Abstract
Classic experiments have indicated that monocular deprivation (MD) for a few days during a critical period of development results in a decrease in the strength of connections mediating responses to the deprived eye, leading to a dramatic breakdown of cortical neuron binocularity. Despite the substantial functional change in the visual cortex, recovery from the effects of MD can be obtained if binocular vision is promptly restored. While great efforts have been made to elucidate the mechanisms regulating loss of deprived eye function, the mechanisms that underlie the recovery of cortical binocularity are poorly understood. Here, we examined whether activation of the N-methyl-d-aspartate receptor (NMDAR) is required for the recovery of cortical binocularity by pharmacologically blocking the NMDAR using d,l-2-amino-5-phosphonopentanoic (APV). Ferrets (n = 10) were monocularly deprived for 6 days, and osmotic minipumps, filled with APV (5.6 mg/ml) or saline, were surgically implanted into the primary visual cortex. One day after surgery, the deprived eye was reopened, and the animals were allowed 24 h of binocular vision. Extracellular recordings showed that intracortical infusion of the NMDAR antagonist, APV, prevented recovery of cortical binocularity while preserving neuronal responsiveness. These findings provide an important new insight for a specific role of NMDARs in the recovery of cortical binocularity from the effects of MD.
INTRODUCTION
One of the defining characteristics of the primary visual cortex is the convergence of monocular inputs at the single neuron level, leading to the establishment of binocular responses. One's ability to perceive three-dimensional depth, as well as the detection of faint patterns, depends on normal cortical binocular integration (Parker 2007). Loss of these functions can occur, however, if one eye is deprived of patterned vision at an early point during development (Gwiazda 1992), perhaps because its field of view was strongly out of focus with respect to the other eye (anisometropia). If not treated early, this can result in a clinical condition called amblyopia, characterized by the loss of cortical binocularity and a significant reduction in visual acuity that cannot be improved by corrective lenses and affects 2–2.5% of the adult population worldwide (Rutstein and Daum 1998).
The loss of cortical binocularity after a brief period of monocular deprivation (MD) has traditionally been regarded as the result of a competitive process in animal models of amblyopia. The deprived eye loses functional cortical representation to its more active, nondeprived counterpart. It is thought that this activity-dependent binocular competition follows a Hebbian process wherein synapses whose activity coincides with target depolarization beyond some threshold level are strengthened (Hebb 1949), and synapses whose activity is not correlated with postsynaptic activity are eliminated (Stent 1973). Contrary to MD, binocular competition is unlikely to drive the recovery of cortical binocularity after deprivation because the previously deprived eye is initially weaker than the nondeprived one. Instead, recovery of cortical binocularity after restoration of binocular vision involves associative mechanisms (Mitchell et al. 2001, 2003) that promote recovery of responses to the deprived eye while preserving the responses to the nondeprived eye (Krahe et al. 2005; Liao et al. 2004). Therefore, absolute levels of correlated activity rather than differences in activity between the two eyes should determine the degree of recovery. In fact, studies have shown that the recovery from MD is greatly reduced if the two eyes are misaligned by surgically induced strabismus (Kind et al. 2002). Thus, despite being mechanistically different (Dadvand et al. 2006; Faulkner et al. 2006; Kaneko et al. 2008; Krahe et al. 2005; Liao et al. 2002), both loss and recovery of cortical binocularity must rely on the correlation of responses from the two eyes.
Evidence suggests that the N-methyl-d-aspartate receptor (NMDAR) may function as the correlation detector that signals whether pre- and postsynaptic activities are synchronous or asynchronous (Bourne and Nicoll 1993). In fact, much attention has been focused on the NMDAR because of its proposed role in brain development (Flint et al. 1997; Hestrin 1992; Roberts and Ramoa 1999), learning and memory (Bliss and Collingridge 1993; Quinlan et al. 2004; Zinebi et al. 2003), and in loss of function after MD (Bear et al. 1990; Constantine-Paton et al. 1990; Fox and Daw 1993; Kleinschmidt et al. 1987; Roberts et al. 1998). Previous studies have also shown that the activation of NMDARs is involved in the recovery of deprived eye responses after reverse deprivation (Bear et al. 1990; Gu et al. 1989). However, reverse deprivation after MD is likely to be mechanistically different from binocular recovery (Faulkner et al. 2006; Liao et al. 2002) and more similar to the effects of MD in terms of its competitive process (Faulkner et al. 2006; Giffin and Mitchell 1978), time course (Gordon and Stryker 1996; Hensch et al. 1998; Mioche and Singer 1989; Mitchell et al. 2001), and structural remodeling (Muller and Griesinger 1998). Therefore, the investigation of the role of the NMDAR specifically in the recovery of cortical binocularity should provide new insight about its mechanisms, as well as have significant implications for the understanding of cortical plasticity and the treatment of amblyopia.
Here, we examined whether the activation of NMDARs is required for the recovery of cortical binocularity after MD. We studied the effects of intracortical infusion of d,l-2-amino-5-phosphonopentanoic (APV) on the recovery of deprived eye responses during the restoration of normal binocular vision in ferrets.
METHODS
Ferrets at postnatal day 45–46 (n = 10), at the peak of the critical period of ocular dominance plasticity (Issa et al. 1999), had the eyelid of their right eye sutured closed to prevent patterned visual stimulation. After 6 days of MD, the animals were surgically implanted with minipumps filled with APV dissolved in sterile saline or with saline alone for control experiments. Minipumps were attached to a 30-gauge stainless steel cannulae, which was inserted in the left visual cortex. One day later, animals were allowed 24 h of binocular vision. At the end of the binocular recovery period, in vivo extracellular recordings were conducted. Five additional age-matched animals included in this study had normal visual experience and were not surgically implanted with minipumps. The ocular dominance of a total of 522 cells was examined.
Implantation of minipumps
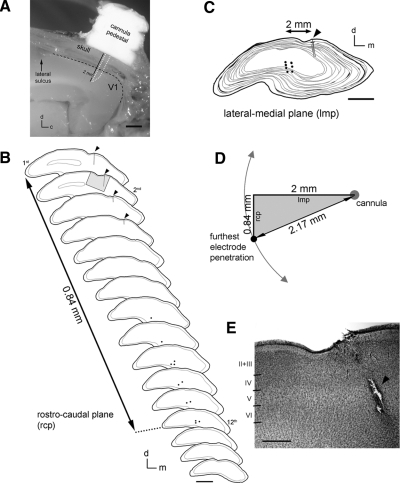
Surgical procedures for minipump implantations were performed as described elsewhere (Krahe et al. 2005). Alzet osmotic minipumps (Alza 1007D, 0.5 μl/h) were filled with APV (5.6 mg/ml; Sigma, St. Louis, MO) (Bear et al. 1990). Cannulas were placed slightly rostral to area 17 to avoid mechanical damage to the recording site. Precise and reliable placement of the cannula across different animals was made by using structural landmarks. After retraction of the cranial muscles, the underlying skull remained transparent enough to permit the visualization of the lateral sulcus, the venous sinus, and major visual cortex vasculature. These structures were used to determine the location of cannula insertion. APV or vehicle was delivered into the left hemisphere (contralateral to the deprived eye). To allow the solution to reach working concentrations, minipump implantations were done 1 day before normal binocular vision was restored (Krahe et al. 2005; Taha and Stryker 2002). After 1 day of binocular recovery, animals were anesthetized, and single unit recordings were performed. At the conclusion of each experiment, ferrets were killed with Euthasol (pentobarbital sodium and phenytoin sodium; 125 mg/kg) and perfused transcardially with cold 0.9% saline (pH 7.2), followed by cold 4% paraformaldehyde (pH 7.2). Vibratome sections were collected at 70 μm intervals and Nissl stained to reconstruct electrode tracks and verify the position of the infusion cannula. In every animal, we certified that the infusion had been successful by verifying the residual volume of solution inside the osmotic minipump during the time of recordings. In one animal, it was determined that the cannula was clogged, and the minipump was completely full. Although recordings made close to the cannula placement site confirmed that neurons in that region displayed recovery of cortical binocularity, the data from this particular animal were not included in this study. Instead, this animal was used to depict the location of the cannula insertion in the striate cortex (Fig. 2A).
Fig. 2.
A: photomicrograph of a ferret brain depicting the location of the cannula insertion in the visual cortex. The cannula pedestal, skull, and brain were carefully cut along the sagital plane. V1, primary visual cortex; d, dorsal; c, caudal. Scale bar 1 mm. B: camera lucida drawings of coronal sections (70 μm) through V1 of an animal after treatment with APV during binocular recovery of deprived eye responses. Sections are organized from rostral to caudal. Black dots indicate electrolytic lesions made during 2 electrode penetrations located near the minipump cannula (arrowheads, vertical black lines). The distance from the first section with a cannula-lesion track (1st) to the last section showing electrolytic lesions (12th) is indicated. d, dorsal; m, medial. Scale bar, 2 mm. C: superimposed sections shown in B depicting the lateral-medial distance between the cannula placement and the electrolytic lesions. d, dorsal; m, medial. Scale bar, 2 mm. D: diagram depicting the distance from the infusion and the recording sites based on the distances shown in B and C. E: photomicrograph of a Nissl-stained coronal section (highlighted in B, 2nd section, gray area) showing normal cortical laminae near the APV injection site. Arrowhead points to the mechanical lesion made by the insertion of the cannula into the ferret primary visual cortex. Scale bar, 500 μm.
Extracellular recordings in vivo
Extracellular single-unit recordings were performed as described elsewhere (Medina et al. 2003). Briefly, animals were anesthetized with pentobarbital sodium (35 mg/kg, ip) and placed in a stereotaxic frame. Nictitating membranes were retracted using phenylephrine hydrochloride (2.5%), the pupils were dilated with atropine sulfate (1%), and contact lenses were placed on the corneas. A craniotomy was performed to expose the caudal most region of the left primary visual cortex (Law et al. 1988; White et al. 1999) where recordings were performed. Single-unit recordings were conducted using a tungsten-microelectrode (0.5–1.5 MΩ, FHC, Bowdoin, ME) lowered into the primary visual cortex at 20° to the vertical. To minimize sampling bias, single-unit recordings used in this study were separated by ∼100 μm along the electrode track. Moreover, for all APV-treated animals, the first recording penetration was always close to the infusion site (1–3.5 mm), and the second penetration was performed at a distal point (internal control recordings, 5–7 mm). This sequence was repeated for the subsequent recording penetrations in each animal. Similar to previous findings (Krahe et al. 2005), we did not notice changes in the ocular dominance profile within a particular region during the duration of single-unit experiments. Supplemental doses of pentobarbital were given every hour throughout the experiment or when heart rate or expired CO2 increased. Body temperature and expired CO2 were monitored continuously, and subcutaneous injections of 10% dextrose and 0.9% saline were given during the experiment as needed. Similar procedures have been previously described and shown to preserve visual responses over time in ferrets (Medina et al. 2003).
After a single unit was isolated, its receptive field, ocular dominance, and preferred orientation, direction, and velocity were determined qualitatively, using a moving bar of light (0.5° wide and 20° long). Ocular dominance was quantitatively determined as previously described (Krahe et al. 2005). Each stimulus presentation consisted of the bar of light moving across the receptive field at the optimal orientation in one direction and back across in the opposite direction. Spikes were collected on a computer during the 10 stimulus presentations using Spike 2 software (Cambridge Electronics Design, Cambridge, UK). The number of spikes was averaged for the 10 stimulus runs. Spontaneous activity was determined by recording in the absence of stimulation for 2 s after each stimulus presentation. The person responsible for the analysis of the raw single-unit data was unaware of the cell's location in relation to the cannula position, sequence of recordings, and minipump treatment. On the conclusion of the experiment, the animal was killed with Euthasol (125 mg/kg).
An ocular dominance index (ODI) was calculated for each cell using the following equation: (LE)/(LE + RE), where LE is the number of spikes in response to stimulation of the left eye, and RE is the response to right eye stimulation. An ODI of 1.0 would indicate responsiveness only to the left eye; an index of 0.0 would indicate responsiveness only to the right eye.
From the cellular ODI values in a specific cortical region (relative to the infusion site), a binocular index (BI) was calculated to reflect the degree of binocularity at that location. The BI index was calculated as follows where PA–B denotes the number of cells with ODI values between A and B, and Ptot denotes the total number of cells. This index is the inverse of the monocular index described originally by Stryker and Harris (1986). A BI value shifted toward 0.0 would reflect that all cells are driven exclusively by one eye or the other, whereas a BI of 1.0 suggests that all individual cells are driven equally by both eyes. Note that an animal would never be expected to have a BI of 1.0 because extracellular recordings were obtained across ocular dominance columns. Thus, monocular responses are expected.
Univariate ANOVAs were performed to determine differences in the ODI and BI data and on visual response properties (number of spikes and spontaneous activity). Differences between experimental groups were also determined using Wilcoxon Mann-Whitney tests and χ2 tests. For all statistical tests, significance was set at P < 0.05 (2-tailed). All procedures described in this study were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Estimation of APV intracortical distribution
In an effort to estimate the APV concentration “close to” (1–3.5 mm) and “far from” (5–7 mm) the infusion site, we relied on previous studies that measured the cortical spread of pharmacological agents at different distances from the cannula using radioactive labeling (Kasamatsu et al. 1998; Shirokawa and Kasamatsu 1986) and in vitro slice techniques (Bear et al. 1990) in the visual cortex of cats. According to the latter, a minipump infusing a 50 mM APV solution into the primary visual cortex for 4 days yields an APV concentration of about 300 μM at 1.0 mm from the infusion site and 250 μM at 3.5 mm away. This concentration decreases to around 150 μM at 5 mm and to ∼100 μM at 7 mm away from the infusion site. On the other hand, estimates based on dilution curves (Kasamatsu et al. 1981; Shirokawa and Kasamatsu 1986) for the same initial APV infused concentration (50 mM) predict that at 1.0 and 3.5 mm away from the infusion site, the APV concentration should be ∼700 and 100 μM, respectively, whereas far from the perfusion center (5–7 mm away), this concentration reaches a plateau of ∼40 μM. Considering that the initial APV concentration used in this study was 28.4 mM and the fact that a steady-state concentration of APV is reached with 18 h of exposure (Bear et al. 1990), the APV concentration at 1.0 and 3.5 mm away should range from 170 to 400 μM and from 60 to 142 μM, respectively; concentrations 5.0 and 7.0 mm away from the infusion site should be between 20 and 85 μM and between 20 and 70 μM, respectively. Previous in vivo (Bear et al. 1990; Kleinschmidt et al. 1987) and in vitro (Artola and Singer 1987) studies suggest that APV concentrations around 100 μM block NMDAR function.
RESULTS
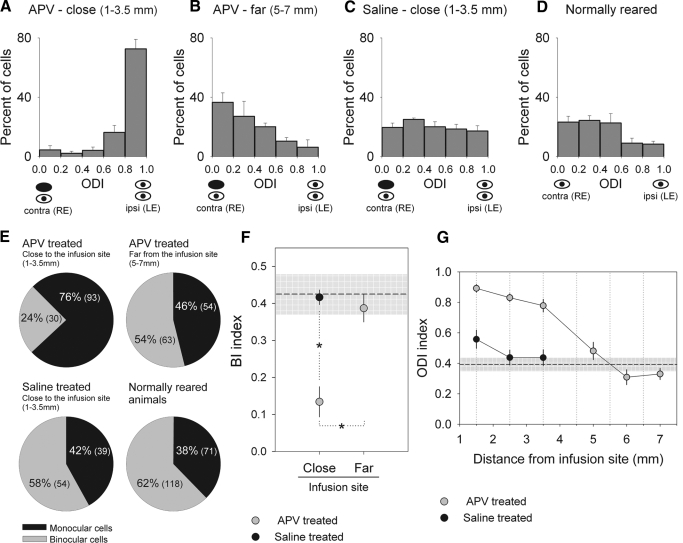
Here, we used intracortical infusion of APV during the period of binocular vision restoration to examine whether ocular dominance plasticity leading to the recovery of cortical binocularity requires the activation of NMDARs. Histograms showing the distribution of neuronal responses sorted into five binocularity ranges based on ODI values (see methods) are shown in Fig. 1 for monocularly deprived animals that received intracortical infusion of APV (Fig. 1, A and B) or saline (Fig. 1C) and were allowed 24 h of binocular vision. The ocular dominance profile of a group of normally reared animals is also shown (Fig. 1D). For all animals, extracellular recordings were made in the left hemisphere (contralateral to the deprived eye). Results show that in APV infused animals, the ocular dominance distribution is markedly shifted toward the nondeprived eye in neurons close to the infusion site (1–3.5 mm, n = 123 cells from 10 recording penetrations in 5 animals), indicating that APV treatment did prevent recovery of deprived eye responses (Fig. 1A). In contrast, the results observed in internal control penetrations located far from the infusion site (5–7 mm, n = 117 cells from 11 recording penetrations) show full recovery of deprived eye responses (Fig. 1B). Similarly, the ocular dominance profile of saline-treated animals in which extracellular recordings were performed close to the infusion site (1–3.5 mm, n = 93 cells from 8 recording penetrations in 4 animals) also show full recovery of deprived eye responses (Fig. 1C). The ODI values for the group of neurons near the APV infusion site (Fig. 1A) differed significantly from those observed for the group of untreated neurons far from the infusion site (Fig. 1B; U = 1,608, P < 0.0001, Mann-Whitney U test) and from those from saline-treated neurons (Fig. 1C; n = 93, U = 1,856.5, P < 0.0001, Mann-Whitney U test) and normally reared animals (Fig. 1D; n = 189, U = 2,558.5, P < 0.0001, Mann-Whitney U test). A significant difference was also found when comparing ODI values between saline and APV control recordings (Fig. 1, B and C; U = 4,285.5, P < 0.01, Mann-Whitney U test) but not when comparing APV control recordings to those obtained from normally reared animals (Fig. 1, B and D; U = 9,920, P = 0.13, Mann-Whitney U test). The reason for such a discrepancy may lie in the fact that APV control recordings were obtained from a more lateral region in the ferret primary visual cortex compared with the other two groups or to possible cortical alterations in the vicinity of the cannula in saline-treated animals. Nonetheless, although significant, this difference is overshadowed by the importance of having both an internal and an across animal control group.
Fig. 1.
Intracortical infusion of d,l-2-amino-5-phosphonopentanoic (APV) in the left primary visual cortex prevented recovery of deprived (right) eye responses for neurons located near the infusion site (A, 1–3.5 mm, n = 123 cells from 10 recording penetrations in 5 animals), whereas neurons located far from the infusion site (B, 5–7 mm, n = 117 cells from 11 recording penetrations) showed recovery of deprived eye responses. Saline treatment during restoration of binocular vision did not prevent recovery of deprived eye responses for neurons located close to the infusion site (C, 1–3.5 mm, n = 93 cells from 8 recording penetrations in 4 animals). Note that the ocular dominance profiles in B and C are similar to the ocular dominance distribution obtained from recordings in normally reared ferrrets (D, 189 cells from 16 recording penetrations in 5 animals). ODI, ocular dominance index; contra, contralateral; ipsi, ipsilateral; RE, right eye; LE, left eye. Error bars indicate SE. E: pie charts showing the percentage of binocular cells (ODIs from 0.2 to 0.79) and monocular cells (ODIs from 0.0 to 0.19, and 0.8 to 1.0) from APV and saline-treated animals and normally reared ferrets. The respective numbers of cells are also shown between brackets. Note the small percentage of binocular cells from recordings close to the APV infusion site compared with percentages of binocular cells from the other experimental groups. F: mean binocular indexes (BIs) plotted based on the proximity of the recordings to the infusion site (close: 1–3.5 mm, far: 5–7 mm) for APV and saline-treated animals. A BI value close to 0.1 indicates that the majority of the cells are driven exclusively by one eye or the other, whereas a BI shifted toward 0.5 suggests that several cells are driven by both eyes. Mean BI values from recordings close to the APV infusion site are significantly different from BI values obtained from saline and APV control sites (univariate ANOVA, F = 17.088, df = 2, P < 0.0001; Bonferroni test, P = 0.001 for both comparisons). Error bars indicate ±SE. Dashed line represents the average BI values of animals with normal visual experience (normally reared ferrets) and gray shaded area ±SE. G: mean ODI plotted by the distance of the recordings from the infusion site (rounded to the nearest 0.5 mm) for APV and saline-treated animals. APV-treated, number (n) of cells: 1.5 mm, n = 25; 2.5 mm, n = 65; 3.5 mm, n = 33; 5 mm, n = 27; 6 mm, n = 34; 7 mm, n = 56 cells. saline-treated, number (n) of cells: 1.5 mm, n = 24; 2.5 mm, n = 36; 3.5 mm, n = 33. Error bars indicate ±SE. Dashed line corresponds to the average ODI values of animals with normal visual experience (n = 189 cells) and gray shaded area ±SD.
To determine whether intracortical infusion of APV prevents recovery of cortical binocularity after restoration of binocular vision, we calculated the percentage of binocular cells (ODI values between 0.2 and 0.79) and monocular cells (ODI values <0.2 and >0.79) in APV and saline-treated animals, as well as in normally reared animals (Fig. 1E). Recordings close to the APV infusion site yielded a significantly lower percentage of binocular cells (24%, n = 30 cells) compared with the percentage in control recordings made far away from APV infusion in the same animals (54%, n = 63 cells, χ2 = 21.92, P < 0.0001). This percentage was also significantly lower than observed in saline-treated animals (58%, n = 54 cells, χ2 = 25.27, P < 0.0001) and animals with normal visual experience (62%, n = 118 cells, χ2 = 43.25, P < 0.0001). On the other hand, the percentage of binocular cells in APV control recordings (far from infusion site), saline-treated, and normal animals were similar (APV far vs. saline, χ2 = 0.37, P = 0.58; APV far vs. normal, χ2 = 2.21, P = 0.13; saline vs. normal, χ2 = 0.50, P = 0.47). The computation of a binocular index (BI, modified from Stryker and Harris 1986; see methods) confirmed that cells located near the APV infusion site (1–3.5 mm) did not show recovery of cortical binocularity after restoration of binocular vision, whereas control recordings in saline- and APV-treated animals showed mean BI values similar to those observed in normally reared animals (Fig. 1F). It is important to note that the lack of cortical binocularity in recordings close to the APV infusion site are not likely caused by tissue damage or inflammatory reactions caused by the cannula because recordings close to the infusion site in saline-treated animals showed full recovery of binocular responses. Taken together, these results indicate that NMDAR activation is necessary for the recovery of binocular function from MD.
To investigate the relationship between recovery of deprived eye responses and APV diffusion, we plotted the mean ODI values based on the cell's distance from the infusion site for APV and saline-treated animals (Fig. 1G). The change in ODI values as a function of the distance in APV treated animals was significant (univariate ANOVA, F = 6.364, df = 5, P < 0.0001). Post hoc comparisons of data points close to the APV infusion site (1.5, 2.5, and 3.5 mm) against data points far from the APV infusion site (5, 6, and 7 mm) were statistically significant (Bonferroni, P < 0.0001, for all comparisons). Moreover, the mean ODI values of cells close to the APV infusion site were significantly different from saline ODI values (saline vs. APV: 1.5 mm, U = 104; 2.5 mm, U = 348; 3.5 mm, U = 177; P < 0.0001 for all comparisons, Mann-Whitney U test). The range of the APV effectiveness (in mm) found in this study (Fig. 1D) is consistent with our previous findings using different pharmacological agents in the ferret primary visual cortex (Krahe et al. 2005). Taken together, our physiological findings and APV concentration estimates based on previous studies (see methods) suggest that intracortical infusion of APV prevented the recovery of cortical binocularity in a ∼3.5 mm radius around the infusion site.
Histological examination of coronal sections through V1 of animals treated with APV during binocular recovery confirmed that the distance of the electrode recordings from the infusion cannula was accurate. Figure 2 depicts the location of the cannula insertion in the ferret visual cortex and the precise location of the extracellular recordings in relation to the cannula placement in a representative APV-treated animal (Fig. 2, A–D). Moreover, the APV treatment at the concentration used in this study did not disrupt the cortical laminae next to the infusion site (Fig. 2E), indicating the absence of gross toxic effects. Overall, our results provide strong evidence that recovery of cortical binocularity depends on the activation of NMDARs.
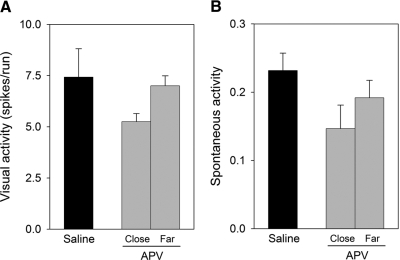
However, the finding that intracortical infusion of APV blocks recovery of cortical binocularity raises the question of whether these effects result from a disruption of visual responses. Previous pharmacological studies have indicated that doses of NMDAR antagonists affecting ocular dominance plasticity lead to the depression of sensory responses and the loss of orientation selectivity of cortical cells (Bear et al. 1990; Daw 1994; Kasamatsu et al. 1998; Miller et al. 1989; Rauschecker et al. 1990). In contrast, qualitative assessment showed that, for the dose and duration of the intracortical infusion used in this study, most neurons examined near the infusion site responded to visual stimulation (only 6 cells of a total of 129 were nonresponsive to visual stimulation, <5%) and were selective to stimulus orientation and direction of movement. Quantitative analysis also showed that APV infusion did not affect the visual responsiveness of the neurons that were studied close to the infusion site (Fig. 3, A and B). Although the maximum responses (in spikes per run, see methods) to stimulation at the optimal orientation close to the APV infusion site were slightly decreased compared with control recordings, no significant differences were observed (univariate ANOVA, F = 2.223, df = 2, P = 0.15). Moreover, our data also show that the infusion of APV did not affect spontaneous activity (univariate ANOVA, F = 2.009, df = 2, P = 0.18), as shown in Fig. 3B. In general, these findings indicate that the effects of APV treatment on recovery of cortical binocularity did not result from a major disruption of sensory responses or spontaneous activity.
Fig. 3.
Mean visual responses (A) and spontaneous activity (B) of V1 cortical cells for saline- and APV-treated animals based on the proximity of the recordings to the infusion site (close: 1–3.5 mm, far: 5–7 mm). Recordings in saline-treated animals were made at 1–3.5 mm from the infusion site. The number of spikes was averaged over the course of the 10 stimulus runs, and spontaneous activity was determined by recording in the absence of stimulation. Error bars indicate SE.
DISCUSSION
Our findings provide important new insight on a specific role of NMDARs in the recovery of cortical binocularity from the effects of MD. Although recent studies indicate that different mechanisms underlie loss and recovery of deprived eye responses (Dadvand et al. 2006; Faulkner et al. 2006; Kaneko et al. 2008; Krahe et al. 2005; Liao et al. 2002), our results suggest that, similarly to MD, NMDAR activation during binocular recovery is necessary for the detection of correlated pre- and postsynaptic activity. The notion that the correlation of input from the two eyes is necessary for the recovery of cortical binocularity is not new (Kind et al. 2002; Mitchell et al. 2001, 2003); neither is the idea that the NMDAR may function as the correlation detector that plays a critical role in activity-dependent neuronal plasticity (Bear et al. 1990; Bourne and Nicoll 1993). However, our results provide experimental evidence that a threshold level of NMDAR activation driven by absolute levels of activity from the two eyes is required for such recovery. Moreover, there is a line of evidence suggesting that MD decreases the threshold for activity dependent synaptic modifications in the primary visual cortex (for a review, see Smith et al. 2009), which is regulated by the ratio between the NMDAR subunits NR2A and 2B (Philpot et al. 2003). Recent findings in the rodent visual cortex suggest that increasing the synaptic proportion of the NR2B subunit, which occurs after MD (Chen and Bear 2007), provides a permissive environment for the strengthening of weak cortical inputs (Cho et al. 2009), such as the inputs from the previously deprived eye are in this study. Future studies will be necessary to investigate whether changes in NMDAR subunits, instead of pharmacological blockade of NMDARs, can also prevent or improve the recovery of cortical binocularity from the effects of MD.
Previous results using pharmacological antagonists of the NMDAR were confounded by a disruption of sensory responses that, by themselves, could be sufficient to cause the loss of plasticity (Bear et al. 1990; Daw 1994; Kasamatsu et al. 1998; Kleinschmidt et al. 1987; Miller et al. 1989; Rauschecker et al. 1990). However, in this study, blockade of NMDAR activation using the selective competitive antagonist APV only slightly affected visual responsiveness of treated cortical neurons, which is consistent with a previous study in cats showing that NMDARs antagonists can block ocular dominance plasticity without specifically affecting neuronal responses (Daw et al. 1999). The explanation for the apparent discrepancy in our results relies on the fact that, contrary to MD, binocular recovery of deprived eye responses in ferrets is extremely fast (Krahe et al. 2005). Therefore we could reduce the amount of time of intracortical APV infusion to 48 h instead of 1 wk as previously described in cats (Bear et al. 1990; Kleinschmidt et al. 1987), thus minimizing potential pharmacological disruption of sensory responses associated with prolonged intracortical infusion of APV. However, we also cannot disregard the possibility that ferrets are more resilient than cats to the effects of APV and/or that, in young ferrets, NMDARs contribute less to visual sensory responses than they do in cats.
In conclusion, we show here that intracortical infusion of an NMDAR antagonist blocks the recovery of cortical binocularty while preserving normal sensory response properties of cortical cells, providing direct evidence for a specific role of NMDARs in the recovery of cortical binocularity after restoration of binocular vision. These findings should have important implications for understanding cortical plasticity as well as for the treatment of amblyopia.
GRANTS
This work was supported by National Institutes of Health Grants EY-011508 to Ary S. Ramoa and AA-13023 to A. E. Medina.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank E. K. Dilger for critical suggestions and careful revision of this manuscript.
REFERENCES
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature 330: 649–652, 1987. [DOI] [PubMed] [Google Scholar]
- Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci 10: 909–925, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Nicoll R. Molecular machines integrate coincident synaptic signals. Cell 72: 65–75, 1993. [DOI] [PubMed] [Google Scholar]
- Chen WS, Bear MF. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology 52: 200–214, 2007. [DOI] [PubMed] [Google Scholar]
- Cho KK, Khibnik L, Philpot BD, Bear MF. The ratio of NR2A/B NMDA receptor subunits determines the qualities of ocular dominance plasticity in visual cortex. Proc Natl Acad Sci USA 106: 5377–5382, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci 13: 129–154, 1990. [DOI] [PubMed] [Google Scholar]
- Dadvand L, Stryker MP, Frank MG. Sleep does not enhance the recovery of deprived eye responses in developing visual cortex. Neuroscience 143: 815–826, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NW. Mechanisms of plasticity in the visual cortex. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 35: 4168–4179, 1994. [PubMed] [Google Scholar]
- Daw NW, Gordon B, Fox KD, Flavin HJ, Kirsch JD, Beaver CJ, Ji Q, Reid SN, Czepita D. Injection of MK-801 affects ocular dominance shifts more than visual activity. J Neurophysiol 81: 204–215, 1999. [DOI] [PubMed] [Google Scholar]
- Faulkner SD, Vorobyov V, Sengpiel F. Visual cortical recovery from reverse occlusion depends on concordant binocular experience. J Neurophysiol 95: 1718–1726, 2006. [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci 17: 2469–2476, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Daw NW. Do NMDA receptors have a critical function in visual cortical plasticity? Trends Neurosci 16: 116–122, 1993. [DOI] [PubMed] [Google Scholar]
- Giffin F, Mitchell DE. The rate of recovery of vision after early monocular deprivation in kittens. J Physiol 274: 511–537, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci 16: 3274–3286, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu QA, Bear MF, Singer W. Blockade of NMDA-receptors prevents ocularity changes in kitten visual cortex after reversed monocular deprivation. Brain Res Dev Brain Res 47: 281–288, 1989. [DOI] [PubMed] [Google Scholar]
- Gwiazda JE. Detection of amblyopia and development of binocular vision in infants and children. Curr Opin Ophthalmol 3: 735–740, 1992. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley, 1949. [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282: 1504–1508, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357: 686–689, 1992. [DOI] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the ferret's visual cortex. J Neurosci 19: 6965–6978, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Hanover JL, England PM, Stryker MP. TrkB kinase is required for recovery, but not loss, of cortical responses following monocular deprivation. Nat Neurosci 11: 497–504, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu T, Iamamura K, Mataga N, Hartveit E, Heggelund U, Heggelund P. Roles of N-methyl-D-aspartate receptors in ocular dominance plasticity in developing visual cortex: re-evaluation. Neuroscience 82: 687–700, 1998. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Itakura T, Jonsson G. Intracortical spread of exogenous catecholamines: effective concentration for modifying cortical plasticity. J Pharmacol Exp Ther 217: 841–850, 1981. [PubMed] [Google Scholar]
- Kind PC, Mitchell DE, Ahmed B, Blakemore C, Bonhoeffer T, Sengpiel F. Correlated binocular activity guides recovery from monocular deprivation. Nature 416: 430–433, 2002. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Bear MF, Singer W. Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science 238: 355–358, 1987. [DOI] [PubMed] [Google Scholar]
- Krahe TE, Medina AE, Bittencourt-Navarrete RE, Colello RJ, Ramoa AS. Protein synthesis independent plasticity mediates rapid and precise recovery of deprived eye responses. Neuron 48: 329–343, 2005. [DOI] [PubMed] [Google Scholar]
- Law MI, Zahs KR, Stryker MP. Organization of primary visual cortex (area 17) in the ferret. J Comp Neurol 278: 157–180, 1988. [DOI] [PubMed] [Google Scholar]
- Liao DS, Krahe TE, Prusky G, Medina AE, Ramoa AS. Recovery of cortical binocularity and orientation selectivity after the critical period for ocular dominance plasticity. J Neurophysiol 92: 2113–2121, 2004. [DOI] [PubMed] [Google Scholar]
- Liao DS, Mower AF, Neve RL, Sato-Bigbee C, Ramoa AS. Different mechanisms for loss and recovery of binocularity in the visual cortex. J Neurosci 22: 9015–9023, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Coppola DM, Ramoa AS. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J Neurosci 23: 10002–10012, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Chapman B, Stryker MP. Visual responses in adult cat visual cortex depend on N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA 86: 5183–5187, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. J Neurophysiol 62: 185–197, 1989. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Gingras G, Kind PC. Initial recovery of vision after early monocular deprivation in kittens is faster when both eyes are open. Proc Natl Acad Sci USA 98: 11667, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE, Kind PC, Sengpiel F, Murphy K. Brief daily periods of binocular vision prevent deprivation-induced acuity loss. Curr Biol 13: 1704–1708, 2003. [DOI] [PubMed] [Google Scholar]
- Muller CM, Griesinger CB. Tissue plasminogen activator mediates reverse occlusion plasticity in visual cortex. Nat Neurosci 1: 47–53, 1998. [DOI] [PubMed] [Google Scholar]
- Parker AJ. Binocular depth perception and the cerebral cortex. Nat Rev Neurosci 8: 379–391, 2007. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci 23: 5588, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Lebel D, Brosh I, Barkai E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron 41: 185–192, 2004. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Egert U, Kossel A. Effects of NMDA antagonists on developmental plasticity in kitten visual cortex. Int J Dev Neurosci 8: 425–435, 1990. [DOI] [PubMed] [Google Scholar]
- Roberts EB, Meredith AM, Ramoa AS. Suppression of NMDA receptor function using antisense DNA blocks ocular dominance plasticity while preserving visual responses. J Neurophysiol 80: 1021–1032, 1998. [DOI] [PubMed] [Google Scholar]
- Roberts EB, Ramoa AS. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of visual cortical plasticity in the ferret. J Neurophysiol 81: 2587–2591, 1999. [DOI] [PubMed] [Google Scholar]
- Rutstein RP, Daum KM. Anomalies of Binocular Vision: Diagnosis and Management. St. Louis, MO: Mosby, 1998. [Google Scholar]
- Shirokawa T, Kasamatsu T. Concentration-dependent suppression by beta-adrenergic antagonists of the shift in ocular dominance following monocular deprivation in kitten visual cortex. Neuroscience 18: 1035–1046, 1986. [DOI] [PubMed] [Google Scholar]
- Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci 364: 357–367, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stent GS. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci USA 70: 997–1001, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci 6: 2117–2133, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha S, Stryker MP. Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron 34: 425–426, 2002. [DOI] [PubMed] [Google Scholar]
- White LE, Bosking WH, Williams SM, Fitzpatrick D. Maps of central visual space in ferret V1 and V2 lack matching inputs from the two eyes. J Neurosci 19: 7089–7099, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinebi F, Xie J, Liu J, Russell RT, Gallagher JP, McKernan MG, Shinnick-Gallagher P. NMDA currents and receptor protein are downregulated in the amygdala during maintenance of fear memory. J Neurosci 23: 10283–10291, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]