Abstract
Aim
To indirectly compare rivaroxaban and dabigatran for prevention of venous thromboembolism (VTE) after total hip or knee arthroplasty (THA, TKA) based on their pivotal efficacy/safety trials embracing a total of 20 618 patients.
Methods
Pooled risk differences (RD) for rivaroxaban vs enoxaparin and dabigatran vs enoxaparin obtained from separate meta-analyses of two sets of trials were used to indirectly estimate RDs for rivaroxaban vs dabigatran.
Results
Primary efficacy (any VTE+all-cause mortality) and safety (major bleeding) outcomes in enoxaparin arms largely differed across similarly designed rivaroxaban and dabigatran trials (differences in venography adjudication and bleeding events definitions). However, incidence of symptomatic VTE and incidence of major/non-major clinically relevant bleeding (including surgical site) were consistent in this respect. RDs (as percentages) for symptomatic VTE were rivaroxaban-enoxaparin = -0.4% (95% confidence interval [CI], -0.9 to 0.05); dabigatran-enoxaparin = -0.09% (95% CI, -1.0 to 0.8); rivaroxaban-dabigatran = -0.3% (95% CI, -1.3 to 0.7; P = 0.275). RDs for major/clinically relevant bleeding were rivaroxaban-enoxaparin = 0.99% (95%CI, 0.29 to 1.69); dabigatran-enoxaparin = 0.02% (95% CI, -1.0 to 1.0); rivaroxaban-dabigatran = 0.97 (95% CI, -0.43 to 2.37; P = 0.085). Mortality rates (all-cause, VTE-related, bleeding-related) were very low not indicating differences between any two of the three treatments.
Conclusion
Methodological differences disable indirect comparisons of rivaroxaban vs dabigatran that would be based on major efficacy/safety outcomes of their pivotal trials. The two drugs do not seem to differ regarding incidence of symptomatic VTE. Risk of a relevant bleeding is higher with rivaroxaban than with enoxaparin and the same tendency exists also vs dabigatran. Direct rivaroxaban vs dabigatran comparisons in this setting are needed.
Total hip and knee arthroplasty (THA and TKA, respectively) are common, routine and effective treatments for a number of conditions affecting the two joints. However, treated patients are at a high risk of venous thromboembolism (VTE), ie, deep venous thrombosis (DVT) potentially resulting in a fatal pulmonary embolism (PE) or other sequelae (pulmonary hypertension, postthrombotic syndrome). Fortunately, the prevailing form of the post-arthroplasty DVT, distal DVT, is rarely associated with PE, but the more ominous proximal DVT may also occur (1-3). Hence, thromboprohylaxis is indicted for all patients undergoing THA/TKA, but choice of the means is still controversial because treatments that effectively prevent VTE (primarily anticoagulants) simultaneously increase the risk of bleeding (3). The prevailing form is surgical site bleeding that might require further interventions (revision, re-operation, transfusion) and/or compromise functional outcomes. Extra-surgical site bleedings are less common but might be critical by localization (intracranial, intraorbital, retroperitoneal) and/or extent, have sequelae, or be fatal (4).
Rivaroxaban (a direct factor Xa inhibitor) and dabigatran etexilate (dabigatran, a direct thrombin inhibitor) are new generation oral anticoagulants. In the European Union, both drugs are approved as effective and acceptably safe in prevention of VTE after THA/TKA (5,6) based on pivotal trials (7-13), in which they were compared with enoxaparin, a low molecular weight heparin preparation. Inherently, they also increase bleeding tendency but provide the convenience of simple dosing (orally, once daily), predictive effects not requiring routine coagulation monitoring, and low potential for drug interactions (5,6). Although the two drugs have never been compared directly, an analysis suggested (14) that rivaroxaban could be more cost-effective than dabigatran due to supposedly better efficacy and lower rate of major bleedings (primary safety concern). However, the analysis (14) referred only to some (and not all) of the pivotal rivaroxaban and dabigatran trials. Furthermore, it used enoxaparin data from a single rivaroxaban trial as a reference for assessment of both rivaroxaban and dabigatran in THA, and enoxaparin data from a single dabigatran trial as a reference for assessment of both drugs in TKA. Two recent reports demonstrated that “mixing” data from different thromboprophylaxis trials in THA/TKA setting in such a manner might not be an appropriate procedure. First, different definitions of “bleeding events” have been used in different trials (4). Second, major efficacy outcomes include counting of, among others, asymptomatic venographically detected VTE based on adjudication. Although the process is standardized, actual event rates in individual trials are largely influenced by the factor “adjudication committee” (15). Therefore, we considered it worthwhile to evaluate whether the existing pivotal trials allowed for indirect assessment of relative efficacy/safety of rivaroxaban vs dabigatran in prevention of VTE after THA/TKA and whether any of the two drugs should be considered superior to the other one.
Materials and methods
Adjusted indirect comparison between treatments
Two treatments (A and B) can be compared indirectly based on their individual comparisons with a common control treatment (C). The treatment effect of A vs B (TAB) is derived from treatment effects of A vs C (TAC) and B vs C (TBC) and the procedure is termed adjusted indirect comparison (16,17). Computationally (18), TAB = TAC – TBC, standard error (SE) of TAB = √[SE(TAC)2 + SE(TBC)2], and z-statistic is used to test the null hypothesis TAB = 0. If there are several A/C and B/C trials, TAC and TBC are derived from appropriate meta-analyses. Adjusted indirect comparisons (A vs B) based on meta-analyses (of A vs C and B vs C) perform well and provide unbiased estimates of TAB (16,17). The basic condition is that A/C and B/C trials are reasonably similar in design (setting, patient characteristics, outcome definitions) and in regard to results for the reference treatment. Also, sets of A/C and B/C trials should be appropriate for meta-analysis (16,17).
Data acquisition and evaluation
Published reports on pivotal rivaroxaban (7-10) and dabigatran (11-13) trials for prevention of VTE after THA/TKA and related documents made public by regulatory agencies (European Medicines Evaluation Agency; Food and Drug Administration, FDA) (5,6,19,20) were retrieved and reviewed. Considering the regulatory requirements (21), it was expected that rivaroxaban and dabigatran trials would be fairly comparable in design, use of the reference treatment, and patient characteristics. To assess feasibility of the major (primary) efficacy and safety outcomes (bleeding events) for indirect comparisons, their definitions and results for enoxaparin arms were compared across corresponding rivaroxaban and dabigatran trials. Other outcomes were also assessed in order to identify retrievable data that would be appropriate for indirect comparisons. Incidence of symptomatic VTE on treatment (includes DVT and/or PE, fatal or not; denominator: patients who underwent surgery and received at least one dose of study drug) was reported in all trials and was considered informative of efficacy, practically relevant – in practice, venograms and other evaluations are typically undertaken when indicated by clinical signs and symptoms – and likely not affected by adjudication. A document supplied by the rivaroxaban sponsor to the FDA (19) provided data on surgical site bleedings that were not explicitly listed in the published reports on rivaroxaban trials (7-10). This allowed that a composite outcome incidence of major or non-major clinically relevant bleeding on treatment be retrieved from all trials and used as a measure of safety (definition of “major” and “non-major clinically relevant” was identical in all trials, but now it included both surgical site and extra-surgical site events; denominator: patients who received at least one dose of study drug). Data on all-cause mortality during trial, bleeding-related mortality on treatment (denominator: patients who received at least one dose of study drug) and VTE-related mortality on treatment (denominator: patients who received at least one dose of study drug and underwent surgery) were also retrieved.
Analysis and data presentation
Dabigatran was used at two dose levels: 220 mg or 150 mg once daily (11-13). Since the efficacy and safety outcomes were practically identical for the two doses, all analyses of dabigatran trials were based on pooled dabigatran data.
Incidence of symptomatic VTE on treatment and incidence of major or non-major clinically relevant bleeding on treatment met the criteria for indirect comparisons. Random-effects meta-analysis was conducted on each outcome separately for the rivaroxaban (7-10) and dabigatran (11-13) trials to obtain pooled treatment effects of each drug vs enoxaparin. Risk difference was used as an effect measure since it was used in the original individual trials (7-13). Cochran Q and I2 statistic were used as indicators of heterogeneity (inconsistency) of trial results. Pooled estimates were then used to calculate rivaroxaban vs dabigatran risk differences, as explained. In the indirect comparison, dabigatran was perceived as a “reference” due to the fact that dabigatran trials (11-13) chronologically preceded the rivaroxaban trials (7-10). All risk differences were low, with 2-4 decimal places. To improve their interpretability, benefit or harm per 1000 treated patients were employed as more intuitive measures. For example, for incidence of VTE, for which risk reduction is beneficial and represents a therapeutic effect, risk difference (RD) between a test (T) and a reference (R) treatment (T-R) with a minus sign “favors” T treatment, say RD (T-R) = -0.005 (95% confidence interval [CI], -0.009 to -0.001); T provides benefit over R, since fewer patients experience VTE. Multiplication by 1000 indicates that “fewer patients” means 5 per 1000, and removal of the minus sign (requiring that the upper and the lower CI limits “switch places”) results in benefit per 1000 patients = 5 (95% CI, 1 to 9). This is interpreted as: for 1000 patients treated with T instead of R, between 1 and 9, and most likely 5 additional would benefit by avoiding VTE. If CI embraces 0 eg, RD = -0.005 (95% CI, -0.013 to 0.003), 95% CI around benefit per 1000 patients extends from benefit to harm (13 benefit to 3 harm): for 1000 patients treated with T instead of R, most likely 5 additional (and up to 13) would benefit by avoiding VTE, however, it could also be that up to 3 more would be harmed by experiencing it. For incidence of bleeding for which higher risk is harmful and represents an adverse effect, RD (T-R) is converted into harm per 1000 patients. Benefit or harm per 1000 patients is rounded to the nearest integer.
Mortality was very low in all trials and most treatment arms had 0 events rendering them impractical for meta-analysis. Instead, event rates were summarized by treatment and by setting (THA, TKA) which were treated as strata. Using enoxaparin-treated patients as “standard population,” two “test populations” (rivaroxaban, dabigatran) were evaluated by calculating standardized mortality ratios (SMR). SMR is an adjusted relative risk that relates test (rivaroxaban, dabigatran) population to the standard population. When two SMRs (relative risks) are derived from similarly structured populations and use the same standard, they can be compared as a ratio of two SMRs to provide information about the relationship between the two test populations. However, two test populations are more explicitly related to each other through a ratio of their directly standardized event rates, ie, standardized rate ratio (SRR). Just as SMR, SRR is an adjusted relative risk. SMR and SRR are based on number of events per observed person-time. VTE-related and bleeding-related mortalities were reported for the period “during treatment” (7-13). Regardless of the treatment (rivaroxaban, dabigatran, enoxaparin), treatment period in thromboprophylaxis after THA/TKA is defined by the setting: 4-5 weeks after THA and 8-15 days after TKA (1-3). Consequently, duration of treatment for the setting was considered a “unit of person-time” and the number of person-time units equaled the number of patients. All-cause mortality was reported for the “entire study period” ie, treatment period + a follow-up period. SMR and SRR were first calculated using the “entire study period” as a unit of person-time. However, rivaroxaban trials (7-10) had shorter follow-up periods (30-35 days after the last dose, ie, till day 45-60 after surgery) than dabigatran trials (11-13) (2 months after the last dose, ie, till day 75-90 after surgery). Mortality (all-cause) between days 60 and 90 after total joint replacement was repeatedly shown to correspond to 1/4 of the cumulative mortality during the first 60 days after the surgery (22,23). Hence, to correct for the imbalance in follow-up periods, number of deaths observed in the rivaroxaban arms and enoxaparin arms from rivaroxaban trials was increased by 25% to yield “corrected all-cause mortality” and SMR and SRR were calculated again. We used StatsDirect statistical software (StatsDirect Ltd, Altrincham, UK).
Results
Characteristics of the pivotal rivaroxaban and dabigatran trials and feasibility of indirect comparisons based on major reported outcomes
There were 4 pivotal rivaroxaban trials (2 in THA, 2 in TKA) and 3 pivotal dabigatran trials (1 THA, 2 TKA). Generally, rivaroxaban trials followed a different statistical concept than dabigatran trials (tested both non-inferiority and superiority hypotheses, whereas dabigatran trials were non-inferiority trials) and had a shorter follow-up (Table 1). By setting, the two groups of trials were largely similar in design. One rivaroxaban trial in THA (RECORD 1) (7) and the single dabigatran trial in this setting (RE-NOVATE) (11) employed the same standard mode of enoxaparin use, whereas the second rivaroxaban trial (RECORD 2) (8) used an atypically short enoxaparin treatment (Table 1). In TKA, one trial with each drug followed the European practice in enoxaparin use (1 × 40 mg/d) (RECORD 3 [9], RE-MODEL [12]) with a slightly shorter duration of treatment in the dabigatran trial (Table 1), whereas the other two followed the North American practice (2 × 30mg/d) (RECORD 4 ([10], RE-MOBILIZE [13]) (Table 1). All rivaroxaban trials concluded comparable safety and superior efficacy vs enoxaparin (Table 1). All dabigatran trials concluded comparable safety vs enoxaparin. Two trials concluded non-inferior efficacy and one concluded inferior efficacy of dabigatran vs enoxaparin (Table 1).
Table 1.
Pivotal efficacy/safety trials (multinational, randomized, double-blind) of rivaroxaban (R) and dabigatran etexilate (D) for prevention of venous thromboembolism after total hip (THA) or knee (TKA) arthroplasty in comparison with enoxaparin sodium (E)*
| Trial/hypothesis | Setting | Test po + placebo injection† | Enoxaparin sc + oral placebo | Efficacy population | Safety population | Main findings |
|---|---|---|---|---|---|---|
| Rivaroxaban | ||||||
| RECORD 1 (7) Non-inferiority Superiority‡ | THA | R 1 × 10 mg/d, 31-39 (33) days Start: 6-8 h after surgery | 1 × 40 mg/d, 31-39 (34) days Start: 12 h before surgery, then 6-8 h after | PP: R = 1537, E = 1492 MITT: R = 1595, E = 1558 | R = 2209, E = 2224 Follow-up 30-35 d after last dose | Efficacy: R>E Safety: R = E |
| RECORD 2 (8) | THA | R 1 × 10 mg/d, 31-39 (34) days Start: as above | 1 × 40 mg/d, 10-14 (12) days Start: as above | PP: R = 812, E = 803 MITT: R = 864, E = 869 | R = 1228, E = 1229 Follow-up as above | Efficacy: R>E Safety: R = E |
| RECORD 3 (9) | TKA | R 1 × 10 mg/d, 10-14 (12) days Start: as above | 1 × 40 mg/d, 10-14 (13) days Start: as above | PP: R = 793, E = 838 MITT: R = 824, E = 878 | R = 1220, E = 1239 Follow-up as above | Efficacy: R>E Safety: R = E |
| RECORD 4 (10) | TKA | R 1 × 10 mg/d, 11-15 (11) days Start: as above | 2 × 30 mg/d 11-15 (11) days Start: 12-24 h after surgery | PP: R = 864, E = 878 MITT: R = 965, E = 959 | R = 1526, E = 1508 Follow-up as above | Efficacy: R>E Safety: R = E |
| Dabigatran | ||||||
| RE-NOVATE (11) Non-inferiority§ | THA | D1 1 × 220, D2 1 × 150 mg/d, 28-35 (32, 33) days; Start: 1-4 h after surgery with 1/2 dose | 1 × 40 mg/d, 28-35 (33) days Start: 12 h before surgery, in some centers after surgery | MITT: D1 = 880, D2 = 874, E = 897 | D1 = 1146, D2 = 1163, E = 1154; Follow-up 2 mo after last dose | Efficacy: D1, D2 = E Safety: D1, D2 = E |
| RE-MODEL (12) | TKA | D1 1 × 220, D2 1 × 150 mg/d, 6-10 (8,8) days; Start: as above | 1 × 40 mg/d, 6-10 (7) days Start: as above | MITT: D1 = 503, D2 = 526, E = 512 | D1 = 679, D2 = 703, E = 694; As above | Efficacy: D1, D2 = E Safety: D1, D2 = E |
| RE-MOBILIZE (13) | TKA | D1 1 × 220, D2 1 × 150 mg/d, 12-15 (14,14) days; Start: 6-12 h after surgery with 1/2 dose | 2 × 30 mg/d, 12-15 (14) days Start: 12-24 h after surgery | MITT: D1 = 604, D2 = 649, E = 643 | D1 = 857, D2 = 871, E = 868; As above | Efficacy: D1, D2<E Safety: D1, D2 = E |
*Abbreviations: po – orally; sc – subcutaneously; PP – per protocol; MITT – modified intent-to-treat.
†Scheduled and actual mean or median (bracketed) treatment duration is presented.
‡Rivaroxaban trials tested two efficacy hypotheses: non-inferiority (PP data) and superiority (MITT data).
§Dabigatran trials tested efficacy non-inferiority (MITT for primary analysis, PP for secondary).
Definitions of primary and main secondary efficacy outcomes were identical in rivaroxaban and dabigatran trials and implicated dependence on adjudication of venography data (Table 2). Most of the events forming these two outcomes were actually asymptomatic (ie, detected solely on venograms) (7-13). Around 23% to 35% of patients across trials (and comparably by treatment arm per trial) who received treatment and underwent surgery were not evaluated for these outcomes since they lacked appropriate venograms (7-13). Data on these two outcomes for the enoxaparin treatment arms (Table 2) clearly indicated the effect of factor “adjudication:” their incidence (and more so for the primary efficacy outcome) was much lower in rivaroxaban than in dabigatran trials with similar designs (RECORD 1 vs RE-NOVATE; RECORD 3 vs RE-MODEL; RECORD 4 vs RE-MOBILIZE) (Table 2). Table 2 also indicates differences between European and North American-style enoxaparin use (RECORD 3 vs RECORD 4, RE-MODEL vs RE-MOBILIZE), and between “standard” and “short-term” enoxaparin use in THA (RECORD 1 vs RECORD 2). The major difference between the rivaroxaban and dabigatran trials, however, was the fact that the main bleeding outcome (“major bleeding”) in the rivaroxaban program did not include “surgical site” bleedings (Table 2). This is of relevance considering that surgical site bleedings were by far the most prevalent bleedings in each trial (7-13). This fact (likely with a contribution of adjudication) resulted in much lower bleeding rates for enoxaparin in rivaroxaban trials than in identically designed dabigatran trials (Table 2). These observations indicated infeasibility of indirect rivaroxaban vs dabigatran comparisons based on reported major efficacy/safety outcomes.
Table 2.
Major efficacy and bleeding (safety) outcomes in pivotal trials of rivaroxaban and dabigatran for prevention of venous thromboemoblism (VTE) after total hip (THA) or knee (TKA) arthroplasty: definitions and results for the enoxaparin (ENOX) treatment arms. Rivaroxaban and dabigatran trials with similar design are aligned side by side*
| Outcomes/definitions | ENOX in Rivaroxaban trials† | ENOX in Dabigatran trials† | Ratio | ||
|---|---|---|---|---|---|
| Primary efficacy (composite)‡ | |||||
| Any DVT (symptomatic or not, proximal/distal), non-fatal PE, all-cause mortality |
RECORD 1 | 3.72% | RE-NOVATE | 6.69% | 0.56 |
| RECORD 2 | 9.32% | — | |||
| RECORD 3 | 18.9% | RE-MODEL | 37.7% | 0.50 | |
| RECORD 4 | 10.1% | RE-MOBILIZE | 25.3% | 0.40 | |
| Main secondary efficacy (composite)‡ | |||||
| Proximal DVT (symptomatic or not), non-fatal PE, VTE-related mortality on treatment |
RECORD 1 | 1.97% | RE-NOVATE | 3.93% | 0.50 |
| RECORD 2 | 5.09% | — | |||
| RECORD 3 | 2.59% | RE-MODEL | 3.52% | 0.74 | |
| RECORD 4 | 1.98% | RE-MOBILIZE | 2.33% | 0.85 | |
| Major bleeding on treatment§ | |||||
|
RECORD trials. Fatal; into a critical organ; leads to reoperation; overt extra-surgical site (Hb fall ≥2 g/dL or requires ≥2 units blood). Dabigatran trials as above + leads to treatment discontinuation; overt surgical site bleeding║ |
RECORD 1 | 0.09% | RE-NOVATE | 1.56% | 0.06 |
| RECORD 2 | 0.08% | — | |||
| RECORD 3 | 0.48% | RE-MODEL | 1.30% | 0.37 | |
| RECORD 4 | 0.27% | RE-MOBILIZE | 1.38% | 0.19 | |
| Non-major, clinically relevant on treatment§ | |||||
| RECORD trials. Multiple source bleeding, spontaneous hematoma ≥25 cm3, excessive wound hematoma.¶Dabigatran trials. Spontaneous hematoma ≥25 cm3, wound hematoma ≥100 cm3, epistaxis >5 min, spontaneous hematuria or a prolonged one after intervention, spontaneous rectal bleeding, gingival bleeding >5 min or any other bleeding judged clinically relevant | RECORD 1 | 2.43% | RE-NOVATE | 3.47% | 0.70 |
| RECORD 2 | 2.69% | — | |||
| RECORD 3 | 2.26% | RE-MODEL | 5.33% | 0.42 | |
| RECORD 4 | 1.99% | RE-MOBILIZE | 2.42% | 0.82 | |
*Abbreviations: DVT – deep venous thrombosis; PE – pulmonary embolism; Hb – hemoglobin; min – minutes.
†See Table 1 for trial details.
‡Modified-intent-to-treat population as a denominator.
§Safety population (received at least one dose of study drug) as a denominator.
║Follows EMEA classification (21) but EMEA includes surgical site bleedings requiring drainage /puncture as “major”. There is a major difference in definition vs rivaroxaban trials indicated in italics.
¶Defined explicitly only in the RECORD 2 trial (8), but presumably used across the RECORD program and most likely embracing events largely similar to the definition used in dabigatran trials.
Incidence of symptomatic VTE on treatment
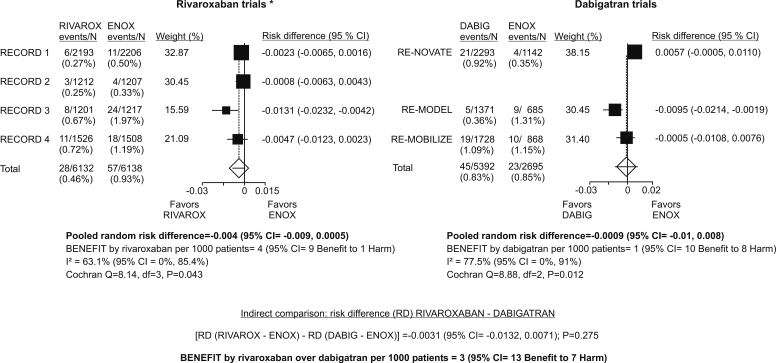
Incidence of symptomatic VTE on treatment (DVT, PE, fatal or not) was comparable across enoxaparin arms in comparably designed rivaroxaban and dabigatran trials (Figure 1). Meta-analysis of rivaroxaban trials indicated that the risk of such events was slightly lower with rivaroxaban than with enoxaparin, but the difference did not attain statistical significance (Figure 1). The estimate is burdened with considerable inconsistency (high I2, significant Cochrane Q statistic), likely reflecting different settings (difference was more in favor of rivaroxaban in two trials in TKA [RECORD 3, RECORD 4] than in the trials in THA) (Figure 1). Meta-analysis of dabigatran trials indicated practically no difference between dabigatran and enoxparin (Figure 1). The estimate is also burdened with inconsistency likely due to a slight trend in favor of enoxaparin in the THA trial (RE-NOVATE) and a significant difference in favor of dabigatran in one of the TKA trials (RE-MODEL) (Figure 1).
Figure 1.
Incidence of symptomatic venous thromboembolism on treatment (deep venous thrombosis, pulmonary embolism, regardless of the outcome) for patients who underwent surgery and received at least one dose of study drug. “On treatment:” between 1st dose and one day (rivaroxaban trials) or 3 days (dabigatran trials) after the last dose. Rivaroxaban and dabigatran trials with similar design are side by side. *In RECORD 2 (4), treatment duration for rivaroxaban was 31-39 days (mean, 34) and for enoxaparin it was 10-14 days (mean, 12.4). Events counted for the enoxaparin treatment arm were those recorded by day 14 (mean treatment duration +1 day).
Indirect comparison of rivaroxaban vs dabigatran indicates no relevant difference between them: per 1000 patients treated with rivaroxaban instead of dabigatran, 3 additional would benefit by not experiencing symptomatic VTE, but the 95% CI extends from 13 who would benefit to 7 additional who would be harmed by experiencing it (Figure 1).
Incidence of major or non-major clinically relevant bleeding on treatment
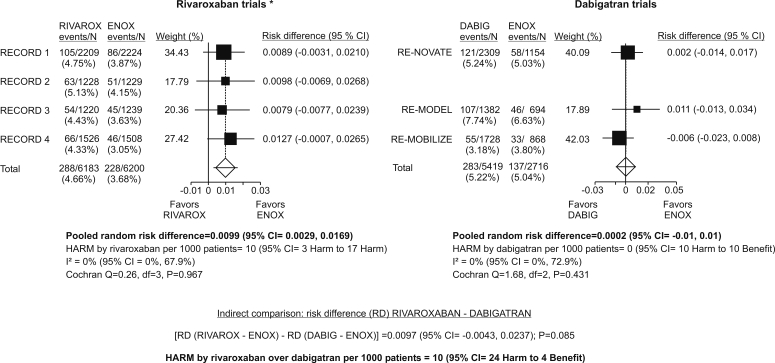
Incidence of major or non-major clinically relevant bleeding (fatal or not) was fairly comparable across enoxaparin arms in comparably designed rivaroxaban and dabigatran trials (Figure 2), ie, differences were slight when compared with differences based on data in published reports (Table 2) and likely represented differences in adjudication and/or slight differences in duration of the “on-treatment” periods. Meta-analysis of rivaroxaban trials consistently indicated a significantly higher risk of such bleedings with rivaroxaban than with enoxaparin: per 1000 patients treated with rivaroxaban instead of enoxaparin, 10 more would have been harmed by experiencing them (95% CI, 3 to 17) (Figure 2). Meta-analysis of dabigatran trials consistently indicated practically no difference between dabigatran and enoxaparin in this respect (Figure 2). Indirect comparison between rivaroxaban and dabigatran indicates that per 1000 patients treated with rivaroxaban instead of dabigatran, 10 more would be harmed by experiencing such bleedings, and 95% CI extends from 24 who would be harmed to 4 who would benefit by not experiencing them. The difference is of borderline significance (P = 0.085) (Figure 2).
Figure 2.
Incidence of major or non-major, clinically relevant bleeding on treatment (regardless of the outcome) for patients who received at least one dose of study drug. “On treatment:” between 1st dose and one day (rivaroxaban trials) or 3 days (dabigatran trials) after the last dose. Rivaroxaban and dabigatran trials with similar design are side by side. *Data include also surgical site bleeding (obtained from unpublished data [19,20], not included in published reports [7-10]). In RECORD 2 (8), events were counted up to 2 days after the last dose.
Mortality
All-cause mortality during entire study periods was low for all treatments. All differences observed between rivaroxaban or dabigatran and enoxaparin (based on SMRs) or between rivaroxaban and dabigatran (based on SRR; particularly with correction for the difference in duration of follow-ups) could have been by chance (Table 3).
Table 3.
Mortality outcomes in pivotal rivaroxaban and dabigatran trials by treatment and setting (total hip [THA] or knee [TKA] arthroplasty). See Table 1 for trial details. Number of deaths (n) is given per number of patients (N) which equals the number of person-time units*
| Mortality outcomes | Rivaroxaban | Dabigatran | Enoxaparin |
|---|---|---|---|
| All-cause mortality (during study)† | |||
| THA cumulative n/N (person-time) | 7/3437 (0.20%) | 8/2309(0.35%) | 14/4607 (0.30%) |
| TKA cumulative n/N (person-time) | 6/2746 (0.22%) | 8/3110 (0.26%) | 18/4309 (0.42%) |
| Total | 13/6183 (0.21%) | 16/5419 (0.29%) | 32/8916 (0.36%) |
| SMR (95% CI) | 0.60 (0.32-1.02) | 0.80 (0.46-1.30) | |
| SMR rivarox/SMR dabig (95% CI) | 0.75 (0.33-1.65) | ||
| SRR rivarox/dabig (95% CI) | 0.70 (0.33-1.45) | ||
| All-cause mortality, follow-up corrected‡ | |||
| THA | 8.75/3437 | 8/2309 | 17.25/4607 |
| TKA | 7.5 /2746 | 8/3110 | 21/4309 |
| SMR (95% CI) | 0.62 (0.36-1.06) | 0.67 (0.39-1.09) | |
| SMR rivarox/SMR dabig (95% CI) | 0.92 (0.44-1.96) | ||
| SRR rivarox/dabig (95% CI) | 0.87 (0.43-1.74) | ||
| PE/DVT-related death on treatment§ | |||
| THA cumulative n/N (person-time) | 2/3405 (0.06%) | 3/2293 (0.13%) | 3/4555 (0.07%) |
| TKA cumulative n/N (person-time) | 1/2727 (0.04%) | 2/3099 (0.06%) | 1/4278 (0.02%) |
| Total | 3/6132 (0.05%) | 5/5392 (0.09%) | 4/8833 (0.045%) |
| SMR (95% CI) | 1.09 (0.22-3.18) | 2.06 (0.67-4.81) | |
| SMR rivarox/SMR dabig (95% CI) | 0.53 (0.08-2.72) | ||
| SRR rivarox/dabig (95% CI) | 0.49 (0.12-2.06) | ||
| Bleeding-related death on treatment§ | |||
| THA cumulative n/N (person time) | 0/3437 | 2/2309 (0.09%) | 0/4607 |
| TKA cumulative n/N (person time) | 1/2746 (0.04%) | 0/3110 | 0/4309 |
| Total | 1/6183 (0.016%) | 2/5419(0.037%) | 0/8916 |
| SRR rivarox/dabig (95% CI) | 0.39 (0.04-4.34) | ||
*Abbreviations: PE – pulmonary embolism; DVT – deep venous thrombosis; SMR – standardized mortality ratio; CI – confidence interval; SRR – standardized rate ratio.
†Between 1st dose of study drug and the end of follow-up. Follow-up was 2 months in dabigatran trials vs 30-35 d in rivaroxaban trials.
‡Correction for the difference in duration of follow-up, see Materials and Methods.
§Between 1st and last dose +1 d (rivaroxaban trials) or +3 d (dabigatran trials).
VTE-related mortality on treatment was very low for all treatments. Crude VTE-related mortality rate in dabigatran-treated patients (5/5392 or 0.09%) was twice higher than the rates in enoxaparin-treated (0.045%) or rivaroxaban-treated (0.05%) patients (Table 3). However, with an average rate of 4 such deaths per 8833 treatment periods (enoxaparin) or with an average rate of 3 deaths per 6132 treatment periods (rivaroxaban), probabilities of observing 5 or more deaths per 5392 treatment periods are 0.101 and 0.127, respectively, and probabilities of observing 5 or fewer deaths are 0.96 and 0.95, respectively. Accordingly, SMR and SRR did not indicate any difference between dabigatran and enoxaparin or between rivaroxaban and dabigatran that could not have been by chance (Table 3).
Only 3 deaths among 20 618 patients who constituted the overall safety population (received at least one dose of study drug) in the 7 trials were adjudicated as related to bleeding, none among enoxaparin-treated patients (Table 3). Crude rate was twice higher for dabigatran-treated patients (2/5419 or 0.037%) than for rivaroxaban-treated patients (0.016%). Also, SRR (rivaroxaban/dabigatran) was <1 but did not indicate a difference that could not have been by chance (Table 3). Indeed, with an average rate of 1 such death per 6183 treatment periods (rivaroxaban), probability of observing 2 or more deaths per 5419 treatment periods is 0.16 and probability of observing 2 or fewer deaths is 0.94.
Discussion
The issue of thromboprophylactic strategy after THA/TKA is rather controversial for at least two reasons (3,24). First, the procedure should achieve an optimum balance between a benefit of avoiding DVT primarily because of the fear of PE and a harm of potential bleeding. In this respect, it should be noted that the association between DVT and PE in THA/TKA patients appears weaker than in some other populations at risk of DVT (eg, patients with thrombophilia, carcinoma, chronic heart failure). Hence, strategies used in these patients might not be automatically applicable to THA/TKA patients (3). Second, a meaningful weighing of benefit vs harm should include information about pre-existing risk factors for either VTE or for bleeding. Pivotal efficacy/safety trials of anticoagulants in THA/TKA settings (conducted primarily for regulatory purposes) usually do not provide such information: patients with pre-existing bleeding tendency or risks factors for DVT are typically not enrolled, and this creates a gap between “clinical trial conditions” and “real life” (3,24). New oral anticoagulants rivaroxaban and dabigatran have valuable properties of simple oral dosing, predictive effects, and low potential for drug interactions. Overwhelmed with daily routine, practicing physicians might be prone to accept such convenient tools despite the actual uncertainties about their real absolute and relative benefits or harms: these treatments yet need to be evaluated in respect to other available means, as well as in respect to each other (24).
In the absence of direct comparative trials, we attempted a formal indirect comparison between rivaroxaban and dabigatran based on their individual comparisons to enoxaparin (7-13). However, a comparison that would be based on major efficacy/safety outcomes reported in pivotal trials was found infeasible due to differences in outcome definitions (bleeding events) and the apparent differences in adjudication procedures (venography data). Therefore, other outcomes were identified that appeared appropriate for indirect comparisons in technical sense (incidence of symptomatic VTE, incidence of major or non-major clinically relevant bleeding, mortality), However, they might not have been fully illustrative of the treatments’ efficacy/safety profiles (at least not in regulatory terms) (21). Hence, the present analysis is only an exploratory effort. Within these limitations, several observations emerged regarding the relationship between rivaroxaban or dabigatran and enoxaparin and between rivaroxaban and dabigatran in prevention of VTE after THA/TKA.
Rivaroxaban vs enoxaparin
In each of the four individual trials (7-10) with overall 12 400 patients, rivaroxaban was found superior to enoxaparin in efficacy and comparable in safety. The present analysis suggests that these conclusions might somewhat overestimate the benefit and underestimate potential harms of rivaroxaban vs enoxaparin. Rivaroxaban was associated with a lower risk of symptomatic VTE (DVT with or without PE, fatal or not) than enoxaparin. Although the difference did not attain statistical significance in the present analysis (benefit per 1000 patients = 4 [95% CI, 9 benefit to 1 harm]), data do suggest that there is a true (population) difference in favor of rivaroxaban. The middle 50% of CI around benefit per 1000 patients (which are most likely to contain the true difference) extend from 2 benefit to 7 benefit, and difference between the treatments most likely would have been significant had there been more trials. However, the size of the difference is small and it does not seem likely that additional trials would meaningfully change the point estimate. At the same time, treatment with rivaroxaban clearly increases the risk of major or non-major clinically relevant bleeding and present results are in agreement with the analysis conducted by FDA (regression-based individual patient data meta-analysis) (20). According to present analysis, there is a numerically negative trade-off between a benefit (avoiding symptomatic VTE) and harm (major or clinically relevant bleeding) for rivaroxaban relative to enoxaparin: per 1000 treated patients, it conveys the benefit of 4 (up to 9) additional patients who avoid symptomatic VTE and the harm of 10 (95% CI, 3 to 17) additional patients who experience bleeding, ie, for each patient who would benefit from switching to rivaroxarban, 2-3 would be harmed. Mortality data (all-cause, VTE-related, bleeding-related) for rivaroxaban and enoxaparin are too scarce to make any meaningful conclusion, primarily due to low event rates. However, at this point it seems plausible to conclude that one should not expect any practically relevant benefit or harm from rivaroxaban in this respect.
Dabigatran vs enoxaparin
In two out of three pivotal trials (11,12) with overall 8150 patients, dabigatran showed non-inferior efficacy and comparable safety to enoxaparin, whereas in one (13) it was comparable in safety but failed, albeit by a small amount, in a formal efficacy non-inferiority test (for the primary efficacy outcome) against the North American-style enoxaparin regimen. In the present analysis, dabigatran was fully comparable to enoxaparin regarding the incidence of symptomatic VTE and incidence of major or non-major clinically relevant bleeding, providing no additional benefit (avoiding VTE) and no additional harm (bleeding). Mortality data are too scarce for a meaningful conclusion. At this point, they do not seem to indicate that one should expect any particular benefit or harm from dabigatran relative to enoxaparin in this respect.
Rivaroxaban vs dabigatran
Indirect comparisons have low power. One randomized trial is as precise as an indirect comparison based on four randomized trials of the same size, or in other words, four times as many similarly sized trials are needed for the indirect approach to have the same power as directly randomized comparisons (17). Having this in mind, the present data suggest that the trade-off between benefit (avoiding symptomatic VTE) and harm (major or non-major clinically relevant bleeding) numerically disfavors rivaroxaban as compared with dabigatran. Regarding the incidence of symptomatic VTE, risk difference between the two drugs indicates a small and insignificant (P = 0.275) difference (benefit) in favor of rivaroxaban (benefit per 1000 patients 3 [95% CI, 13 benefit to 7 harm]). The middle 50% of the CI also extend from benefit to harm (8 benefit to 2 harm) and it does not seem likely that the entire 95% CI around the point-estimate would have been contained within the “benefit area” even with additional trials. At the same time, risk difference regarding major or non-major clinically relevant bleeding indicates a difference (harm) that disfavors rivaroxaban (harm per 1000 patients 10 ([95% CI, 24 harm to 4 benefit]) and which, although of borderline significance (P = 0.085), does indicate a true (population) difference between treatments. Namely, the middle 50% of the CI extend from 3 harm to 17 harm, and the difference most likely would have been significant had there been more trials. Hence, switching from dabigatran to rivaroxaban does not seem likely to confer the benefit of additional patients avoiding symptomatic VTE, but it seems likely to confer the harm of additional patients experiencing bleeding. The “best-case” scenarios favoring one or the other drug (based on “beneficial” limits of the 95% CI of the present estimates) also illustrate the benefit-harm trade-off in favor of dabigatran. In the best-case scenario favoring rivaroxaban, per 1000 patients treated with rivaroxaban instead of dabigatran, 13 more would benefit by not experiencing symptomatic VTE and 4 additional would benefit by not experiencing bleeding. Conversely, in the “best-case” scenario favoring dabigatran, per 1000 patients treated with dabigatran instead of rivaroxaban, 7 more would benefit by not experiencing symptomatic VTE and 24 more would benefit by not experiencing bleeding.
Due to the low observed event rates, mortality data are too scarce to make finite conclusions. The few more VTE-related and bleeding-related deaths among dabigatran-treated than among rivaroxaban-treated patients could have been by chance and do not disqualify dabigatran in comparison with rivaroxaban, particularly as they were subsumed within symptomatic VTE and major or non-major clinically relevant bleeding, respectively.
Conclusion
Rivaroxaban and dabigatran are newly available drugs competing for a market share in the setting of thromboprohylaxis after THA/TKA. Practicing physicians are likely to be exposed to a considerable “marketing pressure.” Although accepted as effective and safe for the purpose, it should be noted that both drugs yet need to be fully evaluated in respect to other available means. As for the relationship between the two, the present analysis suggests that the existing clinical data do not provide grounds for any claims about superiority of one over the other drug. This, of course, does not mean that they should be perceived as “therapeutic equivalents” – evidence of that is also missing. Hence, direct comparative trials of rivaroxaban vs dabigatran in this setting are needed.
References
- 1.Samama CM, Ravaud P, Parent F, Barré J, Mertl P, Mismetti P. Epidemiology of venous thromboembolism after lower limb arthroplasty: the FOTO study. J Thromb Haemost. 2007;5:2360–7. doi: 10.1111/j.1538-7836.2007.02779.x. [DOI] [PubMed] [Google Scholar]
- 2.Rahme E, Dasgupta K, Burman M, Yin H, Bernatsky S, Berry G, et al. Postdischarge thromboprophylaxis and mortality risk after hip-or knee-replacement surgery. CMAJ. 2008;178:1545–54. doi: 10.1503/cmaj.071388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callaghan JJ, Dorr LD, Engh GA, Hanssen AD, Healy WL, Lachiewicz PF, et al. Prophylaxis for thromboembolic disease: recommendations from the American College of Chest Physicians–are they appropriate for orthopaedic surgery? J Arthroplasty. 2005;20:273–4. doi: 10.1016/j.arth.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Hull RD, Yusen RD, Bergqvist D. State-of-the-art review: Assessing the safety profiles of new anticoagulants for major orthopedic surgery thromboprophylaxis. Clin Appl Thromb Hemost. 2009;15:377–88. doi: 10.1177/1076029609338712. [DOI] [PubMed] [Google Scholar]
- 5.European Medicines Evaluation Agency. Committee for Human Medicines (CHMP). CHMP assessment report for Xarelto (INN: rivaroxaban). EMEA/543519/2008. Available from: http://www.ema.europa.eu/humandocs/PDFs/EPAR/xarelto/H-944-en6.pdf. Accessed: April 6, 2010.
- 6.European Medicines Evaluation Agency. Committee for Human Medicines (CHMP). CHMP assessment report for Pradaxa (INN: dabigatran etexilate). EMEA/174363/2008. Available from: http://www.ema.europa.eu/humandocs/Humans/EPAR/pradaxa/pradaxa.htm Accessed: April 6, 2010.
- 7.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–75. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 8.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31–9. doi: 10.1016/S0140-6736(08)60880-6. [DOI] [PubMed] [Google Scholar]
- 9.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–86. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 10.Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–80. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–56. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–85. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 13.RE-MOBILIZE Writing Committee. Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9. doi: 10.1016/j.arth.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 14.McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the irish healthcare setting. Pharmacoeconomics. 2009;27:829–46. doi: 10.2165/11313800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Quinlan DJ, Eikelboom JW, Dahl OE, Eriksson BI, Sidhu PS, Hirsh J. Association between asymptomatic deep vein thrombosis detected by venography and symptomatic venous thromboembolism in patients undergoing elective hip or knee surgery. J Thromb Haemost. 2007;5:1438–43. doi: 10.1111/j.1538-7836.2007.02571.x. [DOI] [PubMed] [Google Scholar]
- 16.Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009;338:b1147. doi: 10.1136/bmj.b1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9:1–134. doi: 10.3310/hta9260. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson & Johnson Pharmaceutical Research & Development. Advisory Committee briefing book. Rivaroxaban for the prophylaxis of deep vein thrombosis (DVT) and pulmonary embolism (PE) in patients undergoing hip or knee replacement surgery. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM138385.pdf Accessed: April 6, 2010.
- 20.Xu Q. Xarelto (Rivaroxaban). Cardiovascular and renal drugs Advisory Committee meting March 19 2009. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM143660.pdf. Accessed: April 6, 2010.
- 21.European Medicines Evaluation Agency. Committee for Human Medicines (CHMP). Guideline on clinical investigation of medicinal products for prophylaxis of high intra- and post-operative venous thromboemoblic risk. CPMP/EWP/707/98 Rev. corr 2007. Available from: http://www.ema.europa.eu/pdfs/human/ewp/70798en_fin.pdf Accessed: April 6, 2010.
- 22.Lie SA, Engesaeter LB, Havelin LI, Gjessing HK, Vollset SE. Mortality after total hip replacement: 0-10-year follow-up of 39,543 patients in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2000;71:19–27. doi: 10.1080/00016470052943838. [DOI] [PubMed] [Google Scholar]
- 23.Lie SA, Engesaeter LB, Havelin LI, Furnes O, Vollset SE. Early postoperative mortality after 67,548 total hip replacements: causes of death and thromboprophylaxis in 68 hospitals in Norway from 1987 to 1999. Acta Orthop Scand. 2002;73:392–9. doi: 10.1080/00016470216312. [DOI] [PubMed] [Google Scholar]
- 24.Merli G, Spyropoulos AC, Caprini JA. Use of emerging oral anticoagulants in clinical practice: translating results from clinical trials to orthopedic and general surgical patient populations. Ann Surg. 2009;250:219–28. doi: 10.1097/SLA.0b013e3181ae6dbe. [DOI] [PubMed] [Google Scholar]