Abstract
We investigated the control of spinal interneurons by corticospinal and medial brain stem descending tracts in two macaque monkeys. Stimulating electrodes were implanted in the left pyramidal tract (PT), and the right medial longitudinal fasciculus (MLF), which contains reticulospinal, vestibulospinal, and some tectospinal fibers. Single unit discharge was recorded from 163 interneurons in the intermediate zone of the right spinal cord (segmental levels C6–C8) in the awake state; inputs from descending pathways were assessed from the responses to stimulation through the PT and MLF electrodes. Convergent input from both pathways was the most common finding (71/163 cells); responses to PT and MLF stimulation were of similar amplitude. Interneuron discharge was also recorded while the animal performed a reach and grasp task with the right hand; the output connections of the recorded cells were determined by delivering intraspinal microstimulation (ISMS) at the recording sites. Convergent input from MLF/PT stimulation was also common when analysis was restricted to cells that increased their rate during grasp (14/23 cells) or to cells recorded at sites where ISMS elicited finger or wrist movements (23/57 cells). We conclude that medial brain stem and corticospinal descending pathways have largely overlapping effects on spinal interneurons, including those involved in the control of the hand. This may imply a more important role for the brain stem in coordinating hand movements in primates than commonly assumed; brain stem pathways could contribute to the restoration of function seen after lesions to the corticospinal tract.
INTRODUCTION
Commands for movement are relayed from the brain to the spinal cord via multiple descending fiber tracts. It is important to understand what information passes over these varied pathways because this will inform concepts of motor control in both health and disease.
The mammalian reticulospinal system takes its origin in the pontomedullary region of the brain stem (Jones 1995; Jones and Yang 1985) with fibers terminating predominantly throughout the cervical and lumbar enlargements of the spinal cord (Kuypers et al. 1960). The system is of considerable importance for the control of motor output in lower vertebrates. Studies in cat, rodent, and lamprey have identified a central reticulospinal role in the gross initiation of movement (Grillner et al. 1997; Mori et al. 2001), postural and gait adjustments during locomotion (Drew et al. 1986; Mori 1987; Orlovsky 1970; Prentice and Drew 2001; Schepens and Drew 2004), and postural control during targeted reaching (Schepens and Drew 2004, 2006).
In primates, most work has focused on the corticospinal tract (CST). In Old World primates, the CST makes monosynaptic connections to motoneurons (Landgren et al. 1962); these are thought to play an important role in dexterous hand and finger movements (Lawrence and Kuypers 1968a; Porter and Lemon 1993). The importance of the CST in man is highlighted by the severity of impairments following stroke. The medial descending systems of the brain stem (including reticulospinal) are thought to control coordinated whole body postural and orienting movements, while the phylogenetically younger corticospinal tract fractionates individual limb movements (Kuypers 1981).
More recent evidence suggests that the primate reticulospinal system may play a more diverse role in motor control than previously assumed. Both single unit recording and stimulation studies have shown an important influence of the reticular formation on proximal muscles (Buford and Davidson 2004; Davidson and Buford 2004, 2006; Davidson et al. 2007), but these reports have also shown some reticulospinal influence over more distal muscles in the forearm. In addition, Riddle et al. (2009) reported that the reticulospinal tract provided input even to motoneurons projecting to intrinsic hand muscles. To understand this influence in more detail, it is important to characterize the spinal connectivity of the reticulospinal tract (RST) because this may constrain the range of functions that it can subserve. Anatomical studies suggest that the terminal distribution of primate RST axons within the cervical and lumbar spinal enlargements is largely restricted to the medial regions of the ventral horn and intermediate zone (Kuypers et al. 1962; Matsuyama et al. 1997, 1999). Combined with evidence from Bernhard and Rexed (1945), this ventromedial termination bias implies an influence over medially located motoneurons that control axial and proximal limb muscles. This would be consistent with a primary role for the RST in postural control (Kuypers et al. 1962).
Two recent studies (Davidson and Buford 2004, 2006) hinted that the RST may have significant access to distal muscle groups. Microstimulation within the pontomedullary reticular formation (PMRF) could produce both facilitation and suppression of ongoing electromyographic (EMG) activity in a variety of arm muscles extending as far distal as the wrist. Furthermore we have recently shown that brain stem descending pathways (including the reticulospinal tract) can influence motoneurons projecting even to intrinsic hand muscles (Riddle et al. 2009). Such effects may be mediated by a more widespread terminal distribution of reticulospinal connections than suggested by previous anatomical work. If the primate RST can influence distal muscles, it is important to define the extent to which this control is parallel to, or separate from, the well characterized inputs to distal motoneuron pools from the CST.
In this study, we compared the functional connections of primate CST and medial brain stem descending pathways to cervical spinal interneurons by documenting responses following stimulation of the pyramidal tract (CST) and medial longitudinal fasciculus (MLF, containing axons of the RST, as well as the medial vestibulospinal tract). By observing the movements produced by intraspinal microstimulation in the vicinity of the recorded cells and by recording the cell's natural firing during a reach-and-grasp task, we were able to characterize interneurons according to whether they were likely to control proximal or distal muscles. We demonstrate a largely convergent activation of interneurons from CST and the brain stem, including those interneurons with more distal projection target muscles involved in hand function. Such connections may underlie greater control of voluntary arm movement by the primate RST than has hitherto been appreciated, and could represent a substrate for functional motor recovery in patients after stroke or spinal cord injury.
METHODS
General
All animal procedures were carried out under appropriate UK Home Office licenses in accordance with the Animals (Scientific Procedures) Act 1986 and were approved by the Local Research Ethics Committee of Newcastle University. Recordings were made from two adult female rhesus macaque monkeys (Macaca mulatta, monkeys L and G) with chronically implanted spinal recording chambers.
Behavioral task
Monkeys were trained to perform a food retrieval task requiring dexterous right hand and finger movements. A clear plastic chamber housing a food well was placed in front of the animal and baited by the experimenter with small pieces of fruit. The well was initially kept out of reach by a pneumatically operated clear plastic door. A trial was initiated by the door rapidly dropping, allowing the animal to reach into the chamber and retrieve the food. The shape and size of the well, and the soft nature of the reward, necessitated accurate hand shaping followed by fine finger movements under visual and proprioceptive guidance for successful retrieval. Hand entry into the food well was registered by infra-red beams, yielding the “pick” event used for alignment of neural activity in this paper. Once the hand left the well, the clear plastic door rose slowly, ending the trial.
EMG implant
EMG recordings were available from patch electrodes (Miller et al. 1993) implanted over the following muscles in the right arm of monkey G: abductor pollicis brevis (AbPB), flexor digitorum superficialis (FDS), and flexor digitorum profundus (FDP). Technical problems prevented similar recordings being available from monkey L.
Surgical preparation
Following behavioral training, animals were implanted with stainless steel headpieces to allow atraumatic head fixation (Lemon 1984) and recording chambers to allow intracranial recordings as part of other studies. All procedures were performed using aseptic technique under general anesthesia comprising 3–5% inhaled sevoflurane in 100% O2, supplemented with a continuous intravenous infusion of alfentanil (25 μg·kg−1·h−1). Postoperative care included broad spectrum antibiotic cover [one of the following: coamoxiclav 140/35 (Synulox): clavulanic acid, 1.75 mg/kg; amoxycillin, 7 mg/kg; Pfizer; cefalexin (Ceporex), 10 mg/kg, Schering-Plough Animal Health; amoxycillin (Clamoxyl LA), 15 mg/kg, Pfizer] and analgesics [buprenorphine (Vetergesic), 10 μg/kg, Reckitt and Colman Products; carprofen (Rimadyl), 5 mg/kg, Pfizer).
Pairs of stimulating electrodes (parylene insulated tungsten, LF501G, Microprobe) were implanted in the left pyramidal tract (PT) after recovery from surgery. Electrodes passed through a small craniotomy made during the surgery, the coordinates of which had been measured in the stereotaxic plane. The approach used the double angle stereotaxic technique described in Soteropoulos and Baker (2006). Correct location was determined with reference to antidromic volleys recorded epidurally from the motor cortex through one of the recording chambers following stimulation through the electrodes. Threshold stimulus currents to elicit an antidromic volley from the final implant site varied from 10 to 30 μA.
In a second surgery several months later, spinal vertebrae C4–T2 were exposed and fused using bone screws placed into the lateral mass. A stainless steel recording chamber was fixed above a laminectomy covering spinal segments C5–T1 (right side). Full details of the spinal chamber and implantation techniques can be found in Perlmutter et al. (1998). All other details of surgical technique and postoperative care were as in the preceding text.
A single tungsten stimulating electrode (LF501G, Microprobe) was implanted in the right MLF, an axon bundle containing many reticulospinal axons (Edgley et al. 2004; Jankowska et al. 2003) as well as fibers from the medial vestibulospinal and tectospinal tracts. Implant of the MLF again used the double angle technique and a craniotomy that was made during the implant surgery; the targeted stereotaxic coordinates were AP0, R0.5, DV0. Electrode positioning was guided by recording epidural volleys from the dorsum of the exposed cord and observation of muscle activation in the right arm. The threshold stimulus current to produce an epidural volley from the final implant site was 20 μA in monkey G and 40 μA in monkey L. To ensure that the electrode had not inadvertently strayed into the pyramidal tract, an occlusion test was performed once a satisfactory location had been reached. Spinal volleys evoked from stimuli (300 μA) delivered through the PT and MLF electrodes were recorded both independently and in combination, the latter with a variable delay (300–600 μs) separating stimuli. Off-line, averaged volleys were examined for evidence of occlusion from combined stimuli. The response to combined PT/MLF stimulation was very similar to that predicted from the algebraic sum of the responses to stimulation through either electrode alone, implying that the responses were mediated by separate fiber tracts. An example of such an occlusion test for spinal volleys is illustrated in Fig. 1 of Riddle et al. (2009).
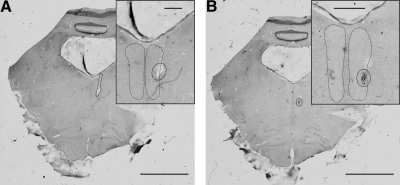
Fig. 1.
Example histological confirmation of medial longitudinal fasciculus (MLF) electrode location. Two sequential pontine sections are shown. Insets: magnified views. A: electrode track (circle) visible entering right MLF (dashed outline). B: electrode tip lesion (circle) with hemorrhage visible within right MLF. Scale bars 5 mm (main); 1 mm (inset).
Recordings
Single neuron extracellular recordings were made from the spinal cord (segments C6–C8) using a five-channel Eckhorn microdrive (Eckhorn and Thomas 1993) loaded with glass insulated platinum tetrodes (Thomas Recording, Giessen, Germany). Unsharpened guide tubes rested on the cord dorsum, and each tetrode was advanced through the spinal dura under visual guidance. We wished to focus on intermediate zone interneurons, and hence most cells were recorded between 1 and 3 mm beyond the depth where clear single unit activity was first observed [avoiding the dorsal horn (<1 mm) and motoneurons in the ventral horn (>3 mm); see Fig. 7]. Neurons were examined for responses to single and trains of three PT and MLF stimuli in turn (biphasic stimuli delivered with constant-current isolated stimulators, 300 μA, 0.2 ms per phase, 1 Hz repetition rate, interstimulus interval for trains 3 ms, n ≈ 100 sweeps per stimulus type) before allowing the animal to perform the behavioral task. Intraspinal microstimulation (ISMS; 13 stimuli at 300 Hz, 5–50 μA) was then applied to each recording site, noting muscle activations and EMG responses (where available). During both PT/MLF stimulation and ISMS the animal was seated quietly and not performing the behavioral task.
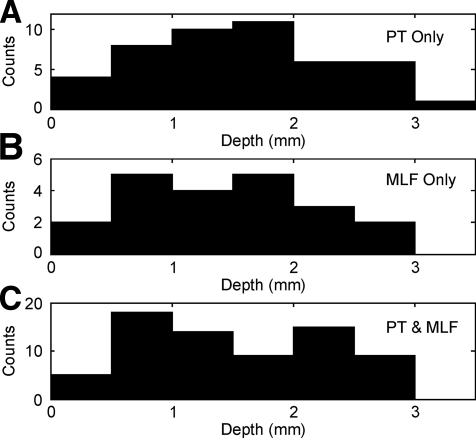
Fig. 7.
Distribution of the depth of cells (relative to the first cellular activity), shown separately for cells responding only to PT stimulation (A), only to MLF stimulation (B), and to both MLF and PT stimulation (C).
Spike waveforms (gain: 2,000–10,000; 300 Hz to 10 kHz band-pass) were sampled continuously at 25 kHz from all four contacts of the tetrodes and stored to hard disk together with behavioral task markers and EMG (sampling rate: 5 kHz, gain: 2,000, 30 Hz to 2 kHz band-pass). Off-line, spike occurrence times were discriminated from the raw waveforms using custom-written cluster cutting routines (Getspike, SN Baker; SpikeLab, Dyball and Bhumbra 2003). Only well-discriminated single units with stable waveforms and an absence of inter-spike intervals <1 ms were used for subsequent analysis.
Some recording sessions with monkey G were carried out under mild sedation (diazepam, maximum intramuscular dose of 2 mg/kg) to improve recording stability. This had no noticeable effect on neuronal activity and did not impair task performance.
Analysis
Analysis primarily involved the construction of peristimulus time histograms (PSTHs) with corresponding cumulative sum plots (CUSUMs) (Ellaway 1978) to assess neuronal responses to stimuli. The on- and offset of responses was determined from inflections in the CUSUM. Putative periods of facilitation or depression of firing were compared with a 50 ms prestimulus baseline period using a Z-test to assess significance with a threshold of P < 0.01. Although cells often exhibited multiple periods of facilitation and/or suppression following stimulus delivery, analysis concentrated on the initial response as likely to be mediated by the most direct pathway. Unless otherwise stated, response latencies are quoted relative to the first stimulus of a train.
To assess the average response across a population of cells, individual PSTHs were first normalized by dividing by the number of stimuli. Response strength was quantified as
where i1 and i2 denote the onset and offset bins of the response, PSTH(i) denotes the normalized PSTH value at the ith bin, and b the baseline measured from a 50-ms-long prestimulus region. The measure s indicates the average number of extra spikes above baseline that are produced in a single cell by a stimulus (Abeles 1982). Population PSTHs (Fig. 4) were generated by averaging normalized PSTHs across all recorded cells.
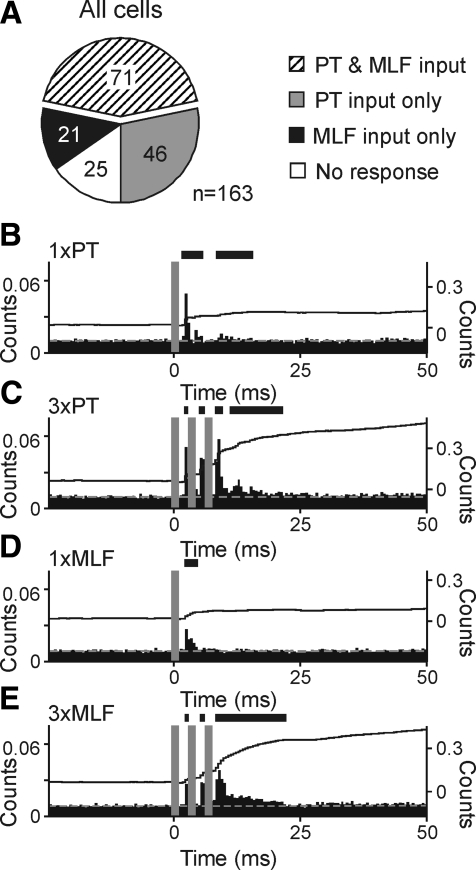
Fig. 4.
Population responses of cervical spinal interneurons to corticospinal and reticulospinal tract activation. A: pie chart illustrating number of neurons across the total sampled receiving convergent excitatory input from PT and MLF stimulation (▨), PT input only ( ), MLF input only (■), or which showed no response to either pathway (☐). Cells were categorized as responding to a stimulus if they showed significant rate increases to either a single stimulus or to trains of three stimuli. B–E: mean normalized responses to corticospinal and reticulospinal tract activation across the complete population of recorded spinal interneurons. Plotting conventions as Fig. 2. Ordinates for both PSTH and CUSUMs are averaged counts per bin per stimulus. Panels represent single PT stimulus (B), 3× PT stimuli (C), single MLF stimulus (D), 3× MLF stimuli (E).
), MLF input only (■), or which showed no response to either pathway (☐). Cells were categorized as responding to a stimulus if they showed significant rate increases to either a single stimulus or to trains of three stimuli. B–E: mean normalized responses to corticospinal and reticulospinal tract activation across the complete population of recorded spinal interneurons. Plotting conventions as Fig. 2. Ordinates for both PSTH and CUSUMs are averaged counts per bin per stimulus. Panels represent single PT stimulus (B), 3× PT stimuli (C), single MLF stimulus (D), 3× MLF stimuli (E).
Stimulus artifacts led to the creation of a “dead time” during which discrimination of spikes was impossible. We estimated the duration of the affected region in the PSTH by examining the raw spike waveform recordings; bins that fell within this region are shaded gray in PSTHs presented here. Because spike occurrence times were taken as the alignment point used in the spike detection process (usually the maximum), the dead time also included a short time prior to the stimulus delivery. For averaged population PSTHs, reasonable mean values for the dead time were used.
Task-related changes in neuronal firing were assessed via the construction of perievent time histograms (PETHs) aligned to the pick event. Baseline firing rate was measured during the period from 1.5 to 2 s prior to pick. Reach phase firing rate was measured during the 0.5-s-long period prior to pick. Measurements of firing rate during the grasping movements used to retrieve the food from the well were made from single trials. For each trial, the time following the pick event when the hand left the infrared beams was measured, and the number of spikes falling between pick and this time counted. Both the number of spikes, and the duration of the grasp phase, were accumulated across trials; dividing the spike count by the total measurement duration provided the firing rate estimate. In all cases, a significant difference from baseline was measured using a Z-test (threshold P < 0.05).
EMG responses during the task in monkey G were examined by the construction of averaged rectified EMG waveforms, smoothed with a Gaussian kernel (width parameter: 200 ms).
Histology
At the end of experiments, monkeys were killed (anesthetic regime described in the preceding text) and perfused through the heart with phosphate-buffered saline followed by 4% paraformaldehyde. Brains were removed and, after immersion in graded sucrose solutions (final strength: 30%) for cryoprotection, sliced at 75 μm on a freezing microtome. Sections were mounted and stained with cresyl violet prior to microscopic examination and reconstruction of the location of stimulating electrodes; all electrodes were correctly located. Figure 1 presents histological images from one animal, showing the location of the electrode tip within the MLF.
Spinal cords were also harvested from each animal and dissected; the segmental level of recordings was confirmed with reference to dorsal root entry points.
RESULTS
Convergence between corticospinal tract and medial brain stem pathways
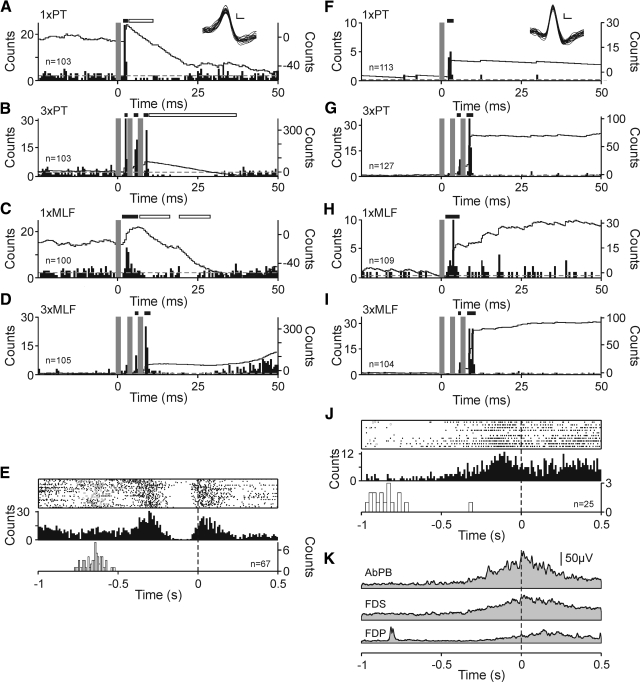
A total of 163 spinal interneurons in two monkeys were tested for responses to stimuli delivered through electrodes implanted in the left PT (corticospinal activation) and right MLF (medial brain stem pathways). Typical single neuron responses from the two animals are illustrated in Fig. 2, A–D and F–I. Single stimuli delivered to PT commonly produced powerful, short latency facilitations in spinal interneurons. In the examples illustrated, onset latencies were 1.7 and 2 ms, and s values were 0.34 and 0.08 (black bar above PSTH, Fig. 2, A and F). This initial excitation was immediately followed by a 7.5-ms-long period of markedly reduced activity for the cell illustrated in Fig. 2A (white bar above PSTH). Across the recorded population, 75 cells (46.0%) responded to single PT stimuli with an initial increase in activity, mean onset latency of 7.7 ± 1.3 ms, and mean s value of 0.27 ± 0.02 (all values given as means ± SE; see distribution across population shown in Fig. 3A). Given the efficacy of a single PT stimulus in activating spinal interneurons, it was unsurprising that trains of three also yielded strong facilitations. In both examples illustrated, three stimuli to the PT gave facilitations at 2 ms (Fig. 2B) and 5 ms (G) latency, with s values of 0.34 and 0.28, respectively. In both cases, neuronal responses followed individual stimuli within a train— this is most obvious in Fig. 2B where three clear peaks are visible (2nd and 3rd peaks at 5 and 8 ms, respectively). In total, 108 neurons (66.3%) responded to a train of three PT stimuli with an initial facilitation, mean onset latency: 8.8 ± 1.0 ms, mean response strength: 0.48 ± 0.05 (see Fig. 3B).
Fig. 2.
Cervical spinal interneurons with activity closely locked to hand movement receive convergent excitatory corticospinal and brain stem input. A–D: single cell example of convergent facilitations from monkey L. Each panel shows peristimulus time histogram (PSTH, left-hand ordinate) with overlain cumulative sum (CUSUM, right-hand ordinate). Stimulus delivery at time 0. Dashed gray line represents mean prestimulus baseline activity. PSTH bin width 0.5 ms. Stimulus artifact dead times are replaced by dark gray bars and corresponding regions of the CUSUM blanked. Significant (P < 0.01, Z-test) changes from baseline are highlighted with black (facilitation) or white (suppression) bars above the PSTH. Overlain spike waveforms shown in inset, scale bars 1 ms, 2 μV. Responses to single 300 μA PT stimulus (A), 3× PT stimuli (B), single 300 μA stimulus to MLF (C); 3× MLF stimuli (D). E: task-related activity for the same example neuron. Panel shows raster and peri-event time histogram (PETH) of neuronal firing aligned to task pick event (time 0, vertical dashed line). PETH bin width 10 ms. Distribution of trial start events (door drop) is shown as open squares in raster and histogram using white bars below PETH. Number of stimuli or trials of task are given by n in each panel. F—J: as A–E for example neuron taken from monkey G. K: mean, rectified, smoothed EMG activity during task performance illustrated in J. Electromyographs (EMGs) are also shown aligned to task pick event (vertical dashed line). Gaussian smoothing kernel width parameter = 200 ms. AbPB, abductor pollicis brevis; FDS, flexor digitorum superficialis; FDP, flexor digitorum profundus.
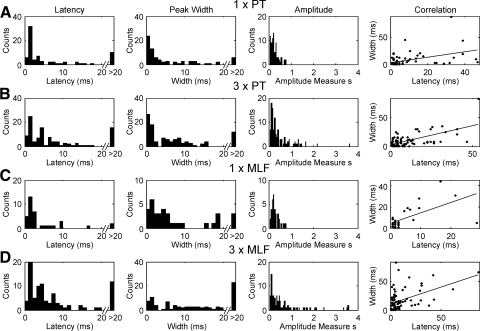
Fig. 3.
Distribution of measures of latency, peak width, and amplitude of facilitations in PSTH following different types of stimulation. Final column shows the correlation between latency and width as a scatter plot and associated regression line (slope significantly different from 0, P < 10−4 for all plots). A: single shock stimulation of pyramidal tract (PT) electrodes (300 μA). B: 3× PT stimuli. C: single 300 μA MLF stimulus. D: 3× MLF stimuli.
As well as receiving powerful excitatory input from the corticospinal tract, the illustrated example neurons also could be activated from the MLF. A single 300 μA stimulus delivered to MLF elicited facilitations at 1.75 ms (black bar above PSTH, Fig. 2C) and 1.5 ms (H) with corresponding s-values of 0.3 and 0.18.
Responses to single MLF stimuli were, however, less common than their pyramidal counterparts. A total of 35 interneurons (21.5%) responded to a single MLF stimulus with an initial facilitation, mean onset latency: 4.6 ± 1.0 ms, mean s value: 0.30 ± 0.03 (Fig. 3C). Trains of three MLF stimuli were more effective at activating the interneuronal pool. For the illustrated cells, facilitations occurred with latencies of 5.3 and 5.5 ms, and s values 0.17 and 0.07 (Fig. 2, D and I). It is likely that the earliest component of the response to three shocks was obscured by stimulus artifact dead time (compare location of gray bars, Fig. 2D with response in C); this probably contributes to the apparently longer latencies and lower s values reported in the preceding text. Once again, responses often followed the train (2 black bars above PSTH, Figs. 2, D and I), and there was response augmentation between the different stimuli of the train (compare 2nd peak heights—single peak s values augment by 112 and 843%, respectively). Across the sampled population, 88 cells (54.0%) were activated by three MLF stimuli with a mean onset latency of 9.5 ± 1.5 ms and mean response strength of 0.67 ± 0.09 (see Fig. 3D). The effective stimulus in a train of three shocks may have been either the second or third, yielding possible maximal reductions in latency of 6 ms from the quoted values. Corticospinal and medial brain stem effects in spinal interneurons clearly occurred at short latency.
There were no significant differences in either the onset latency or strength of facilitations following single PT/MLF stimuli nor between trains of three PT/MLF stimuli. These data therefore imply that, when present, the strength of corticospinal and medial brain stem connections to cervical interneurons were broadly comparable. There was no significant correlation between the amplitude and latency of responses, for any of the different stimulus configurations. By contrast, the peak width was significantly correlated to the onset latency in all cases (P < 10−4, final column of Fig. 3).
In addition to the excitatory responses described in the preceding text, initial suppressions of interneuronal activity were occasionally evoked by PT and MLF stimulation. Such responses were less common than facilitation—trains of three PT stimuli yielded an initial suppression in 27 neurons (17.1%), mean onset latency: 10.1 ± 1.4 ms, mean response strength: s = −0.32 ± 0.05. Similarly, trains of three MLF stimuli depressed activity in 29 neurons (18.4%), mean latency: 7.3 ± 1.4 ms, mean strength: −0.25 ± 0.04. There were no significant differences in suppression amplitude following corticospinal or medial brain stem activation (Mann-Whitney U test, P > 0.05). Facilitations and suppressions did not differ significantly in onset latencies following stimulation of either descending pathway. It is well known that suppression is statistically harder to detect than facilitation in PSTHs, especially when the baseline counts per bin are low as often the case here (Aertsen and Gerstein 1985). It is thus likely that we underestimated the incidence of suppression in our data.
Population data
The preceding data demonstrate that individual interneurons in the primate cervical spinal cord receive convergent excitatory inputs from PT and MLF stimulation. To assess this quantitatively, cells were categorized as receiving input from a given pathway if they were facilitated either following a single stimulus or trains of three stimuli. Across the population of recorded neurons, convergent input was the most common result (71/163 cells, 43.6%; Fig. 4A, ▨). Fewer neurons were excited solely by one descending system [46 cells (28.2%) corticospinal only, gray segment; 21 cells (12.9%) brain stem only, black segment]. A total of 25 neurons (15.3%, ☐) could not be activated from either stimulus site.
Population responses for all recorded cells, normalized by number of stimuli, are illustrated in Fig. 4, B–E, and provide a visual representation of the interneuronal population response to PT and MLF stimulation. For all four stimulus paradigms, cells always responded with an initial, short latency facilitation. Responses to three stimuli closely followed the stimulus train (Fig. 4, C and E).
Interneuron activity during voluntary hand movement
Interneurons were recorded while monkeys performed a food retrieval task; this permitted assessment of whether cells with convergent cortico- and reticulospinal inputs also modulated their activity with hand movements. Such cells are likely to form part of the spinal network for hand control. The task relationship of the two example cells described in the preceding text is shown in Figs. 2, E and J. In both examples, rate increases occurred around the pick event. The cell illustrated in Fig. 2E also increased its firing rate after the trial onset (door drop, open squares in raster); this may be related to the whole-arm reaching movement but could equally reflect hand preshaping, which is known to occur during reach to grasp (Jeannerod 1988). Activity in the cell illustrated in Fig. 2J occurred only just prior to and immediately after the pick event.
Figure 2K shows the modulation of rectified EMG from three hand and forearm muscles recorded simultaneously with the cell illustrated in Fig. 2, F–J. All three muscles showed increased activity around the pick with AbPB demonstrating greatest facilitation. The peak of this increase was close to the pick event (vertical dashed line; time 0), as would be expected since at this point the animal was using fingers and thumb to extract a food morsel from the well. Activity in this muscle was closely mirrored by that in FDS, a muscle primarily concerned with finger flexion at the proximal interphalangeal joints and therefore equally important in the extraction of food. Peak activity in AbPB occurred 13 ms after pick compared with 18 ms for FDS, and in both cases muscle activation started around 360 ms before pick. Activity in FDP occurred later, with peak activation 141 ms after pick and the first increase above baseline levels 241 ms before pick. In this example, it is striking how closely increases in activity in the major thumb/finger flexors (AbPB and FDS) tracked the facilitation of neuronal firing. This suggests an association between this interneuron's firing and activation of the distal arm and hand muscles required for hand shaping and dexterous manipulation of a small object.
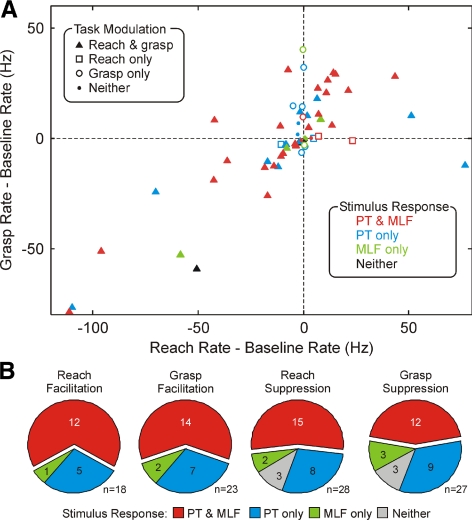
To quantify the cells' task relationship, we measured the firing rate during the reach phase and the grasp phase of the task (see methods). Figure 5A shows a scatter plot of these rates after subtraction of a baseline rate estimate. The different symbols indicate whether the rate was significantly different from baseline during each task phase, as indicated by the key in the top left of the plot. The majority of cells were significantly modulated during both task phases [42/63 cells (67%) recorded during task performance].
Fig. 5.
Comparison of incidence of input from brain stem and corticospinal descending pathways for cells with different task relationships. A: scatter plot of the firing rate measured during the grasp phase of the task vs. the rate during the reach phase. Each rate has been corrected by subtraction of the baseline rate; each point marks data from a single cell. Different symbol shapes (key at top left) show cells with significant modulation of rate in each task phase relative to baseline. Different symbol colors (key at bottom right) show the type of descending input received by that cell. B: pie charts showing the relative incidence of input from the corticospinal or brain stem descending pathways, separately for cells the firing rate of which facilitated or suppressed during the reach or grasp phase of the task.
A particular focus of this study was the potential contribution of the reticulospinal tract to interneurons involved in control of the distal muscles acting on the hand and wrist. Accordingly, in Fig. 5A, the color of the symbols indicates whether cells received excitatory input following PT or MLF stimulation alone or to both types of stimuli, according to the key at the bottom right of the plot. There is a clear preponderance of red symbols, signifying convergent inputs from the two pathways. This is further quantified in Fig. 5B. These pie charts show the proportion of cells receiving convergent or single input (similar to Fig. 4A); the different plots relate to subpopulations of cells classified according to their task relationship. Irrespective of whether interneurons were facilitated or suppressed, and irrespective of which task phase they modulated with, the most common finding was convergent input from brain stem and corticospinal pathways. These pie charts relate to facilitatory input, which as described in the preceding text was the most common finding. Three cells that were suppressed during both the reach and grasp phases of the task are accordingly shown as responding to neither descending input. It should be noted however that the firing of one of these cells was suppressed following both PT and MLF stimulation, while the remaining two showed suppression only after PT stimulation.
Intraspinal microstimulation
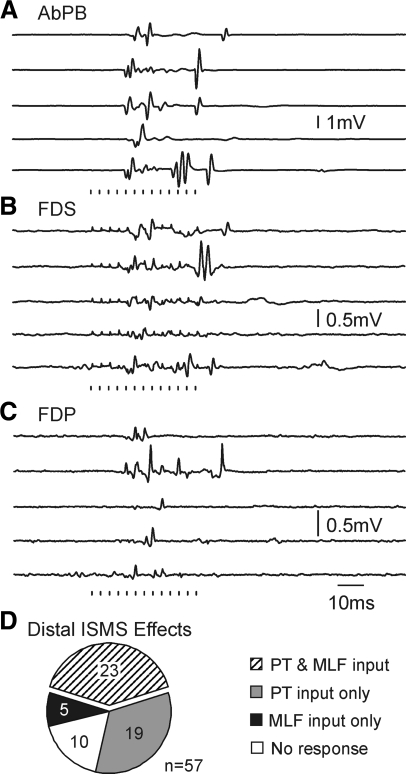
After recordings during task performance had been made, intraspinal microstimulation (ISMS) was delivered at the recording sites, and any EMG responses or overt movements noted. Figure 6 shows single sweep examples of evoked EMG responses following 10 μA ISMS at the location of the recording presented in Fig. 2, F–K. Evoked responses in AbPB were largest (Fig. 6A); a clear thumb flexion was seen. The response commenced 12.7–16.2 ms after the train onset, equivalent to after just five to six shocks each of only 10 μA. This presumably indicates a strong functional connection between interneurons at the stimulus site and motoneurons innervating the AbPB muscle.
Fig. 6.
Sample raw EMG activity evoked by 13-pulse intraspinal microstimulation (ISMS, stimuli indicated below traces) delivered at 10 μA at the location of the neuron in Fig. 2, F–J. Five individual data sweeps are shown for each muscle. Strongest responses were evoked in AbPB (A), with weaker effects in FDS (B) and flexor digitorum profundus (C, FDP). D: pie chart showing the relative incidence of input from corticospinal or brain stem descending pathways, using only cells recorded at sites where ISMS generated movements of the hand or wrist.
ISMS was tested at 154 recording sites. Of these, 63 (40.9%) yielded finger, thumb and wrist movements, whereas 54 (35.1%) produced more proximal responses. Stimulus thresholds for distal responses ranged from 4 to 40 μA (mode: 10 μA), and for proximal activation 5–40 μA (mode: 10 μA).
Figure 6D presents the incidence of different patterns of input from the descending pathways (similar to Figs. 4A and 5B) for cells recorded at sites where ISMS elicited movements of the digits or wrist. Once again, convergent input from both brain stem and corticospinal pathways was the most common finding. Thus regardless of the method used to classify cells, these data imply that spinal interneurons involved in the control of hand movements receive powerful, convergent and largely comparable inputs from corticospinal tract and brain stem descending pathways.
Depth profile of stimulus effects
It was of interest to examine whether the different patterns of stimulus responses exhibited any topographic distribution within the spinal cord. For several technical reasons associated with the chronic spinal recording method, we believe that a full reconstruction of the recording sites and an attempt to assign locations to Rexed's laminae would be unreliable. However, the depth of an electrode during a penetration can be measured with high accuracy; this was recently used by Takei and Seki (2008) to explore topography in chronic spinal recordings. Accordingly Fig. 7 plots the depth distribution, relative to the first recorded single unit activity, separately for cells that showed facilitation after PT or MLF stimuli alone or to both. The three distributions are heavily overlapping; it is clear that both brain stem and corticospinal pathways provide input to interneurons distributed throughout the intermediate zone of the spinal cord (approximate depths of 1–3 mm relative to the 1st recorded cells).
DISCUSSION
The data presented in this paper demonstrate that interneurons in the intermediate zone of the primate cervical spinal cord receive largely convergent excitatory input from the CST and medial brain stem descending pathways. This was the case even when analysis was restricted to cells likely to be involved in the control of hand movements. Together with other recent studies, this forces a reassessment of the role of brain stem descending systems in motor control.
Pathways activated by MLF stimulation
Before considering the different brain stem pathways the axons of which pass through the MLF, it is important to exclude the possibility that other, more distant structures were activated by current spread when stimuli were delivered through the MLF electrode. We first used an occlusion test (Riddle et al. 2009) to demonstrate that the spinal volley produced from MLF stimulation did not activate corticospinal fibers stimulated from the pyramid. The red nucleus is far rostral to the location of the MLF electrode, and the axons of the rubrospinal tract travel laterally in the medulla (Kuypers et al. 1962). Current would thus have to spread further to activate rubrospinal fibers than to the pyramid, making a contribution from this tract to the volley elicited from the MLF also unlikely. Finally, C3/C4 propriospinal interneurons send ascending axons to the lateral reticular nucleus (LRN) of the medulla; antidromic stimulation of these fibers could theoretically produce effects in the cervical enlargement. However, the proportion of these interneurons projecting to the LRN is small in monkey compared with the cat (Isa et al. 2006), and no volley can be recorded from the surface of the cervical cord even following stimulation through an electrode with its tip within the LRN (Nakajima et al. 2000). The LRN is also far lateral in the medulla, compared with the medial location of the MLF stimulating electrodes in this study. Direct stimulation of propriospinal neuron collaterals is thus very unlikely to mediate the effects seen.
Three main descending pathways are known to contribute axons to the MLF. In cat, tectospinal axons pass through the MLF and terminate within the intermediate zone of the cervical cord (Nyberg-Hansen 1964a; Petras 1967). However, in monkey, there are relatively few tectospinal neurons (Harting 1977), making it unlikely that this is a major pathway contributing to the effects of MLF stimulation in our experiments. The medial vestibulospinal tract descends bilaterally via the MLF (Nyberg-Hansen 1964b; Petras 1967; Wilson et al. 1968). Both ipsi- and contralateral projections of the reticulospinal tract pass through the MLF (Kuypers 1981). Either reticulospinal or vestibulospinal fibers could therefore be responsible for the effects that we observed.
Convergent control of spinal motor circuits
The spinal terminations of corticospinal and brain stem descending pathways were investigated using anatomical methods by Kuypers (1960). That work showed that the ventromedial brain stem pathways mostly terminated among the intermediate zone, whereas the CST terminals were located in the intermediate zone and among the motoneuron cell bodies of the ventral horn. Early papers tended to emphasize the differences between the two systems; however, the data presented clearly show a high degree of overlap between the termination regions in the intermediate zone. The present electrophysiological data are thus consistent with previous anatomical work.
The axonal conduction time from the brain stem location of our stimulating electrodes to the cervical enlargement is ∼0.7 ms (Riddle et al. 2009). Response latencies <1.7 ms to single stimuli are thus likely to be generated by monosynaptic input to the recorded interneuron (Fig. 3). The presence of longer latency responses, which were revealed only by a train of stimuli, is compatible with a di- or oligosynaptic linkage from the axons of the descending tract to the recorded cell. It is unsurprising that both types of response could be observed in this relatively heterogeneous population of spinal neurons.
It is difficult to measure accurately the relative strength of input from brain stem versus corticospinal systems. In all cases, stimulating electrodes were placed within the relevant fiber tracts in the brain stem, yielding thresholds ≤40 μA to evoke volleys and with locations confirmed by subsequent histology. The standardized stimulus intensity of 300 μA that we used probably activated most—but not all—fibers; it is possible that the two pathways were activated to different extents. Nevertheless, the finding that response amplitudes were not significantly different following PT and MLF stimuli (Fig. 3) suggests that these two pathways have broadly comparable effects on interneuron firing.
Concepts of spinal interneuron function are best developed for nonprimates, especially the cat. These animals lack direct connections from cortex to motoneurons. Accordingly, interneuron circuits play an important role in relaying descending motor commands to the final output station. Stimulation of the CST in cats produces clear disynaptic responses in motoneurons (Illert et al. 1976); this is transmitted both via segmental interneurons and also by C3–4 propriospinal interneurons (Alstermark and Sasaki 1985; Illert et al. 1977). However, it would be wrong to view spinal interneuron pathways in cat as a simple relay of descending commands; spinal circuitry can autonomously control complex movements such as locomotion (McCrea and Rybak 2008) and the paw-shake response (Carter and Smith 1986).
By contrast, in primates, the CST makes monosynaptic connections to motoneurons (Landgren et al. 1962). This direct access to motor output by the cortex is believed to underlie the enhanced fine motor abilities of primates, especially control of the hand (Porter and Lemon 1993). However, even in primates the majority of corticospinal terminals are located on spinal interneurons. Spinal interneurons show a pronounced modulation of their discharge with hand movements; this includes cells which have a monosynaptic connection to motoneurons assessed by spike triggered averaging of EMG (Perlmutter et al. 1998).
Previous studies of convergence between descending pathways onto spinal interneurons in the cat have yielded different findings, depending on the system studied. The C3–C4 propriospinal neurons receive highly convergent input from the CST, rubrospinal, reticulospinal, and vestibulospinal systems (Illert et al. 1978, 1981). By contrast, descending inputs to Group II activated short propriospinal neurons in cat lumbar spinal cord appear to be segmented. Neurons receive monosynaptic input either from pathways descending in the dorsolateral funiculus (mainly rubrospinal tract) or those in the ventral or ventrolateral funiculus (reticulospinal and vestibulospinal tracts) but rarely from both; corticospinal inputs are sparse (Davies and Edgley 1994). The key difference here may be between the cervical and lumbar cord; our finding of convergence between CST and medial brain stem pathways onto the segmental interneurons of the primate cervical enlargement is consistent with the prior work on cat C3–C4 propriospinal neurons. This in turn could reflect the different functional specializations of the upper and lower limbs. In both cat and monkey, the lower limbs are mainly involved in postural adjustment and the simpler movements of locomotion and scratching. By contrast, the upper limbs additionally perform reaching and grasping movements under visual guidance. This may require more convergent descending inputs, allowing flexible reconfiguration of the spinal circuitry for different tasks (Davies and Edgley 1994).
It is tempting to view primate spinal circuitry as essentially the same as in the cat but with the addition of corticomotoneuronal connections. This is unlikely to be correct. Disynaptic connections from the CST to spinal motoneurons are not normally observed (Maier et al. 1998; Nakajima et al. 2000). Although disynaptic excitability postsynaptic potentials (EPSPs) can be revealed in the cervical enlargement when inhibition is pharmacologically antagonized (Alstermark et al. 1999), many of these appear to come from C3–C4 propriospinal rather than local segmental interneurons. In the awake monkey, motoneuron responses to CST stimulation show no evidence of an appreciable disynaptic pathway (Olivier et al. 2001). In the present study, interneurons received short latency CST input, and ISMS at some recording sites activated muscles with low threshold. It seems very likely that the anatomical connections for di- or oligosynaptic links from the CST to motoneurons exist at the segmental level. An important unresolved issue in the field is therefore why nonmonosynaptic EPSPs in motoneurons after CST stimulation are so hard to detect. One possibility is that primate segmental intermediate zone interneurons are not used in normal movements simply to relay cortical commands received via their CST inputs but rather that the spinal circuitry performs a more complex function.
Schepens and Drew (2006) investigated the firing of PMRF neurons during reaching movements in the cat. They concluded that the RST provided bilateral drive to muscles that coordinated both the movement itself and the associated postural adjustments. This drive was hypothesized to pass through circuits of spinal interneurons, which received converging input from both the RST and CST (see Fig. 16 of Schepens and Drew 2006). An important finding of the Schepens and Drew (2006) study was that the efficacy of the connection from a single RST neuron to a given muscle (assessed by spike triggered averaging) could modulate depending on which limb moved. They suggested that changes in cortical drive would gate the connection from RST to motoneurons by changing the excitability of the interposed interneuronal circuits; this would sculpt a relatively nonspecific RST activity to generate activation of only the subset of muscles appropriate for the current movement.
The present data suggest that spinal circuits in the primate also feature a high degree of convergence from brain stem and cortical descending systems. Although we only tested inputs from the crossed corticospinal tract, and from the ipsilateral MLF, it is possible that such convergence is more widespread—for example, from the ventral (ipsilateral) corticospinal tract and from the contralateral MLF. We have recently shown that many primate cervical motoneurons receive disynaptic EPSPs from the brain stem pathways activated by the MLF (Riddle et al. 2009). It may be that the organization of these interneuron circuits in monkey is similar to that proposed by Schepens and Drew (2006) and that the essential function of the cortical inputs is to gate reticulospinal outputs by altering interneuronal excitability. Such a view would not preclude interneuronal relay of corticospinal commands in some circumstances (e.g., Burke et al. 1994) but would see this as merely an epiphenomenon of cortical control of reticulospinal relay cells.
A different concept of interneuron function was proposed by Bizzi and coworkers (Giszter et al. 1993), who suggested that spinal circuits act to produce “motor primitives.” These elementary patterns of muscle activity can then be combined to generate useful movements by appropriate descending activity. Some support for such an organization in primates has come from Moritz et al. (2007), who demonstrated that complex movements activating multiple muscles could be generated by juxta-threshold stimulation in the cord. Within this conceptual framework, our data would be interpreted as showing that both the medial brain stem pathways and CST have access to the network generating motor primitives and that the two descending systems probably act together to recruit the appropriate component movements.
Brain stem control of the hand
Following the pioneering work of Kuypers, much emphasis has been placed on the role of the corticospinal tract in control of the hand and in particular on the direct corticomotoneuronal connections that are a uniquely primate feature. The existence and strength of such connections correlates with digital dexterity (Bortoff and Strick 1993; Kuypers 1981) and the ability to execute independent finger movements. Lawrence and Kuypers (1968a) showed that following bilateral pyramidal tract lesions at the medulla, animals rapidly recovered gross motor function. Fine control of the hand and the ability to make independent finger movements did not return; however, the animals could generate less fractionated hand movements that were sufficient to climb and grasp food.
By making further lesions of these animals, Lawrence and Kuypers (1968b) suggested that the majority of the recovery of hand function depended on the lateral brain stem pathways (predominantly the rubrospinal tract) rather than medial pathways such as the RST. In support of this, Belhaj-saif and Cheney (2000) demonstrated a strengthening of rubrospinal output to the forearm after pyramidal tract lesion. More recent work has shown that some fine control of the hand can be recovered following lesion of corticomotoneuronal connections; the restoration of function can be mediated partly by C3/C4 propriospinal interneurons (Sasaki et al. 2004) if the corticospinal projection to these cells is spared.
In this paper, we have demonstrated convergent input from the CST and medial brain stem pathways to spinal interneurons. This appeared to be a widespread phenomenon, encompassing not only cells involved in the control of proximal arm muscles but also interneurons involved in hand movement (Fig. 5). The classification of a cell as “hand related” purely on the basis of its discharge during performance of a task poses inevitable difficulties. Although cells fired after the pick event when the animal was performing fine finger movements, we cannot be certain that their activity was not actually related to a more proximal part of the movement synergy required in our reach and grasp task. Equally, cells that modulated their firing during the reaching part of the task may be related to the preshaping of the hand that occurs prior to entry into the food well (Jeannerod 1988).
However, at many of these sites, weak ISMS could elicit activity in muscles acting on the wrist or fingers. This technique also may have problems of interpretation. In the cortex, microstimulation activates both local and distant circuits transynaptically after stimulation of axons (Baker et al. 1998; Bennett et al. 1989; Jankowska et al. 1975). This is also likely to occur in the spinal cord (Gaunt et al. 2006), so that the ability to elicit effects in muscles after ISMS does not unambiguously indicate that cells near the electrode tip are responsible. Nevertheless convergent input from CST and brain stem was seen either when cells were classified as hand related on the basis of their firing behavior during the task or by the ISMS effects. This therefore seems to be a robust finding and supports a role of the medial brain stem pathways in control of the hand in agreement with our recent work on motoneuron responses (Riddle et al. 2009). By implication, this suggests that loss of corticospinal input after brain lesion could be partially compensated by strengthening these preexisting inputs from the brain stem, thereby contributing to functional recovery of hand movements.
We deliberately chose to record from the region of the spinal cord where motoneurons involved in hand control are located because hand movements were a particular focus of this study. As a result, we are unable to provide unbiased quantitative data on the relative incidence of convergent medial brain stem and corticospinal inputs to interneurons involved in hand control versus the control of more proximal arm segments. However, previous work has emphasized the importance of the reticulospinal tract in control of more proximal muscles (Buford and Davidson 2004; Davidson and Buford 2004, 2006; Davidson et al. 2007) as well as demonstrating that the corticospinal tract can influence them (Colebatch et al. 1990; Palmer and Ashby 1992; Phillips and Porter 1964). Responses to transcranial magnetic stimulation suggest shared corticospinal and brain stem control of both hand (Ziemann et al. 1999) and more proximal arm muscles (Colebatch et al. 1990; MacKinnon et al. 2004). In the present study, convergent input was also seen to cells at sites where stimulation produced muscle activation more proximal than the hand. This is therefore likely to be a widespread principle of organization throughout the cervical enlargement.
In the present work, we activated corticospinal and brain stem pathways by electrical stimulation through electrodes placed within their main axon bundles. Although this provides information on the frequency and strength of connections, it cannot address the specificity of the connections from each pathway. It may be that activation of interneurons by CST and brain stem systems are equivalent. Alternatively, connections from individual corticospinal neurons might be directed in a more selective way, allowing greater fractionation of muscle activity than possible from the brain stem. This latter possibility seems likely. Of cells responding to trains of three CST stimuli, 69% also responded to a single shock; by contrast only 40% of cells responding to three MLF stimuli also responded to one stimulus. This implies that connections from the brain stem to interneurons were more indirect than from the CST. In this case, strengthening brain stem connections to interneurons might restore movement after CST lesion but at the cost of a reduced motor repertoire and the obligate coupling of muscles in maladaptive synergies. This is exactly what is observed after recovery from stroke, where unwanted muscle co-contraction often forms the major limitation for recovery of hand function (Lang and Schieber 2004).
It is clear that control of the hand involves not just the CST but also brain stem and spinal circuits. Understanding the relative contributions of these different motor pathways to normal movement and to functional recovery might suggest novel therapies for rehabilitation.
GRANTS
This work was funded by the Wellcome Trust and Medical Research Council (UK). C. N. Riddle was supported by a studentship from Merck Sharp and Dohme.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors thank T. Jackson for animal training, P. Flecknell and C. Richardson for veterinary and anesthesia assistance, and C. Fox for theater support.
REFERENCES
- Abeles M. Quantification, smoothing, and confidence-limits for single-units histograms. J Neurosci Methods 5: 317–325, 1982. [DOI] [PubMed] [Google Scholar]
- Aertsen AMHJ, Gerstein GL. Evaluation of neuronal connectivity: sensitivity of cross correlation. Brain Res 340: 341–354, 1985. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Ohki Y, Saito Y. Disynaptic pyramidal excitation in forelimb motoneurons mediated via C(3)-C(4) propriospinal neurons in the Macaca fuscata. J Neurophysiol 82: 3580–3585, 1999. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. XIII. Corticospinal effects in shoulder, elbow, wrist, and digit motoneurons. Exp Brain Res 59: 353–364, 1985. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. An investigation of the intrinsic circuitry of the motor cortex of the monkey using intra-cortical microstimulation. Exp Brain Res 123: 397–411, 1998. [DOI] [PubMed] [Google Scholar]
- Belhaj-saif A, Cheney PD. Plasticity in the distribution of the red nucleus output to forearm muscles after unilateral lesions of the pyramidal tract. J Neurophysiol 83: 3147–3153, 2000. [DOI] [PubMed] [Google Scholar]
- Bennett KMB, Lemon RN, Werner W. Indirect excitation of corticospinal neurons by intracortical stimulation in the conscious monkey. J Physiol 418: 103P, 1989. [Google Scholar]
- Bernhard CG, Rexed B. The localisation of the premotor interneurons discharging through the peroneal nerve. J Neurophysiol 8: 387–392, 1945. [DOI] [PubMed] [Google Scholar]
- Bortoff GA, Strick PL. Corticospinal terminations in two new-world primates: further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J Neurosci 13: 5105–5118, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159: 284–300, 2004. [DOI] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Non-monosynaptic transmission of the cortical command for voluntary movement in man. J Physiol 480: 191–202, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter MC, Smith JL. Simultaneous control of two rhythmical behaviors. II. Hindlimb walking with paw-shake response in spinal cat. J Neurophysiol 56: 184–195, 1986. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Rothwell JC, Day BL, Thompson PD, Marsden CD. Cortical outflows to proximal arm muscles in man. Brain 113: 1843–1856, 1990. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol 92: 83–95, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173: 25–39, 2006. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci 27: 8053–8058, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurons from descending motor pathways in the cat. J Physiol 479: 463–473, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol 55: 375–401, 1986. [DOI] [PubMed] [Google Scholar]
- Dyball R, Bhumbra G. Digital spike discrimination combining size and shape elements. J Physiol 547P: D9, 2003. [Google Scholar]
- Eckhorn R, Thomas U. A new method for the insertion of multiple microprobes into neural and muscular tissue, including fiber electrodes, fine wires, needles and microsensors. J Neurosci Methods 49: 175–179, 1993. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci 24: 7804–7813, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45: 302–304, 1978. [DOI] [PubMed] [Google Scholar]
- Gaunt RA, Prochazka A, Mushahwar VK, Guevremont L, Ellaway PH. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local alpha-motoneuron responses. J Neurophysiol 96: 2995–3005, 2006. [DOI] [PubMed] [Google Scholar]
- Giszter SF, Mussa-Ivaldi FA, Bizzi E. Convergent force fields organized in the frog's spinal cord. J Neurosci 13: 467–491, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Georgopoulos AP, Jordan LM. (editors). Selection and Initiation of Motor Behavior Cambridge, MA: MIT Press, 1997, p. 3–19 [Google Scholar]
- Harting JK. Descending pathways from the superior collicullus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta). J Comp Neurol 173: 583–612, 1977. [DOI] [PubMed] [Google Scholar]
- Illert M, Jankowska E, Lundberg A, Odutola A. Integration in descending motor pathways controlling the forelimb in the cat. VII. Effects from the reticular formation on C3-C4 propriospinal neurons. Exp Brain Res 42: 269–281, 1981. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Padel Y, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. V. Properties of and monosynaptic connections on C3-C4 propriospinal neurons. Exp Brain Res 33: 101–130, 1978. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. I. Pyramidal effects on motoneurons. Exp Brain Res 26: 509–519, 1976. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. III. Convergence on propriospinal neurons transmitting disynaptic excitation from the corticospinal tract and other descending tracts. Exp Brain Res 29: 323–346, 1977. [DOI] [PubMed] [Google Scholar]
- Isa T, Ohki Y, Seki K, Alstermark B. Properties of propriospinal neurons in the C3-C4 segments mediating disynaptic pyramidal excitation to forelimb motoneurons in the macaque monkey. J Neurophysiol 95: 3674–3685, 2006. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci 23: 1867–1878, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol 249: 617–636, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. The Neural and Behavioural Organisation of Goal-Directed Movements. Oxford University Press, 1988. [Google Scholar]
- Jones B. Reticular Formation: Cytoarchitecture, Transmitters, and Projections. In: The Ral Nervous System, edited by Pasinos G. London: Academic, 1995, p. 155–171 [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol 242: 56–92, 1985. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. Central cortical projections to motor and somato-sensory cell groups. An experimental study in the rhesus monkey. Brain 83: 161–184, 1960. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Fleming WR, Farinholt JW. Descending projections to spinal motor and sensory cell groups in the monkey: cortex versus subcortex. Science 132: 38–40, 1960. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Fleming WR, Farinholt JW. Subcorticospinal projections in the rhesus monkey. J Comp Neurol 118: 107–137, 1962. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Anatomy of the Descending Pathways. In: Handbook of Physiology–The Nervous System, Bethesda, MD, edited by Brookhost JM, Mountcostle VB. Am. Physiol. Soc, 1981, p. 597–666 [Google Scholar]
- Landgren S, Phillips CG, Porter R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurons of the baboon's hand and forearm. J Physiol 161: 91–111, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol 91: 1722–1733, 2004. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HGJM. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91: 1–14, 1968a. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HGJM. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathway. Brain 91: 15–36, 1968b. [DOI] [PubMed] [Google Scholar]
- Lemon RN. (editor). Methods for Neuronal Recording in Conscious Animals. London: Wiley, 1984. [Google Scholar]
- MacKinnon CD, Quartarone A, Rothwell JC. Inter-hemispheric asymmetry of ipsilateral corticofugal projections to proximal muscles in humans. Exp Brain Res 157: 225–233, 2004. [DOI] [PubMed] [Google Scholar]
- Maier MA, Illert M, Kirkwood PA, Nielsen J, Lemon RN. Does a C3-C4 propriospinal system transmit corticospinal excitation in the primate? An investigation in the macaque monkey. J Physiol 511: 191–212, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol 410: 413–430, 1999. [PubMed] [Google Scholar]
- Matsuyama K, Takakusaki K, Nakajima K, Mori S. Multi-segmental innervation of single pontine reticulospinal axons in the cervico-thoracic region of the cat: anterograde PHA-L tracing study. J Comp Neurol 377: 234–250, 1997. [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, Van Kan PLE, Sinkjaer T, Andersen T, Harris GD, Houk JC. Correlation of primate red nucleus discharge with muscle activity during free-form arm movements. J Physiol 469: 213–243, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol 28: 161–195, 1987. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsuyama K, Mori F, Nakajima K. Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv Neurol 87: 25–40, 2001. [PubMed] [Google Scholar]
- Moritz CT, Lucas TH, Perlmutter SI, Fetz EE. Forelimb movements and muscle responses evoked by microstimulation of the cervical spinal cord in sedated monkeys. J Neurophysiol 97: 110–120, 2007. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Maier MA, Kirkwood PA, Lemon RN. Striking differences in transmission of corticospinal excitation to upper limb motoneurons in two primate species. J Neurophysiol 84: 698–709, 2000. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R. The location and termination of tectospinal fibers in the cat. Exp Neurol 9: 212–227, 1964a. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R. Origin and termination of fibers from the vestibular nuclei descending in the medial longitudinal fasciculus. an experimental study with silver impregnation methods in the cat. J Comp Neurol 122: 355–367, 1964b. [DOI] [PubMed] [Google Scholar]
- Olivier E, Baker SN, Nakajima K, Brochier T, Lemon RN. Investigation into non-monosynaptic corticospinal excitation of macaque upper limb single motor units. J Neurophysiol 86: 1573–1586, 2001. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN. Connections of the reticulo-spinal neurons with the “locomotor sections” of the brain stem. Biofizika 15: 171–178, 1970. [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurons in humans. J Physiol 448: 397–412, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter SI, Maier MA, Fetz EE. Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. J Neurophysiol 80: 2475–2494, 1998. [DOI] [PubMed] [Google Scholar]
- Petras JM. Cortical, tectal and tegmental fiber connections in the spinal cord of the cat. Brain Res 6: 275–324, 1967. [DOI] [PubMed] [Google Scholar]
- Phillips CG, Porter R. The pyramidal projection to motoneurons of some muscle groups of the baboon's forelimb. Prog Brain Res 12: 222–245, 1964. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford, UK: Oxford Univ. Press, 1993. [Google Scholar]
- Prentice SD, Drew T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol 85: 679–698, 2001. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Edgley S, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, Seki K, Ohki Y. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol 92: 3142–3147, 2004. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol 96: 2229–2252, 2006. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Baker SN. Cortico-cerebellar coherence during a precision grip task in the monkey. J Neurophysiol 95: 1194–1206, 2006. [DOI] [PubMed] [Google Scholar]
- Takei T, Seki K. Spinomuscular coherence in monkeys performing a precision grip task. J Neurophysiol 99: 2012–2020, 2008. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Wylie RM, Marco LA. Organization of the medial vestibular nucleus. J Neurophysiol 31: 166–175, 1968. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, Wassermann EM. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol 518: 895–906, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]