Abstract
The face distinguishes one human being from another. When the face is disfigured because of trauma, tumor removal, congenital anomalies, or chronic diseases, the patient has a strong desire for functional and esthetic restoration. Current practice of facial reconstruction using autologous grafts, synthetic fillers, and prostheses is frequently below the surgeon's and patient's expectations. Facial reconstruction is yet to take advantage of recent advances in seemingly unrelated fields of stem cell biology, chemical engineering, biomaterials, and tissue engineering. “Biosurgery,” a new concept that we propose, will incorporate novel principles and strategies of bioactive cues, biopolymers, and/or cells to restore facial defects. Small facial defects can likely be reconstructed by cell homing and without cell transplantation. A critical advantage of cell homing is that agilely recruited endogenous cells have the potential to harness the host's innate capacity for regeneration, thus accelerating the rate of regulatory and commercialization processes for product development. Large facial defects, however, may not be restorable without cell delivery per our understanding at this time. New breakthrough in biosurgery will likely originate from integrated strategies of cell biology, cytokine biology, chemical engineering, biomaterials, and tissue engineering. Regardless of cell homing or cell delivery approaches, biosurgery not only will minimize surgical trauma and repetitive procedures, but also produce long-lasting results. At the same time, caution must be exercised against the development of products that lack scientific basis or dogmatic combination of cells, biomaterials, and biomolecules. Together, scientifically derived biosurgery will undoubtedly develop into new technologies that offer increasingly natural reconstruction and/or augmentation of the face.
Introduction
The human face becomes highly individualized during development. Orofacial tissues are arguably the most complex in the human body, accommodating multiple functions of vision, hearing, smell, taste, touch, chewing, speech, swallowing, and breathing. When the face is disfigured because of trauma, tumor resection, infectious diseases, or congenital anomalies, the physical and psychosocial effects are extremely detrimental. In 2000, the U.S. Surgeon General's Report on Oral Health stated that a serious facial and oral disfigurement “may undermine self-image and self-esteem, discourage normal social interaction, and lead to chronic stress and depression as well as to incurring great financial cost.”1
The prevalence of facial defects is summarized in Table 1.2–6 A myriad of congenital anomalies, such as cleft lip, cleft palate, hemifacial microsomia, and craniosynostosis, adversely affect the face. Facial trauma remains one of the most common injuries in war and peacetime and frequently presents as challenges for both esthetic and functional restorations. Chronic diseases result in dental, oral, and craniofacial defects. Postablative head and neck cancer patients frequently have significant functional disabilities and a poor esthetic outcome.
Table 1.
Examples of the Prevalence of Facial Soft Tissue Wounds in the United States
Facial defects are currently restored by the patient's own (autologous) tissue grafts, allogeneic tissue grafts, xenogenic tissue grafts, synthetic materials, or prosthesis.2 Autologous tissue grafts invariably necessitate donor site morbidity. For example, a patient missing a segment of the maxilla or mandible frequently experiences additional trauma, pain, and morbidity because of an autologous bone graft harvested from the donor site such as the ileum. Similarly, a patient's missing portion of the nose after facial trauma or skin cancer resection may be reconstructed by an autologous skin and soft tissue graft from the forehead, which creates additional scarring and disfigurement. Autologous grafts are often considered the clinical gold standard, because allografts, xenografts, and synthetic materials are associated with complications such as pathogen transmission, immune rejection, and suboptimal integration.7–12 However, a key drawback of autologous tissue grafting is donor site morbidity.13–15
In addition to the burden of reconstruction of facial defects, there is an increasing demand for facial augmentation or rejuvenation.13–15 Facial augmentation or rejuvenation is frequently achieved by repetitive injections of fillers of naturally derived tissue analogs or synthetic materials.16 For example, injectables are used to correct contour deformities and rhytids due to loss of soft tissue or aging. Current injectable fillers include collagen (xenografts and allograft), freeze-dried acellular dermal tissue, calcium hydroxyapatite spheres, and polymer beads of poly-l-lactic acid (PLLA) and polymethylmethacrylate (PMMA). Such injectables must be host compatible and not cause immunogenic, inflammatory, or carcinogenic effects. Current fillers are frequently short lasting and require repetitive injections. The long-lasting polymer injectables such as PMMA or PLLA may be associated with clumping and foreign body reaction.17 Moreover, current injectables suffer from suboptimal integration with host tissue, premature degradation, and suboptimal remodeling with surrounding host tissue.
Dermal tissue can potentially be restored by an intricate combination of extracellular matrix fibers including collagen and elastin. Accordingly, extracellular matrix-based materials have been extensively used as acellular soft tissue fillers, including collagen-based homologs such as purified human collagen (CosmoDerm/CosmoPlast, Inamed Gauting, Germany), decellularized processed dermal allograft (Cymetra LifeCell Corporation, Branchburg, NJ), particulate fascia lata allograft (Fascian Fascia Biosystems, Los Angeles, CA), and xenogeneic materials such as bovine collagen (Zyderm/Zyplast [Allergan, Irvine, CA]). Bovine collagen has been widely used as injectable fillers.18 Most of these fillers can be injected readily as an in-office procedure. Given the xenogenic nature of bovine collagen, there are safety considerations including potential pathogen transmission and immunorejection. Even with normal skin tests, approximately 3% of patients develop foreign body reactions.18 Most natural fillers degrade within short periods of time after injection and necessitate repetitive injections.
Other naturally occurring human extracellular matrix substances, such as hyaluronic acid (HA), have also been extensively used in facial augmentation. HA in the skin is commonly depolarized with aging, leading to reduced ability to retain water.18 Thus, replenishing HA may improve skin appearance by rehydrating the subcutaneous tissue. Compared with collagen, HA lasts somewhat longer but still requires repeated injections.
Alloplastic materials may address some of the deficiencies associated with short actions of natural substances. Alloplastic materials include hydroxyapatite particles embedded in a highly viscous gel (Radiesse BioForm Medical, San Mateo, CA), PLLA microparticles (Sculptra Sanofi-aventis U.S., Bridgewater, NJ), and PMMA microspheres (Artecoll Pulmon Medical, Natal, South Africa). The role of tissue engineering in facial reconstruction or rejuvenation is to promote the use of biocompatible materials with or without biomolecules or cells that allow host tissue to remodel and achieve the desired characteristics. Optimally, the injectables should induce cellular ingrowth while undergoing degradation at a well-balanced rate. There has been little progress in the translation of tissue engineering approaches to the clinical setting of soft tissue reconstruction or augmentation, despite meritorious effort. The delivery of chemokines or cytokines that induce cell homing is likely to reach the clinical market before cell-based applications, because cell delivery may require ex vivo manipulations, training of current clinical practitioners on cell handling, and other undesirable features such as excessive cost and potential contamination.
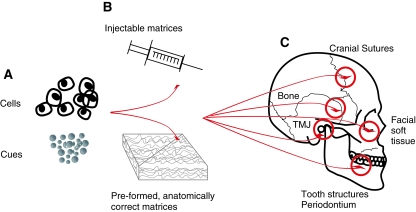
The premise of this review is that collective advances in stem cell biology, cytokine biology, chemical engineering, biomaterials, and tissue engineering, especially in the past decade, have established the foundation for “biosurgery,” a new paradigm for facial reconstruction and augmentation. Biosurgery is based on the principles and practice of the delivery of bioactive cues, biopolymers, and/or cells that are tailored to restore facial defects, circumventing the typically short-term, nonregenerative practice of current facial filler procedures (Fig. 1). It is probable based on the existing experimental data that restoration of small facial defects or augmentation can be achieved by cell homing and without cell transplantation (Fig. 1). At this time, the restoration of large facial defects may still rely on cell delivery (Fig. 1). Biological regeneration of orofacial tissues overcomes most, if not all, of the drawbacks of autologous grafting or artificial materials. Undoubtedly, the end is near for current clinical practice of autologous, allogeneic, and xenogenic grafting. The projected advances in the coming years of facial reconstruction and/or augmentation will likely stem from integrated strategies of cell biology, cytokine biology, chemical engineering, biomaterials, and tissue engineering. A number of challenges need to be further addressed before broad applications of biosurgery in facial reconstruction and augmentation: Would it be possible to heal certain facial defects by cell homing and without cell transplantation in patients? How to induce cell homing? Are autologous cells always necessary? Can allogeneic cells or xenogenic cells be safely transplanted to heal facial defects? What are the scientific and business barriers associated with cell transplantation or cell homing approaches? Here, we will discuss some of these critical questions.
FIG. 1.
Divergence of two biological approaches for facial reconstruction or augmentation. Cells, including stem/progenitor cells, may be injected in soluble matrices or seeded in preformed anatomically correct matrices for the healing or augmentation of dental, oral, and craniofacial defects, as shown in the schematics on the right. However, cell delivery is associated with potential commercialization and regulatory hurdles such as excessive cost of cell harvest, processing, packaging/shipping/storage, contamination, and clinical acceptance rate. In comparison, biological cues can be encapsulated in biocompatible microparticles such as injectables or loaded into preformed, anatomically correct matrices for the healing or augmentation of dental, oral, and craniofacial defects, as shown in the schematics on the right. Biological cues are capable of homing the host's endogenous cells, including stem/progenitor cells, and can be prepackaged and made available in a medical or dental office at the time of clinical need, with several previous products approved for clinical applications. Cell homing and cell delivery combined is the foundation for facial biosurgery that minimizes surgical trauma and yield long-lasting esthetic and functional outcome, in comparison with shortcomings of current practice of tissue grafting and synthetic fillers. Color images available online at www.liebertonline.com/ten.
Cell Transplantation Strategies for Facial Reconstruction: Current Status and Challenges
Cell-based therapies have been considered a key ingredient in the anticipated era of personalized medicine. In this context, diseased, ageing, missing, or traumatized cells or tissues are to be healed by autologous cells, as opposed to autologous tissue grafts that can only be harvested with sizable donor site morbidity. Autologous tissue grafts, the current surgical “gold standard,” are harvested from one part of the patient's body for the reconstruction of another part. Compared with autologous tissue grafts, one of the key advantages of cell-based therapies is to minimize donor site morbidity.19,20 For example, a patient who has a bone graft harvested from the iliac crest for facial reconstruction experiences donor site morbidity of the iliac crest. Early attempts of therapeutic cell delivery have adopted the concept that cells in diseased tissues were to be replaced by like cells that are healthy.19,20 For example, it has been thought for some time that degenerating arthritic cartilage has to be repaired by healthy chondrocytes. A number of drawbacks have become apparent in association with early attempts of autologous cell therapies. First, sufficient numbers of healthy donor cells are scarce and therefore cannot be obtained without inducing donor site trauma that neither the surgeon nor the patient desires to have. A patient with cardiac infarct has no autologous donor site for the restoration of the infarcted area. Second, delivered cells without carriers sometimes migrate away from the intended location or transform into undesirable cells. For the regeneration of structural tissues such as bone or cartilage, cell delivery without carriers fails because of a lack of the obligatory structural and mechanical support. Third, the delivered end-stage cells, because of programmed cell death or necrosis, are often incapable of regenerating or maintaining the volume or the function of target tissues. Allogeneic grafts and xenogenic grafts are derived from human or nonhuman species, respectively. Despite the advantage of no donor site morbidity, the major disadvantages of allogeneic or xenogenic grafts can be substantial, including potential immunorejection, pathogen transmission, and mismatch of color and texture.21–25 Synthetic materials have endless supply but may suffer from the reported suboptimal integration, leakage, foreign body reaction, or extrusion and dislocation.26,27
Autologous adipose tissue has been transplanted to fill soft tissue defects. A drawback of autologous soft tissue grafts, besides donor site morbidity, is postoperative volume reduction.12,28 Volume reduction after autologous fat transfer can be as high as 70%.21–32 Volume reduction following fat grafts or adipose cell transfer procedures may be due to apoptosis of the transplanted mature adipocytes, their low tolerance to ischemia, and slow revascularization rate.32 Single-cell suspension of mature adipocytes isolated from lipectomy or liposuction aspirates has been incorporated in scaffolds and transplanted to fill soft tissue defects. However, postoperative volume reduction of tissues from lipectomy-isolated adipocytes is a major barrier for patient satisfaction.9,12,18,29,30 In addition, adipose grafts from liposuction aspirates may be associated with suboptimal blood supply and necrosis of the graft.32 The yield of adipocytes in lipectomy aspirates is usually low because of a variety of factors including mechanical damage.32 Mature adipocytes are fully differentiated and undergo apoptosis, leading to a common shortage of adipocytes for soft tissue reconstruction.33
Stem/progenitor cells may overcome the deficiencies of autologous tissue grafts or end-stage cells. For example, mesenchymal stem cells, which are progenitors of subcutaneous soft tissue, can be isolated from bone marrow, adipose tissue, or other sources.19,20 These stem/progenitor cells are capable of multiple population doublings, therefore potentially serving as a replenishable source for facial soft tissue reconstruction.14,22,34 The field of facial reconstruction and augmentation has yet to take full benefit of the fruit of stem cell biology, cytokine biology, chemical engineering, materials science, and tissue engineering. Many studies, including those exemplified below, have provided some of the ground works necessary for biosurgery in relation to facial reconstruction and/or augmentation:
Pittenger et al. demonstrated that bone marrow-derived human mesenchymal stem cells differentiated into adipogenic cells.35
Alhadlaq et al. first showed that adipose tissue can be generated in vivo from human mesenchymal stem cells.20
Stosich et al. showed that bioengineered adipose tissue from human mesenchymal stem cells can be vascularized and maintained with predefined shape and dimensions.36
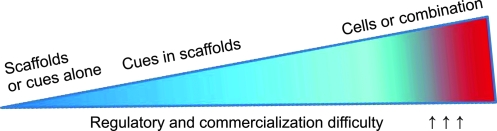
Although the era of biosurgery for facial reconstruction and augmentation is on the horizon, caution must be exercised against product development without sound scientific data or dogmatic combination of cells, biomaterials, and growth factors. Despite temporary hype, products not based on the proof of efficacy and safety are likely short lived.37 Dogmatic combinations of cells, biomaterials, and growth factors have a tendency to yield a level of complexity that gives rise to new ideas for scientific experiments, but, at the same time, may be prohibitive for product development (Fig. 2).
FIG. 2.
Escalating magnitude of regulatory and commercialization challenge of different products for healing dental, oral, and craniofacial defects. The magnitude of regulatory and commercialization difficulty increases from the left to the right. Biocompatible material scaffolds or biological cues alone typically cost the least to develop commercially and have examples of prior regulatory approval for clinical applications. This is followed closely by the incorporation of cues in scaffolds. Cell delivery with or without biological cues and/or scaffolds represents the most costly and challenging approach for regulatory approval and commercialization. Color images available online at www.liebertonline.com/ten.
The process of cell transplantation for generating adipose tissue grafts has been explored.20,36,38–43 A key issue of vascularization in bioengineered soft tissue has been addressed in a number of meritorious studies.39–41 For example, we demonstrated that a biophysical approach of building microchannels and/or delivery of bioactive cues such as basic fibroblast growth factor (bFGF) induces host-derived angiogenesis in bioengineered adipose tissue grafts.36 Remarkably, microchannels in a hydrogel system were shown to serve as conduits for host-derived blood vessels with or without an angiogenic growth factor, bFGF.36 In another example, mesenchymal stem cells were transplanted to the surfaces of the orofacial bone, yielding new bone that changed the contour of the facial appearance in mouse and swine.43 Pending issues for the reconstruction of soft tissues, including the face and breast, by the approach of cell transplantation are as follows:
Create and maintain the shape and dimensions of bioengineered adipose tissue in vivo for long term.
Promote the integration and remodeling of regenerated adipose tissue with host.
Scale up of bioengineered adipose tissue from the current small grafts (up to 6 mm in diameter in Ref.36 for example) to larger grafts that are sufficient for facial defects and breast cancer defects.
Match the structural and mechanical properties of bioengineered soft tissue grafts with the corresponding native tissue.
Ascertain the lack of adverse effects following long-term ex vivo cell manipulation and grafting.
Demonstrate the lack of tumor reoccurrence following soft tissue cancer ablation.
Cell Homing Strategies for Soft Tissue Reconstruction: Can Soft Tissue Be Reconstructed Without Cell Delivery?
Cell homing has been regarded as a process of exit of hematopoietic stem cells from blood vessels by transendothelization and subsequent migration. Here we broadly define cell homing as active recruitment of endogenous cells, including stem/progenitor cells, into an anatomic compartment. Cell homing differs from current filler injections. Most facial fillers do not allow cell homing or even passive cell ingrowth. Fillers further lack mechanisms for active recruitment of cells. In contrast, cell homing-based facial reconstruction or augmentation relies on the recruitment of endogenous cells with novel scaffold characteristics and/or bioactive cues. We postulate that certain facial tissue defects can be healed by cell homing and without cell transplantation (Fig. 2). Cell homing offers a number of advantages over cell transplantation for facial reconstruction and/or augmentation:
Induced homing of host endogenous cells overcomes some of the key scientific, technical, commercialization, and regulatory issues associated with cell transplantation, such as potential contamination, excessive cost, immunorejection, pathogen transmission, and a lack of training of current clinicians to handle cells.
Bioactive cues for cell homing, such as cytokines or chemokines, can be readily packaged as off-the-shelf products and delivered in a single procedure, as opposed to frequent multiple procedures in association with cell transplantation.
Prior instances of regulatory approval for cytokine and chemokine delivery.
Ease of clinical delivery of packaged and stored molecular delivery products.
Maximizes the body's own regenerative capacity.
A number of bioactive cues that participate in adipogenesis have been adopted in soft tissue engineering, including insulin-like growth factors (IGFs), epidermal growth factor, transforming growth factor β, platelet-derived growth factor, insulin, thyroid hormone, and dexamethasone. For example, bFGF adsorbed to Matrigel promotes adipogenesis in vivo.44 Matrigel contains high quantities of laminin-1, an extracellular protein that is preferential for preadipocyte adhesion and may act synergistically with bFGF to induce adipogenesis.45,46 In addition to bFGF, insulin and IGF-I have important functions during adipogenic differentiation. Ectopic islands of adipose tissue in deep muscular fascia of rat abdominal wall are generated upon local delivery of insulin and IGF-I by polylactic glycolic acid (PLGA)/poly (ethylene)-glycol microspheres within 4 weeks.47 We have recently demonstrated the combinatory effects of chemical and physical cues during adipogenic tissue engineering. Delivery of bFGF via poly(ethylene)-glycol-diacrylate hydrogel that contain physical microchannels induced host-derived angiogenesis in adipose tissue grafts by guided host tissue infiltration.36 The delivery of adipogenic and angiogenic factors have promising prospects in transforming otherwise inert and limited reconstructive soft tissue fillers into bioactive and long-lasting substitutes. In development, multiple bioactive cues are expressed in specific temporospatial patterns. Do these temporospatial patterns during natural development need to be recapitulated in therapeutic wound healing or regeneration? This is an open question that warrants serial investigations. Conceptually, it is probably neither necessary nor practical in tissue repair to replicate the temporospatial expression of myriads of bioactive cues that are expressed in development. Nonetheless, microencapsulation and controlled release of bioactive cues offer technical approaches to deliver pivotal bioactive cues in timed courses and in gradients that are most effective.47–50 Delivery of bioactive cues by in vivo injection is associated with rapid denaturation and diffusion.47 For example, we previously showed that controlled release of transforming growth factor β3 over time in vivo restored the morphogenesis of an otherwise synostosed cranial suture and generated a patent mesenchymal/fibrous tissue interface between cranial bones.50
Concluding Remarks
Biosurgery promises not only to minimize surgical trauma and repeated procedures, but also produce native tissue analogs that are long lasting. New breakthrough will likely originate from integrated strategies of cell biology, cytokine biology, biomaterials, chemical engineering, and tissue engineering approaches. Several examples of interdisciplinary approaches have been provided in this review. First, cell homing by bioactive cues offers an unprecedented opportunity for near-term translation of biologically based therapies for facial reconstruction and augmentation. Second, the critical issue of angiogenesis has been addressed by biophysical approaches such as microchannels and biological approaches such as angiogenic growth factors. Third, scale up, another critical issue for soft tissue regeneration, is being addressed by a number of innovative approaches including modularized bioscaffolds that promote angiogenesis and cell survival. The exciting era of human facial reconstruction is yet to come, but the end is near for current widespread clinical practice of tissue grafting, fillers, and prosthesis.
Acknowledgments
The authors thank Sarah Kennedy and Fen Guo for administrative and technical assistance. This research was supported by NIH grants DE015391 and EB006261 (to J.J.M).
Disclosure Statement
No competing financial interests exist.
References
- 1.U.S. Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; 2000. [Google Scholar]
- 2.Kim J. Zemnick C. Mao J.J. Restoration of oral and craniofacial defects by stem cells and bioengineering approaches. In: Nanda R., editor; Kapila S., editor. Science and Practice of Dentofacial Orthopedics. New York: Elsevier; 2009. pp. 134–149. [Google Scholar]
- 3.Tanaka N. Uchide N. Suzuki K. Tashiro T. Tomitsuka K. Kimijima Y. Amagasa T. Maxillofacial fractures in children. J Craniomaxillofac Surg. 1993;21:289. doi: 10.1016/s1010-5182(05)80349-x. [DOI] [PubMed] [Google Scholar]
- 4.Karlson T.A. The incidence of facial injuries from dog bites. JAMA. 1984;251:3265. [PubMed] [Google Scholar]
- 5.Eastridge B.J. Owsley J. Sebesta J. Beekley A. Wade C. Wildzunas R. Rhee P. Holcomb J. Admission physiology criteria after injury on the battlefield predict medical resource utilization and patient mortality. J Trauma. 2006;61:820. doi: 10.1097/01.ta.0000239508.94330.7a. [DOI] [PubMed] [Google Scholar]
- 6.Moro F. de Caro R. de Caro G. Ninfo V. Eyelid basal cell carcinoma with intracranial extension. Ophthal Plast Reconstr Surg. 1998;14:50. doi: 10.1097/00002341-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Erol O.O. Spira M. Reconstructing the breast mound employing a secondary island omental skin flap. Plast Reconstr Surg. 1990;86:510. doi: 10.1097/00006534-199009000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Kononas T.C. Bucky L.P. Hurley C. May J.W., Jr. The fate of suctioned and surgically removed fat after reimplantation for soft-tissue augmentation: a volumetric and histologic study in the rabbit. Plast Reconstr Surg. 1993;91:763. doi: 10.1097/00006534-199304001-00001. [DOI] [PubMed] [Google Scholar]
- 9.Butler D.L. Awad H.A. Perspectives on cell and collagen composites for tendon repair. Clin Orthop Relat Res. 1999;367:S324. doi: 10.1097/00003086-199910001-00031. [DOI] [PubMed] [Google Scholar]
- 10.Eppley B.L. Alloplastic implantation. Plast Reconstr Surg. 1999;104:1761. doi: 10.1097/00006534-199911000-00025. quiz 1784–5. [DOI] [PubMed] [Google Scholar]
- 11.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Benhaim P. Lorenzm H.P. Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 12.Hart D. Overcoming complications of breast implants. Plast Surg Nurs. 2003;23:55. doi: 10.1097/00006527-200323020-00005. [DOI] [PubMed] [Google Scholar]
- 13.Patrick C.W., Jr. Chauvin P.B. Hobley J. Reece G.P. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng. 1999;5:139. doi: 10.1089/ten.1999.5.139. [DOI] [PubMed] [Google Scholar]
- 14.Alster T.S. West T.B. Human-derived and new synthetic injectable materials for soft-tissue augmentation: current status and role in cosmetic surgery. Plast Reconstr Surg. 2000;105:2515. doi: 10.1097/00006534-200006000-00034. discussion 2526–2528. [DOI] [PubMed] [Google Scholar]
- 15.Beahm E.K. Walton R.L. Partick C.W. Progress in adipose tissue construct development. Clin Plast Surg. 2003;30:547, viii. doi: 10.1016/s0094-1298(03)00072-5. [DOI] [PubMed] [Google Scholar]
- 16.Moioli E.K. Hong L. Mao J.J. Inhibition of osteogenic differentiation of human mesenchymal stem cells. Wound Repair Regen. 2007;15:413. doi: 10.1111/j.1524-475X.2007.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eppley B.L. Dadvand B. Injectable soft-tissue fillers: clinical overview. Plast Reconstr Surg. 2006;118:98e. doi: 10.1097/01.prs.0000232436.91409.30. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi N. Toriyama K. Nicodemou-Lena E. Inou K. Torii S. Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA. 1998;95:1062. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alhadlaq A. Mao J.J. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 20.Alhadlaq A. Tang M. Mao J.J. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005;11:556. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 21.Davis G.M. Ringler S.L. Short K. Sherrick D. Bengtson B.P. Reduction mammaplasty: long-term efficacy, morbidity, and patient satisfaction. Plast Reconstr Surg. 1995;96:1106. [PubMed] [Google Scholar]
- 22.Atala A. Future perspectives in reconstructive surgery using tissue engineering. Urol Clin North Am. 1999;26:157, ix. doi: 10.1016/s0094-0143(99)80013-5. [DOI] [PubMed] [Google Scholar]
- 23.Fagien S. Elson M.L. Facial soft-tissue augmentation with allogeneic human tissue collagen matrix (Dermalogen and Dermaplant) Clin Plast Surg. 2001;28:63. [PubMed] [Google Scholar]
- 24.Walgenbach K.J. Shestak K.C. Pedicled TRAM breast reconstruction. Breast Dis. 2002;16:73. doi: 10.3233/bd-2002-16111. [DOI] [PubMed] [Google Scholar]
- 25.Garfein E.S. Orgill D.P. Pribaz J.J. Clinical applications of tissue engineered constructs. Clin Plast Surg. 2003;30:485. doi: 10.1016/s0094-1298(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 26.Ojo-Amaize E.A. Conte V. Lin H.C. Brucker R.F. Agopian M.S. Peter J.B. Silicone-specific blood lymphocyte response in women with silicone breast implants. Clin Diagn Lab Immunol. 1994;1:689. doi: 10.1128/cdli.1.6.689-695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katzin W.E. Feng L.J. Abbuhl M. Klein M.A. Phenotype of lymphocytes associated with the inflammatory reaction to silicone gel breast implants. Clin Diagn Lab Immunol. 1996;3:156. doi: 10.1128/cdli.3.2.156-161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Disa J.J. Chiaramonte M.F. Girotto J.A. Klein M.H. Goldberg N.H. Advantages of autologous fascia versus synthetic patch abdominal reconstruction in experimental animal defects. Plast Reconstr Surg. 2001;108:2086. doi: 10.1097/00006534-200112000-00040. [DOI] [PubMed] [Google Scholar]
- 29.Butler C.E. Orgill D.P. Yannas I.V. Compton C.C. Effect of keratinocyte seeding of collagen-glycosaminoglycan membranes on the regeneration of skin in a porcine model. Plast Reconstr Surg. 1998;101:1572. doi: 10.1097/00006534-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Billings E., Jr. May J.W., Jr. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83:368. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 31.Lee M.C. Lehman J.A., Jr. Tantri M.D. Parker M.G. Wagner D.S. Bilateral reduction mammoplasty in an adolescent population: adolescent bilateral reduction mammoplasty. J Craniofac Surg. 2003;14:691. doi: 10.1097/00001665-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Smahel J. Adipose tissue in plastic surgery. Ann Plast Surg. 1986;16:444. [PubMed] [Google Scholar]
- 33.Kononas T.C. Bucky L.P. Hurley C. May J.W., Jr. The fate of suctioned and surgically removed fat after reimplantation for soft-tissue augmentation: a volumetric and histologic study in the rabbit. Plast Reconstr Surg. 1993;91:763. doi: 10.1097/00006534-199304001-00001. [DOI] [PubMed] [Google Scholar]
- 34.Au P. Tam J. Fukumura D. Jain R.K. Small blood vessel engineering. Methods Mol Med. 2007;104:183. doi: 10.1007/978-1-59745-443-8_11. [DOI] [PubMed] [Google Scholar]
- 35.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 36.Stosich M.S. Bastian B. Marion N.W. Clark P.A. Reilly G. Mao J.J. Vascularized adipose tissue grafts from human mesenchymal stem cells with bioactive cues and microchannel conduits. Tissue Eng. 2007;13:2881. doi: 10.1089/ten.2007.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nature Report of Stem Cells. A Superficial Success. 2009. www.nature.com/stemcells/2009/0901/090115/full/stemcells.2008.163.html. [Feb 17;2010 ]. www.nature.com/stemcells/2009/0901/090115/full/stemcells.2008.163.html
- 38.Moioli E.K. Stosich M.S. Wu J.K. Shah B.S. Lee C.H. Mao J.J. Bioengineered soft tissue for plastic and reconstructive surgeries. In: Mao J.J., editor; Vunjak-Novakovic G., editor; Mikos A.G., editor; Atala A., editor. Translational Approaches in Tissue Engineering and Regenerative Medicine. Boston, MA: Artech House; 2007. pp. 209–230. [Google Scholar]
- 39.Fischbach C. Spruss T. Weiser B. Neubauer M. Becker C. Hacker M. Göpferich A. Blunk T. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res. 2004;300:54. doi: 10.1016/j.yexcr.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 40.Neels J.G. Thinnes T. Loskutoff D.J. Angiogenesis in in vivo model of adipose tissue development. FASEB J. 2004;18:983. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- 41.Hemmrich K. von Heimburg D. Rendchen R. Di Bartolo C. Milella E. Pallua N. Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials. 2005;26:7025. doi: 10.1016/j.biomaterials.2005.04.065. [DOI] [PubMed] [Google Scholar]
- 42.Stosich M.S. Mao J.J. Adipose tissue engineering from human mesenchymal stem cells: clinical implication in plastic and soft tissue reconstructive surgeries. Plast Reconstr Surg. 2007;119:71. doi: 10.1097/01.prs.0000244840.80661.e7. discussion 84–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang D. Seo B.M. Liu Y. Sonoyama W. Yamaza T. Zhang C. Wang S. Shi S. Transplantation of mesenchymal stem cells is an optimal approach for plastic surgery. Stem Cells. 2007;25:1021. doi: 10.1634/stemcells.2006-0576. [DOI] [PubMed] [Google Scholar]
- 44.Yoon E.S. Han S.K. Kim W.K. Advantages of the presence of living dermal fibroblasts within restylane for soft tissue augmentation. Ann Plast Surg. 2003;51:587. doi: 10.1097/01.sap.0000096424.23397.2a. [DOI] [PubMed] [Google Scholar]
- 45.Boss W.K., Jr. Usal H. Fodor P.B. Chernoff G. Autologous cultured fibroblasts: a protein repair system. Ann Plast Surg. 2000;44:536. doi: 10.1097/00000637-200044050-00013. [DOI] [PubMed] [Google Scholar]
- 46.Boss W.K., Jr. Usal H. Chernoff G. Keller G.S. Lask G.P. Fodor P.B. Autologous cultured fibroblasts as cellular therapy in plastic surgery. Clin Plast Surg. 2000;27:613. [PubMed] [Google Scholar]
- 47.Bostwick J., III Breast reconstruction following mastectomy. CA Cancer J Clin. 1995;45:289. doi: 10.3322/canjclin.45.5.289. [DOI] [PubMed] [Google Scholar]
- 48.Moioli E.K. Clark P.A. Xin X. Lal S. Mao J.J. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv Drug Deliv Rev. 2007;59:308. doi: 10.1016/j.addr.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark P.A. Moioli E.K. Sumner D.R. Mao J.J. Porous implants as drug delivery vehicles to augment host tissue integration. FASEB J. 2008;22:1684. doi: 10.1096/fj.07-094789. [DOI] [PubMed] [Google Scholar]
- 50.Moioli E.K. Clark P.A. Sumner D.R. Mao J.J. Autologous stem cell regeneration in craniosynostosis. Bone. 2008;42:332. doi: 10.1016/j.bone.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]