor responses to stimuli, reduced physical struggling against holding/restraint) that is comparable to the “learned helplessness” that afflicts human subjects with clinical depression/depressive symptoms. Further, the results presented in Fig. 9 suggest that there is a level of vascular dysfunction inherent in UCMS and the development of depressive symptoms in mice that is not associated with the measured parameters in the present study, and which may reflect the impact of currently unidentified processes. What makes this particularly compelling is that it is apparent that even though all mice undergoing the UCMS protocol were genetically identical, and each manifest comparable behavioral outcomes, variability in the vascular outcome, and most certainly in the contributing elements to that vascular dysfunction, is present. As a point of speculation, this suggests that the individual responses to chronic exogenous stress may be heterogeneous, even within an inbred strain, and that variability in terms of adaptation in response to imposed external stressors may result in distinct developmental routes through which an integrated pathophysiological outcome develops. We believe that this warrants future investigation.
One area that requires a brief clarification is that of “vascular depression,” a term that describes the development of depressive symptoms that stems from cerebrovascular dysfunction impacting cerebral perfusion distribution (1). In vascular depression, the depressive symptoms are the result of poor perfusion to the prefrontal regions or their modulating pathways owing to an accumulation of vascular lesions that negatively impact behavioral outcomes, leading to a state of clinical depression (2). Given the novelty of using the UCMS model in mice for the study of vascular dysfunction, one area that will require future investigation is the temporal association of the depressive symptom severity and the alterations to cerebrovascular reactivity, as the extent to which these are distinct pathological conditions is presently unclear.
In summary, these results suggest that depressive symptoms of sufficient severity can represent a powerful factor in the development of negative vascular outcomes in a mouse model of chronic stress/depression. The vascular dysfunction that develops with depressive symptoms is characterized by an abrogation of vascular NO bioavailability, although net vascular reactivity is still largely intact owing to the emergence of H2O2 as a compensatory dilator metabolite. We believe that future investigation into the role of depressive symptoms vs. physical activity, the mechanistic bases of the vascular dysfunction, the functional implications for the change in endothelial function, and the potential for ameliorative interventions is well justified.
GRANTS
This study was supported by the American Heart Association (EIA 0740129N), the National Institutes of Health (R01-DK-64668), and a West Virginia University Research Development Grant.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the expertise of Dr. Catherine Belzung of the Université François Rabelais de Tours, France, for assisting us in the developing the UCMS model in our laboratory. Additionally, we acknowledge the expert technical assistance of Barbara Jackson, Milinda James, and the support provided through Center for Cardiovascular and Respiratory Sciences at the West Virginia University Health Sciences Center.
REFERENCES
- 1. Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. “Vascular depression” hypothesis. Arch Gen Psychiatry 54:915–922, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. Am J Psychiatry 154: 562–565, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Brown AD, Barton DA, Lambert GW. Cardiovascular abnormalities in patients with major depressive disorder: autonomic mechanisms and implications for treatment. CNS Drugs 23: 583–602, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Barefoot JC, Brummett BH, Helms MJ, Mark DB, Siegler IC, Williams RB. Depressive symptoms and survival of patients with coronary artery disease. Psychosom Med 62: 790–795, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Barefoot JC, Williams RB. Antidepressant use and the risk of myocardial infarction. Am J Med 108: 87–88, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N; Enhancing Recovery in Coronary Heart Disease Patients Investigators (ENRICHD) Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 289: 3106–3116, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Busija DW, Miller AW, Katakam P, Erdös B. Insulin resistance and associated dysfunction of resistance vessels and arterial hypertension. Minerva Med 96: 223–232, 2005 [PubMed] [Google Scholar]
- 8. Chrapko WE, Jurasz P, Radomski MW, Lara N, Archer SL, Le Mellédo JM. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry 56: 129–134, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Davidson KW, Kupfer DJ, Bigger JT, Califf RM, Carney RM, Coyne JC, Czajkowski SM, Frank E, Frasure-Smith N, Freedland KE, Froelicher ES, Glassman AH, Katon WJ, Kaufmann PG, Kessler RC, Kraemer HC, Krishnan KR, Lespérance F, Rieckmann N, Sheps DS, Suls JM; National Heart, Lung, and Blood Institute Working Group. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 68: 645–650, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Ducottet CC. Belzung, Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res 156: 153–162, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Friedman G, Ben-Yehuda A, Dabach Y, Hollander G, Babaey S, Ben-Naim M, Stein O, Stein Y. Macrophage cholesterol metabolism, apolipoprotein E, and scavenger receptor AI/II mRNA in atherosclerosis-susceptible and -resistant mice. Arterioscler Thromb Vasc Biol 20: 2459–2464, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Gass P, Reichardt HM, Strekalova T, Henn F, Tronche F. Mice with targeted mutations of glucocorticoid and mineralocorticoid receptors: models for depression and anxiety? Physiol Behav 73: 811–825, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, Mclvor M. Sertraline Antidepressant Heart Attack Randomized Trial (SADHEART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 288: 701–709, 2002. [Corrigendum: JAMA 288: 1720, 2002]. [DOI] [PubMed] [Google Scholar]
- 14. Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28: 1982–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res 175: 43–50, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Mineur YS, Prasol DJ, Belzung C, Crusio WE. Agonistic behavior and unpredictable chronic mild stress in mice. Behav Genet 33: 513–519, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Miyoshi T, Matsumoto AH, Shi W. Paradoxical increase in LDL oxidation by endothelial cells from an atherosclerosis-resistant mouse strain. Atherosclerosis 192: 259–265, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Neves VJ, Moura MJ, Tamascia ML, Ferreira R, Silva NS, Costa R, Montemor PL, Narvaes EA, Bernardes CF, Novaes PD, Marcondes FK. Proatherosclerotic effects of chronic stress in male rats: altered phenylephrine sensitivity and nitric oxide synthase activity of aorta and circulating lipids. Stress 12: 320–327, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci 11: 53–58, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Penninx BW, Beekman AT, Honig A, Deeg DJ, Schoevers RA, van Eijk JT, van Tilburg W. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry 58: 221–227, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry 54: 227–240, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Venables MC, Jeukendrup AE. Physical inactivity and obesity: links with insulin resistance and type 2 diabetes mellitus. Diabet Metab Res Rev 25, Suppl 1: S18–S23, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Wiernsperger N, Nivoit P, De Aguiar LG, Bouskela E. Microcirculation and the metabolic syndrome. Microcirculation 14: 403–438, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134: 319–329, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Willner P. Animal models of depression: validity and applications. Adv Biochem Psychopharmacol 49: 19–41, 1995 [PubMed] [Google Scholar]
- 27. Yalcin I, Belzung C, Surget A. Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res 193: 140–143, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Yalcin I, Aksu F, Belzung C. Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol 514: 165–174, 2005 [DOI] [PubMed] [Google Scholar]