Abstract
The cellular properties of long-term potentiation (LTP) following pairing of pre- and postsynaptic activity were examined at a known glutamatergic synapse in the leech, specifically between the pressure (P) mechanosensory and anterior pagoda (AP) neurons. Stimulation of the presynaptic P cell (25 Hz) concurrent with a 2 nA depolarization of the postsynaptic AP cell significantly potentiated the P-to-AP excitatory postsynaptic potential (EPSP) in an N-methyl-d-aspartate receptor (NMDAR)-dependent manner based on inhibitory effects of the NMDAR antagonist MK801 and inhibition of the NMDAR glycine binding site by 7-chlorokynurenic acid. LTP was blocked by injection of bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid (BAPTA) into the postsynaptic (AP) cell, indicating a requirement for postsynaptic elevation of intracellular Ca2+. Autocamtide-2-related inhibitory peptide (AIP), a specific inhibitor of Ca2+/calmodulin-dependent kinase II (CaMKII), and Rp-cAMP, an inhibitor of protein kinase A (PKA), also blocked pairing-induced potentiation, indicating a requirement for activation of CaMKII and PKA. Interestingly, application of AIP during pairing resulted in significantly depressed synaptic transmission. Co-application of AIP with the protein phosphatase inhibitor okadaic acid restored synaptic transmission to baseline levels, suggesting an interaction between CaMKII and protein phosphatases during induction of activity-dependent synaptic plasticity. When postsynaptic activity preceded presynaptic activity, NMDAR-dependent long-term depression (LTD) was observed that was blocked by okadaic acid. Postsynaptic injection of botulinum toxin blocked P-to-AP potentiation while postsynaptic injection of pep2-SVKI, an inhibitor of AMPA receptor endocytosis, inhibited LTD, supporting the hypothesis that glutamate receptor trafficking contributes to both LTP and LTD at the P-to-AP synapse in the leech.
INTRODUCTION
N-methyl-d-aspartate-receptor (NMDAR)-dependent long-term potentiation (LTP) and long-term depression (LTD) have been extensively studied in the mammalian brain as a result of the central role that LTP and LTD play in modifying neural circuits in the context of neural development, sensory processing and learning and memory (Feldman 2009; Massey and Bashir 2007). NMDARs are also present in a wide range of invertebrates including Caernorhabditis elegans, Aplysia, the honey bee, and the leech (Brockie et al. 2001; Grey et al. 2009; Ha et al. 2006; Zannat et al. 2006), and NMDAR-dependent LTP and LTD have been reported in the mollusk Aplysia and the leech Hirudo (Burrell and Sahley 2004; Grey and Burrell 2008; Grey et al. 2009; Li and Burrell 2009; Lin and Glanzman 1994; Murphy and Glanzman 1997, 1999). In vertebrates, many forms of LTP and LTD require activation of NMDARs and increases in postsynaptic Ca2+. In the case of LTP, this increase in Ca2+ activates protein kinase A (PKA) and Ca2+/calmodulin-dependent kinase II (CaMKII), kinases that are thought to stimulate the insertion of additional of glutamate receptors to the postsynaptic surface (Kessels and Malinow 2009; Nayak et al. 1998). In the case of LTD, increases in Ca2+ activate protein phosphatases in hippocampal culture, which ultimately results in the removal of glutamate receptors from the postsynaptic surface (Beattie et al. 2000).
Evidence from Aplysia indicate that LTP in invertebrates also depends on increases in postsynaptic Ca2+ (Lin and Glanzman 1996; Murphy and Glanzman 1996), but it is not known whether invertebrate LTP and LTD utilizes the same cellular signaling processes, e.g., Ca2+/calmodulin kinase or protein phosphatases, as in vertebrates. Understanding the mechanisms that support NMDAR-dependent synaptic plasticity in invertebrates is important given the utility of these animals (especially in regards to their well characterized nervous systems and/or ease of genetic manipulation) in studying fundamental processes in nervous system function, including learning and memory. The leech provides a useful model system for examining the biochemical pathways that mediate pairing-induced synaptic potentiation. The leech possesses a distributed CNS with each ganglion containing ∼400 neurons, many of which can be readily identified, making it possible to test synaptic transmission between that same pair of cells across multiple preparations. In addition, removing individual ganglia for electrophysiological recordings preserves all the synaptic connections between neurons, an advantage not seen in slice preparations used in many vertebrate brain studies.
In this study, activity-dependent plasticity is examined at the synapse formed by the pressure-sensitive (P) neuron onto the anterior pagoda (AP) neuron. This P-to-AP synapse is glutamatergic (Wessel et al. 1999) and has both mono- and polysynaptic components (Gu 1991). In addition, NR1, the obligate NMDA subunit, transcript is present in the postsynaptic (AP) neuron (Grey et al. 2009). Previously, NMDAR-dependent potentiation of the P-to-AP synapse was observed following paired activation of the pre- and postsynaptic neurons (Grey et al. 2009) or following forskolin application (“chemical LTP”) (Grey and Burrell 2008). Here the intracellular signaling mechanisms mediating pairing-dependent synaptic plasticity are examined in more detail. LTP was observed when pre- and postsynaptic activity coincided and this potentiation required postsynaptic increases in intracellular Ca2+ and activation of PKA and CaMKII. LTD was observed when postsynaptic activity preceded presynaptic activity and required activation of protein phosphatases. Interestingly, when protein kinase activity was blocked during LTP induction, LTP was prevented and LTD was observed. This “uncovered” form of LTD could itself be blocked by application of the protein phosphatase inhibitor okadaic acid, indicating that LTP-inducing protocols can initiate both LTP and LTD signal cascades that interact with each other.
METHODS
Leeches, Hirudo verbana (Siddall et al. 2007), weighing 3 g were obtained from a commercial supplier (Leeches USA, Westbury, NY, or Niagra Medicinal Leeches, Niagra Falls, Ontario, Canada) and kept in pond water [0.52 g/l H2O Hirudo salt (Leeches USA)] at 15°C, under a 12 h light/dark cycle. Individual ganglia were dissected and placed in a recording chamber (1 ml) with constant superfusion (∼1 ml/min). Dissections and recordings were carried out in leech saline containing (mM): 115 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, and 10 HEPES.
Dual intracellular recordings were made by impaling individual neurons with a glass microelectrode using a micropositioner (Model 1480; Siskiyou, Grants Pass, OR). Electrodes were pulled from borosilicate capillary tubing (1.0 mm OD, 0.75 mm ID, FHC, Bowdoinham, ME) to a resistance of 25–35 MΩ and filled with 3 M potassium acetate. Signals were amplified with a bridge amplifier (BA-1S; NPI, Tamm, Germany) and then digitally converted (Digidata 1322A A/D converter) for viewing and subsequent analysis (Axoscope; Molecular Devices, Sunnyvale, CA). Individual neurons were identified based on their position, size, and action potential shape. Current pulses were delivered to individual neurons using a programmable stimulator (MultiChannel Systems STG 1004). Excitatory postsynaptic potentials (EPSPs) in the AP cell were elicited by brief, 1.5 nA, 10 ms current injections into a contralateral P cell. To prevent the initiation of action potentials, the AP neuron was hyperpolarized to the same membrane potential during both the pre- and posttests (−75 mV). Input resistance of the postsynaptic AP cell was measured throughout each experiment by injecting negative currents (0.5 nA, 500 ms). Typically, four to six EPSPs and seven to nine input resistance measurements were averaged per recording.
In all experiments, baseline EPSP amplitude and input resistance measurements were taken in normal saline. NMDAR-dependent synaptic plasticity requires the co-agonist glycine, therefore 1 μM glycine was superfused in the bath during pairing sessions (Burrell and Sahley 2004). The pairing protocol was based on studies by Lin and Glanzman (1997) and consisted of 25 Hz P cell stimulation (1.5 nA for 10 ms, 10 pulses) and a simultaneous depolarization of the AP cell (2 nA for 500 ms). Paired activation of the P and AP cells was repeated five times with an intertrial interval of 2 min [0 ms interspike interval (ISI), 2 min intertrial interval (ITI)]. The pairing protocol was followed by a 45 min consolidation period in normal saline, followed by a posttest of the P-to-AP EPSP and AP input resistance. A no stimulation control group consisted of 10 min superfusion of saline +1 μM glycine followed by a 45 min consolidation period in normal saline and posttest of the P-to-AP EPSP. Additional control experiments, such as presynaptic stimulation alone and postsynaptic stimulation alone, were performed and reported in an earlier study (Grey et al. 2009). Bath-applied drugs were delivered via superfusion during pairing. Iontophoretically injected drugs were delivered using 5 nA, 100 ms negative current pulses for 5 min prior to induction of the pairing protocol. For both types of drug treatments, control experiments were performed in the absence of the drug. Across all experiments (approximately 350 P-to-AP synapses), the average AP resting potential was approximately −40 mV, and the average initial input resistance was 12 ± 0.1 MΩ. EPSP amplitude and input resistance measurements were taken at the conclusion of each 60 min experiment, normalized to their initial values (% of baseline), and presented as the means ± SE. Cells were excluded if input resistance changed >30% from baseline. In addition, only cells with initial EPSPs <7 mV were studied, as synapses >7 mV did not potentiate, consistent with observations in both the leech and vertebrates that synapses with an especially large EPSP amplitude do not potentiate (Bi and Poo 1998; Burrell and Sahley 2004; Grey and Burrell 2008; Montgomery et al. 2001).
Solutions
Bath-applied drugs were dissolved in dH2O, except where noted, and superfused in leech saline +1 μM glycine at the following concentrations: autocamtide-2-related inhibitory peptide (AIP; 1 μM, Tocris, Ellisville, MO); cyclopiazonic acid (CPA; 20 μM, Tocris) dissolved in DMSO; 7-chloro-kynurenic acid (7-Cl-KYNA, 20 μM, Tocris); MK-801 (40 μM, Sigma); nimodipine (10 μM, Tocris) dissolved in methanol; okadaic acid (OkA, 1 μM, Sigma) dissolved in DMSO; Rp-cAMP (50 μM, Sigma); ryanodine (20 μM, Tocris) dissolved in ethanol; TMB-8 (100 μM, Sigma) dissolved in DMSO. The following drugs were dissolved in 3 M KAc and iontophoresed directly into the cell: bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid (BAPTA, 1 mM, Sigma); botulinum neurotoxin type B light chain (0.5 μM, List Biological Laboratories, Campbell, CA); EGTA (1 μM-0.5 mM, Sigma); pep2-SVKI (100 μM, Tocris).
Statistics
Statistical tests were conducted using Statistica analysis software (Statsoft). Statistical significance (P < 0.05) was determined using a one-way ANOVA and Tukey's HSD post hoc comparison for all experiments unless otherwise indicated.
RESULTS
Pairing-induced potentiation requires NMDAR activation
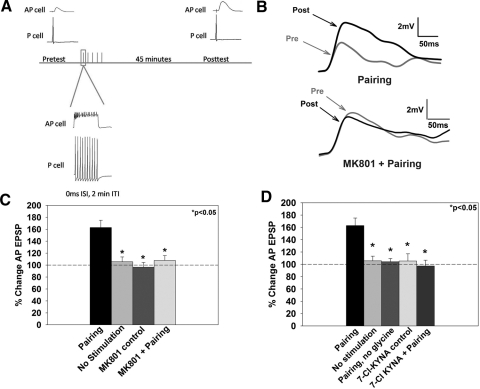
Administration of paired P and AP cell activation resulted in significant potentiation of the P-to-AP EPSP (pairing, 163 ± 12%, n = 15; Fig. 1, B and C) compared with no stimulation control synapses (no stimulation, 106 ± 8%, n = 10; P < 0.05). An earlier study of LTP at this connection also demonstrated that no potentiation was observed when only the P or AP cell was activated (Grey et al. 2009). Previous studies have found that both pairing-dependent LTP and forskolin-induced chemical LTP at the P-to-AP synapse were blocked by the competitive NMDAR antagonist 2-amino-5-phosponopentanoic acid (AP5) (Grey and Burrell 2008; Grey et al. 2009). To confirm that pairing-induced LTP required NMDAR activation, experiments were carried out in the presence of the NMDAR open channel blocker MK801 (40 μM). The amino acids required for MK801 binding are conserved in the leech (Grey et al. 2009), and MK801 has been shown to block currents in C. elegans (Brockie et al. 2001). MK801 blocked the potentiation when applied during pairing (pairing + MK801, 108 ± 8%, n = 6; P < 0.05; Fig. 1C) without an effect on baseline synaptic transmission when applied without pairing [MK801 control, 96 ± 8%, n = 9; F(3,27) = 11.598, P < 0.001; Fig. 1C]. No change in input resistance was observed between pairing and no pairing synapses (P > 0.05).
Fig. 1.
Paired pre- and postsynaptic activity induces potentiation at the pressure (P)-to-anterior pagoda (AP) synapse. A: schematic of the pairing protocol. Pre- and posttest excitatory postsynaptic potential (EPSP) measurements in the postsynaptic AP cell were obtained by eliciting a single presynaptic (P cell) action potential. Pairing consisted of a 10 pulse train (25 Hz, 10 ms) applied to the P-cell coinciding with a 2 nA step depolarization (500 ms) of the AP cell. Posttest EPSP measurements were completed after a 45 min consolidation period. B: representative EPSP traces prior to (pre) and following pairing (post) from synapses that underwent pairing in normal saline (top) or in MK801 (bottom). The gray trace denotes the pretest EPSP and the black trace denotes the posttest EPSP. C: effects of the N-methyl-d-aspartate receptor (NMDAR) antagonist, MK801, on long-term potentiation (LTP). Pairing-induced LTP was blocked by the application of the NMDAR antagonist MK801 (pairing + MK801). No stimulation and MK801 control groups are significantly different from the pairing group, indicating that potentiation only occurs following coordinated activation of the prepostsynaptic neurons. D: role of glycine during LTP. Elimination of glycine from the bath during the training protocol (pairing, no glycine) or application of 7-chlorokynurenic acid (7-Cl KYNA; pairing +7-Cl KYNA), which blocks the NMDAR glycine binding site, prevented pairing-induced potentiation. Administration of 7-Cl KYNA alone (7-Cl-KYNA control) did not significantly affect baseline synaptic transmission. Asterisks indicates statistically significant difference relative to the pairing group.
Glycine is an obligatory co-agonist for NMDAR function and testing glycine-dependence of P-to-AP LTP would provide further support for NMDAR involvement. In previous experiments at the P-to-S synapse, glycine was required during induction of LTP (Burrell and Sahley 2004). Therefore to test whether P-to-AP plasticity showed the same glycine dependence, glycine was omitted from the saline superfusion during the P + AP pairing protocol, and this resulted in no observed potentiation (pairing, no glycine, 104 ± 5%, n = 5; P < 0.05; Fig. 1D). In a second set of experiments, the pairing protocol (with 1 μM glycine) was administered in the presence of 7-Cl-KYNA (20 μM), an inhibitor of the NMDAR glycine-binding site. 7-Cl-KYNA blocked pairing-induced potentiation (pairing + 7-CL KYNA, 97 ± 9%; n = 8; P < 0.05) but did not affect basal synaptic transmission when 7-Cl-KYNA was applied without P + AP pairing [7-Cl-KYNA control, 105 ± 12%, n = 11; F(4,30) = 8.034, P < 0.001; Fig. 1D; 87 total experiments for Fig. 1]. Input resistance was not significantly different between treatments (P > 0.05).
Pairing-induced potentiation requires an increase in postsynaptic intracellular Ca2+
In both mammals and the marine mollusc Aplysia, increases in postsynaptic Ca2+ are necessary for the expression of LTP (Lynch et al. 1983; Murphy and Glanzman 1996) presumably due to Ca2+ influx through NMDARs. To determine whether pairing-induced potentiation requires postsynaptic increases in intracellular Ca2+ in the leech, the Ca2+ chelator BAPTA (1 mM) was iontophoretically injected into the AP (postsynaptic) cell for 5 min prior to the administration of the pairing protocol. Previous studies indicate that BAPTA reaches synaptic regions of leech neurons within this period of time (Grey and Burrell 2008). Injection of BAPTA into the postsynaptic AP cell before pairing blocked LTP (AP BAPTA + pairing, 85 ± 9%, n = 5; P < 0.05; Fig. 2A), indicating that this potentiation depends on an increase in postsynaptic intracellular Ca2+. Injection of BAPTA into the postsynaptic cell alone did not alter baseline synaptic transmission (AP BATPA control, 93 ± 7%, n = 5, P < 0.05; F(2,16) = 30.156, P < 0.001), and there was no significant change in input resistance for any of the BAPTA groups (P > 0.05).
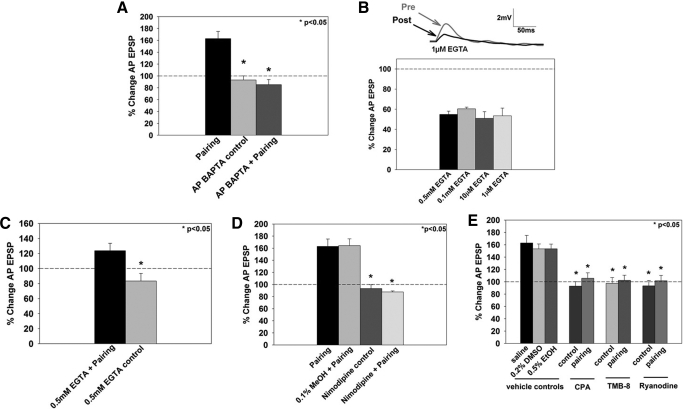
Fig. 2.
Role of multiple sources of postsynaptic Ca2+ during pairing-induced LTP. A: effects of postsynaptic bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid (BAPTA) injection on LTP. No potentiation was observed when BAPTA was injected into the postsynaptic (AP) cell before administration of the pairing protocol (AP BAPTA + pairing). Postsynaptic injection of BAPTA alone did not affect baseline synaptic transmission (AP BAPTA control). B: effects of presynaptic EGTA injection on the P-to-AP EPSP. After 5 min of iontophoretically injected EGTA into the presynaptic (P) cell, evoked P-to-AP synaptic transmission decreased, on average, 50% from preEGTA treatment levels. Top: representative EPSP traces from a P-to-AP synapse treated with 1 μM EGTA. The posttest (post, gray trace) was conducted 1 h after the pretest (pre, black trace). Bottom: averaged change in EPSP size following presynaptic EGTA treatment for concentrations between 1 μM to 0.5 mM. C: effects of presynaptic EGTA injection on LTP. Iontophoresis of 0.5 mM EGTA into the presynaptic (P cell) before administration of the pairing protocol produced potentiation relative to the EGTA control (EGTA treatment, but no pairing). D: effects of nimodipine of LTP. Nimodipine blocked pairing-induced potentiation (nimodipine + pairing). Methanol, the vehicle control for nimodipine, did not affect pairing-induced potentiation (0.1% vol/vol methanol + pairing), and nimodipine administration alone did not affect baseline synaptic transmission (nimodipine control). E: effects of inhibition of release from intracellular Ca2+ stores on LTP. Inhibition of Ca2+ release from intracellular stores also contributed to pairing-induced LTP, which was blocked by depletion of Ca2+ stores by cyclopiazonic acid (CPA), inhibition of IP3 receptors by TMB-8, or by inhibition of ryanodine receptors by ryanodine. The vehicle controls, DMSO for CPA and TMB-8 and ethanol for ryanodine, did not affect potentiation. *, statistically significant difference relative to the pairing group.
The role of presynaptic Ca2+ during pairing-induced LTP was also examined. Another Ca2+ chelator, EGTA, was used to assess Ca2+ increases in the P cell because this chelator has slower kinetics than BAPTA (Nevian and Sakmann 2006) and therefore would be less likely to disrupt synaptic transmission. Nevertheless, presynaptic EGTA did substantially reduce basal P-to-AP synaptic transmission, even at concentrations as low as 1 μM (Fig. 2B; P-to-AP EPSP tested following 5 min EGTA injection), consistent with observations by Ivanov and Calabrese (2006). Despite this reduction in synaptic transmission, significant potentiation was observed in synapses that underwent pairing after EGTA injection compared with synapses that were treated with EGTA but did not undergo pairing [EGTA + pairing, 124 ± 10%, n = 4; EGTA control, 84 ± 10%, n = 7; P < 0.05; F(1,9) = 6.882, P = 0.028; Fig. 2C] even though the level of potentiation in the EGTA-treated synapses was reduced compared with LTP in untreated ganglia (compare black bars in Fig. 2, A and C). One interpretation of these results is that EGTA reduced basal synaptic transmission, but LTP was unaffected and the reduction of potentiation is simply a consequence of EGTA's effect on synaptic transmission. Support for this explanation comes from a previous study of the P-to-AP synapse in which NMDAR-dependent LTP was induced by forskolin application and not by activation of P and AP neurons. In those experiments, presynaptic injection of Ca2+ chelators did not affect this chemical LTP, supporting the idea that potentiation did not involve increases in presynaptic Ca2+ levels (Grey and Burrell 2008).
In addition to NMDARs, other sources of Ca2+ may also contribute to the increase in postsynaptic Ca2+ seen in this pairing-induced potentiation, such as voltage-gated Ca2+ channels (VGCCs) and release of Ca2+ from internal stores, the latter potentially mediated by either IP3 or ryanodine receptors (Balschun et al. 1999; Kapur et al. 1998; Vargas et al. 2007). To test whether LTP requires VGCC activation, nimodipine (10 μM) was superfused during administration of the pairing protocol. Dihydropyridines, such as nimodipine and nifedipine, have been reported to be effective in invertebrates (Dierkes et al. 2004; Elliot et al. 1993; but see Kleinhaus and Angstadt 1995). Because VGCCs are located both pre- and postsynaptically, application of nimodipine could potentially interfere with synaptic transmission during administration of the pairing protocol. To test this possibility, the P-to-AP EPSP was tested directly after 10 min of nimodipine application and was not found to affect the P-to-AP EPSP (100 ± 3%, n = 3, data not shown). Application of nimodipine during pairing blocked potentiation without affecting baseline synaptic transmission or input resistance (nimodipine + pairing, 88 ± 2%, n = 5; nimodipine control, 93 ± 6%, n = 6; P < 0.05; input resistance P > 0.05; Fig. 2D). Nimodipine was dissolved in methanol, yielding a final methanol concentration of 0.1% vol/vol. As a vehicle control for nimodipine, 0.1% vol/vol methanol in saline was applied during pairing, but was not found to disrupt LTP in this synapse [0.1% MeOH + pairing, 164 ± 11%, n = 11; P < 0.05; F(3,24) = 17.043, P < 0.001; Fig. 2D].
The role of Ca2+ release from intracellular stores during LTP was tested by three different drugs. First, CPA inhibits the Ca2+-pump, thereby depleting internal Ca2+ stores and has been shown to be effective in both Hirudo and Helix neurons (Beck et al. 2001; Willoughby et al. 2001). Superfusion of CPA (20 μM) during P + AP pairing blocked LTP (CPA + pairing, 106 ± 9%, n = 8; P < 0.05), whereas CPA application in the absence of pairing did not affect baseline synaptic transmission (CPA control, 93 ± 7%, n = 5; P < 0.05; Fig. 2E). Second, to test the role of IP3-receptor mediated store-released Ca2+, the pairing protocol was administered in the presence of TMB-8, which has been shown to inhibit IP3-induced Ca2+ release in sea urchin eggs (Clapper and Lee 1985). Application of TMB-8 (100 μM) blocked pairing-induced potentiation without affecting baseline synaptic transmission (TMB-8 + pairing, 102 ± 8%, n = 8; TMB-8 control, 98 ± 9%, n = 5; P < 0.05; Fig. 2E). Third, the role of ryanodine receptor (RyR)-mediated release from intracellular Ca2+ stores was also examined. Ryanodine, at micromolar concentrations (Sutko et al. 1997), is a selective inhibitor for RyRs and has been shown to be effective in a variety of invertebrates, including Aplysia, Hirudo, and Drosophila (Geiger and Magoski 2008; Trueta et al. 2004; Xu et al. 2000). Application of ryanodine (50 μM) in conjunction with the pairing protocol blocked LTP [ryanodine + pairing, 102 ± 9%, n = 10; ryanodine control, 93 ± 9%, n = 11; P < 0.05; F(8,68) = 10.898, P < 0.001; Fig. 2E], indicating that RyR-mediated Ca2+ release is necessary for pairing-induced potentiation of the P-to-AP synapse. Neither DMSO (0.2% vol/vol), which was used as a solvent for both CPA and TMB-8, nor ethanol (0.5% vol/vol), the solvent for ryanodine, significantly affected LTP (saline (pairing), 163 ± 12%, n = 15; DMSO + pairing, 153 ± 8%, n = 16; ethanol + pairing, 153 ± 7%, n = 9; both vehicle controls P > 0.05 compared with saline (pairing); Fig. 2E). There was no significant change in input resistance for any of the Ca2+ drug groups (P > 0.05; n = 146 total experiments shown in Fig. 2). These results indicate that IP3-receptor and RyR-mediated release of Ca2+ from intracellular stores contribute to pairing-dependent LTP in the leech. It is possible that these processes contribute to postsynaptic increases in intracellular Ca2+, but additional experiments using injectable, membrane-impermeant blockers of these receptors are needed to confirm this conclusion.
Role of CaMKII and PKA during pairing-induced LTP
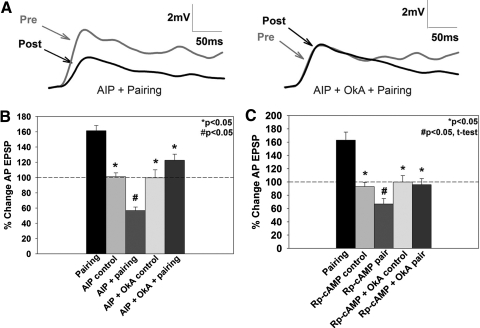
Given the requirement for Ca2+ signaling during P-to-AP LTP, the role of Ca2+-activated biochemical pathways known to mediate LTP in vertebrates, specifically CaMKII and PKA, was investigated (Blitzer et al. 1998; Miyamoto 2006; Yang et al. 2004; Zheng and Keifer 2009). The specific CaMKII inhibitor, AIP, has been shown to block both vertebrate LTP (Yang et al. 2004) and forskolin-induced chemical LTP in the leech (Grey and Burrell 2008). Application of AIP (0.1 μM) during the pairing protocol blocked pairing-induced potentiation in the P-to-AP synapse (Figs. 3, A and B). Interestingly, AIP treatment during pairing resulted in significant depression of the P-to-AP EPSP (AIP + pairing, 57 ± 5%, n = 6, P < 0.05; Fig. 3B). This was observed only when AIP and pairing were combined and was not the result of nonspecific effects of AIP on P-to-AP synaptic transmission. The AIP + pairing group was significantly different from both the pairing group in normal saline and control groups in which AIP was applied but pairing was omitted [AIP control, 101 ± 5%, n = 7, P < 0.05; F(4,29) = 22.751, P < 0.001; Fig. 3B]. In addition, no changes were observed in input resistance as a result of AIP treatment (P > 0.05). This apparent unmasking of synaptic depression in the pairing + AIP group was also seen when AIP was applied during forskolin-induced chemical LTP in the leech (Grey and Burrell 2008).
Fig. 3.
Effects of Ca2+/calmodulin-dependent kinase II (CaMKII) and protein kinase A (PKA) on pairing-induced LTP. A: representative traces showing synaptic depression in the autocamtide-2-related inhibitory peptide (AIP) + pairing group (left). No depression was observed when okadaic acid (OkA) was applied in addition to AIP + pairing (AIP + OkA + pairing; right). B: application of the CaMKII inhibitor, AIP, in conjunction with the pairing protocol significantly depressed the P-to-AP EPSP, as measured by a 1-way ANOVA (see results) compared with the AIP control group where no change in EPSP amplitude was observed. Application of OkA in conjunction with AIP + pairing prevented this depression (AIP + OkA + pairing). C: application of the PKA inhibitor, Rp-cAMP, induced depression (#, P < 0.05) compared with Rp-cAMP controls, as analyzed by an independent t-test. Similar to experiments involving inhibition of CaMKII, OkA combined with Rp-cAMP and pairing (Rp-cAMP + OkA + pairing) prevented this depression. *, statistically significant difference relative to the pairing group.
What is the explanation for this observed depression in AIP + pairing experiments? One possibility is that the molecular signaling pathways associated with both synaptic potentiation and depression are activated by the pairing protocol, but functional expression of synaptic depression is suppressed by CaMKII activity. Protein phosphatases are known to mediate NMDAR-dependent LTD in vertebrates (Anwyl 2006) and are also required for NMDAR-dependent LTD in synapses made by the leech T-to-S synapse (Li and Burrell 2009). In addition, modeling studies by Pi and Lisman (2008) have shown that interactions between protein phosphatase 2A and CaMKII can play a critical role in determining whether synapses express LTP or LTD. Therefore it is possible that the depression observed AIP + pairing synapses could be prevented by blocking the activity of protein phosphatases. To test this hypothesis, OkA (1 μM), which inhibits protein phosphatase 1 and 2A, was co-applied with AIP during pairing. The biochemical properties of protein phosphatases have been characterized in Aplysia, showing similar characteristics to vertebrate protein phosphatases (Endo et al. 1992). Furthermore, OkA has been used to block NMDAR-dependent long-term habituation in Aplysia and LTD at the leech T-to-S synapse (Ezzeddine and Glanzman 2003; Li and Burrell 2009). Co-application of OkA with AIP during pairing blocked the depression observed in AIP + pairing synapses [AIP + OkA + pairing, 123 ± 8%, n = 8; AIP + OkA control, 100 ± 11%, n = 7; P < 0.05; F(4,29) = 22.751, P < 0.001; Fig. 3B] with no change in input resistance (P > 0.05). While a modest level of potentiation was observed in AIP + OkA + pairing synapses, the percent change in EPSP was not statistically different from control preparations that did not undergo pairing.
The potential role of PKA in LTP in the leech was examined using Rp-cAMP, a competitive inhibitor of PKA previously used to block PKA-dependent neuromodulation both in Aplysia and the leech (Burrell and Sahley 2005; Grey and Burrell 2008; Hochner and Kandel 1992). Application of Rp-cAMP (50 μM) during pairing (Rp-cAMP + pairing) blocked LTP and produced depression of the P-to-AP EPSP, similar to the AIP + pairing experiments (Fig. 3, B and C). The level of depression was not statistically significant compared with Rp-cAMP control synapses when analyzed by a one-way ANOVA (Rp-cAMP + pairing, 69 ± 6%, n = 15; P = 0.128; Fig. 3C), but a significant difference was observed between the Rp-cAMP control and Rp-cAMP + pairing groups when analyzed by an independent t-test (P = 0.008, t = 2.864, df = 25). Rp-cAMP alone did not alter P-to-AP synaptic transmission (Rp-cAMP control, 95 ± 6%, n = 12; P < 0.05), indicating that the depression observed in the Rp-cAMP + pairing group was not due to Rp-cAMP acting on basal synaptic transmission. Because the observed depression is similar to the results observed when CaMKII inhibition was combined with pairing (Fig. 3B), the effects of combining OkA with Rp-cAMP + pairing were examined. Co-application of OkA and Rp-cAMP during pairing produced synaptic transmission similar to baseline levels (Rp-cAMP + OkA + pairing, 96 ± 9%, n = 9; nonsignificant trend by ANOVA; Fig. 3, B and C). When the differences between the Rp-cAMP + pairing and Rp-cAMP + OkA + pairing groups were analyzed by an independent t-test, this revealed an apparent reversal of the LTD observed in the Rp-cAMP + pairing by OkA (P < 0.05). Rp-cAMP + OkA did not affect baseline synaptic transmission [Rp-cAMP + OkA control, 100 ± 10%, n = 14; P < 0.05; F(4,51) = 12.252, P < 0.001; Fig. 3C]. No changes in input resistance were observed between drug-treated and control groups (P > 0.05; 93 total experiments for Fig. 3). These results suggest pairing-induced potentiation requires activation of the CaMKII and PKA signaling pathways and that these pathways interact with and likely suppress protein phosphatase activity.
Negative pairing can produce NMDAR-dependent LTD
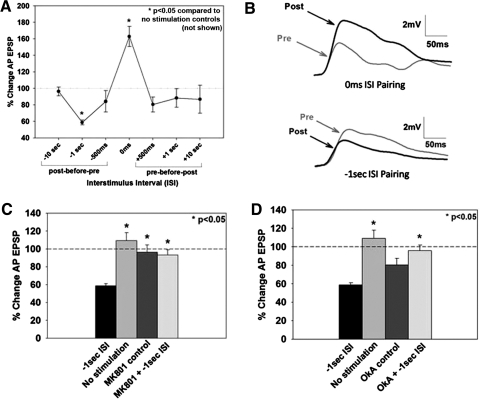
In all of the experiments described so far, LTP has been induced by the simultaneous activation of the pre- (P cell) and postsynaptic (AP cell) neurons. Because alterations in the temporal order of pre- and postsynaptic stimulation can change the polarity of synaptic plasticity (Bi and Poo 1998), the effects of different intervals between the pre- and postsynaptic stimulation during pairing were examined. Specifically, experiments were conducted in which presynaptic stimulation preceded postsynaptic stimulation by 500 ms, 1 s, and 10 s, and postsynaptic preceded presynaptic stimulation by 500 ms, 1 s, and 10 s (referred to as negative intervals). No significant changes in the P-to-AP EPSP were observed at any of the additional intervals tested except when postsynaptic (AP) stimulation preceded presynaptic (P) stimulation by 1 s, which produced significant depression of synaptic transmission [−1 s, post-before-pre, 58 ± 3%, n = 9; P < 0.05 compared with the no stimulation control (not shown in figure); F(7,52) = 7.255, P < 0.001; Fig. 4, A and B]. To investigate whether this form of depression was NMDAR-dependent, MK801 (40 μM) was applied during administration of the −1 s pairing protocol. MK801 blocked −1 s pairing-induced LTD, demonstrating this form of synaptic depression is NMDAR-dependent [MK801 + −1 s ISI, 93 ± 6%, n = 6; P < 0.05; F(3,30) = 10.233, P < 0.001; Fig. 4C]. To assess whether protein phosphatase activation is required for LTD, OkA was applied during −1 s pairing. OkA (1 μM) blocked −1 s pairing-induced LTD (OkA + −1 s ISI, 96 ± 6%, n = 7; P < 0.05), despite significantly depressing baseline synaptic transmission [OkA control, 80 ± 7%, n = 7; F(3,29) = 11.592, P < 0.001; Fig. 4D total number of synapses in Fig. 4 is 89].
Fig. 4.
Effects of the alteration of temporal order of paired P and AP cell activation on synaptic plasticity. A: shifting the relative onset between pre- and postsynaptic stimulation produces 2 windows of plasticity, 1 at the 0 ms ISI time point that resulted in potentiation and a 2nd at the −1 s interspike interval (ISI) that resulted in depression, relative to the no stimulation control group (not shown). B: representative pre- and postsynaptic EPSP traces from synapses that have undergone coincident pairing (0 ms ISI) resulting in LTP and from synapses in which AP activity preceded P cell activity (−1 s ISI) resulting in long-term depression (LTD). C: the LTD produced by −1 s ISI protocol was blocked by the NMDAR antagonist MK801. D: application of OkA during negative pairing blocked depression. OkA by itself yields a slightly depressed baseline compared with no stimulation controls. *, statistically significant difference relative to the no stimulation control group.
Glutamate receptor trafficking
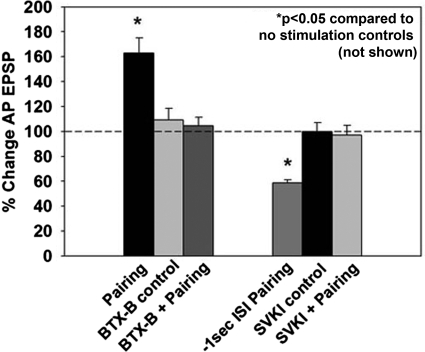
Insertion of AMPA-type glutamate receptors into the postsynaptic membrane is thought to be a critical component of LTP expression (Kessels and Malinow 2009). There are no known antibodies that recognize AMPA receptors in the leech; therefore an alternative approach was employed. Botulinum toxin type B (BTX-B) cleaves SNARE proteins necessary for exocytosis (Montecucco and Schiavo 1995), including those in the leech (Bruns et al. 1997). BTX-B injections inhibit synaptic potentiation, presumably by blocking insertion of glutamate receptors into the postsynaptic membrane, and BTX-B injections have been used to inhibit synaptic potentiation in both vertebrates and invertebrates (Antonov et al. 2007; Chitwood et al. 2001; Frey et al. 2009; Jin and Hawkins 2003; Li et al. 2005). Iontophoresis of BTX-B into the postsynaptic AP-cell prevented pairing-induced LTP (BTX-B + pairing, 104 ± 6%, n = 6; P < 0.05; Fig. 5), while postsynaptic BTX-B treatment by itself did not affect synaptic transmission [BTX-B control, 109 ± 9%, n = 5; P < 0.05; F(3,23) = 11.37, P < 0.001] or input resistance (P > 0.05). These results are consistent with the hypothesis that pairing-induced potentiation in the glutamatergic P-to-AP synapse requires the postsynaptic insertion of glutamate receptors although the possibility that BTX-B is blocking an alternative postsynaptic exocytotic event necessary for LTP cannot be excluded.
Fig. 5.
Effects of inhibition of postsynaptic receptor trafficking on pairing-induced plasticity. Iontophoresis of botulinum toxin type B (BTX-B), which is thought to prevent exocytosis of glutamate receptors into the postsynaptic membrane, into the postsynaptic (AP) cell prior to training completely blocked pairing-induced potentiation. BTX-B iontophoresis into the AP cell by itself did not affect baseline synaptic transmission. Iontophoresis of SVKI, which inhibits endocytosis of AMPA-type glutamate receptors, into the postsynaptic cell prior to administration of the negative pairing protocol blocked pairing-induced LTD. Postsynaptic SVKI treatment without pairing did not affect synaptic transmission.
Removal of glutamate receptors from the postsynaptic membrane is thought to underlie LTD (Beattie et al. 2000; Lüscher et al. 1999). To test whether inhibition of glutamate receptor removal would block LTD in the leech, −1 s pairing was conducted in the presence of SVKI, a peptide that inhibits the endocytosis of AMPA receptors by blocking interactions between the GluR2 subunit and scaffolding proteins (Daw et al. 2000). Invertebrate AMPA-type glutamate receptors do contain PDZ-binding domains that are responsible for interactions with scaffolding proteins and the mechanisms involved in glutamate receptor trafficking appear to be conserved between vertebrates and invertebrates (Chang and Rongo 2005; Walker et al. 2006). Furthermore, SVKI inhibited synaptic depression in leech T-to-T synapses (Li and Burrell 2008). Iontophoresis of SVKI (100 μM) into the postsynaptic AP cell during −1 s pairing blocked LTD (SVKI + pairing, 97 ± 8%, n = 5; P < 0.05; Fig. 5) but did not affect basal synaptic transmission [SVKI control, 100 ± 7%, n = 5; P < 0.05; F(3,25) = 12.44, P < 0.001; Fig. 5] or input resistance (P > 0.05; n = 36 total experiments shown in Fig. 5). These results are consistent with the hypothesis that LTD in the P-to-AP synapse requires the removal of glutamate receptors from the postsynaptic membrane.
DISCUSSION
Paired activation of pre- and postsynaptic spike trains can elicit NMDAR-dependent, bidirectional plasticity in a leech glutamatergic synapse. The pattern of plasticity in the present experiments is similar to what others have observed following pairing of pre- and postsynaptic trains of action potentials in both vertebrates and invertebrates (Butts et al. 2007; Lin and Glanzman 1997). Specifically, pairing of bursts resulted in potentiation only when pre- and poststimulation occurred simultaneously (0 ms ISI); when pre-and postbursts were desynchronized, little or no synaptic plasticity was observed. One difference from these earlier findings is that in the present experiments post-before-pre pairing (that is, AP before P) of 1 s elicits robust synaptic depression.
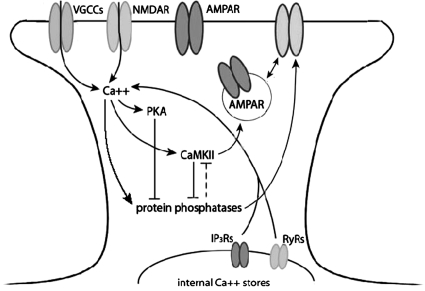
P-to-AP LTP was blocked by the NMDAR antagonist MK801 and by inhibition of the NMDAR glycine binding site, either through application of 7-Cl-KYNA, which blocks the NMDAR glycine binding site, or by omitting glycine during pairing. These findings confirm earlier reports that LTP in the leech P-to-S and P-to-AP synapses was NMDAR-dependent, based on the inhibitory effect of the NMDAR competitive antagonist AP5 (Burrell and Sahley 2004; Grey et al. 2009). LTP in the leech required postsynaptic increases in intracellular Ca2+, given that postsynaptic injection of BAPTA prevented pairing-induced potentiation. The results of the presynaptic (P cell) EGTA injections suggest that increases in presynaptic Ca2+ are not required for pairing-induced LTP, consistent with previous experiments in which increases in presynaptic Ca2+ were not required for NMDAR-dependent LTP induced by forskolin (Grey and Burrell 2008). In addition to NMDARs, increases in intracellular Ca2+ appear to require VGCC activation; release of Ca2+ from internal stores, mediated by both ryanodine and IP3 receptors may contribute to LTP induction as well. PKA and CaMKII activity is also required for LTP at this synapse as is the insertion of additional glutamate receptors into the postsynaptic membrane. A schematic of the cellular mediators of burst-pairing LTP and LTD explored here is summarized in Fig. 6.
Fig. 6.
Model of cellular events in pairing-induced LTP and LTD in the leech. This schematic represents the mechanisms of pairing-induced NMDAR-dependent LTP and LTD examined in this paper. Solid lines represent pathways of pharmacological manipulation reported in this paper, and dashed pathways represent likely events based on evidence reported in the literature. Arrows indicate an activation or increase of the molecule, and a T-junction indicates inhibition of the molecule. Activation of the NMDAR allows for Ca2+ into the postsynaptic cell, which activates PKA, CaMKII, and protein phosphatases. CaMKII and protein phosphatases mutually inhibit each other, and PKA can inhibit PPs. Activation of CaMKII and protein phosphatases have been shown to promote AMPA receptor trafficking, correspondingly inserting and removing receptors from the postsynaptic membrane.
LTD at the P-to-AP synapse was elicited when postsynaptic activity preceded presynaptic activity by 1 s. In addition, P-to-AP LTD was NMDAR-dependent and required protein phosphatase activation and endocytosis of postsynaptic glutamate receptors. These properties have been observed in LTD at other synapses in the leech CNS, indicating that this form of synaptic depression is not limited to the P-to-AP synapse (Burrell and Sahley 2004; Li and Burrell 2008, 2009). These findings indicate that synapses in the leech have the capacity for bidirectional synaptic plasticity.
The evidence for glutamate receptor trafficking during LTP and LTD in the P-to-AP synapse is, admittedly, indirect given that there are no tools to directly label leech glutamate receptors. The ability of BTX-B to block LTP has been used as evidence for glutamate receptor insertion in both vertebrates and invertebrates (Antonov et al. 2007; Chitwood et al. 2001; Frey et al. 2009; Jin and Hawkins 2003; Li et al. 2005). However, it is also possible that BTX-B prevents potentiation via alternative processes, such as blocking the postsynaptic release of a retrograde transmitter necessary for LTP although no evidence of such a retrograde signaling mechanism has yet been reported. Glutamate receptor removal from the postsynaptic membrane contributes to P-to-AP LTD as iontophoresis of SVKI blocked depression following −1 s pairing of P and AP activity. SVKI selectively inhibits endocytosis of AMPA receptors, and has been shown to prevent LTD in vertebrate synapses, by disrupting the GluR2 subunit interaction with glutamate receptor interacting protein (GRIP), AMPA receptor binding protein (ABP), and protein interacting with C kinase (PICK1) at the GluR2 PDZ-binding domain. Although it is not known precisely what AMPA receptor subtype is trafficked in these leech synapses, the SVKI target sequence is found in invertebrate glutamate receptors, and the mechanisms involved in glutamate receptor trafficking appear to be largely conserved between vertebrates and invertebrates (Chang and Rongo 2005; Walker et al. 2006). In addition, both SVKI and general inhibitors of endocytosis (dyanmin inhibitory peptide and concanavalin A) have been found to inhibit LTD in the synapse between touch mechanosensory neurons in the leech (Li and Burrell 2008).
One of the most interesting findings from these experiments is that blocking a signaling pathway that mediates LTP (CaMKII) unmasked LTD in this synapse. These results address a fundamental issue concerning NMDAR-mediated synaptic plasticity. How are NMDAR/ Ca2+ signals able to generate both synaptic potentiation and depression? Recent studies have suggested that this bidirectional plasticity relies on cellular switches that change the balance between potentiating and depressing processes to determine the polarity of synaptic plasticity (Graupner and Brunel 2007; Nishiyama et al. 2000; van Woerden et al. 2009). Specifically, changes in cytosolic Ca2+ levels, mediated by NMDARs and intracellular Ca2+ stores, are sufficient to control both the strength and direction of synaptic plasticity (Bi and Poo 1998). These different cytosolic Ca2+ levels activate kinases (which contribute to LTP) and phosphatases (which contribute to LTD) to differing degrees and modeling studies indicate that the signaling molecule that is more strongly activated inhibits the less-activated molecule (Graupner and Brunel 2007; Pi and Lisman 2008; Zhabotinski 2000). For example, phosphatases that mediate LTD are more sensitive to Ca2+ than kinases that mediate LTP (such as CaMKII), thus lower levels of intracellular Ca2+ would preferentially activate protein phosphates, which would in turn inhibit protein kinases leading to LTD. When intracellular Ca2+ levels are sufficiently high, both the kinases that mediate LTP and the phosphatases that mediate LTD are presumably activated, yet LTP “wins out” because the kinases that mediate potentiation also actively inhibit the phosphatase/LTD pathway. Phosphorylation, particularly autophosphorylation, of CaMKII inhibits protein phosphatase activation and has been shown to be critical to initiate LTP (Fukunaga et al. 2000; Giese et al. 1998). Conversely, active protein phosphatases can dephosphorylate CaMKII as well as initiate processes necessary for LTD (Mulkey et al. 1993; Yoshimura et al. 1999). In addition to CaMKII, PKA also inhibits protein phosphatases, specifically protein phosphatase 1, and by this mechanism, PKA is said to “gate” the induction of LTP (Blitzer et al. 1998). The data from both the CaMKII (AIP) and the PKA (Rp-cAMP) experiments presented in this study support this hypothesis. Inhibition of CaMKII or PKA during pairing not only blocked potentiation but also revealed a form synaptic depression in the P-to-AP connection. This unmasked depression was blocked when the protein phosphatase inhibitor okadaic acid was applied.
The findings from this study have a number of important implications in terms of evolution and phylogenetic relationship of synaptic function and plasticity between vertebrates and invertebrates. It is well established that invertebrate nervous systems possess many of the molecules necessary for activity-dependent synaptic plasticity in vertebrates (e.g., NMDAR subunits, CaMKII, protein phosphatases) (also see Moroz et al. 2006), but there have been relatively few detailed studies of the cellular properties of invertebrate LTP and LTD. Furthermore, bioinformatic studies comparing the complement of synaptic proteins in vertebrates and invertebrates have suggested a reduced capacity for synaptic plasticity in invertebrates (Ryan and Grant 2009; Ryan et al. 2008). The present results demonstrate that, as in vertebrates, invertebrate synapses are capable of bidirectional modification governed by the relative activation of the same compliment of protein kinases and phosphatases that regulate bidirectional synaptic plasticity in vertebrates. These findings indicate that a number of fundamental processes of activity-dependent synaptic plasticity are conserved between in invertebrates and are likely to play a functional role in processes of learning and memory, development and sensory processing.
GRANTS
This work was supported by a subproject (B. Burrell, project director) of the Division of Research Resources Grant P20 RR-015567 (J. Keifer, PI), which is designated as a Center of Biomedical Research Excellence (COBRE).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Drs. Joyce Keifer, Cliff Summers, Pat Manzerra, and Yi-Fan Li for helpful comments and suggestions during the preparation of this manuscript.
Present address of K. B. Grey, Dept. of Medicine, Div. of Geriatric Medicine, University of California, San Diego, La Jolla, CA 92093-0746.
REFERENCES
- Antonov I, Ha T, Antonova I, Moroz LL, Hawkins RD. Role of nitric oxide in classical conditioning of siphon withdrawal in Aplysia. J Neurosci 27: 10993–11002, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog Neurobiol 78: 17–37, 2006. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Bertocchini F, Barone V, Conti A, Zuschratter W, Missiaen L, Lipp HP, Frey JU, Sorrentino V. Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. EMBO J 18: 5264–5273, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci 3: 1291–1300, 2000. [DOI] [PubMed] [Google Scholar]
- Beck A, Lohr C, Deitmer JW. Calcium transients in subcompartments of the leech Retzius neuron as induced by single action potentials. J Neurobiol 48: 1–18, 2001. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18: 10464–10472, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 280: 1940–1942, 1998. [DOI] [PubMed] [Google Scholar]
- Brockie PJ, Mellem JE, Hills T, Madsen DM, Maricq AV. The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron 31: 617–630, 2001. [DOI] [PubMed] [Google Scholar]
- Bruns D, Engers S, Yang C, Ossig R, Jeromin A, Jahn R. Inhibition of transmitter release correlates with the proteolytic activity of tetanus toxin and botulinus toxin A in individual cultured synapses of Hirudo medicinalis. J Neurosci 17: 1898–1910, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Multiple forms of long-term potentiation and long-term depression converge on a single interneuron in the leech CNS. J Neurosci 24: 4011–4019, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Serotonin mediates learning-induced potentiation of excitability. J Neurophysiol 94: 4002–4010, 2005. [DOI] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol 5: , 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Rongo C. Cytosolic tail sequences and subunit interactions are critical for synaptic localization of glutamate receptors. J Cell Sci 118: 1945–1956, 2005. [DOI] [PubMed] [Google Scholar]
- Chitwood RA, Li Q, Glanzman DL. Serotonin facilitates AMPA-type responses in isolated siphon motor neurons of Aplysia in culture. J Physiol 534: 501–510, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper DL, Lee HC. Inositol trisphosphate induces calcium release from nonmitochondrial stores in sea urchin egg homogenates. J Biol Chem 260: 13947–13954, 1985. [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28: 873–886, 2000. [DOI] [PubMed] [Google Scholar]
- Dierkes PW, Wende V, Hochstrate P, Schlue WR. L-type Ca2+ channel antagonists block voltage-dependent Ca2+ channels in identified neurons. Brain Res 1013: 159–167, 2004. [DOI] [PubMed] [Google Scholar]
- Endo S, Shenolikar S, Eskin A, Zwartjes RE, Byrne JH. Characterization of neuronal protein phosphatases in Aplysia californica. J Neurochem 58: 975–982, 1992. [DOI] [PubMed] [Google Scholar]
- Ezzeddine Y, Glanzman DL. Prolonged habituation of the gill-withdrawal reflex in Aplysia depends on protein synthesis, protein phosphatase activity, and postsynaptic glutamate receptors. J Neurosci 23: 9585–9594, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot LS, Kandel ER, Siegelbaum SA, Blumeneld H. Imaging terminals of Aplysia sensory neurons demonstrates role of enhanced Ca2+ influx in presynaptic facilitation. Nature 361: 634–637, 1993. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci 32: 33–55, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey MC, Sprengel R, Nevian T. Activity pattern-dependent long-term potentiation in neocortex and hippocampus of GluA1 (GluR-A) subunit-deficient mice. J Neurosci 29: 5587–5596, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Muller D, Ohmitsu M, Bako E, DePaoli-Roach AA, Miyamoto E. Decreased protein phosphatase 2A activity in hippocampal long-term potentiation. J Neurochem 74: 807–817, 2000. [DOI] [PubMed] [Google Scholar]
- Geiger JE, Magoski NS. Ca2+-induced Ca2+ release in Aplysia bag cell neurons requires interaction between mitochondrial and endoplasmic reticulum stores. J Neurophysiol 100: 24–37, 2008. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279: 870–873, 1998. [DOI] [PubMed] [Google Scholar]
- Graupner M, Brunel N. STDP in a bistable synapse model based on CaMKII and associated signaling pathways. PLoS Comput Biol 3: , 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey KB, Burrell BD. Forskolin induces NMDA receptor-dependent potentiation at a central synapse in the leech. J Neurophysiol 99: 2719–2724, 2008. [DOI] [PubMed] [Google Scholar]
- Grey KB, Moss BL, Burrell BD. Molecular identification and expression of the NMDA receptor NR1 subunit in the leech. Invert Neurosci 9: 11–20, 2009. [DOI] [PubMed] [Google Scholar]
- Gu XN. Effect of conduction block at axon bifurcations on synaptic transmission to different postsynaptic neurones in the leech. J Physiol 441: 755–778, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TJ, Kohn AB, Bobkova YV, Moroz LL. Molecular characterization of NMDA-like receptors in Aplysia and Lymnaea: relevance to memory mechanisms. Biol Bull 210: 255–270, 2006. [DOI] [PubMed] [Google Scholar]
- Hochner B, Kandel ER. Modulation of a transient K+ current in the pleural sensory neurons of Aplysia by serotonin and cAMP: implications for spike broadening. Proc Natl Acad Sci USA 89: 11476–11480, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Calabrese RL. Spike-mediated and graded inhibitory synaptic transmission between leech interneurons: evidence for shared release sites. J Neurophysiol 96: 235–251, 2006. [DOI] [PubMed] [Google Scholar]
- Jin I, Hawkins RD. Presynaptic and postsynaptic mechanisms of a novel form of homosynaptic potentiation at Aplysia sensory-motor neuron synapses. J Neurosci 23: 7288–7297, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Yeckel MF, Gray R, Johnston D. L-Type calcium channels are required for one form of hippocampal mossy fiber LTP. J Neurophysiol 79: 2181–2190, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron 61: 340–350, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus AL, Angstadt JD. Diversity and modulation of ionic conductances in leech neurons. J Neurobiol 27: 419–433, 1995. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Jr, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol 76: 279–327, 2005. [DOI] [PubMed] [Google Scholar]
- Li Q, Burrell BD. CNQX and AMPA inhibit electrical synaptic transmission: a potential interaction between electrical and glutamatergic synapses. Brain Res 1228: 43–57, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Burrell BD. Two forms of long-term depression in a polysynaptic pathway in the leech CNS: one NMDA receptor-dependent and the other cannabinoid-dependent. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195: 831–841, 2009. [DOI] [PubMed] [Google Scholar]
- Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: dependence on release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis, and modulation of postsynaptic AMPA receptor efficacy. J Neurosci 25: 5623–5637, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL. Hebbian induction of long-term potentiation of Aplysia sensorimotor synapses: partial requirement for activation of an NMDA-related receptor. Proc Biol Sci 255: 215–221, 1994. [DOI] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL. Effect of interstimulus interval on pairing-induced LTP of Aplysia sensorimotor synapses in cell culture. J Neurophysiol 77: 667–674, 1997. [DOI] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron 24: 649–658, 1999. [DOI] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305: 719–721, 1983. [DOI] [PubMed] [Google Scholar]
- Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci 30: 176–184, 2007. [DOI] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci 100: 433–442, 2006. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Schiavo G. Structure and function of tetanus and botulinum neurotoxins. Q Rev Biophys 28: 423–472, 1995. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Pavlidis P, Madison DV. Pair-recordings reveal all-silent synaptic connections and the postysnaptic expression of long term potentiation. Neuron 29: 691–701, 2001. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, Jezzini S, Lovell P, Iannucculli W, Chen M, Nguyen T, Sheng H, Shaw R, Kalachikov S, Panchin YV, Farmerie W, Russo JJ, Ju J, Kandel ER. Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell 127: 1453–67, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science 261: 1051–1055, 1993. [DOI] [PubMed] [Google Scholar]
- Murphy GG, Glanzman DL. Enhancement of sensorimotor connections by conditioning-related stimulation in Aplysia depends upon postsynaptic Ca2+. Proc Natl Acad Sci USA 93: 9931–9936, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GG, Glanzman DL. Mediation of classical conditioning in Aplysia californica by long-term potentiation of sensorimotor synapses. Science 278: 467–471, 1997. [DOI] [PubMed] [Google Scholar]
- Murphy GG, Glanzman DL. Cellular analog of differential classical conditioning in Aplysia: disruption by the NMDA receptor antagonist DL-2-amino-5-phosphonovalerate. J Neurosci 19: 10595–10602, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A, Zastrow DJ, Lickteig R, Zahniser NR, Browning MD. Maintenance of late-phase LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature 394: 680–683, 1998. [DOI] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci 26: 11001–11013, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature 408: 584–588, 2000. [DOI] [PubMed] [Google Scholar]
- Pi HJ, Lisman JE. Coupled phosphatase and kinase switches produce the tristability required for long-term potentiation and long-term depression. J Neurosci 28: 13132–13138, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TJ, Emes RD, Grant SGN, Komiyama NH. Evolution of NMDA receptor interaction domains: implications for organisation of synaptic signaling complexes. BMC Neurosci 9: 6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TJ, Grant SGN. The origin and evolution of synapses. Nat Rev Neurosci 10: 701–712 [DOI] [PubMed] [Google Scholar]
- Siddall ME, Trontelj P, Utevsky SY, Nkamany M, Macdonald KS. Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proc Biol Sci 274: 1481–1487, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko L, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev 49: 53–98, 1997. [PubMed] [Google Scholar]
- Trueta C, Sanchez-Armass S, Morales MA, De Miguel FF. Calcium-induced calcium release contributes to somatic secretion of serotonin in leech Retzius neurons. J Neurobiol 61: 309–316, 2004. [DOI] [PubMed] [Google Scholar]
- van Woerden GM, Hoebeek FE, Gao Z, Nagaraja RY, Hoogenraad CC, Kushner SA, Hansel C, De Zeeuw CI, Elgersma Y. betaCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat Neurosci 12: 823–825, 2009. [DOI] [PubMed] [Google Scholar]
- Vargas R, Cifuentes F, Morales MA. Differential contribution of extracellular and intracellular calcium sources to basal transmission and long-term potentiation in the sympathetic ganglion of the rat. Dev Neurobiol 67: 589–602, 2007. [DOI] [PubMed] [Google Scholar]
- Walker CS, Brockie PJ, Madsen DM, Francis MM, Zheng Y, Koduri S, Mellem JE, Strutz-Seebohm N, Maricq AV. Reconstitution of invertebrate glutamate receptor function depends on stargazin-like proteins. Proc Natl Acad Sci USA 103: 10781–10786, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel R, Kristan WB, Jr, Kleinfeld D. Supralinear summation of synaptic inputs by an invertebrate neuron: dendritic gain is mediated by an “inward rectifier” K(+) current. J Neurosci 19: 5875–5888, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D, Thomas R, Schwiening C. The effects of intracellular pH changes on resting cytosolic calcium in voltage-clamped snail neurones. J Physiol 530: 405–416, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bhat MB, Nishi M, Takeshima H, Ma J. Molecular cloning of cDNA encoding a drosophila ryanodine receptor and functional studies of the carboxyl-terminal calcium release channel. Biophys J 78: 1270–1281, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Chen RQ, Gu QH, Yan JZ, Wang SH, Liu SY, Lu W. Metaplastic regulation of long-term potentiation/long-term depression threshold by activity-dependent changes of NR2A/NR2B ratio. J Neurosci 29: 8764–8773, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HW, Hu XD, Zhang HM, Xin WJ, Li MT, Zhang T, Zhou LJ, Liu XG. Roles of CaMKII, PKA, and PKC in the induction and maintenance of LTP of C-fiber-evoked field potentials in rat spinal dorsal horn. J Neurophysiol 91: 1122–1133, 2004. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Sogawa Y, Yamauchi T. Protein phosphatase 1 is involved in the dissociation of Ca2+/calmodulin-dependent protein kinase II from postsynaptic densities. FEBS Lett 446: 239–242, 1999. [DOI] [PubMed] [Google Scholar]
- Zannat MT, Locatelli F, Rybak J, Menzel R, Leboulle G. Identification and localisation of the NR1 sub-unit homologue of the NMDA glutamate receptor in the honeybee brain. Neurosci Lett 398: 274–279, 2006. [DOI] [PubMed] [Google Scholar]
- Zhabotinsky AM. Bistability in the Ca(2+)/calmodulin-dependent protein kinase-phosphatase system. Biophys J 79: 2211–2221, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Keifer J. PKA has a critical role in synaptic delivery of GluR1- and GluR4-containing AMPARs during initial stages of acquisition of in vitro classical conditioning.. J Neurophysiol 101: 2539–2549, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]