Abstract
Accurate diagnosis of spinal cord injury (SCI) severity must be achieved before highly aggressive experimental therapies can be tested responsibly in the early phases after trauma. These studies demonstrate for the first time that axial diffusivity (λ||), derived from diffusion tensor imaging (DTI) within 3 h after SCI, accurately predicts long-term locomotor behavioral recovery in mice. Female C57BL/6 mice underwent sham laminectomy or graded contusive spinal cord injuries at the T9 vertebral level (5 groups, n = 8 for each group). In-vivo DTI examinations were performed immediately after SCI. Longitudinal measurements of hindlimb locomotor recovery were obtained using the Basso mouse scale (BMS). Injured and spared regions of ventrolateral white matter (VLWM) were reliably separated in the hyperacute phase by threshold segmentation. Measurements of λ|| were compared with histology in the hyperacute phase and 14 days after injury. The spared normal VLWM determined by hyperacute λ|| and 14-day histology correlated well (r = 0.95). A strong correlation between hindlimb locomotor function recovery and λ||-determined spared normal VLWM was also observed. The odds of significant locomotor recovery increased by 18% with each 1% increase in normal VLWM measured in the hyperacute phase (odds ratio = 1.18, p = 0.037). The capability of measuring subclinical changes in spinal cord physiology and murine genetic advantages offer an early window into the basic mechanisms of SCI that was not previously possible. Although significant obstacles must still be overcome to derive similar data in human patients, the path to clinical translation is foreseeable and achievable.
Key words: axial diffusion, diffusion tensor imaging, hyperacute, magnetic resonance imaging, recovery, spinal cord injury
Introduction
The first 6 hours following traumatic spinal cord injury (SCI), the hyperacute phase, represents a window of time when medical and surgical interventions are most likely to be effective (Bracken et al., 1997; Fehlings and Perrin, 2006). After both human and experimental SCI, the initial trauma leads to an instantaneous, often complete, neurological deficit below the injury level. The initial flaccid paralysis and subsequent return of reflexes observed in the hours and days after SCI are collectively referred to clinically as “spinal shock” (Ditunno et al., 2004). Axons that remain intact immediately after the trauma are unable to conduct action potentials until the delicate microenvironment and axonal membrane potentials are restored. During spinal shock, neurological exams and clinical electrophysiological measurements do not accurately predict degrees of long-term functional impairment (Little et al., 1999).
The first 6 h immediately after SCI also present some unique challenges for the basic neuroscientist. Random cross-sections used to quantify spared normal white matter do not necessarily represent numbers of intact axons during the hyperacute phase because the removal of axonal debris by macrophages does not ensue until much later (Blight, 1992). We have previously demonstrated that myelin basic protein (MBP) immunostains remain essentially unchanged in the hyperacute phase (Loy et al., 2007), although damage to oligodendrocytes has been well documented with electron microscopy studies (Keirstead and Blakemore, 1997).
Routine clinical MRI is useful for predicting complete versus incomplete SCI outcomes after surgery and demonstrates the extent of tissue edema (Miyanji et al., 2007). However, it provides no direct measurement of axonal integrity (Miyanji et al., 2007; Shepard and Bracken, 1999). Diffusion tensor imaging (DTI) provides detailed micro-structural information about myelinated axons and the associated pathophysiological changes with quantifiable parameters (Pierpaoli and Basser, 1996). Our lab has previously demonstrated the potential for DTI-derived axial diffusivity (λ||), describing water diffusion parallel to the principal axis of the white matter tract, and radial diffusivity (λ⊥ ), describing water diffusion perpendicular to the principal axis of the white matter tract, to differentially detect myelin and axon abnormalities after SCI (Kim et al., 2007), experimental autoimmune encephalitis (EAE) (Budde et al., 2008), cuprizone-induced axonal injury and demyelination (Sun et al., 2006), dysmyelination in shiverer mice (Song et al., 2002), and wallerian degeneration of the optic nerve resulting from retinal ischemia (Song et al., 2003). Others have also reported the utility of λ|| and λ⊥ in rodent SCI resulting from inflammation (DeBoy et al., 2007), acute ischemia (Gaviria et al., 2006), and contusion (Schwartz and Hackney, 2003; Schwartz et al., 2003) with histological validation.
Herein, we conducted in-vivo DTI measurements on mouse SCI of varying injury severity to non-invasively determine the extent of spared normal white matter hyperacutely. Our findings demonstrate that the hyperacute spared normal white matter extent defined in vivo with DTI correlates with long-term neurological function as assessed using the Basso mouse scale (Basso et al., 2006). In-vivo DTI-derived axial diffusivity, λ||, appears to be a reliable biomarker of axonal injury, and may be used as the surrogate end-point to predict long-term outcome of traumatic SCI in mice.
Methods
Spinal cord injury
All surgical intervention and pre- and post-surgical care was provided in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, 1996), and with the approval of the Washington University Animal Studies Committee.
Forty 10-week-old female C57BL/6 mice weighing 19–21 g (Harlan-Sprague Dawley, Inc. Indianapolis, IN) were used. The mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) intraperitoneally. A modified OSU impacting device (1.3-mm-diameter round tip, 0.2 m/sec impact speed) was employed for contusion injury (Jakeman et al., 2000; Kim et al., 2009b). Mice received 0.6-, 0.7-, 0.8-, or 0.9-mm (n = 8 for each group) contusive SCIs with a bipolar electromagnetic wave form after dorsal laminectomy at the T8 and T9 vertebral level. The zero point was determined by a touch sensor (Brody et al., 2007). Eight mice received laminectomy only as sham controls. The surgical site was closed in layers with 5-0 polyglactin 910 and nylon sutures. Enrofloxacin (2.5 mg/kg) and lactated Ringer's solution (2 mL) were injected subcutaneously. In-vivo DTI measurements were performed immediately after surgery for all mice.
For each group including controls, two mice were randomly selected for in-vivo DTI and histology comparison immediately after injury. The rest of the animals were used for longitudinal evaluations after acute DTI. Post-operative care including bladder expression was performed twice daily until the animals recovered bladder function.
Animal preparation for in-vivo diffusion tensor imaging
Following laminectomy and SCI, all mice immediately underwent in-vivo DTI (Kim et al., 2006). The surgery and acute in-vivo MR examination took 3 h, including 30 min for surgery and 2.5 h for in-vivo MRI planning and DTI acquisition. An isoflurane and oxygen mixture (0.2–1.5%) anesthesia was used during all DTI. A circulating warm water pad was used to maintain core body temperatures at 37°C. The respiratory exhaust line was connected to a pressure transducer to synchronize DTI data acquisition with the animal's respiratory rate (Kim et al., 2006).
A 9-cm inner diameter Helmholtz coil was employed as the RF transmitter. An inductively-coupled surface coil covering the T8–T11 vertebral segments (15 mm × 8 mm) was used as the RF receiver. The whole preparation was placed in an Oxford Instruments 200/330 magnet (4.7 T, 33-cm clear bore) equipped with a 15-cm inner diameter, actively-shielded Oxford gradient coil (18 G/cm, 200-μsec rise time). The Varian UNITY-INOVA console controlled by a Sun Blade 1500 workstation (Sun Microsystems, Santa Clara, CA) was used to interface the magnet, gradient coils, and Techron gradient power supply.
In-vivo diffusion tensor imaging
A conventional spin-echo diffusion imaging sequence, modified by adding Stejskal-Tanner diffusion weighting gradients (Stejskal and Tanner, 1965) was employed for in-vivo DTI measurements. Due to respiratory gating, the repetition time (TR ∼ 1.2 sec) was slightly varied among animals according to the period of individual breathing cycle (∼270 msec), with fixed echo time (TE) at 38 msec. Images, three slices per breath, were obtained with diffusion-sensitizing gradients applied in six orientations: (Gx,Gy,Gz) = (1,1,0), (1,0,1), (0,1,1), (–1,1,0), (0,–1,1), and (1,0,–1). Two diffusion-sensitizing b values, 0 and 0.750 msec/μm2, were used with time between application of gradient pulses (Δ) = 17 msec, and diffusion gradient on time (δ) = 8 msec. The chosen b value, 0.750 msec/μm2, represents optimal experimental conditions within the gradient performance of our system for diffusion imaging of rodent model spinal cord (Budde et al., 2008; Hasan and Narayana, 2006; Kim et al., 2006, 2007; Loy et al., 2007; Madi et al., 2005). Eight scans were averaged per k-space line, with field of view 10 × 10 mm2, and data matrix 128 × 256 (zero filled to 256 × 256). Nine transverse images (slice thickness 0.75 mm) were collected covering vertebral segments T8–T10 in 2.5 h, with 0.5 h for MR imaging set-up/planning, and 2.0 h for in-vivo DTI.
In-vivo diffusion tensor imaging data analyses
Lab-developed software written in Matlab (MathWorks, Natick, MA) was used to derive the diffusion tensors independently for each voxel from the diffusion-weighted images using a weighted linear least-squares method (Koay et al., 2006). The eigenvalue decomposition was then applied to each tensor, yielding a set of eigenvalues (λ1 ≥ λ2 ≥ λ3) and eigenvectors for each voxel. Maps of diffusion indices of the axial diffusivity (λ||), radial diffusivity (λ ⊥), and relative anisotropy (RA), and mean diffusivity (<D>) were generated by applying the following equations for each voxel:
 |
(1) |
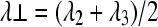 |
(2) |
 |
(3) |
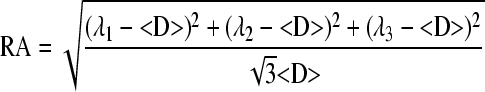 |
(4) |
For both sham-operated control and contusion-injured animals, the manual segmentation of ventrolateral white matter (VLWM) was performed based on tissue contrast (gray-white matter contrast) using RA, and λ⊥ maps for region-of-interest (ROI) analysis. The gray-white matter tissue contrast was largely preserved in RA and λ⊥ maps acutely for contusion-injured cords. The manual segmentation of total VLWM was readily achieved for both control and injured cords using RA (bright) and radial (dark) maps. The delineated ROI was applied to quantify λ||. Acutely after injury, the gray-white matter contrast was preserved in RA and λ⊥ maps. Thus, the manual segmentation using RA and λ⊥ maps provides the total VLWM volume for both control and injured cords.
Animal behavior assessments
After acute in-vivo DTI observations, all contusion-injured and sham-operated mice, except the 10 sacrificed immediately after hyperacute in-vivo DTI for histology, underwent open-field voluntary motor function assessment. Each animal's hindlimb motor function was assessed from 1 day to 2 weeks after injury with the Basso mouse scale (BMS) (Basso et al., 2006) instead of the Basso, Beattie, and Bresnahan (BBB) scale (Basso et al., 1996). Briefly, two blinded raters observed the hindlimb motor function of each mouse within 4 min. Only voluntary movement was selected for evaluation. The deficit rule was applied to determine each category of assessment. If two scores did not match, the lowest score was chosen, not the average (Basso et al., 2006).
Quantification of normal (or spared) ventrolateral white matter
The VLWM from 6 control mice were manually delineated based on RA and λ ⊥ maps and applied to the λ|| map. The λ|| values from all VLWM pixels were grouped for histogram analysis. The mean and standard deviation was determined and a threshold was set at mean ± 2 × standard deviations for the segmentation of the spared normal VLWM in the injured cords. The threshold was applied to the entire cord in the λ|| map. The dorsal white matter was excluded manually, since the dorsal column is physically separated from VLWM. The segmented ROI was then used to calculate the spared normal VLWM volume. The spared normal VLWM volume from each mouse was then normalized to mean control VLWM volume expressed as a percentage of spared normal VLWM. The correlation between acutely measured spared normal VLWM volume and the chronic hindlimb motor function (2 weeks after injury) was then investigated.
Tissue preparation and histological analysis
At the end of hyperacute in-vivo DTI examination, 10 randomly selected mice (two from each group) were perfusion fixed through the left cardiac ventricle under deep anesthesia with 100 mL of 0.1 M PBS (pH 7.4), followed by 100 mL of 0.1 M PBS containing 4% paraformaldehyde (pH 7.4). After fixation, the spine was harvested and left in the fixative (4% paraformaldehyde) for 24 h. A 2-mm segment of spinal cord centered at the contusion epicenter was cut, decalcified with the vertebral column intact, and embedded in paraffin. The embedded tissue was sectioned on a sliding microtome (3-μm thick). For microscopic examination of axons, silver staining (modified Bielschowsky method) was performed. Samples were placed in sodium thiosulfate followed by incubation with AgNO3 (4°C). Development was quenched with 1% acetic acid. Tissue was mounted for microscopic inspection.
Sections immediately adjacent to those utilized for silver staining were used for immunohistochemistry. The labeling of myelin basic protein and phosphorylated neurofilament were performed as previously reported (Loy et al., 2007). Briefly, deparaffinized and rehydrated sections were placed in 1 mM EDTA in a 95–100°C water bath for antigen retrieval. After rinsing with 0.01 M PBS, all sections were incubated with 0.01 M PBS containing a 2% blocking solution (Invitrogen, Carlsbad, CA) for 1 h. The sections were then incubated at 4°C overnight with one or more of the following primary antibodies: polyclonal anti-myelin basic protein (MBP, 1:1000; Sigma Chemical Co., St. Louis, MO) and monoclonal anti-neurofilament (SMI-31, 1:5000; Sternberger Monoclonals, Inc., Lutherville, MD) to label MBP and phosphorylated neurofilament, respectively. After rinsing with 0.01 M PBS, the sections were incubated with fluorescein isothiocyanate (FITC)-conjugated Fab’ fragments (goat anti-mouse or rabbit, 1:300; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). After washing with PBS, the sections were cover-slipped using the Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA). All images were captured within 1 week following completion of immunohistochemistry and compared to in-vivo DTI maps.
At the end of longitudinal BMS observation, all mice were perfusion-fixed, paraffin-embedded, and sectioned as performed for the acute cords. A phosphorylated neurofilament (pNF) immunohistochemical stain was employed to evaluate the residual spared white matter. The extent of pNF-positive axons was quantified by manual intensity threshold using Image J software (Rasband, 1997–2005). Briefly, to improve the consistency of spared VLWM estimation, an intensity threshold obtained from the pNF of control VLWM was applied to all injured cords. The pNF-positive region was further investigated using particle analysis in ImageJ. The pNF-positive axons with size greater than two times that of normal peripheral nerve were excluded. The percentage of normal VLWM was obtained by normalizing individual normal VLWM volume to those of control mice.
Statistical analysis
All statistical analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC) on the Linux platform. The acutely quantified DTI indices from VLWM were compared between study groups, and p < 0.05 was taken as statistically significant. Median BMS scores were compared between the five injury severity groups at each day of the 14-day follow-up period. Nonparametric Kruskal-Wallis tests were used because the BMS values are continuous and unimodel, but clearly non-gaussian in distribution. p-Values were adjusted for multiple testing using a Holm (step-down Bonferroni) correction. The weighted sample variance (or mean and standard deviation) of control VLWM λ|| distribution was produced from accumulated histograms of six control VLWM λ||. The prediction of percentage normal-appearing VLWM for “Recovery defined as BMS = 9” was tested by receiver operating characteristic (ROC) curve.
Results
Diffusion tensor imaging in the hyperacute phase after spinal cord injury
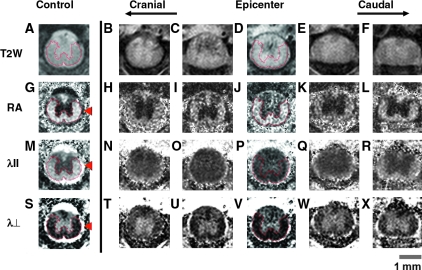
T2-weighted (T2W) images obtained with parameters comparable to standard human clinical scans demonstrated extensive edema within SCI epicenters (Fig. 1). The epicenter was easily detected with abnormally increased T2 signal intensity of VLWM and dark hemorrhage in gray matter (Fig. 1C and D). Contrast normally evident between central gray matter and peripheral white matter long tracts (Fig. 1A) was essentially lost within the epicenter (Fig. 1C and D), as reported previously (Bilgen et al., 2001; Narayana et al., 1999). This limitation of standard T2W sequences is currently an obstacle to the interpretation of clinical MR images after human SCI.
FIG. 1.
Hyperacute, 3 h after insult, in-vivo diffusion tensor imaging (DTI) maps of sham control (left column) and 0.9-mm contusion spinal cord injury (SCI; columns 2–6) animals. All images had 750-μm thickness extending rostral and caudal to the epicenter. Standard T2-weighted (T2W) images (top row) demonstrate extensive edema obscuring the gray-white matter junction, and small punctuate foci of hemorrhage (dark signal) within the central gray matter. Relative anisotropy (RA) maps (scale 0–1.414) demonstrate excellent central gray matter (isotropic, dark signal) and peripheral white matter (anisotropic, bright signal) contrast in regions obscured by edema on standard T2W sequences (compare to top row). RA maps are essential for manual identification of ventrolateral white matter (VLWM) long tracts in the hyperacute phase after experimental SCI. The arrowhead indicates cerebrospinal fluid. Axial diffusion (λ||) represents the relative movement of protons parallel to the long axis of the spinal cord after the application of DTI gradients. Quantitative λ|| maps (scale 0–3 μm2/msec) demonstrate dark signal intensity within injured white matter long tracts. Decreases in λ|| indicate axonal injury. Radial diffusion (λ⊥ ) represents the relative movement of protons perpendicular to the long axis of the spinal cord. In the hyperacute phase after SCI, qualitative reductions in λ ⊥ (0–1 μm2/msec) are likely the result of cytotoxic edema. Color image is available online at www.libertonline.com/neu.
The cerebrospinal fluid appears dark on anisotropy maps; however, it appears bright on both λ|| and λ ⊥ maps, a useful property for separating cord parenchyma from surrounding bone (arrowheads in Fig. 1G, M, and S). RA maps (Fig. 1G, sham control) provide distinct contrast between gray (dark) and white (bright) matter, respectively. The gray-white matter contrast on RA maps remained after SCI in the hyperacute phase regardless of injury severity, enabling manual segmentation of total VLWM (Figs. 1 H–L and 3a). The acutely preserved anisotropy of the white matter was consistent with a previous report (Loy et al., 2007).
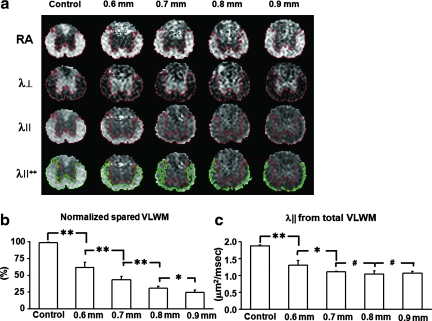
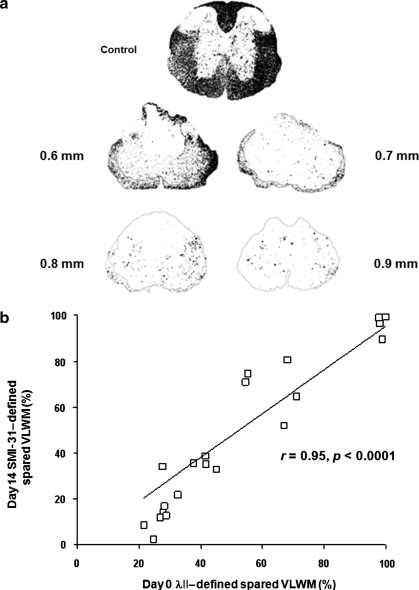
FIG. 3.
Maps of λ|| demonstrate the decrease in spared ventrolateral white matter (VLWM) associated with greater injury severity. RA, λ⊥ , and λ|| maps are displayed in scales of 0–1.414, 0–1, and 0–3 μm2/msec, respectively. (a) Representative hyperacute in-vivo diffusion tensor imaging (DTI) maps of sham control animals and animals with all four SCI severities are shown. Injury grades are displayed as millimeters of dorsal spinal cord displacement. The classification of total VLWM was manually performed, and spared VLWM was performed though threshold segmentation based on normal values obtained from sham-operated VLWM animals. λ||++ contains both total (manual) and spared (threshold) VLWM regions of interest. (b) The spared VLWM obtained threshold segmentation is normalized with manually segmented total VLWM. Across all mice (n = 8 for all groups), the percentage of spared VLWM determined by threshold segmentation correlated with injury severity, and significantly differentiated all groups from one another (r = –0.97 and p < 0.0001). (c) Manual segmentation of total VLWM λ|| is not sufficient to detect significant differences between moderate and severe spinal cord injury grades in the hyperacute phase. Data in b and c are presented as mean and standard deviation (*p < 0.001; **p < 0.0001; #p > 0.1). Color image is available online at www.liebertonline.com/neu.
Segmentation of normal (or spared) ventrolateral white matter using λ||
Hyperacutely injured VLWM tracts lose signal intensity (become dark relative to controls) on λ|| and λ⊥ maps (Fig. 1O–Q, 1V, and 3a). The threshold value of λ|| at T9 for automatically segmenting the normal VLWM was 1.82 ± 0.36 μm2/msec (mean ± 2 × standard deviations; n = 6, Fig. 2). The ROIs of normal-appearing VLWM delineated by λ|| threshold, and total VLWM delineated manually using RA and λ ⊥ maps were comparable (>95% overlap) in the control mice, as shown in Fig. 2. However, λ|| threshold segmented normal-appearing VLWM only identifies spared tissues in injured spinal cords. Thus the total VLWM extent was not determined using λ|| threshold. Total (Fig. 3a, red outlines) and normal-appearing white matter (Fig. 3a, green outline) was readily identified in all injury groups. The ratio of the normal-appearing VLWM volume normalized to total white matter volume was statistically significant between all groups with a strong linear correlation (Fig. 3b; r = –0.97; p < 0.0001).
FIG. 2.
Normal ventrolateral white matter (VLWM) λ|| histogram. Data graphically represent the normal range of VLWM λ|| values acquired from 6 sham control mice with manual segmentation (red outline). The total VLWM was manually delineated from RA and λ ⊥ maps and applied to λ|| maps. The distribution of each control mouse VLWM λ|| is shown with mean (solid squares) and standard deviation (open squares). The final histogram shown as a graph is a combination of each mouse's VLWM λ|| distribution. Automatic segmentation using threshold values did not differ significantly from regions segmented manually. λ||++ contains both total and spared VLWM regions of interest. This relatively simple method was used to identify spared normal VLWM non-invasively in vivo during the first 3 h post-SCI. Color image is available online at www.libertonline.com/neu.
Histological evaluation of ventrolateral white matter injury
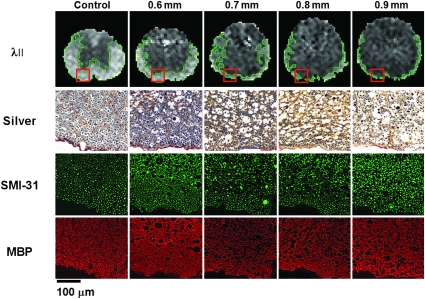
Representative silver-stained sections and the immunostain directed at phosphorylated neurofilament (SMI-31) and MBP were examined (Fig. 4). The histological stains were performed on the same animals shown as λ|| maps in Fig. 4 (each column represents a single animal). In the hyperacute phase, the silver stains best demonstrated white-matter necrosis and cytotoxic edema, as evidenced by progressively increasing axonal caliber and periaxonal ovoid lucencies with injury severity. However, separation of spared normal axons from damaged or transected axons within these histological sections obtained soon after injury was not possible with the silver stain preparation.
FIG. 4.
Sensitivity of in-vivo λ|| compared to histology for assessing hyperacute ventrolateral white matter (VLWM) integrity. Hyperacute λ|| maps are displayed with representative histological sections obtained from animals sacrificed immediately after diffusion tensor imaging (DTI) scans. Each column of histological sections is from the same animal. Outlines on the λ|| maps encompass image voxels displaying normal threshold values (mean ± 2 standard deviations) within regions of spared normal VLWM. Histological regions of interest are represented by boxes in the VLWM of each corresponding MR image. Silver staining and SMI-31 (neurofilament immunostaining) both depict progressively increasing axonal swelling and vacuolation indicative of cytotoxic edema as injury severity increases. Aside from the obvious morphological changes, no changes are evident in sections immunostained with myelin basic protein (MBP) over the range of injury grades (scale bar = 100 μm). Color image is available online at www.liebertonline.com/neu.
The intensity of immunohistochemical staining, SMI-31, and MBP did not show a significant difference between control and injured cords (Fig. 4). However, the abnormal size of the axons in the contused cord was clearly seen with SMI-31 staining. Swollen myelinated axons were also clearly seen in MBP surrounded by enlarged myelin rings. As shown in the silver-stained preparation, both SMI-31 and MBP staining showed abnormal morphology of myelinated axons in the vicinity of the central canal, while relatively normal-appearing axons were seen peripherally, consistent with the posterior-to-anterior contusion model.
The margins of injured and spared normal VLWM were difficult to visualize with all the employed histological staining methods. However, the general trend of peripheral VLWM sparing after dorsal impact injuries seemed to hold for both histology and DTI (for example, compare λ|| and silver stain).
Hindlimb motor function evaluation with the Basso mouse scale
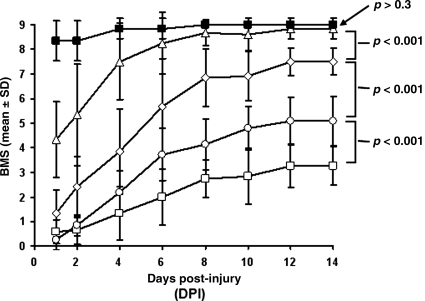
Longitudinal measurements of overground hindlimb locomotion were performed daily using the BMS scale (Fig. 5) for a period of 14 days after injury, because overground locomotor scores in untreated C57BL/6 mice did not significantly change beyond this time point (Basso et al., 2006). The first scoring session was not performed on the day of surgery and hyperacute DTI (day 0) in order to allow the animals to fully recover from general anesthesia. Deficits were observed in all experimental groups beginning on day 1. Sham-operated controls displayed only minor deficits (BMS score 8.4 ± 0.8), that essentially resolved by day 4 (BMS score 9 ± 0.3). The 0.6-mm displacement impact resulted in occasional to frequent plantar-stepping with no fore-and-hindlimb coordination (BMS score 4.3 ± 1.5), that improved rapidly during the first 6 days after injury. Measurements after day 6 revealed no significant difference between the 0.6-mm injury and the sham-control groups. The impact of 0.7-, 0.8-, and 0.9-mm displacement resulted in complete loss of weight support (BMS scores 0.6, 0.8, and 1.3, respectively) during the first 2 days. No significant differences between these groups could be measured during the early acute phase after injury (p > 0.05). After a period of rapid recovery during the first 6 days, significant differences were evident between these injury groups (p < 0.001). The 0.7- and 0.8-mm injury groups regained weight support and coordinated stepping at day 14 with BMS scores of 7.5 ± 0.9 and 5.1 ± 0.5, respectively. The 0.9-mm-injured animals showed recovery of joint movement, but did not regain weight support (BMS score 3.3 ± 0.8) at day 14.
FIG. 5.
Basso mouse scale (BMS) scores from control (solid squares), 0.6- (triangles), 0.7- (diamonds), 0.8- (circles), and 0.9-mm-injured mice (open squares) obtained from 24 h to 14 days after spinal cord injury demonstrate corresponding degrees of hindlimb dysfunction. Data are presented as mean and standard deviation. At 24 h, no significant differences are seen between the 0.7-mm, 0.8-mm, and 0.9-mm injury groups (p > 0.1). All these animals essentially demonstrate hindlimb paralysis. Similarly to previous reports, the most rapid rates of recovery are seen during the acute phase (the first 7 days) after injury in all injury groups. In the early subacute phase (7–14 days), significant differences were demonstrated between all injury groups (p < 0.001). Animals receiving mild (0.6 mm) injuries were not significantly different from sham controls (p > 0.3).
Predicting long-term outcome
Hyperacute λ|| measured the spared normal VLWM extent, showing strong correlation with BMS scores at 14 days post-SCI (Fig. 6a). Complete recovery of hindlimb locomotor function (BMS 9) was observed only in animals exhibiting > 50% preservation of VLWM, as determined by λ||. Below this VLWM preservation threshold, hindlimb function as examined with open-field BMS scoring was permanently impaired, similarly to previous reports (Basso et al., 1996; Nishi et al., 2007). Diagnostic accuracy of λ|| based on the threshold of 50% spared normal VLWM at 3 h post-SCI yielded the following performance measures for prediction of significant locomotor recovery: sensitivity = 1.0, specificity = 0.85, positive predictive value = 0.85, and negative predictive value = 1.0. An ROC curve analysis was also performed using terminal BMS scores as the gold standard for assignment of animals to a normal (BMS = 9) or injured (BMS < 9) status (Table 1 and Fig. 6b). The criterion of ≥50% identified all mice that did recover, and 90% of the mice that did not recover. There was a strong relationship between normal VLWM as determined by λ|| and recovery (Fig. 6). The odds of recovery increased by 18% with each increase of 1% in normal white matter (odds ratio = 1.18; p = 0.037). The area under the ROC curve was 0.98, suggesting that spared normal VLWM as measured by λ|| predicts recovery with a high degree of accuracy.
FIG. 6.
In-vivo λ|| maps obtained 3 h after traumatic spinal cord injury (SCI) predict Basso mouse scale (BMS) locomotor scores at 14 days. (a) BMS scores are plotted against the percentage of spared normal ventrolateral white matter (VLWM) as measured with λ|| diffusion tensor imaging (DTI) scans in the hyperacute phase after injury in control (solid squares) animals, and those with 0.6- (triangles), 0.7- (diamonds), 0.8- (circles), and 0.9-mm displacement injury (open squares). A threshold value of 50% hyperacute spared VLWM optimally (but not completely) separated mice with complete (BMS = 9) versus incomplete (BMS <9) recovery. (b) Receiver operating characteristic (ROC) curve analysis (recovery defined as BMS = 9) revealed that hyperacute spared VLWM was highly predictive of day 14 BMS score. The odds of significant recovery increase by 18% with each 1% increase in normal VLWM demonstrated with λ|| in the first 3 h after SCI (odds ratio = 1.18; p = 0.037).
Table 1.
The True-Positive and False-Positive Rates and the True-Negative Rate
| Percentage of spared white matter indicating recovery to BMS 9 | True-positive rate (sensitivity) | True-negative rate (specificity) | False-positive rate |
|---|---|---|---|
| ≥40% | 100% | 68% | 32% |
| ≥45% | 100% | 84% | 16% |
| ≥50% | 100% | 90% | 10% |
| ≥55% | 82% | 95% | 5% |
| ≥60% | 73% | 95% | 5% |
| ≥65% | 73% | 95% | 5% |
| ≥70% | 64% | 100% | 0% |
BMS, Basso mouse scale.
At the conclusion of the study, the extent of spared VLWM was estimated for each animal using pNF stains. Entire regions of VLWM characterized as injured tissue by λ|| were eliminated by 14 days post-injury, presumably by the inflammatory response (Fig. 7a). Hyperacute measurements of λ|| showed a linear, high correlation with final histological measurements of VLWM volumes in all experimental groups (r = 0.95, p < 0.0001; Fig. 7b).
FIG. 7.
Correlation with hyperacute and chronic spared ventrolateral white matter (VLWM). (a) The number of phosphorylated neurofilament (SMI-31)-immunopositive axons 14 days after contusive spinal cord injury decreased with increasing injury severity. (b) In-vivo λ||-defined hyperacute spared VLWM was highly correlated with histological SMI-31-defined spared VLWM at 14 days post-injury.
Discussion
These studies demonstrate for the first time that the previously reported non-invasive biomarker of axonal injury, λ|| (Budde et al., 2008; Kim et al., 2006, 2007; Loy et al., 2007; Song et al., 2002, 2003), depicted the extent of axonal damage within critical VLWM long tracts both visually and quantitatively as early as 3 h after injury. The extent of spared normal VLWM measured using λ|| hyperacutely predicted long-term behavioral recovery as assessed by BMS scores after SCI. The odds of significant behavioral recovery increased by approximately 18% with each 1% increase in hyperacute normal VLWM (odds ratio = 1.18, p = 0.037). Histological studies demonstrated excellent correlation (r = 0.95) between hyperacute λ|| and chronic spared normal VLWM content.
We recently reported that in-vivo measurements of directional diffusivity derived from DTI provided excellent contrast between gray and white matter during the hyperacute phase after SCI, especially within edematous regions typically obscured on T2W images (Loy et al., 2007). The axial diffusivity, λ||, was shown to be a sensitive and specific biomarker of axonal damage in many murine models of CNS injury and disease (Kim et al., 2006, 2007; Song et al., 2003). The λ|| reference value for normal VLWM at T9, the standardized injury level for locomotor behavioral assessments, was established in these experiments. Mean values of 1.82 μm2/msec were not significantly different from measurements previously reported at the T12–T13 vertebral level, or within the cervical spinal cord, indicating that diffusion characteristics of descending long tracts do not change significantly along the spinal axis (Kim et al., 2009a, 2007; Loy et al., 2007).
Initially, λ|| measurements including the entire VLWM demonstrated significant differences between sham, 0.6 mm (mild), and 0.7 mm (moderate) injuries, but failed to demonstrate differences among 0.7 mm, 0.8 mm (moderately severe), and 0.9 mm (severe) injuries (Fig. 3c). Upon closer inspection, it became apparent that the ratios of normal to injured VLWM decreased to an extent in moderate and severe SCI that differences in total VLWM λ|| changes could no longer be reliably demonstrated between these injury groups using our standard techniques. This additional, relatively simple, post-processing step revealed significant differences between all injury groups that were later confirmed convincingly with quantitative histological measurements of spared normal white matter (Figs. 3c and 7). Our data show unequivocally that spared regions of VLWM can be accurately identified in the hyperacute phase with MR imaging. Furthermore, standardized BMS evaluations of overground locomotor function demonstrated strong correlations between cross-sectional measurements of spared normal VLWM, as measured with λ|| approximately 3 h after SCI, and behavioral outcomes at 14 days (vide supra).
The descending tracts responsible for locomotor function in mice are unknown. In these experiments, all of the VLWM, including the ventral, lateral, and ventrolateral funiculi, was included in the region of interest based on previous reports in rats describing a diffuse arrangement of axons responsible for the initiation of locomotion within these regions (Loy et al., 2002a, 2002b). Dorsal contusion injuries produce disproportionate axonal damage in VLWM compared to DC at severe displacements, a finding predicted by the viscoelastic properties of spinal cord (Blight and Decrescito, 1986; Loy et al., 2007). We previously reported that λ|| does not normalize, but does improve significantly (approximately 20%) within regions of interest encompassing the entire VLWM during the first 7 days after severe SCI (Kim et al., 2007). No temporal improvement in λ|| could be demonstrated between 7 and 14 days. Importantly, the high degree of correlation (r = 0.95) between λ|| measurements of normal epicenter VLWM in the hyperacute phase and histological measurements of spared normal VLWM 14 days later presented here (Fig. 7), suggest that the axons completely spared from the initial mechanical trauma are primarily responsible for the observed locomotor recovery seen after SCI.
Our finding that acute λ||-determined extent of spared VLWM linearly correlated with locomotor function recovery is consistent with previous reports (Basso et al., 1996; Ma et al., 2001). Further investigations reported that spared white matter volume is the parameter correlating with functional outcome the most (McEwen and Springer, 2006; Schucht et al., 2002; You et al., 2003). This also holds true for mice of different genetic backgrounds (Hashimoto et al., 2007). The normal hindlimb motor function in both rats (Basso et al., 1996) and mice (Nishi et al., 2007) have been reported, with greater than 50–60% of spared white matter measured using post-mortem histology. The present study demonstrated the feasibility of the accurate and non-invasive acute estimation of spared white matter extent correlating with chronic locomotor recovery.
The mechanisms of λ|| decrease after SCI are unknown, but likely result from a combination of intracellular and extracellular changes yet to be resolved experimentally. Rapid, possibly instantaneous, changes in λ|| after SCI suggest that the mechanical disruption of axons may be a primary contributor to diffusion restriction. Disorganization of white matter tracts alone would be expected to present extracellular barriers to diffusion along the longitudinal axis of the spinal cord. However, dynamic cytoskeletal reorganization also begins rapidly within the axon after SCI (Gallyas et al., 2002). Activation of the Ca2+-dependent protease calpain results in neurofilament compaction and membranous organelles begin to accumulate in paranodal regions as fast axonal transport mechanisms fail. Mitochondrial swelling has also been documented in the hyperacute phase (Banik et al., 1997; Maxwell, 1996). Combinations of these factors likely comprise significant intracellular contributions to λ|| restriction after SCI.
Due to the interfering “spinal shock” present acutely after SCI, there are limited means that physicians can use to accurately assess the functional integrity of the injured spinal cord. One of the most clinically relevant aspects of the current study is the accurate assessment of white matter integrity 3 h after SCI, offering a window of opportunity that is currently not available for treating SCI patients. Specifically, the acute and non-invasive in-vivo DTI-determined λ|| is capable of correctly predicting remote hindlimb motor function 2 weeks after SCI, suggesting that the successful translation of this technique to human SCI could help determine acute interventions, monitor efficacy of treatment, and allow more accurate prognosis of SCI patients. However, the translation of this technique for use in human SCI presents many challenges, including (1) the low signal-to-noise ratio and image resolution of human spinal MRI may prevent the accurate anatomical definition of the injured tissues; (2) the more challenging respiratory motion that complicates human spinal MRI, since the spine immobilization device used in rodents is not applicable in humans; (3) the severely degraded echo planar diffusion-weighted MRI quality due to severe susceptibility effects that prevent as high-quality spinal DTI in humans compared to that of rodents within a reasonable time period; and (4) the limited time frame to perform the acute MRI before stabilizing hardware placement in SCI patients. Despite these challenges, there have been some human spinal cord DTI reports in the literature (Ellingson et al., 2008a, 2008b; Facon et al., 2005; Guleria et al., 2008; Shanmuganathan et al., 2008; Yeatman et al., 2009).
In summary, these studies demonstrate that λ|| measurements in the hyperacute phase after traumatic SCI accurately predict eventual locomotor recovery in mice. The combination of MR instruments capable of measuring subclinical changes in spinal cord physiology and murine genetic advantages offer an interesting, early window into the basic mechanisms of SCI that was not previously attainable. Although significant obstacles remain to be overcome to derive similar data in human patients, the eventual clinical application of this technique appears promising.
Acknowledgments
This study was supported in part by National Institute of Health (grant NS047592), and the University of Missouri Spinal Cord Injuries Research Program.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Banik N.L. Matzelle D.C. Gantt-Wilford G. Osborne A. Hogan E.L. Increased calpain content and progressive degradation of neurofilament protein in spinal cord injury. Brain Res. 1997;752:301–306. doi: 10.1016/s0006-8993(96)01488-6. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Fisher L.C. Anderson A.J. Jakeman L.B. McTigue D.M. Popovich P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Bilgen M. Abbe R. Narayana P.A. Dynamic contrast-enhanced MRI of experimental spinal cord injury: in vivo serial studies. Magn. Reson. Med. 2001;45:614–622. doi: 10.1002/mrm.1083. [DOI] [PubMed] [Google Scholar]
- Blight A.R. Macrophages and inflammatory damage in spinal cord injury. J. Neurotrauma. 1992;9(Suppl. 1):S83–S91. [PubMed] [Google Scholar]
- Blight A.R. Decrescito V. Morphometric analysis of experimental spinal cord injury in the cat: the relation of injury intensity to survival of myelinated axons. Neuroscience. 1986;19:321–341. doi: 10.1016/0306-4522(86)90025-4. [DOI] [PubMed] [Google Scholar]
- Bracken M.B. Shepard M.J. Holford T.R. Leo-Summers L. Aldrich E.F. Fazl M. Fehlings M. Herr D.L. Hitchon P.W. Marshall L.F. Nockels R.P. Pascale V. Perot P.L., Jr. Piepmeier J. Sonntag V.K. Wagner F. Wilberger J.E. Winn H.R. Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- Brody D.L. Mac Donald C. Kessens C.C. Yuede C. Parsadanian M. Spinner M. Kim E. Schwetye K.E. Holtzman D.M. Bayly P.V. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J. Neurotrauma. 2007;24:657–673. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde M.D. Kim J.H. Liang H.F. Russell J.H. Cross A.H. Song S.K. Axonal injury detected by in vivo diffusion tensor imaging correlates with neurological disability in a mouse model of multiple sclerosis. NMR Biomed. 2008;21:589–597. doi: 10.1002/nbm.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoy C.A. Zhang J. Dike S. Shats I. Jones M. Reich D.S. Mori S. Nguyen T. Rothstein B. Miller R.H. Griffin J.T. Kerr D.A. Calabresi P.A. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007;130:2199–2210. doi: 10.1093/brain/awm122. [DOI] [PubMed] [Google Scholar]
- Ditunno J.F. Little J.W. Tessler A. Burns A.S. Spinal shock revisited: a four-phase model. Spinal Cord. 2004;42:383–395. doi: 10.1038/sj.sc.3101603. [DOI] [PubMed] [Google Scholar]
- Ellingson B.M. Ulmer J.L. Kurpad S.N. Schmit B.D. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am. J. Neuroradiol. 2008a;29:1976–1982. doi: 10.3174/ajnr.A1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson B.M. Ulmer J.L. Schmit B.D. Morphology and morphometry of human chronic spinal cord injury using diffusion tensor imaging and fuzzy logic. Ann. Biomed. Eng. 2008b;36:224–236. doi: 10.1007/s10439-007-9415-6. [DOI] [PubMed] [Google Scholar]
- Facon D. Ozanne A. Fillard P. Lepeintre J.F. Tournoux-Facon C. Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am. J. Neuroradiol. 2005;26:1587–1594. [PMC free article] [PubMed] [Google Scholar]
- Fehlings M.G. Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine. 2006;31:S28–S35. doi: 10.1097/01.brs.0000217973.11402.7f. discussion S36. [DOI] [PubMed] [Google Scholar]
- Gallyas F. Farkas O. Mazlo M. Traumatic compaction of the axonal cytoskeleton induces argyrophilia: histological and theoretical importance. Acta Neuropathol. 2002;103:36–42. doi: 10.1007/s004010100424. [DOI] [PubMed] [Google Scholar]
- Gaviria M. Bonny J.M. Haton H. Jean B. Teigell M. Renou J.P. Privat A. Time course of acute phase in mouse spinal cord injury monitored by ex vivo quantitative MRI. Neurobiol. Dis. 2006;22:694–701. doi: 10.1016/j.nbd.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Guleria S. Gupta R.K. Saksena S. Chandra A. Srivastava R.N. Husain M. Rathore R. Narayana P.A. Retrograde Wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. J. Neurosci. Res. 2008;86:2271–2280. doi: 10.1002/jnr.21664. [DOI] [PubMed] [Google Scholar]
- Hasan K.M. Narayana P.A. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magn. Reson. Med. 2006;56:130–137. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- Hashimoto M. Sun D. Rittling S.R. Denhardt D.T. Young W. Osteopontin-deficient mice exhibit less inflammation, greater tissue damage, and impaired locomotor recovery from spinal cord injury compared with wild-type controls. J. Neurosci. 2007;27:3603–3611. doi: 10.1523/JNEUROSCI.4805-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakeman L.B. Guan Z. Wei P. Ponnappan R. Dzwonczyk R. Popovich P.G. Stokes B.T. Traumatic spinal cord injury produced by controlled contusion in mouse. J. Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- Keirstead H.S. Blakemore W.F. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J. Neuropathol. Exp. Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Kim J.H. Budde M.D. Liang H.F. Klein R.S. Russell J.H. Cross A.H. Song S.K. Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiol. Dis. 2006;21:626–632. doi: 10.1016/j.nbd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kim J.H. Haldar J. Liang Z.P. Song S.K. Diffusion tensor imaging of mouse brain stem and cervical spinal cord. J. Neurosci. Methods. 2009a;176:186–191. doi: 10.1016/j.jneumeth.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H. Loy D.N. Liang H.F. Trinkaus K. Schmidt R.E. Song S.K. Noninvasive diffusion tensor imaging of evolving white matter pathology in a mouse model of acute spinal cord injury. Magn. Reson. Med. 2007;58:253–260. doi: 10.1002/mrm.21316. [DOI] [PubMed] [Google Scholar]
- Kim J.H. Tu T.W. Bayly P. Song S.K. Impact speed does not determine severity of spinal cord injury in mice with fixed impact displacement. J. Neurotrauma. 2009b. [DOI] [PMC free article] [PubMed]
- Koay C.G. Chang L.C. Carew J.D. Pierpaoli C. Basser P.J. A unifying theoretical and algorithmic framework for least squares methods of estimation in diffusion tensor imaging. J. Magn. Reson. 2006;182:115–125. doi: 10.1016/j.jmr.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Little J.W. Ditunno J.F., Jr. Stiens S.A. Harris R.M. Incomplete spinal cord injury: neuronal mechanisms of motor recovery and hyperreflexia. Arch. Phys. Med. Rehabil. 1999;80:587–599. doi: 10.1016/s0003-9993(99)90204-6. [DOI] [PubMed] [Google Scholar]
- Loy D.N. Kim J.H. Xie M. Schmidt R.E. Trinkaus K. Song S.K. Diffusion tensor imaging predicts hyperacute spinal cord injury severity. J. Neurotrauma. 2007;24:979–990. doi: 10.1089/neu.2006.0253. [DOI] [PubMed] [Google Scholar]
- Loy D.N. Magnuson D.S. Zhang Y.P. Onifer S.M. Mills M.D. Cao Q.L. Darnall J.B. Fajardo L.C. Burke D.A. Whittemore S.R. Functional redundancy of ventral spinal locomotor pathways. J. Neurosci. 2002a;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy D.N. Talbott J.F. Onifer S.M. Mills M.D. Burke D.A. Dennison J.B. Fajardo L.C. Magnuson D.S. Whittemore S.R. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp. Neurol. 2002b;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- Ma M. Basso D.M. Walters P. Stokes B.T. Jakeman L.B. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp. Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- Madi S. Hasan K.M. Narayana P.A. Diffusion tensor imaging of in vivo and excised rat spinal cord at 7 T with an icosahedral encoding scheme. Magn. Reson. Med. 2005;53:118–125. doi: 10.1002/mrm.20304. [DOI] [PubMed] [Google Scholar]
- Maxwell W.L. Histopathological changes at central nodes of Ranvier after stretch-injury. Microsc. Res. Tech. 1996;34:522–535. doi: 10.1002/(SICI)1097-0029(19960815)34:6<522::AID-JEMT4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- McEwen M.L. Springer J.E. Quantification of locomotor recovery following spinal cord contusion in adult rats. J. Neurotrauma. 2006;23:1632–1653. doi: 10.1089/neu.2006.23.1632. [DOI] [PubMed] [Google Scholar]
- Miyanji F. Furlan J.C. Aarabi B. Arnold P.M. Fehlings M.G. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome—prospective study with 100 consecutive patients. Radiology. 2007;243:820–827. doi: 10.1148/radiol.2433060583. [DOI] [PubMed] [Google Scholar]
- Narayana P. Abbe R. Liu S.J. Johnston D. Does loss of gray- and white-matter contrast in injured spinal cord signify secondary injury? In vivo longitudinal MRI studies. Magn. Reson. Med. 1999;41:315–320. doi: 10.1002/(sici)1522-2594(199902)41:2<315::aid-mrm15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Nishi R.A. Liu H. Chu Y. Hamamura M. Su M.Y. Nalcioglu O. Anderson A.J. Behavioral, histological, and ex vivo magnetic resonance imaging assessment of graded contusion spinal cord injury in mice. J. Neurotrauma. 2007;24:674–689. doi: 10.1089/neu.2006.0204. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C. Basser P.J. Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Rasband W.S. Image processing and Analysis in Java 1997–2005.
- Schucht P. Raineteau O. Schwab M.E. Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Schwartz E.D. Hackney D.B. Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Exp. Neurol. 2003;184:570–589. doi: 10.1016/S0014-4886(03)00295-4. [DOI] [PubMed] [Google Scholar]
- Schwartz E.D. Shumsky J.S. Wehrli S. Tessler A. Murray M. Hackney D.B. Ex vivo MR determined apparent diffusion coefficients correlate with motor recovery mediated by intraspinal transplants of fibroblasts genetically modified to express BDNF. Exp. Neurol. 2003;182:49–63. doi: 10.1016/s0014-4886(03)00036-0. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan K. Gullapalli R.P. Zhuo J. Mirvis S.E. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am. J. Neuroradiol. 2008;29:655–659. doi: 10.3174/ajnr.A0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M.J. Bracken M.B. Magnetic resonance imaging and neurological recovery in acute spinal cord injury: observations from the National Acute Spinal Cord Injury Study 3. Spinal Cord. 1999;37:833–837. doi: 10.1038/sj.sc.3100927. [DOI] [PubMed] [Google Scholar]
- Song S.K. Sun S.W. Ju W.K. Lin S.J. Cross A.H. Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song S.K. Sun S.W. Ramsbottom M.J. Chang C. Russell J. Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Stejskal E.O. Tanner J.E. Spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965;42:288–292. [Google Scholar]
- Sun S.W. Liang H.F. Trinkaus K. Cross A.H. Armstrong R.C. Song S.K. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D. Ben-Shachar M. Bammer R. Feldman H.M. Using diffusion tensor imaging and fiber tracking to characterize diffuse perinatal white matter injury: a case report. J. Child. Neurol. 2009;24:795–800. doi: 10.1177/0883073808331080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S.W. Chen B.Y. Liu H.L. Lang B. Xia J.L. Jiao X.Y. Ju G. Spontaneous recovery of locomotion induced by remaining fibers after spinal cord transection in adult rats. Restor. Neurol. Neurosci. 2003;21:39–45. [PubMed] [Google Scholar]