Abstract
Smooth pursuit (SP) eye movements are used to maintain the image of a moving object on or near the fovea. Visual motion signals aid in driving SP and are necessary for its adaptation. The sources of visual error signals that support SP adaptation are incompletely understood but could involve neurons in cortical and brain stem areas with direction selective visual motion responses. Here we focus on the pretectal nucleus of the optic tract (NOT), which encodes retinal error information during SP. The aim of this study was to characterize the role of the NOT in SP adaptation. SP adaptation is typically produced using a double step of velocity ramp (double-step paradigm), where target speed either increases or decreases 100 ms after the beginning of a trial. In our study, we delivered a brief (200 ms) train of microelectrical stimulation (ES) in the left NOT to introduce directional error signals at the point in time where a second target speed would appear in a double-step paradigm. The target was extinguished coincidentally with the onset of the ES train. Initial eye acceleration (1st 100 ms) showed significant increases after 100 trials, which included left NOT stimulation during ongoing pursuit in an ipsiversive (leftward) direction. In contrast, initial eye acceleration showed significant decreases after repeated left NOT stimulation during contraversive (rightward) SP. Control studies performed using the same periodicity of NOT stimulation as in the preceding text but without accompanying SP did not induce changes in eye acceleration. In contrast, ES of the NOT paired with active SP produced gradual changes in eye acceleration similar to that observed in double-step paradigm. Therefore our findings support the suggestion that the NOT is an important source of visual error information for guiding motor learning during horizontal SP.
INTRODUCTION
Learning new motor behaviors is aided by error signals that occur in association with each attempted behavior. Error signals are carried in specific sensory feedback pathways to modify incorrect responses during the learning process. Especially for motor learning, interaction between sensory and motor-related signals could be necessary to improve the desired motor behavior. The visual-oculomotor system and the vestibular ocular reflex have proven to provide ideal model systems to study neural mechanisms associated with learning because visual error and eye motion signals are quantifiable. Although visual error signals are known to be essential for learning in these systems, the nature and source of error signals are incompletely understood. To further our understanding of this question, we used a smooth pursuit adaptation paradigm to examine the neural mechanisms underlying visually guided motor learning. Visual (retinal) error signals are necessary for smooth pursuit to maintain the image of a moving object on or near the fovea. During smooth pursuit, retinal error signals are defined as the difference between target and eye motion signals. Previous studies have demonstrated that significant adaptive changes of initial eye motion during smooth pursuit occur using a double step of velocity ramp (double-step paradigm), where the target begins moving at one speed for first 100 ms and then changes to either a higher or lower speed (e.g., Fukushima et al. 1996; Kahlon and Lisberger 1996; Ono and Mustari 2007; Takagi et al. 2000). This double-step paradigm is designed to introduce larger retinal error motion than single velocity ramp tracking.
Neurons in the accessory optic system (AOS) and pretectal nucleus of the optic tract (NOT) are highly sensitive to the direction of visual motion (see Mustari and Ono 2007 for review) and have appropriate connections to provide the floccular complex and oculomotor vermis of the cerebellum (Glickstein et al. 1994; Kitazawa et al. 2009; Nagao et al. 1997) with retinal error signals through the ipsilateral dorsal cap of Kooy (dcK) and medial accessory (MAO) region of the inferior olive (e.g., Büttner-Ennever et al. 1996; Mustari et al. 1994). In earlier studies, Mustari and Fuchs (1990) reported that some neurons in the primate NOT were modulated during horizontal smooth pursuit of a small diameter (<0.2°) target spot moving over a dark background. This study has demonstrated that smooth pursuit-related neurons in NOT showed a clear drop in firing rate during a target blink testing while pursuit is maintained. Furthermore, using multiple-linear regression modeling, we showed that smooth pursuit-related NOT neurons, which have foveal and parafoveal visual receptive fields, encode mainly retinal error velocity (Das et al. 2001). In fact, virtually all of the smooth pursuit-related neurons in the NOT are sensitive to ipsiversive retinal image motion (Mustari and Fuchs 1990). It is unknown whether NOT neurons could play a role in visually guided motor learning in smooth pursuit. In this study, we used brief trains of microelectrical stimulation (ES) of the left NOT to artificially introduce directional error information during smooth pursuit. If the NOT plays a significant role in smooth pursuit adaptation, we would expect to create gain increases or decreases with ES, according to the direction of smooth pursuit. If the NOT plays no role in guiding motor learning, we would expect no change in smooth pursuit gain across repeated trials. Our results indicate that the NOT is a potential source of visual error signals leading to adaptive plasticity in smooth pursuit.
METHODS
Surgical procedures
A detailed description of our surgical procedures can be found in earlier publications (e.g., Mustari et al. 2001; Ono and Mustari 2007). All surgical procedures were performed in strict compliance with National Institutes of Health Guide for the Care and Use of Laboratory Animals, and protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Emory University. Two juvenile rhesus (Macaca mulatta) monkeys weighing 5–8 kg, born in captivity at the Yerkes National Primate Research Center, were used in this study. Surgical procedures were performed in a dedicated facility using aseptic techniques under isoflurane anesthesia (1.25–2.5%). Vital signs including blood pressure, heart rate, blood oxygenation, body temperature, and CO2 in expired air were monitored with a Surgivet Instrument (Waukesha, WI) and maintained in normal physiological limits. Postsurgical analgesia (Buprenorphine, 0.01 mg/kg im) and antiinflammatory [Banamine (fluxinin meglumine), 1.0 mg/kg im] treatment were delivered every 6 h for several days. We used stereotaxic methods to implant a titanium head stabilization post and titanium recording chambers (Crist Instruments) aimed at the location of the NOT. In the same surgery, a scleral search coil for measuring eye movements (Fuchs and Robinson 1966) was implanted underneath the conjunctiva of one eye using the technique of Judge et al. (1980).
Localization of NOT
Our protocol for conducting microelectrical stimulation studies was to first extensively map the smooth pursuit regions of NOT using functional criteria as described in our earlier studies (Das et al. 2001; Mustari and Fuchs 1990). We initially localized the NOT by stereotaxic position (anterior = 0 mm, angle = 20°, lateral = 3–5 mm for an aiming point in the depth of pretectal region) with respect to the oculomotor nucleus and by finding neurons that were modulated during horizontal motion of either a large-field (75 × 75°) visual stimulus or during horizontal smooth pursuit of a small-diameter (0.2°) target spot moving over a dark background. The neuronal responses of both large-field and parafoveal NOT neurons were modulated during high-frequency oscillation (typically 3–5 Hz, ±0.5°) of large- or small-diameter visual stimuli, respectively. Direction preference for the majority of neurons was ipsiversive (e.g., Das et al. 2001; Mustari and Fuchs 1990).
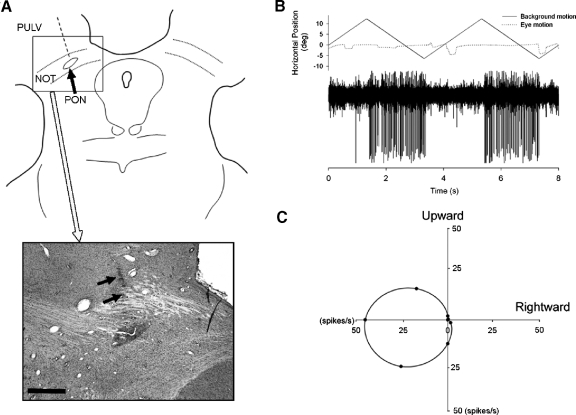
At the conclusion of our experiments, animals were killed using IACUC approved methods and perfused, transcardially, with physiological saline followed by 4% paraformaldehyde. The brain was blocked in the coronal stereotaxic plane and prepared for histology as described elsewhere (e.g., Mustari et al. 1994). Frozen sections were cut at 50 μm, mounted on microscope slides, and stained for Nissl substance to allow histological reconstruction of representative electrode tracks. The location of the NOT relative to our recording and ES positions is shown in Fig. 1A. The arrows in Fig. 1A (bottom) showed part of an electrode track that was located in the NOT at the level of the pretectal olivary nucleus (PON). The response of a well-isolated left-NOT neuron during horizontal visual motion is shown in Fig. 1B. The monkey is fixating a stationary target during this testing. This representative neuron and most other NOT neurons prefer ipsiversive visual motion (Fig. 1C). Direction preference was tested using eight cardinal directions of visual motion separated by 45°. Tuning curves were computed as described in earlier publications (Das et al. 2001; Mustari and Fuchs 1990).
Fig. 1.
Histological reconstruction of recording sites. A: line drawing and representative histological section stained for Nissl substance to demonstrate location of electrode tracks. An electrode track reconstructed from histological section is indicated (- - -). → (bottom), an electrode track that was located in the rostrocaudal extent of the pretectum at the level of the pretectal olivary nucleus (PON). NOT, nucleus of the optic tract; PULV, pulvinar. Scale bar = 1 mm. B: response properties of a representative NOT neuron during horizontal large-field visual motion presented on a tangent screen while the monkey (Macaca mulatta) fixated a stationary target spot. A strong direction-selective response occurs at short latency (50 ms) after the start of leftward visual motion. C: directional tuning data for a representative NOT neuron. Large-field visual motion was presented in different directions while the monkeys fixated a stationary target. The average firing rate is indicated at 45° intervals (●), with a curve fit to estimate the direction preference for the unit.
Experimental protocol and microelectrical stimulation in NOT
During all experiments, monkeys were seated in a primate chair (Crist Instruments) with the head stabilized in the horizontal stereotaxic plane. Experiments were conducted in a sound attenuated and lightproof room. Testing was conducted in a customized room that was made completely dark, extinguishing all light sources during ES. A complete dark environment was verified by having a dark-adapted and observer sits in the enclosure for 30 min to check for any possible light leaks. Visual stimuli were rear projected on a tangent screen 57 cm distant from the monkey. Smooth pursuit targets were delivered using appropriate optic bench hardware and computer controlled two-axis mirror galvanometers (General Scanning, Watertown) as described in detail in previous publications (e.g., Mustari et al. 2001; Ono and Mustari 2007).
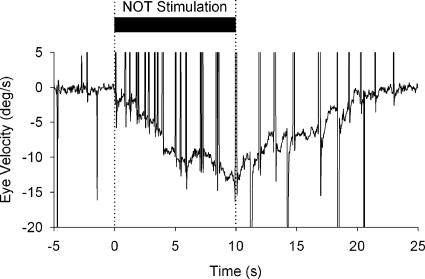
To aid in verifying that our NOT stimulation electrodes are well placed during ES experiments, we delivered stimulus trains (biphasic pulse; 200 μs duration; 200 Hz; 20–80 μA) to generate optokinetic-like nystagmus (Fig. 2) with slow phases directed toward the side of stimulation. Such relatively long durations of ES (Mustari and Fuchs 1990; Schiff et al. 1988), which charge the so-called velocity storage mechanism (Cohen et al. 1977), were not used during our smooth pursuit adaptation experiments. Once we verified that our electrode was well placed, we presented adaptation trials, which included brief (200 ms duration) trains of microelectrical stimulation delivered in the left NOT. Stimulation onset started 100 ms after onset of target motion (Fig. 3). Controlling ES pulse train delivery was achieved using a CED-Power1401 hardware interface (Cambridge Electronic Designs, Cambridge, UK) connected to a stimulus isolation unit (Bak Electronics, Mount Airy, MD).
Fig. 2.
Electrical stimulation (ES) of the left NOT using a 10 s train of low current (50 μA) short-duration (200 μs) biphasic pulses at 200 Hz. Nystagmus was elicited at short latency following stimulation at the site indicated in Fig. 1. Immediately following the start of the ES (thick bar, top traces), leftward optokinetic nystagmus (OKN)-like nystagmus begins with slow-phase eye velocity building up during stimulation. First · · · , the onset of the ES.
Fig. 3.
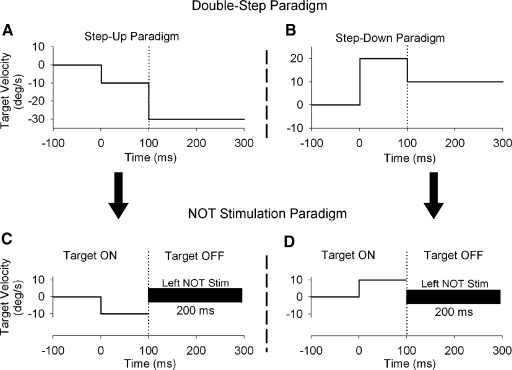
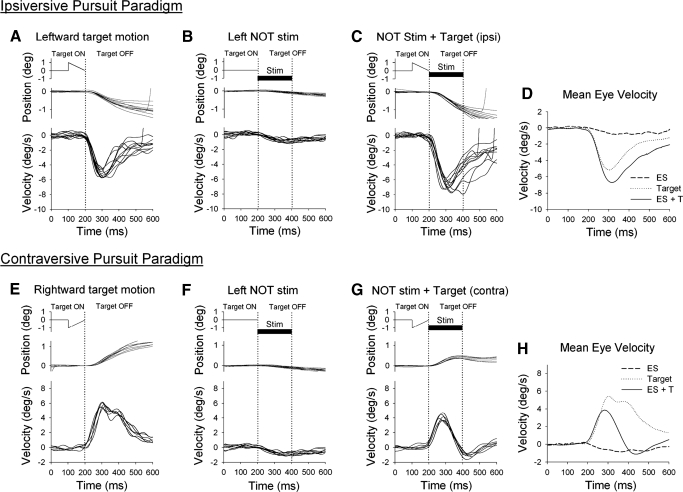
Smooth pursuit adaptation paradigm using microstimulation of NOT. The standard double-step paradigm (top), which was like that used previous studies (e.g., Kahlon and Lisberger 1996; Ono and Mustari 2007; Takagi et al. 2000), has the target moving at one speed for 1st 100 ms followed by either a higher (A) or lower (B) speed. Smooth pursuit adaptation with NOT stimulation (bottom) delivered at the point in time where a second target speed would appear in standard double-step paradigms. The target was extinguished coincidentally with the onset of the ES train to eliminate actual retinal error motion associated with the target. C and D: ipsi- and contraversive target motion, respectively.
The timing and duration of NOT electrical stimulation during ongoing pursuit was designed to artificially introduce directional error information (e.g., Das et al. 2001) in place of actual visual error signals associated with the double-step paradigm (Fig. 3, A and B). The advantage of this approach is that we activate a known source of horizontal (ipsiversive) visual motion information. Figure 3, C and D, shows our experimental design for smooth pursuit combined with NOT stimulation. Figure 3, A and B, shows the timing during step-up and -down paradigms, which was similar to that reported in other studies of visually guided motor learning of smooth pursuit (e.g., Kahlon and Lisberger 1996; Ono and Mustari 2007). The vertical dotted lines show the onset of the speed change in target motion for step-up and -down paradigms. Figure 3, C and D, shows NOT stimulation paradigms with the onset of NOT stimulation indicated by vertical dotted lines. We applied brief (200 ms) trains of microelectrical stimulation in the left NOT to introduce leftward error signals at the point in time where a second target speed would appear in double-step paradigms. The target was extinguished coincidentally with onset of the electrical stimulation train to eliminate actual retinal image motion. Figure 3C shows left NOT stimulation during leftward smooth pursuit and target motion. In this case, both ES introduced error information and pursuit motion are in the same direction. This condition mimics the challenge to the smooth pursuit system experienced during the step-up paradigm. Figure 3D shows left NOT stimulus during rightward target motion. In this condition, ES introduced error motion (leftward) and pursuit eye motion are in opposite directions; this mimics conditions in the step-down paradigm. Because the NOT carries only ipsiversive visual motion signals, electrical stimulation of NOT would be expected to produce different effects during left- and rightward pursuit directions. We used 100 trials of smooth pursuit coupled with ES, simulating the double-step adaptation paradigm with real target motion. We conducted one set of adaptation or control trials in a given experimental session. Therefore we conducted 19 experimental sessions in total, which include NOT stimulation paradigm with ipsi- and contraversive directions and control testing of NOT stimulation alone and target motion alone in two monkeys (see Fig. 8).
Fig. 8.
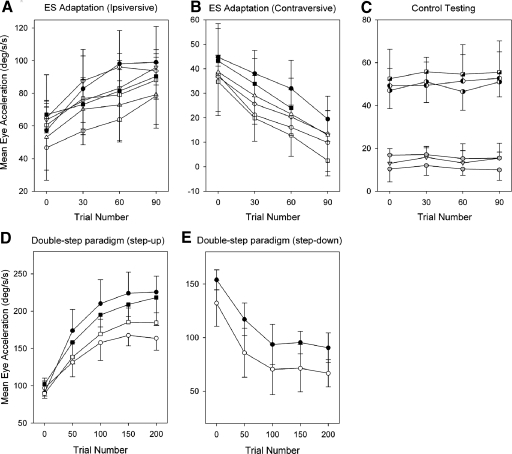
Mean eye acceleration during the 1st 100 ms of tracking for 10 trials are shown at 30-trial intervals for 2 monkeys. Ipsi- (A) and contraversive adaptation paradigms (B) and control testing (C) are shown. Eye acceleration during double-step paradigm of step-up (D) and step-down (E) are shown at 50-trial intervals in same subjects. Filled and open circles in A, B, D, and E indicate different monkeys. Half filled symbols and gray symbols in C indicate control testing using target motion alone and NOT stimulation alone, respectively. Different half filled symbols (circle and square) and gray symbols (circle and triangle) indicate different monkeys.
Data collection and analysis
Eye movements were detected using standard electromagnetic methods (Fuchs and Robinson 1966) using precision hardware (CNC Electronics, Seattle, WA). Eye and target position signals were processed with anti-aliasing filters at 200 Hz using six-pole Bessel filters prior to digitization at 1 kHz with 16-bit precision using CED-Power1401 hardware (Cambridge Electronic Designs). Velocity data were generated by digital differentiation of the position arrays using a central difference algorithm in Matlab (Mathworks, Natick, MA). Pursuit initiation during step-ramp tracking was taken as the time that average eye speed reached ≥3 SD above the pretrial values during fixation. Initial acceleration was calculated as the average eye acceleration in the first 100 ms period of pursuit. ≥10 trials of right- or leftward tracking were averaged to quantify initial acceleration and velocity values. We have used the convention of representing rightward eye position as positive values in our plots unless stated otherwise.
RESULTS
NOT stimulation during ongoing pursuit
Figure 4 documents preadaptation testing results with and without electrical stimulation of the NOT. In Fig. 4 and similar figures to follow (Figs. 5–7), target position, eye position and eye velocity are all aligned at the point in time where the target is extinguished or ES onset (1st dotted vertical line). Figure 4 (top) shows results related to ipsiversive (leftward) smooth pursuit during target motion alone (Fig. 4A), NOT stimulation alone (B), or combined pursuit and NOT stimulation (C). Results related to contraversive (rightward) smooth pursuit direction are shown in a similar format (Fig. 4, bottom). We used two different control paradigms to help evaluate the potential roles of smooth pursuit and ES in gain adaptation. First, we applied short-duration (100 ms) target motion alone (Fig. 4A), which elicited smooth pursuit at a mean latency of 106.3 ± 7.9 ms. However, eye velocity (peak eye velocity = 5.3 ± 0.48°/s) was lower than target velocity (10°/s) because short duration of target motion. Second, we applied microelectrical stimulation in left NOT without target motion (Fig. 4B). Left NOT stimulation produced leftward eye movement (peak eye velocity = 1.25 ± 0.16°/s) at short latency (∼10 ms). The evoked eye movement did not persist after the short-duration (200 ms) ES period. Furthermore, no buildup in eye velocity was observed after repeated trials (see discussion). We then applied combined target motion and NOT stimulation in a manner that possibly mimicked natural smooth pursuit adaptation using double-step paradigm (Fig. 4C). In this paradigm, leftward eye velocity increased (peak eye velocity = 6.95 ± 0.67°/s, latency = 104.2 ± 11.2 ms, Fig. 4D) beyond that observed during target motion alone.
Fig. 4.
Representative eye and target motion traces in ipsiversive (top) and contraversive (bottom) directions of smooth pursuit. A and E: control testing using target motion alone. Target motion began moving at 10°/s for 100 ms, and then target was extinguished. B and F: control testing using left NOT stimulation without target motion. The stationary target was extinguished coincidentally with the onset of the NOT stimulation train (200 ms duration; 50 μA). C and G: coupled NOT stimulation with target motion. D and H: comparison of mean eye velocity during three different testing conditions.
Fig. 5.
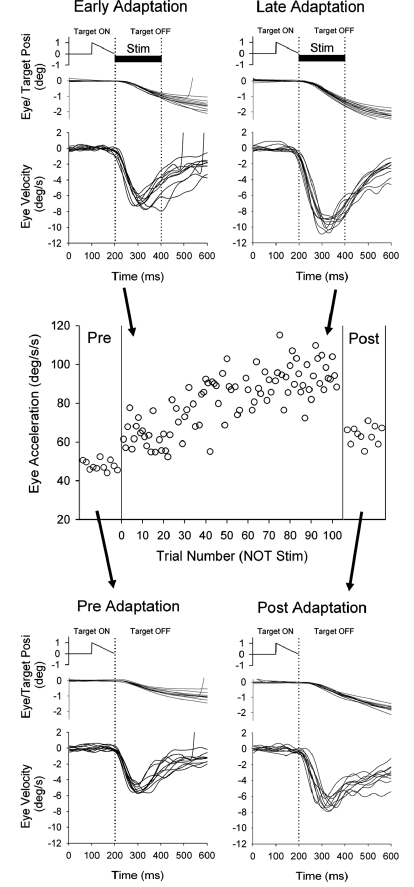
Adaptation paradigm (top) during left NOT stimulation (200 ms duration; 50 μA) and leftward (ipsiversive) smooth pursuit of a moving target. Average eye acceleration of the 1st 100 ms of tracking in the adaptation paradigm of ipsiversive pursuit were shown as a function of trial number for a representative experiment. Control trials using leftward target motion alone are shown before and after adaptation paradigm. The eye traces in pre- and early adaptation are the same as those illustrated during target motion and ES plus target shown in Fig. 4.
Fig. 7.
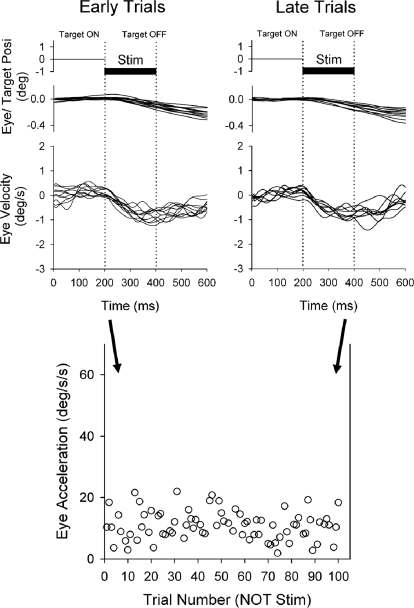
Control paradigm using NOT stimulation without target motion. Average eye acceleration of the 1st 100 ms of tracking in the control paradigm using left NOT stimulation alone (200 ms duration) are shown as a function of trial number for a representative experiment. Eye velocity and acceleration did not change in an adaptive manner across repeated trials of NOT stimulation.
Figure 4, bottom, shows results associated with left NOT stimulation during rightward (contraversive) smooth pursuit. During rightward pursuit and target motion followed by left NOT stimulation (Fig. 4G), rightward eye velocity showed a rapid reduction after ES. This is likely due to the ES induced eye motion (leftward) subtracting from rightward pursuit eye motion. Therefore rightward eye velocity was significantly decreased during combined target and ES trials (peak eye velocity = 3.9 ± 0.5°/s, latency = 100.5 ± 11.0 ms) compared with that during target motion alone (peak eye velocity = 5.4 ± 0.6°/s, latency = 105.1 ± 10.4 ms, Fig. 4H).
Smooth pursuit adaptation
GAIN-INCREASE PARADIGM.
We hypothesize that ES of the left NOT generates error signals similar to those associated with leftward smooth pursuit in a step-up paradigm, where actual visual motion activates NOT neurons. To test this idea, we presented adaptation trials in which electrical stimulation was delivered in NOT for 200 ms duration starting 100 ms after onset of target motion. Figure 5 (top) shows eye position and velocity traces during leftward target motion for 100 ms followed by left NOT stimulation in a representative experimental session. In this case, artificial retinal motion and pursuit eye motion are same direction as occurs in a step-up (gain-increase) condition. In 100 trials of smooth pursuit plus ES paradigm, eye velocity later in adaptation (last 10 trials of 100 trials) showed significant increase compared with early trials (1st 10 trials of 100 trials). To provide an estimate of adaptation, we calculated initial acceleration as the average eye acceleration in the first 100 ms of eye motion. Trial-by-trial eye acceleration data during adaptation across 100 trials are plotted in Fig. 5, middle. We used the absolute value of acceleration to produce positively sloped plots for gain increase, regardless of the direction of smooth pursuit. Initial eye acceleration showed significant increases in late trials compared with early trials (63.7 ± 7.9°/s2, 1st 10 trials; 95.9 ± 10.8°/s2, last 10 trials; P < 0.001, unpaired t-test). We plotted eye acceleration data pre- and postadaptation to show the magnitude of change by testing smooth pursuit without ES. Similarly, initial eye acceleration showed significant increases after 100 trials of adaptation compared with the preadapted state (49.7 ± 3.7°/s2, preadapt; 63.5 ± 8.6°/s2, postadapt; P < 0.001, unpaired t-test). Therefore ipsiversive smooth pursuit (relative to the NOT stimulation site) coupled with ES produced significant adaptation in the absence of actual visual motion at the point in time where ramp-speed change would normally occur.
GAIN-DECREASE PARADIGM.
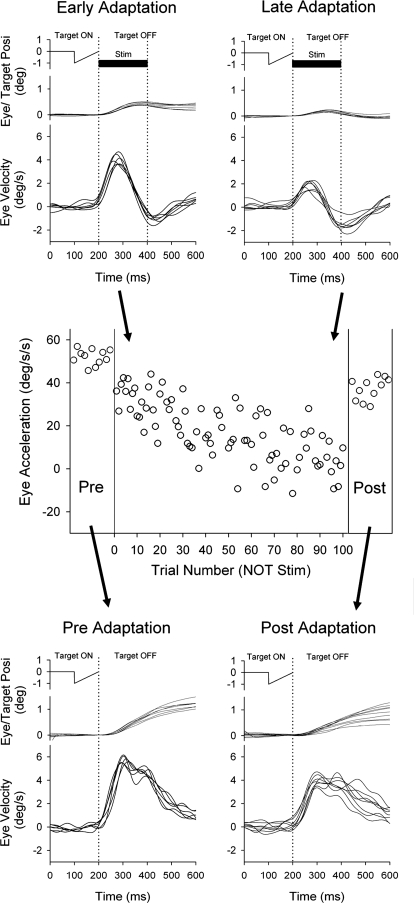
We also examined the effects of ES in NOT during contraversive smooth pursuit. Figure 6 shows eye motion traces during rightward target motion for 100 ms followed by left NOT stimulation in a representative experimental session. In this condition, artificial leftward error signals and pursuit eye motion are in opposite directions as occurs in a step-down (gain-decrease) condition. During repetition of this paradigm, rightward eye motion traces showed significant decrease in late adaptation trial (last 10 trials of 100 trials) compared with early trials (1st 10 trials; Fig. 6, top). To estimate the degree of adaptation, we calculated initial acceleration as described in the preceding text (Fig. 6, middle). Initial eye acceleration across trials produced negatively sloped plots and evinced significant decreases in late adaptation trials compared with early trials (33.8 ± 6.8°/s2, 1st 10 trials; 2.98 ± 7.8°/s2, last 10 trials; P < 0.001, unpaired t-test). In pre- and postadaptation testing (without electrical stimulation), initial eye acceleration also showed significant decrease after 100 trials (52.0 ± 3.6°/s2, preadapt; 37.3 ± 5.2°/s2, postadapt; P < 0.001, unpaired t-test).
Fig. 6.
Adaptation paradigm (top) during left NOT stimulation (200 ms duration; 50 μA) and rightward (contraversive) smooth pursuit of a moving target. Average eye acceleration during the 1st 100 ms of tracking in the adaptation paradigm associated with contraversive pursuit shown as a function of trial number for a representative experiment. Control trials using rightward target motion alone are shown before and after adaptation paradigm. The eye traces in pre- and early adaptation are same as the trace during target motion and ES plus target shown in Fig. 4.
Controls for effects of NOT stimulation during adaptation
In control experiments, we wished to test the possibility that repeated trials of NOT stimulation alone might produce an ongoing eye velocity. Therefore we applied microelectrical stimulation in the left NOT without target motion using the same time period between 200 ms duration ES trains as we used in our adaptation paradigms described in the preceding text. Figure 7 (top) shows eye position and velocity traces during left NOT stimulation (200 ms duration) in a representative experimental session. Eye position and velocity traces showed small amounts of induced eye motion in the ipsiversive (leftward) direction with short-duration NOT stimulation. However, initial eye acceleration did not change during repeated trials of ES (10.4 ± 6.1°/s2, first 10 trials; 9.8 ± 4.9°/s2, last 10 trials; P = 0.87, unpaired t-test). This result indicates that short-duration NOT stimulation alone did not induce velocity storage in eye motion (see discussion). Similarly, in pre- and posttesting with smooth pursuit of target motion for 100 ms without ES, initial eye acceleration did not show significant changes across trials (50.5 ± 3.2°/s2, pre-; 51.3 ± 4.1°/s2, post 100 trials; P = 0.63, unpaired t-test) delivered at the same periodicity as in our smooth pursuit plus ES paradigm. Therefore repeated trials of short-duration ES alone do not induce smooth pursuit adaptation.
Comparison of each adaptation paradigm and control testing
The results of 19 experiments using ES paradigm of gain-increase adaptation, gain-decrease adaptation and control testing in two monkeys are shown in Fig. 8, A–C. Furthermore, the time courses of adaptation using double-step paradigms are shown in Fig. 8, D and E. In ES paradigm, average eye acceleration during first 100 ms of tracking for 10 trials is plotted at 30 trial intervals for each experiment. Eye acceleration showed changes within 100 trials in both gain-increase (Fig. 8A) and gain-decrease (B) adaptation paradigms. The plots in Fig. 8, A and B, document consistent changes in eye acceleration found in repeated adaptation experiments in two monkeys (opened and closed symbols). Following gain-increase adaptation (Fig. 8A), mean values of initial eye acceleration (7 experiments of 2 monkeys) showed significant increases in late adaptation trials compared with values of early trials (59.0 ± 7.2°/s2, 1st 10 trials; 89.3 ± 7.9 °/s2, last 10 trials; P < 0.001). Similarly, in gain-decrease adaptation (Fig. 8B), initial eye acceleration (6 experiments of 2 monkeys) showed significant decreases in late adaptation trials compared with early trials (39.3 ± 3.9°/s2, 1st 10 trials; 13.7 ± 7.5°/s2, last 10 trials; P < 0.001). In contrast, results from control experiments (Fig. 8C), where we applied NOT stimulation alone (gray symbols) or target motion alone (half filled symbols) showed no adaptation. Specifically, initial eye acceleration did not show significant adaptive change even after 100 trials for neither target motion alone (49.6 ± 2.7°/s2, 1st 10 trials; 53.1 ± 2.2°/s2, last 10 trials; P = 0.16) nor NOT stimulation alone (13.4 ± 3.3°/s2, 1st 10 trials; 13.6 ± 3.1°/s2, last 10 trials; P = 0.94) for two monkeys. These results indicate that electrical stimulation of the NOT paired with smooth pursuit of target motion was most effective in producing adaptive changes of initial eye acceleration.
Time course of smooth pursuit adaptation
Figure 8, D and E, shows the time course of double-step paradigm of step-up and -down in same subjects. In step-up paradigm (Fig. 8D), the target moves from 10 to 30°/s. In step-down paradigm (Fig. 8E), the target moves from 25 to 5°/s (scales for eye acceleration in Fig. 8, D and E, are different from Fig. 8, A and B, because eye acceleration values in double-step paradigm are larger than those in ES paradigm). In step-up paradigm, eye acceleration reached 157–210°/s2 within 100 trials, whereas maximum eye acceleration in ES paradigm (gain-increase) were 78–98°/s2. In step-down paradigm, eye acceleration in early trials were 137–153°/s2, whereas in ES paradigm (gain-decrease), eye acceleration in early trials were 35–45°/s2. Even though absolute values of eye acceleration are different, percentages of change in eye acceleration during adaptation within 100 trials showed similar values (P > 0.05, paired t-test) between two different paradigms. In ES paradigm producing gain-increase, mean acceleration increased by 36.0 ± 8.9% and eye acceleration in step-up paradigm increased by 41.3 ± 8.4%. In ES paradigm producing gain-decrease, mean acceleration decreased by 58.4 ± 10.2% and eye acceleration in step-down paradigm decreased by 51.8 ± 12.6%. Furthermore, eye acceleration continued increasing or decreasing over 100 trials in ES paradigm. Similarly, eye acceleration during the double-step paradigm increased or decreased until 100–200 trials. Therefore most of the adaptation in both ES and double-step paradigm seemed to occur within, at least, 100 trials. Similar time courses of pursuit adaptation during a double-step paradigm have been reported in the other studies (Kahlon and Lisberger 1996; Takagi et al. 2000).
DISCUSSION
Our study was designed to consider whether retinal error signals carried in the NOT neurons could play a role in motor learning in smooth pursuit. Therefore we applied brief (200 ms) trains of microelectrical stimulation, unilaterally, in the left NOT during ongoing smooth pursuit in ipsi- and contraversive direction. Our results showed that electrical stimulation in NOT, which provides ipsiversive retinal error signals, produced different effects for a given direction of smooth pursuit. Here we discuss whether artificial error information (simulated retinal image motion) produced by NOT stimulation paired with smooth pursuit produced guided motor learning like that observed in natural conditions with actual retinal error information such as in a double-step paradigm.
Physiological and anatomical properties in NOT neurons
Neurons in the terminal nuclei of the AOS and NOT on one side of the brain project heavily to the ipsilateral dcK and MAO region of the inferior olive even though qualitatively the assigned score of labeling density over the MAO was lower than that in the dcK (e.g., Büttner-Ennever et al. 1996; Mustari et al. 1994; see Giolli et al. 2006 for review). The dcK is a major source of visual climbing fibers to the contralateral flocculus and ventral paraflocculus, while MAO sends climbing fiber inputs to the contralateral vermal lobule VII (Glickstein et al. 1994; Kitazawa et al. 2009; Nagao et al. 1997). Those cerebellar regions including floccular complex and oculomotor vermis are known to play a role in smooth pursuit adaptation (Kahlon and Lisberger 2000; Takagi et al. 2000). Therefore the NOT might be an important source of horizontal retinal error signals destined for the cerebellum through the inferior olive (Hoffmann et al. 1976). The direction selective neurons in the NOT (Hoffmann and Schoppmann 1981; Mustari and Fuchs 1990) drive left inferior olivary neurons that project to the right cerebellum producing leftward visually contingent complex spikes in Purkinje cells. Visual complex spikes have been reported in studies of the floccular complex during smooth pursuit (Stone and Lisberger 1990) and ocular following (e.g., Kobayashi et al. 1998).
Previous studies have reported that some neurons in the primate NOT, which have foveal and parafoveal visual receptive fields, were modulated during smooth pursuit (Mustari and Fuchs 1990) and encode mainly ipsiversive retinal error velocity (Das et al. 2001). Those studies suggest that the retinal error-related neurons could respond during smooth pursuit adaptation using double-step paradigm according to the amount and direction of retinal error velocity. Furthermore, ES of the NOT produced optokinetic-like nystagmus with slow phases directed toward the side of stimulation (Mustari and Fuchs 1990; Schiff et al. 1988). This electrically generated nystagmus with an ipsiversive direction is comparable to the optokinetic nystagmus (OKN) and after nystagmus (OKAN) produced by unidirectional retinal image motion (e.g., Cohen et al. 1977). Therefore microelectrical stimulation in left NOT could provide leftward retinal error motion information destined for the left inferior olive and subsequently the right flocculus, ventral paraflocculus, and oculomotor vermis. Although the NOT sends modest projections to a number of other centers including the pregeniculate nucleus, nucleus prepositus hypoglossi, and medial vestibular nucleus (Buettner-Ennever et al. 1996; Mustari et al. 1994), their potential role in smooth pursuit adaptation has not been studied.
The NOT also sends a relatively modest projection to the ipsilateral dorsolateral pontine nucleus (DLPN) (e.g., Mustari et al. 1994). However, there is some reason to consider that NOT-DLPN pathway might not play a role in ES paradigm as used in the current study. This is because ES of the NOT produces optokinetic-like eye movements (Fig. 2) but without an initial rapid rise in eye velocity (Mustari and Fuchs 1990; Schiff et al. 1988). This initial rapid rise in eye velocity during OKN has been attributed to so-called “direct” pathways involved in SP and dependent on the DLPN per se (e.g., Schiff et al. 1990). In contrast, activation of the “indirect” pathway involving the NOT is essential for slow buildup in eye velocity during OKN (see Mustari and Ono 2007 for review). Currently we do not have any definitive data regarding the role of NOT-DLPN pathway in SP or optokinetic eye movements.
Single unit recording studies have demonstrated that neurons in the NOT could be classified into at least two types (Mustari and Fuchs 1990). One type preferentially responds to large-field visual motion (large-field neurons) as shown in Fig. 1B, whereas the other type responds to motion with a smaller receptive field (parafoveal neurons), which are modulated during smooth pursuit. Furthermore, both types of NOT neurons are located in the same pretectal region. Therefore when we delivered ES in the NOT, both parafoveal neurons and large-field neurons might be activated even though low stimulus currents (<80 μA) were used. Because ES adaptation paradigm showed similar effect to the double-step paradigm, parafoveal neurons activated by ES could play a role in adaptation in same form as double-step paradigm. We do not yet know a functional role of large-field neurons in smooth pursuit adaptation. Because the large-field neurons are not modulated during tracking of a small target spot, they might not be involved in smooth pursuit. Further studies employing combined paradigm using a small target spot and large-field visual motion are necessary to resolve this question.
Role of the NOT inputs in smooth pursuit adaptation
One possibility is that smooth pursuit adaptation requires interaction between mossy (MF) and climbing fibers (CF) of the cerebellum, which includes floccular complex and oculomotor vermis. Signals in MF and CF pathways might play a role in smooth pursuit adaptation in conditions where significant amounts of retinal error motion exist during attempted smooth pursuit. For example, during leftward smooth pursuit in the gain-increase condition, the left NOT could provide leftward retinal error signals (complex spikes) to the right cerebellum, while the right cerebellum receives leftward smooth-pursuit-related MF inputs from pontine nuclei (Fig. 9). In contrast, during rightward smooth pursuit in the gain-decrease condition, leftward retinal error signals from CF inputs and rightward smooth pursuit signals from MF inputs might interact in the right cerebellum (Fig. 9). We know that the NOT is essential for some forms of visually guided motor learning including VOR adaptation in visual-vestibular mismatch paradigms (Yakushin et al. 2000) and in the process of vestibular compensation following injury to the vestibular labyrinth (Stewart et al. 2005). Interactions between visual CF-derived complex spikes and vestibular MF-derived simple spikes may be most important for VOR plasticity (e.g., Raymond and Lisberger 1998). In contrast, during smooth pursuit adaptation, interactions between visual CFs carrying retinal error information and MFs carrying smooth-pursuit-related signals (e.g., pontine nuclei derived) might be most important.
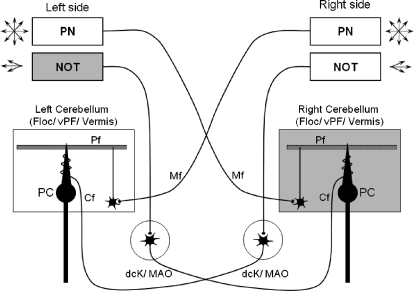
Fig. 9.
Simplified diagram to indicate some of the pathways for NOT and pontine nuclei (PN) derived visual and eye motion signals to the contralateral cerebellum. Horizontal directional retinal error information for smooth pursuit adaptation is available in the NOT. NOT neurons respond preferentially to ipsiversive visual motion and drive neurons in the ipsilateral dorsal cap of Kooy (dck) and the medial accessory olive (MAO) of the inferior olive. For leftward pursuit and left NOT stimulation (ipsiversive pursuit paradigm), the right floccular complex and vermis receive leftward complex spikes and multidirectional simple spikes derived from the PN (e.g., NRTP and DLPN). The arrows indicate that the PN provides multi-directional eye and visual motion signals, while the NOT provides only horizontal visual error signals. Abbreviations; cf., climbing fiber; Floc, flocculus; Mf, mossy fiber; PC, Purkinje cell; Pf, parallel fiber; vPF, ventral paraflocculus.
According to some theories of cerebellar learning, simple spike activity from MF inputs provide signals for moment-by-moment motor control, whereas complex spike activity from CFs provides error signals that could guide motor learning in the cerebellar cortex (e.g., Albus 1971; Ito 1972; Marr 1969; Raymond and Lisberger 1998). Figure 9 provides a simplified anatomical wiring diagram to indicate potential sources of directional signals that could play a role in smooth pursuit adaptation. The pontine nuclei [e.g., DLPN and nucleus reticularis tegmenti pontis (NRTP)] contain neurons coding all directions of eye motion during smooth pursuit. The NRTP contains neurons sensitive to both eye acceleration and eye velocity and weaker visual sensitivity during smooth pursuit (Ono et al. 2005; Suzuki et al. 2003). The DLPN contains eye velocity and visual velocity sensitive neurons (Mustari et al. 1988; Ono et al. 2005; Suzuki and Keller 1984). Those pontine nuclei are known to have anatomical connections with the dorsal, ventral paraflocculus, and the vermal lobules VI, Vll (Brodal 1982; Glickstein et al. 1994; Nagao et al. 1997). In contrast, the NOT is a visual center and contains neurons that code mostly ipsiversive directions of large- or small-field visual motion (Mustari and Fuchs 1990). Our results support the hypothesis that interactions between CF inputs carrying retinal error information derived by NOT and MF inputs carrying smooth pursuit signals (e.g., pontine nuclei derived) might be essential for adaptation. Furthermore, the time course of the adaptation showed increase or decrease gradually until ≥100 trials (Fig. 8); this is important when considering cerebellar motor learning. This is because repeated coincident inputs of error signals and motor output are thought to be necessary for cerebellar motor learning processes, such as long term depression (LTD) (e.g., Ito 1972). LTD could cause gradual changes in eye acceleration over the adaptation period by changing the response properties of cerebellar Purkinje cells and their influence on distal centers such as the vestibular and fastigial nuclei.
Possible cortico-brain stem pathways involving smooth pursuit adaptation
Recent studies using electrical stimulation of middle temporal cortex (MT) during step-ramp smooth pursuit indicate that MT could be a source of visual, direction selective signals appropriate for guiding smooth pursuit adaptation (Carey et al. 2005). This was demonstrated by delivering microelectrical stimulation trains in locations where MT neurons with vertical sensitivity were recorded. Repeated trials of this ES coupled with horizontal step-ramp tracking led to induction of a vertical component in the attempted horizontal pursuit after a training session with similar structure to that used here. These MT effects are likely to be exerted through connections with visual centers in the brain stem. Anatomical and functional connectivity studies have shown that MT sends strong inputs to the DLPN, NOT, and accessory optic system (e.g., Distler et al. 2002; Hoffmann et al. 1992, 2002; Mustari et al. 1994; see Gamlin 2006 for review). Early studies showed that MT neurons with foveal/parafoveal visual receptive fields were modulated during smooth pursuit (Komatsu and Wurtz 1988; Newsome et al. 1988). The responses of these MT neurons were shown to be visually contingent because neuronal response dropped when retinal image motion was reduced by target stabilization or blinking during pursuit.
Different directions of visual motion are carried in different brain stem centers that inputs from MT. For example, the population of neurons in the DLPN represents all directions of visual motion and could therefore support smooth pursuit in any direction as has been previously discussed (e.g., Mustari et al. 1988; Suzuki et al. 1990; Thier et al. 1988). We found that unilateral DLPN lesions impaired smooth pursuit in mostly the ipsilesional direction (May et al. 1988; Ono et al. 2003). Similarly, smooth pursuit adaptation was reduced in specific directions following unilateral DLPN inactivation with muscimol (Ono and Mustari 2007). We suggested that this impairment of motor learning was produced by altering the interaction between simple and complex spikes in the cerebellum contralateral to the lesion. MT also has highly specific projections to brain stem centers coding specific directions of visual motion. For example, the MT projection to NOT features only horizontal, direction selective neurons (Hoffmann et al. 1992). Similarly, the lateral terminal nucleus (LTN) of the AOS contains only vertical, visual direction selective neurons (Mustari and Fuchs 1989) and receives projections from MT. It is possible that the pursuit adaptation produced by Carey and colleagues (2005) following ES in vertical subregions of MT produced its effect by way of the LTN, DLPN, or perhaps other pathways. Similarly, the MT may use projections to the NOT to provide horizontal (ipsiversive) retinal error information for smooth pursuit adaptation. Therefore MT could influence motor learning for all directions of smooth pursuit by connections with NOT, LTN, and DLPN.
Conclusion and future studies
In conclusion, our findings support the suggestion that the NOT is a major source of error information that could provide instructive signals for visually guided motor learning during horizontal smooth pursuit. Smooth pursuit adaptation for gain-increase and -decrease could depend on direction sensitive retinal error signals coded by NOT neurons for distribution to the cerebellum. We suggest that our paradigm for artificial activation of a major source of retinal error information may provide an effective means for studying visually guided motor learning. It is still unknown whether ES of the NOT produces complex spike activity in Purkinje cells of cerebellar regions active during smooth pursuit. There might be alternative pathways involving the NOT or AOS including projections to ipsilateral nucleus prepositus hypoglossi, medial vestibular nucleus, and DLPN (Mustari et al. 1994) that could alter smooth pursuit adaptation. Further studies will be necessary to completely determine the neural sites and pathways essential for visual guided motor learning during smooth pursuit.
GRANTS
This work was supported by National Institutes of Health Grants EY-06069 to M. J. Mustari, EY-019266 to S. Ono, RR-00165, and RR-00166.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- Albus JS. A theory of cerebellar function. Math Biosci 10: 25–61, 1971. [Google Scholar]
- Brodal P. Further observations on the cerebellar projections from the pontine nuclei and the nucleus reticularis tegmenti pontis in the rhesus monkey. J Comp Neurol 204: 44–55, 1982. [DOI] [PubMed] [Google Scholar]
- Buttner-Ennever JA, Cohen B, Horn AK, Reisine H. Efferent pathways of the nucleus of the optic tract in monkey and their role in eye movements. J Comp Neurol 373: 90–107, 1996. [DOI] [PubMed] [Google Scholar]
- Carey MR, Medina JF, Lisberger SG. Instructive signals for motor learning from visual cortical area MT. Nat Neurosci 8: 813–819, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Matsuo V, Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol 270: 321–344, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das VE, Economides JR, Ono S, Mustari MJ. Information processing by parafoveal cells in the primate nucleus of the optic tract. Exp Brain Res 140: 301–310, 2001. [DOI] [PubMed] [Google Scholar]
- Distler C, Mustari MJ, Hoffmann KP. Cortical projections to the nucleus of the optic tract and dorsal terminal nucleus and to the dorsolateral pontine nucleus in macaques: a dual retrograde tracing study. J Comp Neurol 444: 144–158, 2002. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Tanaka M, Suzuki Y, Fukushima J, Yoshida T. Adaptive changes in human smooth pursuit eye movement. Neurosci Res 25: 391–398, 1996. [DOI] [PubMed] [Google Scholar]
- Gamlin PD. The pretectum: connection and oculomotor-related roles. Prog Brain Res 151: 379–405, 2006. [DOI] [PubMed] [Google Scholar]
- Giolli RA, Blanks RH, Lui F. The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function. Prog Brain Res 151: 407–440, 2006. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Gerrits N, Kralj-Hans I, Mercier B, Stein J, Voogd J. Visual pontocerebellar projections in the macaque. J Comp Neurol 349: 51–72, 1994. [DOI] [PubMed] [Google Scholar]
- Hoffmann KP, Behrend K, Schoppmann A. A direct afferent visual pathway from the nucleus of the optic tract to the inferior olive in the cat. Brain Res 115: 150–153, 1976. [DOI] [PubMed] [Google Scholar]
- Hoffmann KP, Bremmer F, Thiele A, Distler C. Directional asymmetry of neurons in cortical areas MT and MST projecting to the NOT-DTN in macaques. J Neurophysiol 87: 2113–2123, 2002. [DOI] [PubMed] [Google Scholar]
- Hoffmann KP, Distler C, Ilg U. Callosal and superior temporal sulcus contributions to receptive field properties in the macaque monkey's nucleus of the optic tract and dorsal terminal nucleus of the accessory optic tract. J Comp Neurol 321: 150–162, 1992. [DOI] [PubMed] [Google Scholar]
- Hoffmann KP, Schoppmann A. A quantitative analysis of the direction-specific response of Neurons in the cat's nucleus of the optic tract. Exp Brain Res 42: 146–157, 1981. [DOI] [PubMed] [Google Scholar]
- Ito M. Neural design of the cerebellar motor control system. Brain Research 40: 81–84, 1972. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980. [DOI] [PubMed] [Google Scholar]
- Kahlon M, Lisberger SG. Coordinate system for learning in the smooth pursuit eye movements of monkeys. J Neurosci 16: 7270–7283, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa H, Xiong G, Hiramatsu T, Ohki M, Nagao S. Difference of climbing fiber input sources between the primate oculomotor-related cerebellar vermis and hemisphere revealed by a retrograde tracing study. Neurosci Lett 462: 10–13, 2009. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kawano K, Takemura A, Inoue Y, Kitama T, Gomi H, Kawato M. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys. II. Complex spikes. J Neurophysiol 80: 832–848, 1998. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol 60: 580–603, 1988. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol 202: 437–470, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JG, Keller EL, Suzuki DA. Smooth-pursuit eye movement deficits with chemical lesions in the dorsolateral pontine nucleus of the monkey. J Neurophysiol 59: 952–977, 1988. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF. Response properties of single units in the lateral terminal nucleus of the accessory optic system in the behaving primate. J Neurophysiol 61: 1207–1220, 1989. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF. Discharge patterns of neurons in the pretectal nucleus of the optic tract (NOT) in the behaving primate. J Neurophysiol 64: 77–90, 1990. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF, Kaneko CR, Robinson FR. Anatomical connections of the primate pretectal nucleus of the optic tract. J Comp Neurol 349: 111–128, 1994. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF, Wallman J. Response properties of dorsolateral pontine units during smooth pursuit in the rhesus macaque. J Neurophysiol 60: 664–686, 1988. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Ono S. The optokinetic system. In: The New Encyclopedia of Neuroscience (4th ed), edited by Squire L. New York: Elsevier, 2007. [Google Scholar]
- Mustari MJ, Tusa RJ, Burrows AF, Fuchs AF, Livingston CA. Gaze-stabilizing deficits and latent nystagmus in monkeys with early-onset visual deprivation: role of the pretectal not. J Neurophysiol 86: 662–675, 2001. [DOI] [PubMed] [Google Scholar]
- Nagao S, Kitamura T, Nakamura N, Hiramatsu T, Yamada J. Differences of the primate flocculus and ventral paraflocculus in the mossy and climbing fiber input organization. J Comp Neurol 382: 480–498, 1997. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol 60: 604–620, 1988. [DOI] [PubMed] [Google Scholar]
- Ono S, Das VE, Economides JR, Mustari MJ. Modeling of smooth pursuit-related neuronal responses in the DLPN and NRTP of the rhesus macaque. J Neurophysiol 93: 108–116, 2005. [DOI] [PubMed] [Google Scholar]
- Ono S, Das VE, Mustari MJ. Role of the dorsolateral pontine nucleus in short-term adaptation of the horizontal vestibuloocular reflex. J Neurophysiol 89: 2879–2885, 2003. [DOI] [PubMed] [Google Scholar]
- Ono S, Mustari MJ. Horizontal smooth pursuit adaptation in macaques after muscimol inactivation of the dorsolateral pontine nucleus (DLPN). J Neurophysiol 98: 2918–2932, 2007. [DOI] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J Neurosci 18: 9112–9129, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff D, Cohen B, Büttner-Ennever J, Matsuo V. Effects of lesions of the nucleus of the optic tract on optokinetic nystagmus and after-nystagmus in the monkey. Exp Brain Res 79: 225–239, 1990. [DOI] [PubMed] [Google Scholar]
- Schiff D, Cohen B, Raphan T. Nystagmus induced by stimulation of the nucleus of the optic tract in the monkey. Exp Brain Res 70: 1–14, 1988. [DOI] [PubMed] [Google Scholar]
- Stewart CM, Mustari MJ, Perachio AA. Visual-vestibular interactions during vestibular compensation: role of the pretectal not in horizontal VOR recovery after hemilabyrinthectomy in rhesus monkey. J Neurophysiol 94: 2653–2666, 2005. [DOI] [PubMed] [Google Scholar]
- Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. II. Complex spikes. J Neurophysiol 63: 1262–1275, 1990. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, Keller EL. Visual signals in the dorsolateral pontine nucleus of the alert monkey: their relationship to smooth-pursuit eye movements. Exp Brain Res 53: 473–478, 1984. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, May JG, Keller EL, Yee RD. Visual motion response properties of neurons in dorsolateral pontine nucleus of alert monkey. J Neurophysiol 63: 37–59, 1990. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, Yamada T, Yee RD. Smooth-pursuit eye-movement-related neuronal activity in macaque nucleus reticularis tegmenti pontis. J Neurophysiol 89: 2146–2158, 2003. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol 83: 2047–2062, 2000. [DOI] [PubMed] [Google Scholar]
- Thier P, Koehler W, Buettner UW. Neuronal activity in the dorsolateral pontine nucleus of the alert monkey modulated by visual stimuli and eye movements. Exp Brain Res 70: 496–512, 1988. [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Reisine H, Buttner-Ennever J, Raphan T, Cohen B. Functions of the nucleus of the optic tract (NOT). I. Adaptation of the gain of the horizontal vestibulo-ocular reflex. Exp Brain Res 131: 416–432, 2000. [DOI] [PubMed] [Google Scholar]