Abstract
Since free radicals play a role in the mechanisms of brain injury after hemorrhagic stroke, the effect of melatonin (a potent antioxidant and free-radical scavenger) on outcomes was investigated after intracerebral hemorrhage (ICH) in rats. ICH was induced by clostridial collagenase infusion into the right caudate putamen, and several time points and doses of melatonin were studied. Brain edema and neurological function at 24 h were unchanged in comparison with vehicle-treated groups, in spite of oxidative stress reductions. Repeated treatment with the lower dose of melatonin (5 mg/kg) given at 1 h and every 24 h thereafter for 3 days after ICH, led to normalization of striatal function and memory ability over the course of 8 weeks, and less brain atrophy 2 weeks later. These results suggest that melatonin is safe for use after ICH, reduces oxidative stress, provides brain protection, and could be used for future investigations of free radical mechanisms after cerebral hemorrhage.
Key words: behavior, free radical, histopathology, intracerebral hemorrhage, melatonin
Introduction
Intracerebral hemorrhage (ICH) accounts for 15–20% of strokes (Broderick et al., 1999; Qureshi et al., 2001), affects approximately 1 in 6000 people every year (Woo et al., 2002), and there is no effective treatment available for it. Although hemorrhagic transformations will convert up to 30% of ischemic strokes into ICH (Lyden and Zivin, 1993), much less research has been done in this field compared with that done for cerebral ischemia (Sacco et al., 2009). Only one-half of patients will survive ICH, and these individuals will be afflicted with significant brain atrophy and lifelong neurological deficits (Broderick et al., 1999; Skriver and Olsen, 1986). Tragically, around 80% of rehabilitating patients will not be considered “independent” by 6 months after ICH ictus (Gebel and Broderick, 2000).
In addition to motor deficits after ICH, cognitive impairments evolve that are most troubling (King et al., 2006; Nys et al., 2007; Thajeb et al., 2007). ICH most commonly occurs in the basal ganglia (striatum), and lesions there are reported to cause significant learning and memory deficits (Bhatia and Marsden, 1994; Hochstenbach et al., 1998; Su et al., 2007; Werring et al., 2004). Neuropsychological studies confirm the role of the striatum in cognition (Benke et al., 2003; El Massioui et al., 2007; Ragozzino, 2007; Sridharan et al., 2006), and ICH into the caudate putamen of rats produces a similar effect (Hartman et al., 2009).
Free radicals are known to be an effective mechanistic target for reducing brain injury after ICH (Nakamura et al., 2008; Peeling et al., 2001a, 1998, 2001b). The intracerebral accumulation of erythrocytes will lead to cell lysis and the release of hemoglobin, which is degraded into the neurotoxins heme and iron (Wagner et al., 2003; Wu et al., 2003; Xi et al., 2006). The injection of either lysed erythrocytes or purified hemoglobin into the brains of rats will produce oxidative brain damage (to proteins, lipid, and DNA), considerable neurological deficits, and extensive brain edema within the first day (Huang et al., 2002; Nakamura et al., 2005, 2006; Xi et al., 1998a; Zhao et al., 2007). Thrombin released from the blood clot will also contribute to free radical–mediated cytotoxic injury (Lee et al., 1996; Nakamura et al., 2005; Xi et al., 1998b).
Melatonin (5-methoxy-N-acetyl-tryptamine) and its metabolites are potent antioxidants and free-radical scavengers (Cervantes et al., 2008; Peyrot and Ducrocq, 2008; Tan et al., 2007). Regarding erythrocytes, melatonin has been shown to inhibit free radical–associated red blood cell lysis (Tesoriere et al., 1999) and hemoglobin degradation (Tesoriere et al., 2001). Melatonin pre-treatment at daily doses of 5 mg/kg was able to reduce neuronal death after injections of iron into the parietal cortex of rats (Hayter et al., 2004). The hippocampal co-infusion of iron with melatonin (10 mg/kg), or its metabolite 6-hydroxymelatonin, was also protective against neurotoxicity and free-radical formation (Maharaj et al., 2006). Furthermore, nigrostriatal co-infusion of iron with melatonin, and repetitive intraperitoneal injections of melatonin (10 mg/kg) were able to suppress striatal neurodegeneration (Lin and Ho, 2000).
ICH shares several pathophysiological mechanisms with ischemic stroke (Xi et al., 2006), and the treatment of cerebral ischemia with melatonin has repeatedly been shown to exert cytoprotective effects (Chen et al., 2006a, 2006b; Kilic et al., 2005; Kondoh et al., 2002; Lee et al., 2007; Pei et al., 2003, 2002; Torii et al., 2004). In light of the available evidence, we hypothesized that melatonin treatment would ameliorate free-radical generation, decrease brain edema, reduce brain injury, and improve functional outcomes after intracerebral hemorrhage in rats.
Methods
Animals
Seventy-six adult male Sprague-Dawley rats (290–395 g; Harlan, Indianapolis, IN) were used in this study. All the procedures were in compliance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee at Loma Linda University.
General procedures
Aseptic technique was used for all surgeries. Rats were anesthetized with isoflurane (4% induction, 2% maintenance, 70% N2O and 30% O2). The animals were allowed free access to food and water after recovery from anesthesia. The neurological testing time points were based on functional outcomes from published studies characterizing rats after ICH (Hartman et al., 2009; MacLellan et al., 2009).
Intracerebral hemorrhage
The anesthetized animals were placed prone in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). A midline incision was made over the scalp under which the following stereotactic coordinates localized the right basal ganglia: 0.2 mm anterior, 5.6 mm ventral, and 2.9 mm lateral to the bregma. A posterior cranial burr hole (1 mm) was drilled over the right cerebral hemisphere, and a 27-gauge needle was inserted at a rate of 1 mm/min, attached to a microinfusion pump (Harvard Apparatus, Holliston, MA) for infusion of bacterial collagenase (VII-S, 0.2 U in 1 μL saline; Sigma-Aldrich, St. Louis, MO) through a Hamilton syringe at a rate of 0.2 μL/min. The needle remained in place for an additional 10 min after injection to prevent back-leakage. To maintain a core temperature of 37.0 ± 0.5°C, an electronic thermostat-controlled warming blanket was used throughout the operation. After needle removal, the burr hole was sealed with bone wax, the incision was sutured closed, and the animal was allowed to recover. Sham surgeries consisted of needle insertion alone.
Melatonin treatment
Melatonin (5-methoxy-N-acetyltryptamine) was purchased from Sigma-Aldrich . This compound was dissolved in 10% ethanol and diluted with 0.9% normal saline. Doses of 5 mg/kg and 15 mg/kg were determined according to the body weight of the animal. All injections were administered by intraperitoneal injections at 15 min or 3 h for the short-term experiments (24-h sacrifice), and 1, 24, 48, and 72 h for the long-term experiments (sacrificed 10 weeks after ICH induction). Vehicle-treated animals received the same volume via intraperitoneal injection. Animals were randomly divided into groups of six each by a blinded observer.
Experiment 1: Short-term outcome
Brain water content
One day after ICH, all groups (n = 6 in each of six groups) were re-anesthetized and brain samples were collected. Brain edema was measured using methods described previously (Tang et al., 2005). Briefly, the rats were decapitated and the brains were removed immediately, and then divided into five parts: the ipsilateral and contralateral basal ganglia, the ipsilateral and contralateral cortex, and the cerebellum. These tissue samples were weighed with an electronic analytical balance (model AE 100; Mettler Instrument Co., Columbus, OH) to the nearest 0.01 mg to obtain the wet weight (WW), and then the tissue was dried at 100°C for 24 h to determine the dry weight (DW). Finally, the brain water content (%) was calculated as (WW – DW)/WW × 100.
Forelimb placing score
Vibrissae-elicited forelimb placing testing (Hua et al., 2002) was performed in all groups (n = 6 in each of six groups) at 23 h after ICH by a highly experienced and blinded examiner. The animals were held by their torsos, allowing the forelimbs to hang free. Next the animal was moved gently up and down before testing in order to facilitate muscle relaxation and minimize any struggling movements (those trials in which the examiner encountered high levels of resistance, muscle straining or struggling, or placing of any limbs onto the examiner's hand were not counted). Each forelimb received independent testing by brushing the respective vibrissae (to that limb) on the edge of a tabletop. Most animals are able to place the ipsilateral (to cerebral injury) vibrissae-stimulated forelimb quickly onto the tabletop. Depending on the amount of cerebral injury, there should be impairment of placement of the forelimb contralateral to cerebral injury in response to tabletop contact with the contralateral vibrissae. For each experiment the rats were tested a total of 10 times per forelimb, and the percentage of successful placements onto the tabletop were calculated.
Neuroscore
Neurological evaluation (Garcia et al., 1995) was performed in all groups (n = 6 in each of six groups) at 23 h after ICH by a highly experienced and blinded examiner. The testing consisted of six tests with a possible score of 0–3 for each test (0 = worst and 3 = best), thus the minimum neurological score was 0 and the maximum was 18. These individual tests included: (1) spontaneous activity, (2) symmetry of movement, (3) forepaw outstretching, (4) climbing, (5) body proprioception, and (6) response to vibrissae touch. The score given to each rat at the completion of the evaluation was a summation of all six individual test scores.
Experiment 2: Free-radical stress
MDA assay
One day after ICH, all groups (n = 4 in each of four groups) were re-anesthetized and cardiac perfused with ice-cold PBS, then brain samples were collected and stored at −80°C. The level of lipid peroxidation products (malondialdehyde [MDA]) was measured (Kusaka et al., 2004) in the right cerebral cortex using LPO-586 kits (OxisResearch, Portland, OR). Homogenized brain tissue was placed in 20 mmol/L phosphate buffer (pH 7.4) with 0.5 M butylated hydroxytoluene in acetonitrile. These homogenates were then centrifuged at 20,800g for 10 min at 4°C and the supernatants were collected. The protein concentration was determined using a DC protein assay (Bio-Rad Laboratories, Inc., Hercules, CA), and these samples were reacted with a chromogenic reagent at 45°C for 60 min. After incubation, the samples were centrifuged at 20,800g for 10 min at 4°C and the supernatants were measured at 586 nm. The level of MDA was calculated as picomoles per milligram of protein according to the derived standard curve.
Experiment 3: Striatal functional outcome
Forelimb placing score
Vibrissae-elicited forelimb placing testing (Hua et al., 2002) was performed in all groups (n = 6 in each of four groups) over the first 3 days, and at 10 weeks post-ICH, by a highly experienced and blinded examiner. The animals were held by their torsos, and impairment of placement of the forelimb contralateral to cerebral injury in response to vibrissae tabletop contact was observed, as described above.
Rotarod
This is a test of coordination and balance. The rats were tested at baseline (prior to ICH), and on days 1, 3, and 5 of the 10-day battery at weeks 2 and 8 after ICH. The rotarod consists of a rotating horizontal cylinder (7 cm diameter) that is divided into 9.5-cm-wide lanes. When placed into a lane, an animal must continuously walk forward to avoid falling off the cylinder. Latency to fall off was detected and recorded by a photobeam circuit. Two consecutive trials were administered per day, in which the cylinder started turning at 4 rpm, and was accelerated by 2 rpm every 5 sec. To control for any potential learning effect due to previous rotarod exposure, an additional set of more difficult trials was added to the 8-week time point, in which the cylinder started turning at 10 rpm, and was accelerated by 2 rpm every 5 sec.
Experiment 4: Learning and memory outcome
Water maze
This rodent learning and memory test (Hartman et al., 2005a, 2005b, 2006, 2001) was administered on test days 6–10 of the 10-day battery at weeks 2 and 8 after ICH. Briefly, this test of spatial navigation learning requires finding a hidden (submerged) platform in a pool of water using visual cues from around the room. It consists of a metal pool (110 cm diameter) in a well-lit room filled to within 15 cm of the upper edge with water made opaque by the addition of white non-toxic tempera paint. The pool contains a platform (11 cm diameter) that the animal can step onto. For each trial, the animal was placed with its nose against the wall into the water at one of four release points and allowed to find the platform. All trials lasted a maximum of 60 sec, at which point the animal was manually guided to the platform. An overhead camera recorded the swim path, allowing quantification of swim distance, escape latency, proximity to the target, swim speed, and left/right turn bias by a computerized tracking system. Generally, as performance improves, escape latency and swim path length decrease. The cued water maze was tested on day 6 of the 10-day battery at weeks 2 and 8 after ICH. This is the “visible-platform” task, used primarily as a control to assess sensorimotor and/or motivational deficits that could affect performance during the spatial water maze task. For this task, the surface of the escape platform was visible (5 mm above the surface of the water), and a 20-cm-tall pole capped by a tennis ball was placed on top of the platform to make its location even more obvious. The animals were tested for 10 trials per day in five blocks of two consecutive trials with a 10-min inter-block interval. The location of the platform was changed for each block of trials. The animals were released into the pool opposite the location of the platform for that trial, and allowed to remain on the platform for 5 sec after finding it (or being guided to it). None of the animals displayed behaviors inappropriate for spatial water maze testing (including spinning, thigmotaxic navigation around the perimeter of the pool, or inability to swim). The spatial water maze test was given on days 7–10 of the 10-day battery at weeks 2 and 8 after ICH. For this task, the surface of the escape platform was submerged 1 cm below the surface of the water, requiring the animal to find the platform based on its relationship to spatial cues in the room rather than direct visualization. The animals were given 10 trials per day in five blocks of two consecutive trials with a 10-min inter-block interval. The location of the platform remained the same throughout each day, and was changed for the next day. Three locations were tested. After finding (or being guided to) the platform, the animals were allowed to remain on it for 10 sec. A different set of platform locations and spatial cues was used for the early and late time points. At the beginning of the next day, each animal was given a 60 sec “probe” trial, in which the platform was removed from the water maze. The total number of times that the animal crossed over the former location of the platform was recorded, as well as the amount of time spent searching the target quadrant. An hour later, the platform was placed back into the pool in a new location, and the next set of 10 trials was administered.
Experiment 5: Histopathology
Neuropathological analysis was performed in all groups (n = 6 in each of four groups) at 10 weeks after ICH. Animals were euthanized by isoflurane overdose followed by transcardiac perfusion of ice-cold PBS (60 mL, 0.01 mol/L, pH 7.4). The brains were quickly removed, post-fixed in fresh 4% formaldehyde solution at 4°C overnight, and then immersed in 30% sucrose until sinking. Three coronal sections (10 μm) were cut with a cryostat (CM3050S; Leica Microsystems, Inc., Bannockburn, IL) through the needle site (identifiable on the brain surface), and at sites 1.0 mm anterior and 1.0 mm posterior to that plane, placed on poly-lysine-coated glass slides (Richard-Allen Scientific, Kalamazoo, MI), and then cresyl violet staining was performed (Kusaka et al., 2004; Ostrowski et al., 2006).
Mophometric analysis involved computer-assisted (ImageJ 4.0 software; Media Cybernetics Inc., Silver Spring, MD) hand delineation of the following areas: the caudate putamen, cerebral cortex, ventricle, and corpus callosum. The borders of the cerebral structures were based on criteria previously defined from a stereologic study using optical dissector principles (Oorschot, 1996). The volumetric lesion area (cavity and cellular debris) and brain atrophy (ventriculomegaly) were calculated using the following equations (MacLellan et al., 2006a, 2006b, 2008):
 |
The loss of neuronal density at the striatum was estimated in accordance with established methods (Felberg et al., 2002; MacLellan et al., 2008; Peeling et al., 2001a) with modification. Striatal neurons were counted at 400× magnification in five areas (250 μm × 250 μm grids) per hemisphere of the section with maximal hematoma diameter. The relative loss of neurons was calculated by subtraction of averaged neuronal counts from the ipsilateral and contralateral sides.
The quantification of white-matter injury was approximated by measuring the corpus callosum area in each hemisphere, then calculating its volume from three coronal sections at 1-mm intervals (volume = average corpus callosum area × section interval × number of sections), and then comparing the two sides.
Statistical analysis
An α-level of .05 was used for all statistical significance tests, and all data are presented as ± SEM. Brain water content, MDA assay, histological quantification, and all neurobehavioral tests were analyzed using analysis of variance (ANOVA). The Student's t-test and Mann-Whitney rank-sum test were used when appropriate. To avoid violating the assumption that differences between levels of repeated measures must not be correlated across subjects, the p-values for such repeated-measures analyses reflected the conservative Huynh-Feldt adjustment to the degrees of freedom. Rotarod fall latency data were analyzed with a treatment (sham, vehicle, melatonin 5 mg/kg, or melatonin 15 mg/kg ) × time point (2 weeks or 8 weeks) × day (first or second) × trial (first or second) repeated-measures ANOVAs. The more difficult set of rotarod trials (administered at 8 weeks) was analyzed with a treatment (sham, vehicle, melatonin 5 mg/kg, or melatonin 15 mg/kg) × trial (first or second) repeated-measures ANOVAs. Water maze probe trial data (percentage of the trial spent searching the probe quadrant, and the number of target location crossings) were analyzed using treatment (sham, vehicle, melatonin 5 mg/kg, or melatonin 15 mg/kg) × time point (2 weeks or 8 weeks) × spatial location (first, second, or third) repeated-measures ANOVAs. Significant ANOVA interactions were further explored using the conservative Scheffe post-hoc test.
Results
Experiment 1: Short-term outcome
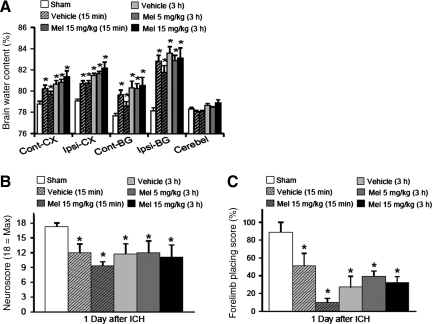
There was a very significant increase in neurological deficits (p < 0.05) and cerebral edema (p < 0.05) at both the basal ganglia and cerebral cortex of all rats subjected to ICH (Fig. 1 A–C). Melatonin failed to show any significant effect (15-min or 3-h delay; low dose 5 mg/kg, or high dose 15 mg/kg) on brain edema and neurological deficits at 1 day post-ICH (p > 0.05 compared to vehicle). Although high-dose (15 mg/kg) melatonin showed a trend toward ameliorating basal ganglia edema when administered 15 min after injury, this was mitigated by a small, insignificant neurological deficit increase 1 day later (Fig. 1C; p > 0.05 compared to vehicle).
FIG. 1.
Effect of melatonin upon brain edema and early neurological deficits. (A–C) Bar graphs showing the effects of melatonin (Mel) at doses of 5 and 15 mg/kg, at immediate (15 min) and delayed (3 h) time points, on brain water content and neurologic deficits (neuroscore and forelimb placing scores) at 24 h post-ICH. The measurements were obtained from brains of rats infused with 0.2 U collagenase (injured) or needle insertion without infusion (sham controls) (Cont-CX, contralateral cortex; Ipsi-CX, ipsilateral cortex; Cont-BG, contralateral basal ganglia; Ipsi-BG, ipsilateral basal ganglia; Cerebel, cerebellum; ICH, intracerebral hemorrhage; values are expressed as mean ± standard error of the mean, n = 6 per group; *p < 0.05 compared with sham animals).
Experiment 2: Free-radical stress
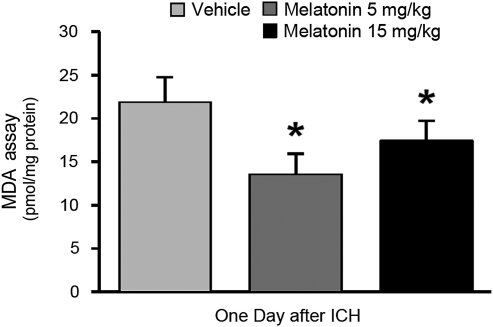
Lipid peroxidation assays were performed by measuring the level of malondialdehyde (MDA) activity in the right cortex of the rats at 1 day post-injury (Fig. 2). ICH led to a high level of free-radical stress that was still elevated 24 h later (p < 0.05). Three-hour-delayed treatment with melatonin (5 mg/kg and 15 mg/kg) led to a significant reduction in lipid peroxidation compared to vehicle-treated animals (p < 0.05; n = 4 per group).
FIG. 2.
Three-hour delayed melatonin treatment reduces free-radical stress. Graph showing the effect of melatonin at doses of 5 and 15 mg/kg, given as delayed (3 h) treatment, on the level of lipid peroxidation. The level of malondialdehyde (MDA) activity was measured in the right cortex of the rats at 24 h post-ICH. All values are expressed as mean ± standard error of the mean, n = 4 per group; *p < 0.05 compared with vehicle treatment; ICH, intracerebral hemorrhage).
Experiment 3: Striatal functional outcome
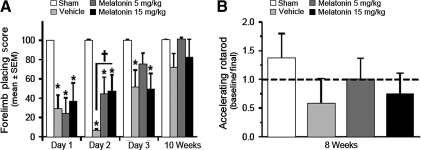
Forelimb placing and rotarod performance were used to evaluate striatal ability over time (Fig. 3). Treated animals received repeated melatonin (5 mg/kg or 15 mg/kg) or vehicle over the first 3 days post-injury. Compared to sham-treatment, all injured animals had significant forelimb placing deficits over the first 2 days after injury (p < 0.05). Melatonin treatment (5 mg/kg or 15 mg/kg) significantly improved forelimb function at day two compared to vehicle-treated animals (p < 0.05). Low-dose melatonin (5 mg/kg) normalized forelimb ability by day 3, when it was no longer significantly different from sham treatment (p > 0.05). The vehicle- and high-dose-treated animals did not experience the same level of amelioration at 72 h (p < 0.05 compared to sham treatment).
FIG. 3.
Repeated melatonin treatment improves striatal function after ICH. Graphs showing the effect of repeated melatonin treatments (5 mg/kg or 15 mg/kg) given at 1, 24, 48, and 72 h after ICH, upon striatal function (forelimb placing and rotarod testing). (A) Bar graph demonstrating the effect of melatonin treatment on forelimb placing scores measured at 1–3 days and 10 weeks post-ICH. (B) Bar graph showing the change in performance on rotarod testing between baseline measurements (pre-ICH) and 8 weeks after injury (values are expressed as mean ± standard error of the mean, n = 6 per group; *p < 0.05 compared with sham animals; †p < 0.05 compared with vehicle treatment; ICH, intracerebral hemorrhage).
In the days prior to ICH induction all animals were trained and blindly tested for baseline rotarod ability. Eight weeks later was the final training point for the rotarod test, and the animals were re-scored as previously tested. Low-dose melatonin (5 mg/kg) showed a large qualitative difference in returning rotarod performance back to baseline (1.0), while high-dose melatonin (15 mg/kg) only partially ameliorated this effect (0.75); in comparison the vehicle-treated animals had a 42% drop in performance. However, the difference between the groups failed to reach statistical significance (p = 0.177 by ANOVA).
Experiment 4: Learning and memory outcome
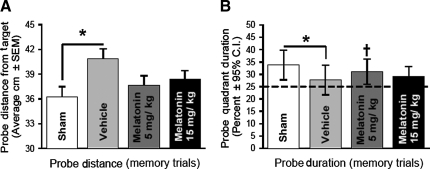
The animals were evaluated in the water maze for learning and memory function at 2 and 8 weeks after ICH. For both time points, there were no spatial learning deficits among the groups, and all of the animals learned the task of platform searching equally well (p > 0.05; data not shown). In the probe trials (when the platform was removed), the vehicle-treated animals had a significantly greater distance from the target (Fig. 4A; p < 0.05 compared to sham treatment), and they spent less time in the probe quadrants (Fig. 4B; p < 0.05 compared to sham treatment). Those animals receiving repeated melatonin treatments (5 mg/kg or 15 mg/kg) for 3 days post-ICH did not experience a loss of memory (Fig. 4 A and B; p > 0.05 compared to sham treatment), and melatonin at 5 mg/kg significantly improved the amount of time in the probe quadrant (Fig. 4B; p < 0.05 compared to vehicle treatment), while animals receiving both doses experienced only positive trends compared to vehicle treatment for the probe distance from target (Fig. 4A’ p > 0.05 compared to vehicle treatment). Thus melatonin treatment improved long-term memory deficits after ICH.
FIG. 4.
Repeated melatonin treatment normalizes memory ability after ICH. (A and B) Bar graphs demonstrating the effects of repeated melatonin treatments (5 mg/kg or 15 mg/kg) given at 1, 24, 48, and 72 h after ICH, on probe trials (memory) during water-maze testing at 2 weeks (early) and 8 weeks (late) after injury (values are expressed as mean ± standard error of the mean; [probe distance] and mean ± 95% confidence interval [probe duration], n = 6 per group; *p < 0.05 compared with sham animals; †p < 0.05 compared with vehicle treatment; ICH, intracerebral hemorrhage).
Experiment 5: Histopathology
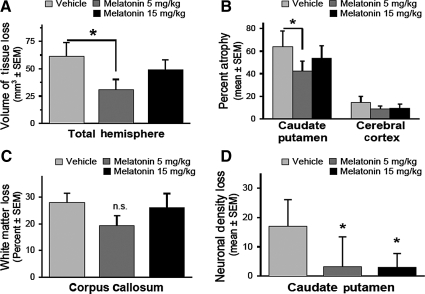
At 10 weeks after ICH, the animals suffered a large amount of cerebral damage, primarily to the striatum, but also to the internal capsule, corpus callosum, cerebral cortex, thalamus, and globus pallidus. Compared to vehicle-treated animals the total volume of tissue loss was reduced by low-dose melatonin treatments (5 mg/kg = 30.8 ± 10.4 mm3, vehicle = 61.3 ± 13.5 mm3, Fig. 5A; p < 0.05 versus vehicle treatment). High-dose melatonin's tendency to reduce brain injury was not statistically significant (Fig. 5A; melatonin 15 mg/kg = 49.1 ± 9.3 mm3, p > 0.05 versus vehicle treatment).
FIG. 5.
Repeated melatonin treatment improves cerebral histopathology 10 weeks after ICH. (A) Volume of tissue loss of the ipsilateral cerebral hemisphere. (B) Atrophy of the caudate putamen and cerebral cortex based on percentage volumetric difference between the ipsilateral and contralateral sides. (C) White-matter loss measured as percentage volumetric difference at the corpus callosum between the injured and uninjured sides. (D) The loss of neuronal density at the ipsilateral (peri-hematomal) striatum expressed as the difference in cell counts between the ipsilateral and contralateral regions (values are expressed as mean ± standard error of the mean, n = 6 per group; *p < 0.05 compared with vehicle treatment; ICH, intracerebral hemorrhage).
Low-dose melatonin was mostly effective at reducing atrophy in the striatum (Fig. 5B; p < 0.05 compared to vehicle treatment), but also tended to protect the cerebral cortex (Fig. 5B, p = 0.068 versus vehicle treatment) and white matter (Fig. 5C; p = 0.122 versus vehicle treatment) from long-term injury. The higher dose of melatonin did not produce much of a reduction in even striatal tissue loss (Fig. 5B; p = 0.266 compared to vehicle treatment).
To evaluate the effects of melatonin treatment on neuronal density loss after ICH in the striatum, the neurons were counted using automated software, and then the totals were calculated by averaging the neuronal density from all five locations, and then subtracted from either side of the brain (ipsilateral and contralateral hemispheres). Both high-dose and low-dose melatonin were able to significantly reduce the long-term neuronal cell loss in the striatum compared to vehicle-treated animals (Fig. 5D; p < 0.05, vehicle = 17.0 ± 4.5, melatonin 5 mg/kg = 3.2 ± 5.0, melatonin 15 mg/kg = 2.9 ± 2.4).
Discussion
The findings of this study indicate that systemic melatonin treatment can reduce long-term brain atrophy and can reverse striatal and cognitive functional deficits back to near-normal levels. As have others, we have demonstrated the impact of oxidative stress–mediated mechanisms on outcomes after ICH (Nakamura et al., 2008; Peeling et al., 2001a, 1998). The fact that short-term outcomes as evaluated by brain edema and neurological deficits were unchanged emphasizes the importance of performing long-term functional studies across several neurobehavioral domains, as was recommended by the stroke therapy academic industry roundtable (Recommendations for standards regarding preclinical neuroprotective and restorative drug development, 1999). Functional outcomes are major end-points of clinical trials, and lesion size does not always correlate particularly well with clinical functional impairments (Furlan et al., 1999; Tissue plasminogen activator for acute ischemic stroke, 1995).
MDA is a product of lipid peroxide decomposition and an indicator of oxidative stress and free-radical-mediated cell and tissue damage. We have demonstrated that ICH led to an accumulation of MDA, and 3-h-delayed melatonin treatment significantly reduced the levels of lipid peroxide decomposition products present at 24 h after injury. Therefore, melatonin would be an effective treatment for use in future investigations of the role of reactive oxygen species after ICH.
In agreement with our findings, previous studies using the collagenase model of striatal ICH have shown improvements in long-term neurological deficits after treatment with a scavenger of hydroxyl radicals (DMTU; 1,3-dimethyl-2-thiourea; Peeling et al., 1998), and with free-radical-trapping agents (PBN; α-phenyl-N-tert-butyl nitrone; Peeling et al., 1998, and NXY-059; disodium 4-[(tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide; Peeling et al., 2001a). The functional benefit was presumably due to either inflammation reduction or attenuation of other free-radical-mediated mechanisms. Unlike our findings, in these other studies there was no amelioration of pathological outcomes. We found that melatonin (5 mg/kg) ameliorated both long-term brain atrophy, and peri-hematomal neuronal density counts (5 mg/kg and 15 mg/kg) at 10 weeks after ICH. While the extent of volumetric brain tissue loss is a reliable finding, the neuronal density measurements could be an overestimation of neuronal viability, since our methods could not differentiate between living and dead neurons, and this should be addressed in future studies. The difference between the findings of our study and those of previous reports, however, may be related to the dual actions of melatonin (and its metabolites), as both a potent antioxidant and a free-radical scavenger (Cervantes et al., 2008; Peyrot and Ducrocq, 2008; Tan et al., 2007), suggesting broader reductions of oxidative stress and subsequently less cell and tissue damage with melatonin treatment. This possibility is further corroborated by the fact that we saw a significant reversal of oxidative stress, which has not been previously shown after striatal collagenase-induced ICH (Peeling et al., 2001a, 1998).
In another study researchers investigated the systemic administration of edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a free-radical scavenger of hydroxyl radicals, in a rat model of striatal autologous blood injection (Nakamura et al., 2008). They found a dose-dependent reduction in brain edema and neurological deficits (forelimb placing and corner turn). In comparison, our results failed to show any brain edema amelioration, except for a slight tendency with early (15 min) treatment. Forelimb placement in our study was also transiently ameliorated at 48 h, but this occurred only in the multiple-dosing groups. To explain this finding, several differences between the collagenase and autologous blood injection models would need to be addressed. Most notably, with regard to brain edema and factors affecting acute functional outcome, the collagenase model is known to cause far more lasting and severe blood–brain barrier rupture and neurological deficits than autologous blood injection (MacLellan et al., 2008). Neither model mimics clinical conditions perfectly, but both may be necessary since human ICH also presents in a wide spectrum of severity (Jaffe et al., 2009). Nonetheless, the study of short-term free-radical-mediated pathophysiological mechanisms may yet yield significant results in future studies using the autologous blood infusion model, since melatonin's effects may simply be inadequate to overcome the severity of acute-phase collagenase injury. Alternatively, combined treatment of melatonin with a more specific anti-inflammatory drug could synergistically overcome these pathophysiological mechanisms (Li et al., 2009), and this possibility needs further exploration.
Melatonin (5 mg/kg) has been shown to reduce blood-brain barrier permeability and the hemorrhagic transformation into ICH after ischemic stroke in mice (Chen et al., 2006b), and this reduction in bleeding is likely to be mediated by attenuation of matrix metalloproteinase (MMP)-9 activation (Hung et al., 2008). Similar findings relating to MMP-9 and hemorrhagic transformation have been reported after edaravone treatment in rats (Yagi et al., 2009). While there is little question that MMP-9 inhibition is cytoprotective after striatal autologous blood injection in rodents (Tejima et al., 2007; Xue et al., 2006), it has been argued that MMP-9 downregulation could lead to aggravation of brain injury after collagenase-induced ICH (Grossetete and Rosenberg, 2008; Tang et al., 2004). In fact, MMP-9-null mice experienced greater hemorrhagic volume, brain edema, and diminished neurological function than wild-type controls (Tang et al., 2004). The downregulation of MMP-9 by melatonin (Hung et al., 2008) may account for the differences between our equivocal short-term findings (i.e., brain edema and neurological function) and the benefits reported after free-radical reduction with edaravone in an autologous blood injection model (Nakamura et al., 2008).
In this study we found that melatonin improved memory after ICH. There is likely some mechanistic benefit beyond the reductions in peri-hematomal free-radical injury. Hippocampal neurons have receptors for melatonin (Morgan et al., 1994; Musshoff et al., 2002), and the administration of this hormone is known to alter excitability and synaptic transmission within the hippocampus (Hogan et al., 2001; Musshoff et al., 2002; Wan et al., 1999), and melatonin has been shown to alter hippocampal synaptic plasticity through the MT2-mediated regulation of the adenylate cyclase–protein kinase A (AC–PKA) pathway (Wang et al., 2005). The synaptic plasticity in the hippocampus and other brain regions has recently gained attention as an important means by which melatonin may augment its neuroprotective effects beyond reductions in oxidative stress alone (Baydas et al., 2005; Bob and Fedor-Freybergh, 2008; Fukunaga et al., 2002; Gorfine and Zisapel, 2007; Larson et al., 2006; Talaei et al., 2009; Wang et al., 2005). In light of these findings, and those of this study, there is an urgent need for more high-quality mechanistic studies to further investigate these plasticity processes as a possible means of improving outcomes after ICH.
Melatonin has also been shown to increase the expression of the NMDA receptor (NMDAR) in the rat hippocampus (Sutcu et al., 2006). The NMDAR is a known critical factor involved with induction of both long-term potentiation and long-term depression, events believed to be directly related to learning and memory formation (Martin et al., 2000; Rison and Stanton, 1995; Stanton, 1996). The modulation of NMDAR function has been shown to enhance the learning and memory of cognitively impaired rats (Burgdorf et al., 2009). The massive release of glutamate seen after ICH (Castillo et al., 2002; Miller et al., 2007; Qureshi et al., 2003) leads to the downregulation of the NMDAR complex (Carmichael et al., 2008). This could play a role in the delayed cognitive deficits seen after ICH, and the normalization of the NMDAR could be another means of melatonin's cytoprotection.
Melatonin has been widely tested and is well known to induce improvements in animal models of cerebral ischemia (Macleod et al., 2005), and translational work may soon allow advancement to clinical trials for human stroke (Korkmaz et al., 2009). Although ICH shares several mechanisms with ischemic stroke, not all therapeutic benefits apply to the same degree to ICH and ischemic stroke (Xi et al., 2006). Nonetheless, the effects of ischemic stroke therapies should be rigorously tested in models of ICH prior to clinical trials to determine their safety profile, since the stroke type (i.e., ischemic versus hemorrhagic) is not always known in patients soon enough to allow administration of neuroprotective drugs. This being said, the findings of this study indicate that melatonin has no adverse affects, and its use leads to improvements in striatal function, memory, and brain atrophy after ICH.
Acknowledgments
This work was funded by National Institutes of Health grant NS53407 to J.H.Z.
Author Disclosure Statement
No competing financial interests exist.
References
- Baydas G. Ozer M. Yasar A. Tuzcu M. Koz S.T. Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res. 2005;1046:187–194. doi: 10.1016/j.brainres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Benke T. Delazer M. Bartha L. Auer A. Basal ganglia lesions and the theory of fronto-subcortical loops: neuropsychological findings in two patients with left caudate lesions. Neurocase. 2003;9:70–85. doi: 10.1076/neur.9.1.70.14374. [DOI] [PubMed] [Google Scholar]
- Bhatia K.P. Marsden C.D. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117(Pt. 4):859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- Bob P. Fedor-Freybergh P. Melatonin, consciousness, and traumatic stress. J. Pineal Res. 2008;44:341–347. doi: 10.1111/j.1600-079X.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- Broderick J.P. Adams H.P., Jr. Barsan W. Feinberg W. Feldmann E. Grotta J. Kase C. Krieger D. Mayberg M. Tilley B. Zabramski J.M. Zuccarello M. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- Burgdorf J. Zhang X.L. Weiss C. Matthews E. Disterhoft J.F. Stanton P.K. Moskal J.R. The N-methyl-d-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.012. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S.T. Vespa P.M. Saver J.L. Coppola G. Geschwind D.H. Starkman S. Miller C.M. Kidwell C.S. Liebeskind D.S. Martin N.A. Genomic profiles of damage and protection in human intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2008;28:1860–1875. doi: 10.1038/jcbfm.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J. Davalos A. Alvarez-Sabin J. Pumar J.M. Leira R. Silva Y. Montaner J. Kase C.S. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology. 2002;58:624–629. doi: 10.1212/wnl.58.4.624. [DOI] [PubMed] [Google Scholar]
- Cervantes M. Morali G. Letechipia-Vallejo G. Melatonin and ischemia-reperfusion injury of the brain. J. Pineal Res. 2008;45:1–7. doi: 10.1111/j.1600-079X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Chen H.Y. Chen T.Y. Lee M.Y. Chen S.T. Hsu Y.S. Kuo Y.L. Chang G.L. Wu T.S. Lee E.J. Melatonin decreases neurovascular oxidative/nitrosative damage and protects against early increases in the blood-brain barrier permeability after transient focal cerebral ischemia in mice. J .Pineal Res. 2006a;41:175–182. doi: 10.1111/j.1600-079X.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- Chen T.Y. Lee M.Y. Chen H.Y. Kuo Y.L. Lin S.C. Wu T.S. Lee E.J. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J .Pineal Res. 2006b;40:242–250. doi: 10.1111/j.1600-079X.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- El Massioui N. Cheruel F. Faure A. Conde F. Learning and memory dissociation in rats with lesions to the subthalamic nucleus or to the dorsal striatum. Neuroscience. 2007;147:906–918. doi: 10.1016/j.neuroscience.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Felberg R.A. Grotta J.C. Shirzadi A.L. Strong R. Narayana P. Hill-Felberg S.J. Aronowski J. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Ann. Neurol. 2002;51:517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- Fukunaga K. Horikawa K. Shibata S. Takeuchi Y. Miyamoto E. Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J. Neurosci. Res. 2002;70:799–807. doi: 10.1002/jnr.10400. [DOI] [PubMed] [Google Scholar]
- Furlan A. Higashida R. Wechsler L. Gent M. Rowley H. Kase C. Pessin M. Ahuja A. Callahan F. Clark W.M. Silver F. Rivera F. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- Garcia J.H. Wagner S. Liu K.F. Hu X.J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- Gebel J.M. Broderick J.P. Intracerebral hemorrhage. Neurol. Clin. 2000;18:419–438. doi: 10.1016/s0733-8619(05)70200-0. [DOI] [PubMed] [Google Scholar]
- Gorfine T. Zisapel N. Melatonin and the human hippocampus, a time dependent interplay. J. Pineal Res. 2007;43:80–86. doi: 10.1111/j.1600-079X.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- Grossetete M. Rosenberg G.A. Matrix metalloproteinase inhibition facilitates cell death in intracerebral hemorrhage in mouse. J. Cereb. Blood Flow Metab. 2008;28:752–763. doi: 10.1038/sj.jcbfm.9600572. [DOI] [PubMed] [Google Scholar]
- Hartman R.E. Izumi Y. Bales K.R. Paul S.M. Wozniak D.F. Holtzman D.M. Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer's disease. J. Neurosci. 2005a;25:6213–6220. doi: 10.1523/JNEUROSCI.0664-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman R.E. Lee J.M. Zipfel G.J. Wozniak D.F. Characterizing learning deficits and hippocampal neuron loss following transient global cerebral ischemia in rats. Brain Res. 2005b;1043:48–56. doi: 10.1016/j.brainres.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Hartman R.E. Shah A. Fagan A.M. Schwetye K.E. Parsadanian M. Schulman R.N. Finn M.B. Holtzman D.M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol. Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Hartman R.E. Wozniak D.F. Nardi A. Olney J.W. Sartorius L. Holtzman D.M. Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer's-like neuropathology. Exp. Neurol. 2001;170:326–344. doi: 10.1006/exnr.2001.7715. [DOI] [PubMed] [Google Scholar]
- Hartman R. Lekic T. Rojas H. Tang J. Zhang J.H. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res. 2009;1280:148–157. doi: 10.1016/j.brainres.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayter C.L. Bishop G.M. Robinson S.R. Pharmacological but not physiological concentrations of melatonin reduce iron-induced neuronal death in rat cerebral cortex. Neurosci. Lett. 2004;362:182–184. doi: 10.1016/j.neulet.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Hochstenbach J. van Spaendonck K.P. Cools A.R. Horstink M.W. Mulder T. Cognitive deficits following stroke in the basal ganglia. Clin. Rehabil. 1998;12:514–520. doi: 10.1191/026921598666870672. [DOI] [PubMed] [Google Scholar]
- Hogan M.V. El-Sherif Y. Wieraszko A. The modulation of neuronal activity by melatonin: in vitro studies on mouse hippocampal slices. J. Pineal Res. 2001;30:87–96. doi: 10.1034/j.1600-079x.2001.300204.x. [DOI] [PubMed] [Google Scholar]
- Huang F.P. Xi G. Keep R.F. Hua Y. Nemoianu A. Hoff J.T. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J. Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Hua Y. Schallert T. Keep R.F. Wu J. Hoff J.T. Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Hung Y.C. Chen T.Y. Lee E.J. Chen W.L. Huang S.Y. Lee W.T. Lee M.Y. Chen H.Y. Wu T.S. Melatonin decreases matrix metalloproteinase-9 activation and expression and attenuates reperfusion-induced hemorrhage following transient focal cerebral ischemia in rats. J. Pineal Res. 2008;45:459–467. doi: 10.1111/j.1600-079X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- Jaffe J. AlKhawam L. Du H. Tobin K. O'Leary J. Pollock G. Batjer H.H. Awad I.A. Outcome predictors and spectrum of treatment eligibility with prospective protocolized management of intracerebral hemorrhage. Neurosurgery. 2009;64:436–445. doi: 10.1227/01.NEU.0000330402.20883.1B. discussion 445–436. [DOI] [PubMed] [Google Scholar]
- Kilic U. Kilic E. Reiter R.J. Bassetti C.L. Hermann D.M. Signal transduction pathways involved in melatonin-induced neuroprotection after focal cerebral ischemia in mice. J. Pineal Res. 2005;38:67–71. doi: 10.1111/j.1600-079X.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- King J,T., Jr. DiLuna M.L. Cicchetti D.V. Tsevat J. Roberts M.S. Cognitive functioning in patients with cerebral aneurysms measured with the mini mental state examination and the telephone interview for cognitive status. Neurosurgery. 2006;59:803–810. doi: 10.1227/01.NEU.0000232666.67779.41. discussion 810–801. [DOI] [PubMed] [Google Scholar]
- Kondoh T. Uneyama H. Nishino H. Torii K. Melatonin reduces cerebral edema formation caused by transient forebrain ischemia in rats. Life Sci. 2002;72:583–590. doi: 10.1016/s0024-3205(02)02256-7. [DOI] [PubMed] [Google Scholar]
- Korkmaz A. Reiter R.J. Topal T. Manchester L.C. Oter S. Tan D.X. Melatonin: an established antioxidant worthy of use in clinical trials. Mol. Med. 2009;15:43–50. doi: 10.2119/molmed.2008.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka I. Kusaka G. Zhou C. Ishikawa M. Nanda A. Granger D.N. Zhang J.H. Tang J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H2442–H2451. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- Larson J. Jessen R.E. Uz T. Arslan A.D. Kurtuncu M. Imbesi M. Manev H. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci. Lett. 2006;393:23–26. doi: 10.1016/j.neulet.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Lee K.R. Colon G.P. Betz A.L. Keep R.F. Kim S. Hoff J.T. Edema from intracerebral hemorrhage: the role of thrombin. J. Neurosurg. 1996;84:91–96. doi: 10.3171/jns.1996.84.1.0091. [DOI] [PubMed] [Google Scholar]
- Lee M.Y. Kuan Y.H. Chen H.Y. Chen T.Y. Chen S.T. Huang C.C. Yang I.P. Hsu Y.S. Wu T.S. Lee E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res. 2007;42:297–309. doi: 10.1111/j.1600-079X.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- Lin A.M. Ho L.T. Melatonin suppresses iron-induced neurodegeneration in rat brain. Free Radic. Biol. Med. 2000;28:904–911. doi: 10.1016/s0891-5849(00)00169-6. [DOI] [PubMed] [Google Scholar]
- Li Z.Q. Liang G.B. Xue Y.X. Liu Y.H. Effects of combination treatment of dexamethasone and melatonin on brain injury in intracerebral hemorrhage model in rats. Brain Res. 2009;1264:98–103. doi: 10.1016/j.brainres.2009.01.055. [DOI] [PubMed] [Google Scholar]
- Lyden P.D. Zivin J.A. Hemorrhagic transformation after cerebral ischemia: mechanisms and incidence. Cerebrovasc. Brain Metab. Rev. 1993;5:1–16. [PubMed] [Google Scholar]
- MacLellan C.L. Auriat A.M. McGie S.C. Yan R.H. Huynh H.D. De Butte M.F. Colbourne F. Gauging recovery after hemorrhagic stroke in rats: implications for cytoprotection studies. J. Cereb. Blood Flow Metab. 2006a;26:1031–1042. doi: 10.1038/sj.jcbfm.9600255. [DOI] [PubMed] [Google Scholar]
- MacLellan C.L. Gyawali S. Colbourne F. Skilled reaching impairments follow intrastriatal hemorrhagic stroke in rats. Behav. Brain Res. 2006b;175:82–89. doi: 10.1016/j.bbr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- MacLellan C.L. Langdon K.D. Churchill K.P. Granter-Button S. Corbett D. Assessing cognitive function after intracerebral hemorrhage in rats. Behav. Brain Res. 2009;198:321–328. doi: 10.1016/j.bbr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- MacLellan C.L. Silasi G. Poon C.C. Edmundson C.L. Buist R. Peeling J. Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J. Cereb. Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Macleod M.R. O'Collins T. Horky L.L. Howells D.W. Donnan GA. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J. Pineal Res. 2005;38:35–41. doi: 10.1111/j.1600-079X.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Maharaj D.S. Maharaj H. Daya S. Glass B.D. Melatonin and 6-hydroxymelatonin protect against iron-induced neurotoxicity. J. Neurochem. 2006;96:78–81. doi: 10.1111/j.1471-4159.2005.03532.x. [DOI] [PubMed] [Google Scholar]
- Martin S.J. Grimwood P.D. Morris R.G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Miller C.M. Vespa P.M. McArthur D.L. Hirt D. Etchepare M. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduced levels of extracellular cerebral glutamate and unchanged lactate pyruvate ratios. Neurocrit. Care. 2007;6:22–29. doi: 10.1385/NCC:6:1:22. [DOI] [PubMed] [Google Scholar]
- Morgan P.J. Barrett P. Howell H.E. Helliwell R. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem. Int. 1994;24:101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Musshoff U. Riewenherm D. Berger E. Fauteck J.D. Speckmann E.J. Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus. 2002;12:165–173. doi: 10.1002/hipo.1105. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Keep R.F. Hua Y. Hoff J.T. Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res. 2005;1039:30–36. doi: 10.1016/j.brainres.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Keep R.F. Hua Y. Nagao S. Hoff J.T. Xi G. Iron-induced oxidative brain injury after experimental intracerebral hemorrhage. Acta Neurochir. Suppl. 2006;96:194–198. doi: 10.1007/3-211-30714-1_42. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Kuroda Y. Yamashita S. Zhang X. Miyamoto O. Tamiya T. Nagao S. Xi G. Keep R.F. Itano T. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008;39:463–469. doi: 10.1161/STROKEAHA.107.486654. [DOI] [PubMed] [Google Scholar]
- Nys G.M. van Zandvoort M.J. de Kort P.L. Jansen B.P. de Haan E.H. Kappelle L.J. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23:408–416. doi: 10.1159/000101464. [DOI] [PubMed] [Google Scholar]
- Oorschot D.E. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical dissector methods. J. Comp. Neurol. 1996;366:580–599. doi: 10.1002/(SICI)1096-9861(19960318)366:4<580::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ostrowski R.P. Tang J. Zhang J.H. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke. 2006;37:1314–1318. doi: 10.1161/01.STR.0000217310.88450.c3. [DOI] [PubMed] [Google Scholar]
- Peeling J. Del Bigio M.R. Corbett D. Green A.R. Jackson D.M. Efficacy of disodium 4-[(tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (NXY-059), a free radical trapping agent, in a rat model of hemorrhagic stroke. Neuropharmacology. 2001a;40:433–439. doi: 10.1016/s0028-3908(00)00170-2. [DOI] [PubMed] [Google Scholar]
- Peeling J. Yan H.J. Chen S.G. Campbell M. Del Bigio M.R. Protective effects of free radical inhibitors in intracerebral hemorrhage in rat. Brain Res. 1998;795:63–70. doi: 10.1016/s0006-8993(98)00253-4. [DOI] [PubMed] [Google Scholar]
- Peeling J. Yan H.J. Corbett D. Xue M. Del Bigio M.R. Effect of FK-506 on inflammation and behavioral outcome following intracerebral hemorrhage in rat. Exp. Neurol. 2001b;167:341–347. doi: 10.1006/exnr.2000.7564. [DOI] [PubMed] [Google Scholar]
- Pei Z. Fung P.C. Cheung R.T. Melatonin reduces nitric oxide level during ischemia but not blood-brain barrier breakdown during reperfusion in a rat middle cerebral artery occlusion stroke model. J. Pineal Res. 2003;34:110–118. doi: 10.1034/j.1600-079x.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- Pei Z. Pang S.F. Cheung R.T. Pretreatment with melatonin reduces volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. J. Pineal Res. 2002;32:168–172. doi: 10.1034/j.1600-079x.2002.1o847.x. [DOI] [PubMed] [Google Scholar]
- Peyrot F. Ducrocq C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J. Pineal Res. 2008;45:235–246. doi: 10.1111/j.1600-079X.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- Qureshi A.I. Ali Z. Suri M.F. Shuaib A. Baker G. Todd K. Guterman L.R. Hopkins L.N. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit. Care Med. 2003;31:1482–1489. doi: 10.1097/01.CCM.0000063047.63862.99. [DOI] [PubMed] [Google Scholar]
- Qureshi A.I. Tuhrim S. Broderick J.P. Batjer H.H. Hondo H. Hanley D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Ragozzino M.E. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann. N.Y. Acad. Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Rison R.A. Stanton P.K. Long-term potentiation and N-methyl-D-aspartate receptors: foundations of memory and neurologic disease? Neurosci. Biobehav. Rev. 1995;19:533–552. doi: 10.1016/0149-7634(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Sacco S. Marini C. Toni D. Olivieri L. Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40:394–399. doi: 10.1161/STROKEAHA.108.523209. [DOI] [PubMed] [Google Scholar]
- Skriver E.B. Olsen T.S. Tissue damage at computed tomography following resolution of intracerebral hematomas. Acta Radiol. Diagn. (Stockh.) 1986;27:495–500. doi: 10.1177/028418518602700502. [DOI] [PubMed] [Google Scholar]
- Sridharan D. Prashanth P.S. Chakravarthy V.S. The role of the basal ganglia in exploration in a neural model based on reinforcement learning. Int. J. Neural Syst. 2006;16:111–124. doi: 10.1142/S0129065706000548. [DOI] [PubMed] [Google Scholar]
- Stanton P.K. LTD, LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6:35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Su C.Y. Chen H.M. Kwan A.L. Lin Y.H. Guo N.W. Neuropsychological impairment after hemorrhagic stroke in basal ganglia. Arch. Clin. Neuropsychol. 2007;22:465–474. doi: 10.1016/j.acn.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Sutcu R. Yonden Z. Yilmaz A. Delibas N. Melatonin increases NMDA receptor subunits 2A and 2B concentrations in rat hippocampus. Mol. Cell Biochem. 2006;283:101–105. doi: 10.1007/s11010-006-2385-4. [DOI] [PubMed] [Google Scholar]
- Talaei S.A. Sheibani V. Salami M. Light deprivation improves melatonin related suppression of hippocampal plasticity. Hippocampus. 2009 doi: 10.1002/hipo.20650. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Tan D.X. Manchester L.C. Terron M.P. Flores L.J. Reiter R.J. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- Tang J. Liu J. Zhou C. Alexander J.S. Nanda A. Granger D.N. Zhang J.H. MMP-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J. Cereb. Blood Flow Metab. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- Tang J. Liu J. Zhou C. Ostanin D. Grisham M.B. Neil Granger D. Zhang J.H. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J. Neurochem. 2005;94:1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- Tejima E. Zhao B.Q. Tsuji K. Rosell A. van Leyen K. Gonzalez R.G. Montaner J. Wang X. Lo E.H. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J. Cereb. Blood Flow Metab. 2007;27:460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- Tesoriere L. Allegra M. D'Arpa D. Butera D. Livrea M.A. Reaction of melatonin with hemoglobin-derived oxoferryl radicals and inhibition of the hydroperoxide-induced hemoglobin denaturation in red blood cells. J. Pineal Res. 2001;31:114–119. doi: 10.1034/j.1600-079x.2001.310204.x. [DOI] [PubMed] [Google Scholar]
- Tesoriere L. D'Arpa D. Conti S. Giaccone V. Pintaudi A.M. Livrea M.A. Melatonin protects human red blood cells from oxidative hemolysis: new insights into the radical-scavenging activity. J. Pineal Res. 1999;27:95–105. doi: 10.1111/j.1600-079x.1999.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Thajeb P. Thajeb T. Dai D. Cross-cultural studies using a modified mini mental test for healthy subjects and patients with various forms of vascular dementia. J. Clin. Neurosci. 2007;14:236–241. doi: 10.1016/j.jocn.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N. Engl. J. Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Torii K,. Uneyama H. Nishino H. Kondoh T. Melatonin suppresses cerebral edema caused by middle cerebral artery occlusion/reperfusion in rats assessed by magnetic resonance imaging. J. Pineal Res. 2004;36:18–24. doi: 10.1046/j.1600-079x.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- Wagner K.R. Sharp F.R. Ardizzone T.D. Lu A. Clark J.F. Heme and iron metabolism: role in cerebral hemorrhage. J. Cereb. Blood Flow Metab. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Wang L.M. Suthana N.A. Chaudhury D. Weaver D.R. Colwell C.S. Melatonin inhibits hippocampal long-term potentiation. Eur. J. Neurosci. 2005;22:2231–2237. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q. Man H.Y. Liu F. Braunton J. Niznik H.B. Pang S.F. Brown G.M. Wang Y.T. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat. Neurosci. 1999;2:401–403. doi: 10.1038/8062. [DOI] [PubMed] [Google Scholar]
- Werring D.J. Frazer D.W. Coward L.J. Losseff N.A. Watt H. Cipolotti L. Brown M.M. Jager H.R. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265–2275. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- Woo D. Sauerbeck L.R. Kissela B.M. Khoury J.C. Szaflarski J.P. Gebel J. Shukla R. Pancioli A.M. Jauch E.C. Menon A.G. Deka R. Carrozzella J.A. Moomaw C.J. Fontaine R.N. Broderick J.P. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190–1195. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]
- Wu J. Hua Y. Keep R.F. Nakamura T. Hoff J.T. Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- Xi G. Keep R.F. Hoff J.T. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J. Neurosurg. 1998a;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- Xi G. Keep R.F. Hoff J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Xi G. Wagner K.R. Keep R.F. Hua Y. de Courten-Myers G.M. Broderick J.P. Brott T.G. Hoff J.T. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998b;29:2580–2586. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- Xue M. Hollenberg M.D. Yong V.W. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J. Neurosci. 2006;26:10281–10291. doi: 10.1523/JNEUROSCI.2806-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K. Kitazato K.T. Uno M. Tada Y. Kinouchi T. Shimada K. Nagahiro S. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40:626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- Zhao X. Sun G. Zhang J. Strong R. Song W. Gonzales N. Grotta J.C. Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann. Neurol. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]