Abstract
Nucleus laminaris (NL) neurons encode interaural time difference (ITD), the cue used to localize low-frequency sounds. A physiologically based model of NL input suggests that ITD information is contained in narrow frequency bands around harmonics of the sound frequency. This suggested a theory, which predicts that, for each tone frequency, there is an optimal time course for synaptic inputs to NL that will elicit the largest modulation of NL firing rate as a function of ITD. The theory also suggested that neurons in different tonotopic regions of NL require specialized tuning to take advantage of the input gradient. Tonotopic tuning in NL was investigated in brain slices by separating the nucleus into three regions based on its anatomical tonotopic map. Patch-clamp recordings in each region were used to measure both the synaptic and the intrinsic electrical properties. The data revealed a tonotopic gradient of synaptic time course that closely matched the theoretical predictions. We also found postsynaptic band-pass filtering. Analysis of the combined synaptic and postsynaptic filters revealed a frequency-dependent gradient of gain for the transformation of tone amplitude to NL firing rate modulation. Models constructed from the experimental data for each tonotopic region demonstrate that the tonotopic tuning measured in NL can improve ITD encoding across sound frequencies.
INTRODUCTION
Sensory systems often process different stimulus components in separate neural channels. In the auditory system, tonotopic organization arises from a spectral-to-spatial decomposition that occurs in the inner ear (Fettiplace and Fuchs 1999; Robles and Ruggero 2001). In addition to the frequency mapping, precisely timed action potentials in the auditory nerve encode the fine structure (Anderson et al. 1971) and envelope fluctuations (Joris and Yin 1992) of sound. Brain stem circuits involved in sound localization use this timing information (Goldberg and Brown 1969), relying on a binaural computation known as coincidence detection that requires specific synaptic and intrinsic neuronal properties.
Nucleus laminaris (NL) neurons, the first sites of binaural convergence in the avian auditory system, receive afferent projections from nucleus magnocellularis (NM) and perform coincidence detection on synaptic input (Carr and Konishi 1990; Jeffress 1948; Koppl and Carr 2008; Nishino et al. 2008). NL neurons encode interaural time difference (ITD), the primary cue used to localize low-frequency sounds (Brand et al. 2002; Carr and Konishi 1990; Goldberg and Brown 1969; Koppl and Carr 2008; Mills 1958; Nishino et al. 2008; Rayleigh 1907; Yin and Chan 1990). NL is tonotopically organized; characteristic frequencies of neurons increase from the caudolateral to the rostromedial pole (Rubel and Parks 1975). Previous investigations found tonotopic differences in the dendritic length, membrane conductance, and synaptic input of NL neurons (Kuba et al. 2005; Reyes et al. 1996; Smith and Rubel 1979). The measurement of a nonmonotonic tonotopic distribution of a low threshold K+ current led Kuba et al. (2005) to conclude that the middle frequency neurons of NL are best suited for sound localization. However, a subsequent in vivo study in chicks showed that NL neurons maintain good sensitivity to ITDs over the entire tonotopic range (Nishino et al. 2008). Thus we hypothesized that the mechanisms underlying coincidence detection should be tuned differently in neurons that respond to different sound frequencies.
In the present study, we used a systems analysis approach (Marmarelis and Marmarelis 1978; Rieke et al. 1997) to characterize the tonotopic differences across NL and to investigate their functional consequences. Our strategy was to determine the linear filters that best characterize 1) the transformation from presynaptic spikes to postsynaptic current and 2) the transformation from postsynaptic current to the output spike train. The first, the synaptic filter, was estimated by whole cell voltage-clamp recording of unitary EPSCs (uEPSCs) in NL neurons. The second, the intrinsic filter, was estimated based on the spike-triggered average (STA) current during noise stimulation in current-clamp mode. The uEPSCs and intrinsic filtering showed large differences between tonotopic regions. The functional importance of these differences was investigated by constructing a simple, data-based model of an NL neuron in each region, comprised of the two linear filters in series followed by a step nonlinearity to generate the spike output. Using these models, driven by simulated NM spike trains, we tested the hypothesis that frequency-specific tuning improves ITD encoding in NL.
METHODS
Brain slice preparation
Details of the slicing procedure have been described elsewhere (Reyes et al. 1996). Briefly, chicks [embryonic day 21 (E21) to posthatching day 1 (P1)] were decapitated and a 1 cm section of the skull containing the brain stem was removed with a razor blade and quickly submerged in ice-cold artificial cerebrospinal fluid (ACSF) consisting of (in mM) 130 NaCl, 26 NaHCO3, 3 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, and 10 dextrose and bubbled with 95% O2-5% CO2 to maintain pH at 7.4. An 8 mm transverse section of the brain stem containing NL was dissected and transferred to a Vibratome tissue slicer (TPI, St. Louis, MO). Six 200 μm coronal slices were cut, placed in separate compartments in a holding chamber filled with ACSF, incubated at 34°C for 30 min, and then cooled to room temperature.
Whole cell recordings
Individual slices were transferred to a recording chamber (∼0.5 ml volume) mounted on an upright Zeiss microscope (Carl Zeiss MicroImaging, Thornwood, NY) with a fixed stage and perfused with warmed ACSF (35°C unless otherwise noted) at a rate of about 1.5 ml/min. Individual neurons were viewed with a charge-coupled device camera with a ×40 water immersion lens using differential interference contrast optics and infrared illumination.
The electrodes were drawn from 75 μl borosilicate hematocrit tubing (VWR, San Francisco, CA) and usually had resistances ranging from 2 to 3 MΩ. In voltage-clamp recordings, the electrodes were filled with (in mM) 5 EGTA, 2.7 CaCl2, 123 Cs-methanesulfonate, 5 QX314-Cl, 2 MgCl2, 10 HEPES, 2 Na2ATP, 0.5 Na3GTP, and 0.5–1% biocytin; pH was adjusted to 7.2 with CsOH. The Cs-based internal solution was used to block K+ and Ih conductances and QX314-Cl was used to block Na+ conductance. During all voltage-clamp experiments, 100 μM picrotoxin was included in the ACSF to block inhibitory neurotransmission. Command voltages during experiments were corrected for a calculated liquid junction potential of −8.5 mV (JPcalc, Axon Instruments, Foster City, CA).
In current-clamp recordings, the electrodes were filled with (in mM) 5 EGTA, 2.7 CaCl2, 27 KCl, 103 K-gluconate, 2 MgCl2, 10 HEPES, 4 Na2ATP, 10 phosphocreatine, and 0.5–1% biocytin; pH was adjusted to 7.2 with KOH. The osmolality of both solutions was about 285 mOsm. During all current-clamp experiments, 20 μM 6,7-dinitroquinoxaline-2,3-dione (DNQX) and 100 μM picrotoxin were included in the ACSF to block excitatory and inhibitory neurotransmission, respectively. Data shown in all figures with current-clamp recordings were corrected for a calculated liquid junction potential of −3.6 mV (JPcalc, Axon Instruments). All chemicals were obtained from Sigma unless otherwise noted.
Voltage-clamp recordings were performed with an Axopatch 200A amplifier (Axon Instruments) in whole cell mode. Series resistance and whole cell capacitance were estimated and compensated electronically by 40–75%. Recordings in which the series resistance exceeded 10 MΩ showed distortion in the uEPSC time course and were not included in the analysis. The range of series resistances for data included in the analysis was 1.0–9.5 MΩ.
Current-clamp recordings were performed with a Multiclamp 700A or an Axoclamp 2A amplifier (Axon Instruments) in continuous bridge mode. In some experiments an analog dynamic-clamp amplifier (custom made) was used to simulate synaptic conductance in the neuron. In this case the command current took the form
| (1) |
where Is is the injected synaptic current, gs is the time-varying synaptic conductance waveform, Vm is the membrane voltage, and Erev is the reversal potential for the synaptic conductance (Reyes et al. 1996; Robinson and Kawai 1993; Sharp et al. 1993). Current and voltage traces were low-pass filtered at 10 kHz and digitized at 20 kHz with an InstruTECH ITC-16 A/D converter (InstruTECH, Port Washington, NY) and stored on a G3 Power Macintosh computer.
Anatomical reconstruction
NL is tonotopically organized; characteristic frequencies (CFs) of the neurons increase from the caudolateral to the rostromedial pole (Rubel and Parks 1975). The nucleus was divided into three roughly equal sections defined as low-CF (0.2–1 kHz), middle-CF (1–2.2 kHz), and high-CF (2.2–3.3 kHz) (Fig. 2A). Typically, NL was contained in five 200 μm coronal brain slices. The most caudal slice was defined as all low-CF. The next, more rostral slice was defined as low-CF on the lateral third of the slice, where the neuropil showed widening and there was less uniformity to the monolayer of cells. The medial two thirds were defined as middle-CF. In the third slice from the caudal pole, the lateral two thirds were defined as middle-CF while the medial third was defined as high-CF. Both the fourth and fifth slices (most rostral) were defined as high-CF. This division of the nucleus was similar to the division reported in a previous investigation (Kuba et al. 2005). In a prior study from our lab (Reyes et al. 1996) the relation of uEPSP amplitude and time course to tonotopic position showed less consistent results then what we report below. In the prior study we only used the rostral to caudal position of neurons in NL for assignment of tonotopic location and chicks aged E19 to E21 were used. The refinement of the procedures used for this study likely account for the different results.
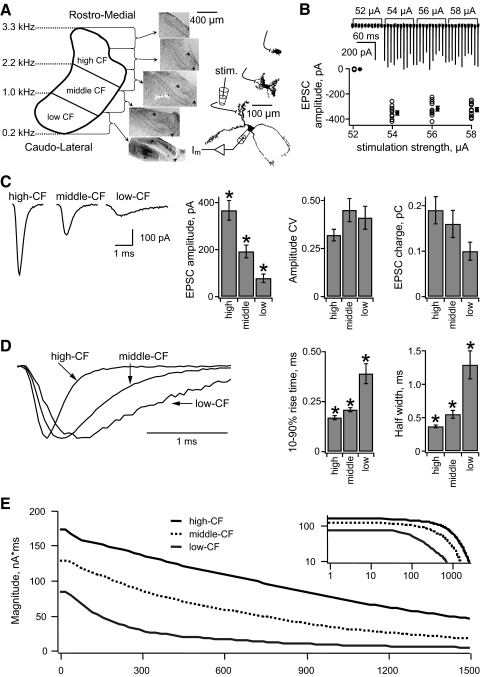
Fig. 2.
A tonotopic gradient of unitary excitatory postsynaptic currents (uEPSCs) in NL neurons. A, left: a schematic diagram of the tonotopic axis of NL, showing the anatomical sectioning of the nucleus into high-, middle-, and low-characteristic frequency (CF) regions, with approximate CFs at the borders indicated at the left. Middle: photomicrographs of the sequential 200 μm thick horizontal brain slices containing NL from one chick (top 4 slices, Nissl stained; bottom slice contains a biocytin filled low-CF neuron above the *, diaminobenzidine [DAB] reaction). NL is outlined by dotted line. NM is indicated by arrows. Right: representative biocytin-labeled neurons from each of the 3 frequency regions are shown. The patch electrodes show the configuration for somatic voltage clamp and stimulation of the ipsilateral NM fibers. B, top: an example (high-CF neuron) of typical synaptic currents recorded during the minimal stimulation protocol. Ipsilateral NM axons were stimulated at a range of current amplitudes (numbers above the trace) once every 10 s. Each sweep is 15 ms long. The holding potential was −60 mV. Bottom: plot of the individual uEPSC amplitudes vs. stimulation strength. Filled symbols with error bars (offset for clarity) indicate the mean ± SE. C, left: representative examples of average uEPSCs recorded in a single neuron from each tonotopic position. Bar plots (right) of the average uEPSC amplitude (left), coefficient of variation (CV) of uEPSC amplitudes (middle), and average uEPSC charge (right) in NL neurons from each frequency group. D, left: representative examples of average uEPSCs from a neuron in each tonotopic position. Amplitudes are normalized to compare the time courses. Bar plots (right) show the average 10–90% rise time (left) and average uEPSC half-width (right) in the neurons from each frequency group. E: Fourier magnitude spectrum of the average uEPSC recorded in each frequency region. The inset shows the same data on logarithmic axes, over the entire frequency range represented in NL. In all figures the error bars indicate SE and an asterisk (*) indicates statistically significant pairwise differences (P < 0.05).
To identify the frequency region of each neuron, biocytin was included in the recording pipette solution. Brain slices were fixed overnight in 4% paraformaldehyde and later visualized using the Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA), followed by development with diaminobenzidine and H2O2 (Sigma Fast kit; Sigma, St. Louis, MO). Following this reaction, slices were counterstained with 0.05% cressyl violet to identify the boundaries of NL. The frequency region of the neuron was identified by locating the recording site relative to the poles of the nucleus (Fig. 2A).
Electrical stimulation of nucleus magnocellularis fibers
Electrical stimulation of the ipsilateral nucleus magnocellularis (NM) fibers was performed using a monopolar glass pipette (pulled to 0.5–3 MΩ when filled with ACSF). The electrode was placed in the NM fiber bundle ventral to the NL neuropil. Stimuli were generated by a constant current stimulator (World Precision Instruments). The stimulus waveform in all experiments was a monophasic current pulse with a duration of 100 μs.
In minimal stimulation experiments, the stimulation intensity was gradually increased from 30 μA in 4 μA steps until EPSCs were evoked in an NL neuron. The stimulus intensity was then decreased by 4 μA. Responses to 10 stimuli at 0.1 Hz were measured at this level and at higher levels in 2 μA intervals. The average response at each level was calculated. A minimal stimulation experiment was judged successful if the EPSCs had a sharp threshold and constant average amplitude for ≥3 levels (2, 4, 6 μA) above threshold (see Fig. 2). Only recordings that met these criteria (34/38) were included in the analysis and classified as a unitary EPSC (uEPSC). The average uEPSC and coefficient of variation (CV) of the uEPSC amplitude were calculated from 30 to 40 responses over three to four stimulation levels.
In synaptic depression experiments, the stimulation intensity was increased from 40 μA in 10 μA steps until EPSCs >500 pA were evoked in an NL neuron. Responses to 10 stimuli at 0.1 Hz were measured at this level and at a level roughly 10% higher (see following text). If the EPSC amplitude was judged to be stable by eye, the depression protocol commenced. If the EPSC amplitude increased, the stimulation amplitude was turned down in 2 μA increments until the amplitude returned to the previous level. The depression protocol consisted of 50 stimuli at 200 Hz followed by a single stimulus at one of seven intervals (3, 10, 30, 100, 300, 1,000, or 3,000 ms) presented in random order. Each stimulation epoch lasted 3.26 s and was repeated 7–70 times at 0.1 Hz.
In a control experiment, antidromic spikes were recorded in NM neurons (n = 12) while the axon bundle was stimulated with the synaptic depression protocol described above. At the threshold level for antidromic spikes at 0.1 Hz, about 50% of NM neurons had at least one failure during 50 stimuli at 200 Hz, in which an antidromic spike was not recorded at the soma. This may indicate that a spike was not evoked and therefore would not activate the NM-to-NL synapse. However, it was determined empirically that increasing the stimulus strength by roughly 10% above threshold resulted in antidromic NM spikes on every cycle at 200 Hz. This result suggests that stimulation failures did not occur during the measurement of synaptic depression in NL.
Gaussian-distributed current and conductance stimulation
The noise stimulus used in current-clamp experiments was created by passing Gaussian white noise through an exponential filter with a 0.2 ms time constant. In all experiments (unless otherwise indicated) the noise stimulus had a constant CV (SD/mean) equal to 0.5. A continuous noise stimulus was presented to the neuron for 250 s, typically eliciting 5,000–10,000 spikes (20–40 spikes/s). In other experiments measuring the effects of temperature, conductance, or pharmacology, the noise stimulus was presented for 100 s. This noise stimulus was either current, current added to steady conductance, or noisy conductance alone. Only cells that fired spikes and had stable resting potentials below −55 mV were accepted for analysis.
Sinusoidal stimulation
In five neurons, responses were measured to broadband noise and sinusoids to test for linearity (Fig. 5). Before both stimuli, neurons were depolarized to approximately −46 mV using holding current. A zero-mean noise stimulus was then added on top of the holding current. The noise was scaled in each neuron to produce an average firing rate of 5–30 Hz. Following the noise stimulus, a sine wave (10 cycles) ranging from 50 to 800 Hz in 50 Hz increments was scaled in amplitude until the neuron began to fire. The smallest amplitude at which any spike(s) occurred was defined as the threshold for a given stimulus frequency.
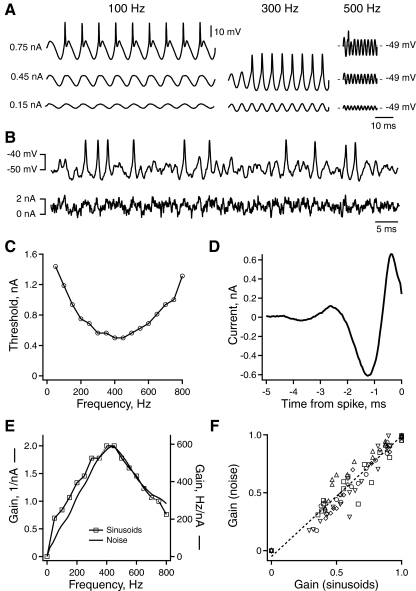
Fig. 5.
Linearity of intrinsic band-pass filtering in NL neurons. A: membrane voltage response of an NL neuron to sinusoidal current injected at increasing frequency (left to right) and amplitude (bottom to top). B: membrane voltage response of an NL neuron to stimulation with broadband noise current. C: amplitude of sinusoidal current required to reach spike threshold vs. current frequency. D: the spike-triggered average (STA) current vs. time relative to spikes (isolated by 10 ms) computed from the response to current noise stimulation. E: the relative gain of spike output vs. frequency calculated from the spike response to sinusoidal (gray) and noise (black) current (see methods). F: the gain of spike output at each frequency calculated from responses to noise vs. gain calculated in response to sinusoids. Each set of markers indicates the responses of the same neuron to different frequencies (n = 5). The dashed line is a linear fit to the data [y(x) = 1.04x − 0.05, R2 = 0.92].
Stimulation with simulated binaural input
In 14 neurons, spike responses were measured to both noise and simulated binaural EPSC trains at several phase delays (Fig. 6). The noise stimulus was the same as described above except that it had a CV of 0.75 (rather than 0.5). The simulated binaural input consisted of 20 NM spike trains from the ipsi- and contralateral sides of the brain, each having a mean spike rate of 250 Hz and phase-locked to a sound frequency of 400 Hz. The spike trains were added on each side, which resulted in a train of impulses, each one point in width. Each train of impulses was then convolved with an uEPSC waveform with rise and fall time constants of 0.2 and 0.25 ms, respectively. The contralateral EPSC train was given one of 21 phase delays ranging from 0 to 144° in steps of 7.2° and then summed with the ipsilateral input. The phase delayed input and the noise had the same mean, which was scaled in each neuron to elicit a high firing rate in response to the EPSC train at 0° phase delay. Each stimulus was presented in alternating blocks of 230 ms with 250 ms of rest. Each IPD stimulus was repeated 5–10 times.
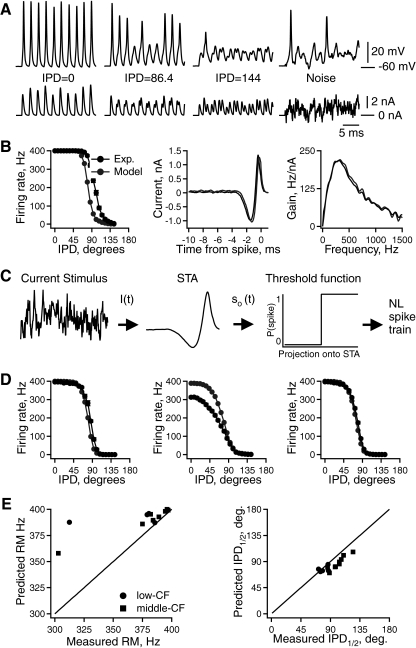
Fig. 6.
Intrinsic frequency tuning predicts interaural phase difference (IPD) tuning in vitro. A: responses of a middle-CF NL neuron to simulated phase-delayed EPSC trains (left 3 panels) and noise (right panel). B, left: average firing rate vs. IPD for experimental (black) and model (gray) responses to the same stimuli. STA (middle) and gain (right) calculated from experimental (black) and model (gray) responses to noise. C: schematic diagram of a linear–nonlinear (LN) cascade model of an NL neuron. D: 3 examples of average firing rate vs. IPD for experimental (black) and model (gray) responses to the same stimuli. E, left: predicted vs. measured rate modulation (firing rate at IPD = 0° − firing rate at IPD = 180°). Right: predicted vs. measured IPD corresponding to half the maximal firing rate.
Temperature dependence of intrinsic frequency tuning
Intrinsic frequency tuning was determined in the same neuron at two to three temperatures (25, 30, and 35°C) in increasing order (Fig. 10). The temperature was adjusted by regulating the current supply to the bath perfusion line heater and monitored with a thermistor in the bath adjacent to the slice. After the temperature stabilized at a given level, spike responses to the noise stimulus were recorded and used to calculate the STA and gain (see following text). The peak gain and gain half-width at 40°C were estimated by extrapolating a linear fit to data at lower temperatures. The peak frequency at 40°C was estimated similarly but with a quadratic fit.
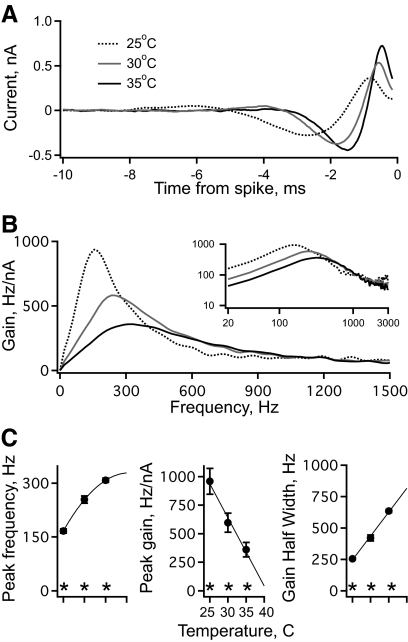
Fig. 10.
The effect of temperature on intrinsic frequency tuning. A: the average STA current vs. time relative to each isolated spike in a population of middle-CF neurons with a bath temperature of 25°C (dashed black, n = 11), 30°C (gray, n = 11), and 35°C (solid black, n = 12). B: the gain of spike output vs. frequency computed from the STAs in A. The inset shows the same data on logarithmic axes, over the entire frequency range represented in NL. C: peak frequency (left), peak gain (middle), and gain half-width (right) vs. bath temperature. The stars indicate that all 3 of these measurements differed significantly with temperature. The peak frequency was fit with a quadratic and the peak gain and gain half-width were fit with a line to extrapolate to values at 40°C.
Spike-triggered average measurement
During the noise stimulus, denoted by s(t), the occurrence time of every spike {ti} was recorded and used to select the stimulus waveforms preceding spikes si(τ) = s(ti − τ), where τ denotes the time index relative to the spike time. The spike-triggered current was calculated from τ = 0 to 10 ms before each spike. The mean s̄i(τ) is the STA and gives a linear estimate of the temporal filtering interposed between the current input and spike output of the neuron. At high firing rates, the STA includes the influences of interspike interactions due to spike-triggered conductance changes, which may alter the STA waveform (Aguera y Arcas et al. 2003). To minimize this effect, spikes that were isolated from the preceding spike by <10 ms were excluded. The STA calculated from isolated spikes predicted the threshold magnitude of sine wave current required for spike triggering more accurately than the STA calculated from all spikes (see Supplemental Fig. S1).1
During the standard noisy current stimulus, two distinct forms of firing rate adaptation were observed. NL neurons showed a rapid increase in rate over several seconds and then a decrease in rate over tens of seconds. The increase in firing rate is similar to that seen in NM neurons, where it has been shown to result from inactivation of low-threshold voltage-gated potassium conductance (Kuznetsova et al. 2008). The mechanism underlying the slower decrease in firing rate is not currently known. The STA was calculated from isolated spikes evoked during the entire stimulation period (250 s). Therefore the STA likely contains contributions across all levels of firing rate adaptation.
The average membrane potential of the neuron in response to a stimulus composed of a mean direct current (DC) input in addition to broadband noise was found to change the time course of the STA. In the same neuron, a stimulus causing greater depolarization resulted in a faster STA time course compared with a stimulus causing less depolarization (data not shown). This factor was controlled for across neurons by scaling the stimulus (with a constant CV) to produce a similar average membrane potential in each cell (−46 ± 0.4 mV, n = 44).
Gain calculation
The intrinsic frequency tuning of NL neurons was determined by reverse correlation. We measured gain based on the contribution of each input frequency to the triggering of spikes. Gain G(f) was calculated by first correlating the broadband noise stimulus s(t) and spike response r(t), obtaining the correlation csr as a function of the time difference τ
| (2) |
Next the stimulus autocorrelation css was determined. For Gaussian noise filtered with an exponential exp(−t/τrelaxation), css can be calculated based on the noise variance (σ2) and τrelaxation
| (3) |
The spectra of csr(τ) and css(τ) were computed by fast Fourier transform (FFT). G(f) was then obtained as the ratio of their magnitudes
| (4) |
Models of binaural phase-locked input from NM
Simulations of NM input to NL were used in Figs. 1, 4, 6, 8, and 11. In Figs. 1 and 4, A–D NM spike trains were modeled as comb functions (a train of equal amplitude impulses) with a frequency equal to the sound frequency. The amplitude spectrum of the NM input in Fig. 1B was computed from the time dependent histogram of spike counts using FFT.
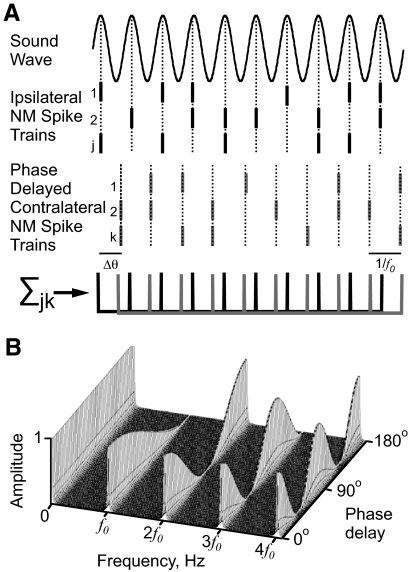
Fig. 1.
A simulation of binaural input to nucleus laminaris (NL) neurons. A: simulated spike trains from ipsi- (black) and contralateral (gray) nucleus magnocellularis (NM) neurons phase-locked to the sound frequency f0. The contralateral NM spike trains were given a phase delay, θ. The histogram of spike counts vs. time approximates the sum of 2 phase-delayed comb functions (bottom). B: the frequency spectrum of the phase-delayed comb function. Amplitudes are plotted as a function of both frequency and phase delay.
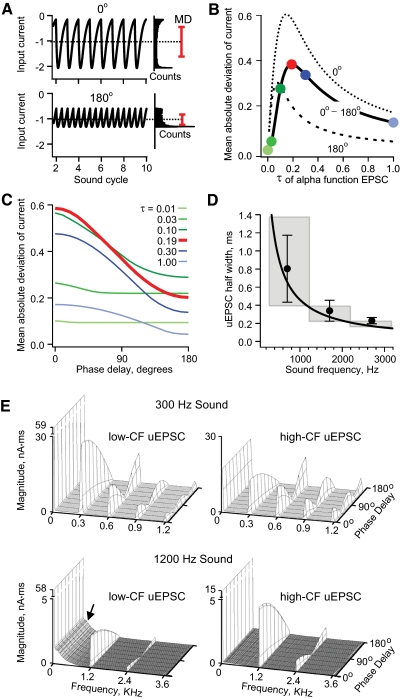
Fig. 4.
Effect of the uEPSC gradient on the magnitude of input fluctuation as a function of interaural time difference (ITD). A, top: a computed EPSC train created by convolving the sum of 2 in-phase, 1 kHz comb functions with an alpha function. The input current is plotted as a function of the sound cycle. The distribution of current values is shown to the right. The dotted line indicates the mean and the red bar indicates the mean ± 1 mean absolute deviation (MD). Bottom: the same as the top but with the comb function 180° out of phase. B: the MD of current vs. the time constant τ of the alpha function for in-phase (dotted line) and out-of-phase (dashed line) comb functions and the difference between these curves (solid black line). C: the MD of current vs. phase delay of the comb functions. Each curve corresponds to the MD computed from an EPSC train with a different alpha function time course (colored circles in B correspond to colored lines in C). D: the solid line is the theoretical optimum uEPSC half-width (ms) as a function of sound frequency. Symbols are experimentally measured mean uEPSC half-width in each frequency region, corrected for the difference between experimental and physiological temperature (see text) and positioned at the estimated center frequency of each region. Error bars are SD. Gray boxes indicate the range of CF across each region and the associated range of theoretically optimum uEPSC half widths. E: Fourier analysis (magnitude vs. frequency) of simulated synaptic current input to an NL neuron responding to 300 Hz sound (top panels) or 1,200 Hz sound (bottom panels). The presynaptic impulse trains were convolved with the average experimentally measured low-CF uEPSC (left panels) or the average measured high-CF uEPSC (right panels). See text for details.
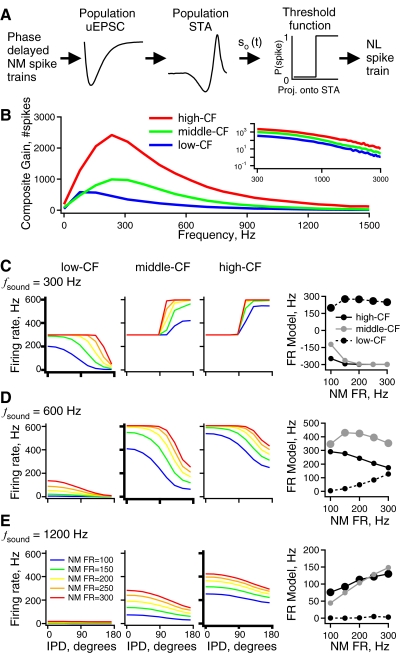
Fig. 8.
NL model responses to simulated NM spike trains at different sound frequencies and intensities. A: a schematic diagram of the LN cascade model of an NL neuron with both synaptic and intrinsic linear filtering stages (similar to Fig. 6). The NM spike trains are passed through experimentally derived synaptic and intrinsic linear filters and the result is thresholded to produce output spikes. A different model was used based on measured responses in each CF region. B: the composite gain vs. frequency due to the combined synaptic and intrinsic filtering in the high- (red), middle- (green), and low-CF (blue) regions. The inset shows the same data on logarithmic axes to illustrate differences in gain at the highest frequencies represented in NL. C: firing rate vs. IPD of the 3 NL models (3 left panels) for input simulated at a sound frequency of 300 Hz. For a given plot, each curve is the model response to a different sound intensity [average NM firing rate = 100 (blue), 150 (green), 200 (yellow), 250 (orange), 300 (red) spikes/s]. Right panel: plot of spike rate modulation (firing rate at IPD = 0° − firing rate at IPD = 180°) at each intensity for the high-CF (black, solid line), middle-CF (gray, solid line), and low-CF (black, dotted line) model responses. D: same as C but for 600 Hz sound frequency. E: same as C but for 1,200 Hz sound frequency.
Fig. 11.
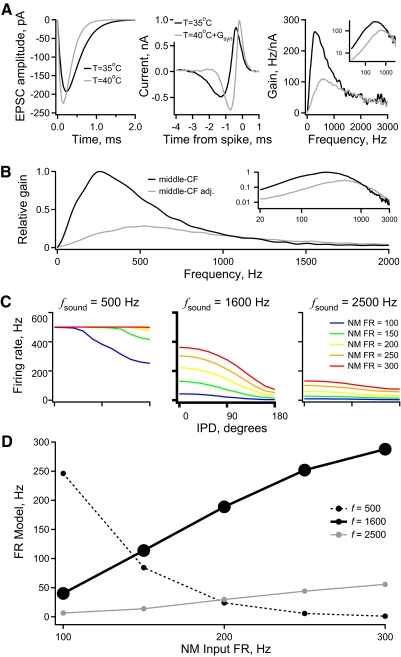
Middle-CF LN model responses with parameters adjusted for physiological temperature and synaptic conductance. A: the uEPSC (left) used in the middle-CF model before (black) and after (gray) adjustment. The same color assignment applies to all panels of A and B. The STA (middle) and gain (right) calculated from model responses to noise. The inset in the gain plot shows the same data on logarithmic axes, over the entire frequency range represented in NL. B: the relative gain vs. frequency due to the combined synaptic and intrinsic filtering in both middle-CF models. The inset shows the same data on logarithmic axes, over the entire frequency range represented in NL. C: firing rate vs. IPD of the adjusted middle-CF model for input simulated at sound frequencies of 500 Hz, 1,600 Hz (bold), and 2,500 Hz. For a given plot, each curve is the model response to a different sound intensity [average NM firing rate = 100 (blue), 150 (green), 200 (yellow), 250 (orange), 300 (red) spikes/s]. D: spike rate modulation (firing rate at IPD = 0° − firing rate at IPD = 180°) at each intensity for the model responses to stimuli at 500, 1,600 (bold), and 2,500 Hz.
The simulated input modeled in Figs. 4E, 8, and 11 consisted of 40 NM spike trains on the ipsi- and contralateral sides of the brain, phase-locked to a simulated sound frequency (300 or 1,200 Hz in Fig. 4E; 300, 600, or 1,200 Hz in Fig. 8; and 500, 1,600, or 2,500 Hz in Fig. 11). In Fig. 4E the NM spike times were jittered based on draws from a Gaussian distribution with SD of 100 μs. The contralateral NM spike trains were given one of nine IPDs (0, 21.6, 43.2, 64.8, 86.4, 108, 129.6, 151.2, and 172.8°). In Figs. 8 and 11, this procedure was repeated with five different NM spike rates (100, 150, 200, 250, and 300 Hz) to simulate different sound intensities (Warchol and Dallos 1990). In Fig. 4E the NM spike rate was 300 Hz. The spike trains were added across all NM cells to create a histogram of spike count versus time with a bin size of 0.05 ms. We used every combination of sound frequency, IPD, and NM firing rate, for a total of 270 simulated spike trains. In Fig. 4 the NM spike trains were convolved with an alpha function of (see Eq. 5) to create an EPSC train. In Figs. 8 and 11 NM spike trains were convolved with uEPSCs measured from NL neurons.
Linear–nonlinear cascade models of NL> neurons
We constructed two types of linear–nonlinear (LN) cascade models of NL neurons (Meister and Berry 2nd 1999; Simoncelli et al. 2004). The first type of LN model simulated the transformation from the summed synaptic current input to the output spike train and was calculated for individual neurons from spike responses to noise (see Fig. 6C). The second type of model simulated the transformation from the afferent spike input to the output spike train. The current-to-spikes model consisted of a linear filtering stage acting on the input current followed by a static nonlinearity to implement a spike threshold. We used the STA as the filter and a simple step threshold for the nonlinearity. This threshold function produces a spike with probability 1 whenever the filtered input is above the threshold value and with probability 0 otherwise. We also imposed a 1.5 ms refractory period after each output spike. For each neuron model, the threshold value of the nonlinearity was chosen to produce the same spike rate in response to the noise stimulus as was experimentally measured in the real neuron (see Fig. 6). This threshold value produces a model with the same STA and gain as those of the real neuron (Fig. 6B).
The STA calculated from the noise stimulus includes the exponential filtering of the stimulus. To remove this effect, each STA was deconvolved by the autocorrelation of the noise stimulus. The STA was then normalized to a magnitude of 1 (in the vector sense). This normalization is simply a choice of gain; we could equally well have scaled the threshold value needed to match model and experimental firing rates to noise (as described earlier).
The second type of LN model, which takes the afferent spikes as its input, was constructed based on population responses in each of the three CF regions (Figs. 7 and 10). This model adds to the first type of LN model a linear filter simulating the transformation from input spikes into current prior to filtering by the STA (Fig. 8A). The synaptic filter is represented by an alpha function of the form
| (5) |
where A is amplitude, τ is the time constant, and t is time. The alpha function varied in time course and amplitude to match the average amplitude and half-width measured in each CF region (Fig. 2). For high-CF: A = 357 pA, τ = 0.15 ms; for middle-CF: A = 192 pA, τ = 0.225 ms; and for low-CF: A = 78 pA, τ = 0.53 ms. For all alpha functions the amplitudes varied randomly around their mean with a CV of 0.39 (the average measured across all CFs).
Fig. 7.
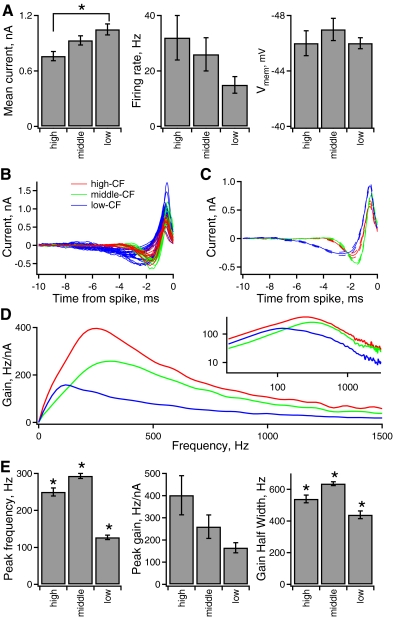
Tonotopic variation of intrinsic frequency tuning. A, left to right: statistics of the noise stimulus, firing rate, and membrane response measured in the different tonotopic groups. B: STA current vs. time relative to each isolated spike for a population of individual NL neurons in different tonotopic regions. (high-CF, red; middle-CF, green; low-CF, blue). The same color-coding applies in B and C. C: the average STA of neurons within each tonotopic position in NL. The solid line indicates the average, and the broken lines indicate the average ± the standard error of the mean. D: the average gain of spike output at each current frequency computed from the STAs. The inset shows the same data on logarithmic axes, over the entire frequency range represented in NL. E, left to right: the peak frequency (frequency at maximal gain), maximal gain, and gain half-width in the different frequency groups.
The STA filter was the average STA measured in each CF region. The threshold value in each CF model was set to produce the average firing rate measured in each CF region in response to the average scaling of the noise stimulus (see Fig. 7A). This procedure gave each CF model a gain that matched the average gain measured in each CF region (Fig. 7D). Threshold values of 2.22, 2.91, and 3.11 (in units of nA2·s) were used in the high-, middle-, and low-CF models, respectively. These models were driven with simulated NM spike trains (see Models of binaural phase-locked input from NM).
Statistics
All experimental errors are expressed as SE. Comparisons among groups of three were made by ANOVA analysis. Post hoc multiple comparisons were made with two-tailed Student's t-test with Bonferroni adjustment. Comparisons between two groups were made with two-tailed Student's t-test. Values of P < 0.05 were considered significant.
Comparisons of combined gain (synaptic gain × intrinsic gain) between frequency regions were made using Monte Carlo permutation tests, which are valid for data that do not have a normal distribution. For each comparison, we estimated the distribution of a test statistic (T) under the null hypothesis of no difference between regions, based on 2,000 random reassignments of the group labels (low-, mid-, and high-frequency). The test statistic for the original data (Tobserved) or the randomly relabeled data (Ti, where i = 1, … , 2,000) was a function of the expected values of combined gain, 〈Gcombined〉, in the groups compared. 〈Gcombined〉 depends on the means, SDs, and correlation of the synaptic and intrinsic gains. An initial test was performed using T = variance (〈Gcombined〉) among the three groups. Because these gains were measured in different neurons, their correlation is not known. Thus tests were performed under the extreme assumptions of no correlation and perfect correlation. For no correlation, 〈Gcombined〉 = 〈synaptic gain〉〈intrinsic gain〉, whereas with perfect correlation, 〈Gcombined〉 = 〈synaptic gain〉〈intrinsic gain〉 + SDsynapticSDintrinsic. To compare the three groups the P value was taken as the fraction of relabeled data sets for which Ti > Tobserved. If the variance among groups was significant, three pairwise tests were performed using the difference of 〈Gcombined〉 between groups as the test statistic and the two-tailed P value was taken as the fraction of the relabeled data sets for which |Ti| > Tobserved.
RESULTS
Simulation of binaural input to nucleus laminaris
The anatomical and electrophysiological properties of the avian ITD encoding circuit allow one to model the excitatory signals a nucleus laminaris (NL) neuron would receive in response to binaural tones in vivo. To clearly illustrate a feature of the input, we will assume that there is no temporal jitter in NM spike timing. Given this assumption, consider spike trains from ipsilateral NM neurons converging onto an individual NL neuron (Fig. 1A). In response to a sound of frequency f0, each NM neuron fires a spike with some probability on each cycle, with a fixed phase with respect to the input (Fukui et al. 2006; Warchol and Dallos 1990). The total ipsilateral signal is the sum of spikes from all NM neurons. A large number of NM neurons will reduce the spike count variability on each cycle and will produce a signal that resembles a comb function of frequency f0 (Fig. 1A, bottom). The contralateral input will be of the same form but may have a different phase relative to the ipsilateral input. The phase difference Δθ depends on interaural phase difference (IPD) in the sound wave as well as differences in neural transmission time. The sum of the two comb functions gives a very simple mathematical approximation of the time-varying input to an NL neuron.
The simulated NL input can be decomposed into its frequency components by Fourier transformation (Fig. 1B; see methods). When Δθ = 0, the signal is a simple comb function with frequency f0. In this case the amplitude has components at zero frequency (or the signal mean), the sound frequency f0, and its higher harmonics (2f0, 3f0, … ). As Δθ increases, the amplitude at zero frequency remains constant, whereas the amplitude at f0 declines monotonically. The amplitude at 2f0 changes nonmonotonically, first decreasing from 0 to 90° phase delay and then increasing from 90 to 180° phase delay. The amplitude of the higher harmonics fluctuates more rapidly with Δθ. When the two comb functions are 180° out of phase, the input signal is a single comb function of frequency 2f0. In this case the amplitude of the net input is concentrated at zero frequency and all the even harmonics of f0. In general, the amplitude at zero frequency is constant with respect to changes in phase delay, whereas each harmonic component k moves through k/2 cycles as Δθ increases from 0 to 180° (Ashida et al. 2007).
This simple model illustrates one important point: phase delay in the binaural input from NM to NL produces changes in amplitude at harmonics of the sound frequency. Only the changes at f0 are monotonic, such that the amplitude unambiguously represents the phase delay. Therefore one expects that NL neurons should be sensitive to signals at sound frequency f0, to encode IPD monotonically in their output firing rate, whereas it may be advantageous to filter out the nonmonotonic components at higher harmonics.
The signal encoded by NM spike trains is filtered by the NM-to-NL synapses and postsynaptically by active and passive membrane properties. In this study we pose one major question: How are the synaptic and postsynaptic filtering properties tuned to encode IPD at different sound frequencies? To address this question, neurons from NL were classified anatomically into three tonotopic regions corresponding to ranges of characteristic frequency (CF). We refer to these regions as high-CF, middle-CF, and low-CF (Fig. 2A, similar to the regions of Kuba et al. 2005).
Tonotopic gradient of synaptic frequency tuning
The strength and temporal filtering properties of the synaptic connections from NM axons to NL neurons were measured using minimal stimulation of the ipsilateral NM fiber tract to evoke unitary EPSCs (uEPSCs) (see Fig. 2B and methods). The uEPSCs were recorded from neurons in each of three CF regions of NL (Fig. 2A) and showed significant differences in amplitude between the three frequency groups (Fig. 2C). High-CF neurons had the largest amplitudes (367 ± 42 pA, n = 11), followed by middle-CF (192 ± 27 pA, n = 12) and low-CF (78 ± 18 pA, n = 11). The variability of uEPSC amplitude in each recording was quantified by calculating the CV, which revealed no significant differences among the three frequency regions.
The uEPSC kinetics also showed a tonotopic gradient, from fast at high CF to slow at low CF (Fig. 2D). This gradient was significant for both the 10–90% rise time and the width at half height. This tonotopic gradient of uEPSC kinetics is consistent with a previous report of the EPSC kinetics recorded after stimulation of multiple NL inputs (Kuba et al. 2005). The combined effect of the gradients of amplitude and time course led to a smaller gradient of total uEPSC charge. Because the amplitude gradient was steeper than the opposing kinetic gradient, the uEPSC charge was largest at high CF and smallest at low CF.
Tonotopic differences in filtering by the NM-to-NL synapses were further analyzed by computing the Fourier spectrum of the average uEPSC in each frequency region (Fig. 2E). The uEPSC spectra are all low-pass. As expected, the faster the uEPSC, the slower the Fourier components decrease with increasing frequency. The tonotopic differences at zero frequency correspond to those of the uEPSC charge.
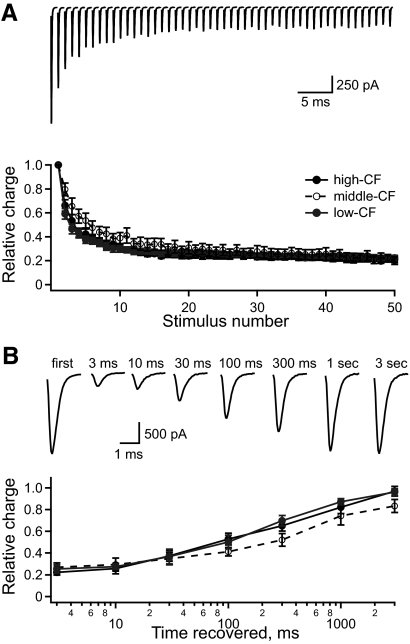
Synaptic depression and recovery are similar across the tonotopic map
The tonotopic gradient of uEPSC amplitude was measured at 0.1 Hz, which is much lower than physiological NM firing rates (∼100–400 Hz) (Fukui et al. 2006; Warchol and Dallos 1990). At physiological rates, excitatory input to NL neurons undergoes short-term synaptic depression (Cook et al. 2003; Kuba et al. 2002b). Tonotopic variation in the degree or time course of depression and its recovery could alter the relative amplitudes of uEPSCs in vivo. This possibility was tested by measuring EPSCs while stimulating multiple NM axons with trains of 50 pulses at 200 Hz. The EPSC amplitude depressed to <50% of its initial value over the first five stimuli and more slowly thereafter (Fig. 3A, top). Because EPSCs tend to broaden during depression (Brenowitz and Trussell 2001), we measured the relative EPSC charge on each stimulus trial. Figure 3A (bottom) shows the average EPSC charge in each CF region for each stimulus pulse. The ratio of depressed charge to initial charge did not vary significantly among the three frequency regions (high-CF = 0.20 ± 0.03, n = 6; middle-CF = 0.23 ± 0.03, n = 6; and low-CF = 0.23 ± 0.02, n = 7).
Fig. 3.
Synaptic depression in NL neurons is similar across the tonotopic map. A, top traces: the EPSCs in response to 50 stimuli at 200 Hz (average of 42 repeats in one neuron). Bottom plot: the average relative EPSC charge vs. stimulus number for NL neurons in the high- (filled), middle- (open), and low-CF (gray) regions. B, top traces: the EPSCs recorded in a single neuron in response to the first stimulus and at variable times (indicated above the traces) after the 50th stimulus in the depression train (average of 6 repeats). Bottom plot: the average relative EPSC charge vs. time recovered for NL neurons in the high- (filled), middle- (open), and low-CF (gray) regions.
The time course of recovery from synaptic depression was measured based on the EPSC charge (relative to the nondepressed EPSC) for single EPSCs evoked at different times after the 200 Hz train (Fig. 3B). The average recovery in each CF region had a similar time course. The synaptic depression and recovery data suggest that the relative gradient of uEPSC size will persist when NM neurons fire at physiological rates.
Gradient of uEPSC properties provides frequency-appropriate filtering of input to NL
NL neurons require rapid fluctuation of their input to generate output action potentials (Higgs et al. 2006; Reyes et al. 1996). Given that constraint, we reasoned that a large change in the fluctuation of the summed synaptic current as a function of phase delay would favor a large modulation of output firing rate as a function of phase delay. Specifically, we tested the effect of the uEPSC time course on the amount of input fluctuation. NM spike trains were modeled as phase-delayed comb functions, as described earlier (shown at the bottom of Fig. 1A; also see methods). This spike input was convolved with alpha functions to simulate uEPSCs of different durations but identical peak amplitudes. Figure 4A, top shows an example of an EPSC waveform created by this process. In this example the NM spike trains are phase locked to a 1 kHz tone, in phase with each other, and the alpha function has a time course defined by the parameter τ = 0.19 ms (see methods). The amount of fluctuation in the input current was quantified by measuring the mean absolute deviation (MD) of the stimulus (see methods). Figure 4A (top right) shows the distribution of stimulus values and the mean stimulus value (dashed line) ±1MD (red line). The bottom panel of Fig. 4A is the same as the top panel, except that the NM spike trains are 180° out of phase. In this case the amount of current fluctuation, as quantified by the MD, is less.
Figure 4B plots the MD of the stimulus versus the time course of the alpha function for NM spike trains (phase-locked to 1 kHz) that are in phase (dotted line) and out of phase (dashed line). The difference between these curves (thick black line) gives the change in MD for in-phase inputs relative to out-of-phase inputs. This function is maximal for an alpha function with τ = 0.19 ms (red dot). Figure 4C plots the MD versus phase delay for different time courses of the alpha function. The colored lines correspond to the colored points in Fig. 4B. The thick red line (τ = 0.19 ms) has the largest change in MD with phase delay.
This analysis revealed that there is an optimal alpha function (or uEPSC) duration for a given sound frequency, which produces the largest change in the input MD as a function of phase delay. This worked out to an EPSC half-width equal to 0.46 of the sound period. Figure 4D (solid black curve) shows this optimal alpha function half-width (in ms) versus sound frequency. The experimentally measured mean uEPSC half-widths from the low-, middle-, and high-CF regions of NL (corrected from 35 to 40°C using a Q10 of 2.2; Zhang and Trussell 1994) (filled black circles) agree very well with the theoretical prediction for the timescale that provides a maximal change in input MD as a function of phase delay.
To get further insight about the combined effect of the gradients of uEPSC amplitude and time course, we convolved simulated binaural impulse trains (i.e., as shown at the top of Fig. 1A) with the measured mean uEPSC from either the low- or the high-frequency region of NL. We simulated 40 inputs per side, each having an average impulse rate of 300 spikes/s, phase-locked to a tone of either 300 or 1,200 Hz. The resulting EPSC trains from the two sides were then combined at different phase delays, from 0 to 180°. The resulting input current waveform for each phase delay was then Fourier transformed to evaluate the magnitude of the frequency components of the synaptic input to NL as a function of phase delay (as was done for the presynaptic impulse trains in Fig. 1B).
Figure 4E shows the plots for four conditions: 300 Hz (Fig. 4E, top) and 1,200 Hz (Fig. 4E, bottom) sound, each filtered with the experimentally measured low-CF uEPSC (Fig. 4E, left) or the high-CF uEPSC (Fig. 4E, right). The plots demonstrate three points.
First, when the 300 Hz tone is combined with the low-CF uEPSC, the monotonic change in the first harmonic as a function of phase delay is relatively large compared with the nonmonotonic changes that occur at higher harmonics. This difference in the magnitude of the change is much smaller when the 300 Hz tone is combined with high-CF uEPSC. Thus for an NL neuron with a low CF, slower uEPSCs may help to reduce the confounding influence of nonmonotonic changes at the second and higher harmonics.
Second, we find that the absolute magnitude of the change in the first harmonic with phase delay is larger when the 300 Hz input is filtered by the low-CF uEPSC, compared with the high-CF uEPSC. Up to a point, a broader uEPSC of given peak amplitude more effectively transmits a periodic input, simply because the integrated synaptic current, or “charge transfer,” is greater. In contrast, for the 1,200 Hz input the absolute magnitude change at the first harmonic is larger after filtering with the high-CF uEPSC. In this case, the width of the low-CF uEPSC exceeds the optimum and the resulting low-pass filtering strongly attenuates the first harmonic. In addition, the larger peak amplitude of the high-CF uEPSC boosts the absolute magnitude of input at all frequencies.
Finally, the summed EPSC signal is not entirely restricted to the harmonics, but shows finite magnitude between zero frequency and the first harmonic produced by the variability in amplitude on each cycle due to the probabilistic firing of each input (see methods). This is particularly apparent for the 1,200 Hz input filtered by the low-CF uEPSC (Fig. 4E, bottom left, arrow). The low-frequency input shows little variation with phase delay and thus cannot contribute directly to IPD encoding. This input component is reduced by sufficiently fast uEPSCs (compare Fig. 4E, bottom left and bottom right).
Intrinsic frequency tuning of NL neurons
Although the gradients of uEPSC amplitude and time course (low-pass filtering) enhance frequency-specific monotonic changes in input magnitude, further band-pass filtering could reduce the influence of input components at frequencies below and above the first harmonic. Such band-pass filtering may be provided by intrinsic properties of NL neurons that influence the transformation of summed synaptic current to output spikes. To test this hypothesis we experimentally measured the intrinsic frequency tuning of neurons in the low-, middle-, and high-frequency regions of NL.
We determined the intrinsic frequency tuning of NL neurons in response to sine wave and broadband noise current injections (see methods). Figure 5A shows typical responses to sinusoidal current. As previously reported, the subthreshold membrane potential response showed resonance (Strohmann et al. 1995), which was at about 300 Hz input frequency in this neuron (Fig. 5A, bottom row). At slightly larger stimulus amplitudes (Fig. 5A, middle row) the neuron fired only in response to input frequencies around 300 Hz. As the amplitude was increased (Fig. 5A, top row) the neuron responded to a wider range of frequencies. We constructed a tuning curve given by the threshold current magnitude at which a sine wave of each frequency first elicited at least one spike (Fig. 5C). All NL neurons had a minimum current threshold for some nonzero frequencies; in other words, these cells had band-pass frequency tuning. The threshold at zero frequency (or DC) was infinite because no spikes were evoked at any amplitude tested. This behavior was consistent with previous work demonstrating that most NL neurons fire only once at the onset of a current step (Kuba et al. 2005; Reyes et al. 1996).
The intrinsic tuning of NL neurons was also measured using broadband noise (Fig. 5B). The spike train recorded while injecting noise was used to find the spike-triggered average (STA) current. The STA in NL neurons (Fig. 5D) was oscillatory and damped to zero within two or three cycles, at 5–10 ms before the spike time. The damped oscillation of the STA is characteristic of a neuron that functions as a band-pass filter. Below the preferred frequency, such a neuron acts like a differentiator, transmitting higher-frequency input oscillations more strongly than lower-frequency input components. Consistent with this idea, the shapes of the STAs measured in NL neurons were qualitatively similar to those of NM neurons, which were previously shown to act as differentiators of their synaptic input (Slee et al. 2005). In NM neurons, the previous study showed that band-pass filtering was eliminated by α-dendrotoxin, which blocks low-threshold voltage-gated potassium conductance (GKlt) (Kuba et al. 2005). However, our present results indicate that band-pass filtering in NL neurons persists during bath application of α-dendrotoxin (see Supplemental Fig. S2). This result demonstrates that GKlt is not necessary for band-pass filtering in NL. It is possible that the ionic mechanisms that regulate band-pass filtering in NL neurons differ substantially from those in NM cells. However, differences in current stimuli (in particular, the large DC offset applied in the present study but not in the previous NM experiments) may also contribute to these findings.
To examine the intrinsic frequency tuning of NL neurons in the frequency domain, the responses to sine waves and noise were used to calculate the sensitivity of spike output to each input frequency, which we will refer to as gain (Fig. 5E; see methods). Different, but closely related, measures of gain were obtained for the subthreshold-to-threshold sine wave currents and the broadband noise. For sinusoidal input, the frequency-dependent “threshold crossing gain” was defined as the inverse of the current magnitude threshold. For noise, gain was defined conventionally as the mean magnitude of sinusoidal firing rate modulation in response to each Fourier component of the stimulus, divided by the magnitude of the corresponding stimulus component. The gain in response to noise is proportional to the Fourier amplitude spectrum of the STA divided by the magnitude of the noise autocorrelation at corresponding frequencies. Figure 5E shows gain measured in a single NL neuron for both noise and sinusoids. We found that the threshold-crossing gain was highly correlated with the gain computed in response to noise (R2 = 0.92; Fig. 5F; also see Supplemental Material for further discussion).
Intrinsic frequency tuning predicts IPD tuning
In another set of experiments we compared intrinsic frequency tuning to IPD tuning (in vitro) in the same neuron. NL neurons were driven by a stimulus comprised of interleaved epochs of noise and simulated phase-delayed EPSC trains (see methods). The EPSC trains were phase-locked to a 400 Hz tone. Figure 6A shows an example of spike responses to the IPD stimuli (three left panels) and the noise stimulus (right panel). As reported previously, the spike response decreased with increased IPD (Reyes et al. 1996). The IPD tuning of the neuron is summarized in Fig. 6B (black trace in left panel) and the STA and gain measured from spike responses to noise are also shown in Fig. 6B (black traces in middle and right panels, respectively).
We modeled IPD tuning in this NL neuron using the STA in a linear–nonlinear (LN) cascade model (Fig. 6C) (Meister and Berry 2nd 1999; Simoncelli et al. 2004). This class of model breaks spike generation into a linear filter operation on the input current (Fig. 6C, middle) followed by a static nonlinearity (Fig. 6C, right) to implement the spike threshold. We used a simple step threshold (Keat et al. 2001), chosen such that the model output matched the experimentally measured firing rate in response to the noise stimulus (see methods).
The gray traces in Fig. 6B show the model responses to the same IPD and noise stimuli presented in the experiment. The model's IPD tuning function closely matched the experiment, but its firing rate fell off at a slightly lower IPD. The STA and gain computed from the model responses to noise were in close agreement with the experiment. This is the expected result since the model was fit based on the noise responses.
Figure 6D illustrates the range of agreement between the model and experiment in three different NL neurons. In general, the model accurately predicted the experimental IPD tuning but in some neurons it predicted too high a firing rate at low IPD (Fig. 6D, middle) or a decreased rate at a lower IPD as in Fig. 6B. These discrepancies may arise from sources of variability in the spike threshold of the real neurons that are not included in step threshold of the model, such as fluctuations in membrane potential. At lower stimulus levels this variability could reduce firing on some sound cycles as in Fig. 6B (middle panel and the two outliers in the left panel of Fig. 6E). Figure 6E summarizes experimental and model IPD tuning in 14 NL neurons (9 middle-CF, 5 low-CF), showing the total rate modulation (Fig. 6E, left) and the IPD at which the rate fell to half its maximum value (Fig. 6E, right). The proximity of most points to the line of unity demonstrates that the model successfully predicted experimental IPD tuning.
Tonotopic variation of intrinsic frequency tuning
Based on the preceding results, we characterized the intrinsic tuning of NL neurons across the tonotopic axis using the STA and gain measured from spike responses to noise. To facilitate comparison of noise responses across an inhomogeneous population of neurons, the noise stimulus applied to each neuron was scaled to produce an average membrane potential of approximately −46 mV, whereas the coefficient of variation (CV = SD/mean) was held fixed at 0.5 (see methods). Figure 7A (left) shows that it was necessary to inject a significantly larger stimulus in low-CF neurons compared with high-CF neurons to achieve the same average membrane potential (Fig. 7A, right). With this stimulus scaling, there was a trend indicating higher firing rates in higher CF neurons. This trend was not statistically significant, possibly due to the large variation in firing rate within each CF group.
Intrinsic frequency tuning varied significantly across the tonotopic axis of NL. Figure 7, B and C shows STAs measured in high-CF (n = 10), middle-CF (n = 12), and low-CF (n = 22) neurons. The STAs were used to calculate the average gain in each CF region (Fig. 7D). The frequency at which maximal gain occurs (peak frequency) differed significantly between the groups: 250 ± 11 Hz in high-CF neurons, slightly higher in middle-CF neurons (293 ± 7 Hz), and lowest in low-CF neurons (127 ± 6 Hz). The maximal value of gain (peak gain) showed a tonotopic gradient (Fig. 6E, middle): highest in high-CF neurons, 402 ± 88 Hz/nA; lower in middle-CF neurons, 260 ± 53 Hz/nA; and lowest in low-CF neurons, 165 ± 23 Hz/nA (ANOVA, P = 0.005). However, a post hoc paired t-test with Bonferroni correction for multiple comparisons indicated that the difference between groups was not significant. This result could be due to the large variability in peak gain within each CF group. Nonetheless, it was interesting that the peak gain of NL neurons showed the same trend as the uEPSC amplitude, suggesting that synaptic and intrinsic gradients of response magnitude may serve a common function. The width at half the maximal gain (gain half-width) was significantly different across frequency groups, being widest in middle-CF neurons (635 ± 12 Hz), narrower in high-CF neurons (539 ± 24 Hz), and narrowest in low-CF neurons (439 ± 24 Hz).
Although the middle- and high-CF NL neurons showed consistent intrinsic tuning, some low-CF neurons fired single spikes to current steps, others fired two spikes, and occasionally neurons fired several spikes, consistent with previous reports (Kuba et al. 2005). Sufficient data for a statistical analysis were accumulated from low-CF neurons with singlet (n = 12) and doublet (n = 10) firing to current steps. The peak frequency was significantly lower in doublet-firing (107 ± 3 Hz) compared with singlet-firing low-CF neurons (143 ± 8), whereas the peak gain (200 ± 40 Hz/nA in doublet-firing vs. 135 ± 25 Hz/nA in singlet-firing) and gain half-width (402 ± 33 Hz in doublet-firing vs. 470 ± 32 Hz in singlet-firing) did not differ significantly.
Modeling the role of tonotopic tuning for encoding IPD across sound frequencies
An effective encoding of IPD requires that the system both show a monotonic dependence of firing rate on phase and avoid firing rate saturation. To investigate how tonotopic tuning affects IPD encoding at different sound frequencies, the measured low-pass synaptic filtering and band-pass intrinsic filtering were combined in an LN model. Three population LN models (similar to Fig. 6C) were constructed from the filtering properties measured in the three CF regions (Fig. 8A). Each LN model consisted of three stages in series: a linear synaptic filter (average uEPSC within each CF region), a linear intrinsic filter (average STA within each CF region), and a nonlinear threshold to implement spike generation.
Figure 8B shows the combined gain functions from the two filtering stages. The high-CF population model, with the largest uEPSC and intrinsic gain, has the greatest gain, followed by the middle- and low-CF models, respectively. The expected values of combined gain at 300, 600, 1,200, and 2,400 Hz for each region of NL were compared using Monte Carlo permutation tests (see methods). Because the correlation between synaptic and intrinsic gain within each frequency region is not known, tests were performed under the extreme assumptions of no correlation and perfect correlation. Under both assumptions, the difference among NL regions was significant at each tested frequency. Post hoc comparisons using paired Monte Carlo permutation tests showed P < 0.05 for each pair of regions at each frequency, except for the middle- versus high-frequency region at 600 Hz under the assumption of perfect correlation (P = 0.07).
The models were driven with simulated binaural NM spike trains in response to phase-modulated pure tones, as in Fig. 1A (Reyes et al. 1996). The simulated input consisted of 40 NM spike trains per side, which were phase-locked to one of three sound frequencies (300, 600, or 1,200 Hz). The contralateral NM spike trains were given one of nine IPDs (0, 21.6, 43.2, 64.8, 86.4, 108, 129.6, 151.2, and 172.8°). Five NM spike rates (100, 150, 200, 250, and 300 Hz) were used to simulate different sound intensities (Fukui et al. 2006; Warchol and Dallos 1990). The spike trains were added across all NM cells to create a histogram of spike count versus time. All combinations of region-specific NL model, sound frequency, IPD, and NM firing rate were simulated to test the hypothesis that the region-specific tuning of NL synapses and intrinsic neuronal properties improves IPD encoding. In Fig. 8, C–E the mean firing rate of each NL model is plotted as a function of IPD for each simulated sound intensity.
We first consider the responses of the three models to a low-frequency, 300 Hz tone. As expected, the low-CF model decreased its firing rate with phase delay at all sound intensities (Fig. 8C, left, bold axes), although at the highest intensity the model was saturated at most phase delays. In contrast, the middle- and high-CF models show an increased firing rate with phase delay for all simulated 300 Hz tone stimuli (Fig. 8C, middle). The middle- and high-CF models have a higher peak gain and a slower fall-off in gain from 300 to 600 Hz and thus fail to attenuate the harmonics that lead to a nondecreasing firing rate.
The total rate modulation (firing rate in phase − out of phase) for each model as a function of the firing rate of the NM afferents (simulated sound intensity) is shown in Fig. 8C (right panel). For the 300 Hz input, the low-CF model had the greatest rate modulation over the entire range of sound intensities. This is due both to the lower total gain of the combined intrinsic and synaptic filters and to their attenuation of high frequencies (Fig. 8B). At this sound frequency, relatively low gain is required to prevent the NL firing rate from reaching saturation, where a spike is generated on every sound cycle, at all phase delays. Saturation is a particular problem for low sound frequencies because the NM spikes are phase-locked to a relatively small number of cycles, which results in many input spikes to NL on each cycle. The low gain of the low-CF model solves this problem by reducing the probability of spiking for a given input strength. In sum, this analysis indicates that larger, faster responses are not always better and appropriately low gain and frequency cutoffs are best suited for encoding IPD at low sound frequencies.
When the three models were driven at 600 Hz sound frequency, the middle-CF model, better tuned to match to the input, had the most rate modulation over all sound intensities (Fig. 8D, right and bold axes). The high-CF model also reduced firing rate with IPD at all sound intensities. However, at high intensities the high-CF model was driven to fire at rates exceeding 400 Hz by the out-of-phase inputs, suggesting that its gain at twice the sound frequency (1,200 Hz) was too high for this input. The low-CF model was not driven as well by the 600 Hz tone, especially at low intensities (Fig. 8D, left), suggesting that its gain at the sound frequency (600 Hz) was too low for this input.
At 1,200 Hz sound frequency, both the high- and middle-CF models were driven at high rates by in-phase input. However, the amount of rate modulation with phase delay was less than that obtained for the 600 Hz sound frequency (Fig. 8E). In real neurons, the degree of rate modulation may be increased by additional mechanisms not included in the present models (see discussion). For the three lowest sound intensities, the high-CF model fired at the highest rates and had the greatest total rate modulation (Fig. 8E, right). The superior performance of the high-CF model for these relatively weak inputs may be attributed to its high gain. At the two highest intensities, the middle-CF model had lower rates than those of the high-CF model but had greater total rate modulation. The low-CF model fired at <50 Hz at any sound intensity and thus did a poor job of encoding phase delay at 1,200 Hz sound frequency. This analysis indicates that fast tuning and a large gain are required to encode phase delay at 1,200 Hz sound frequency (or higher).
The results of these simulations suggest that tonotopic differences in temporal filtering provide each model with the best IPD tuning over a range of sound frequencies. However, the range of sound frequencies over which these models showed strong IPD tuning was limited to about 300 to 1,200 Hz. This range covers only the middle/low portion of the tonotopic axis, which extends out to about 3,300 Hz (Rubel and Parks 1975). The LN models did not respond well at higher frequencies (data not shown), although recent in vivo studies report IPD sensitivity across the entire CF range (Koppl and Carr 2008; Nishino et al. 2008). These results suggested that additional factors not included in the model influence IPD tuning in NL. In the following two sections, we investigate the roles of synaptic conductance and physiological temperature on the intrinsic frequency tuning of NL neurons.
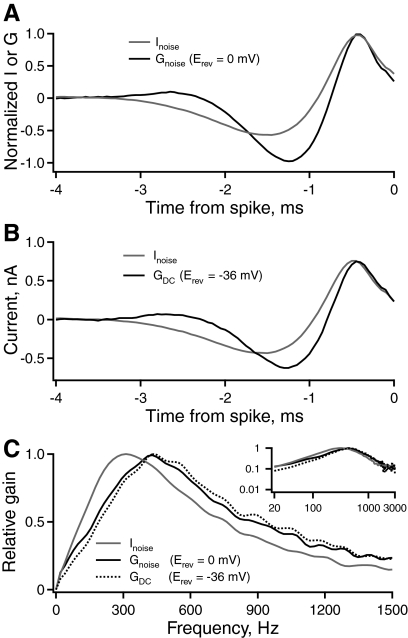
Synaptic conductances increase the peak frequency of gain
NL neurons have bipolar dendrites that vary in length across the tonotopic axis (Smith and Rubel 1979). Cells in the middle- and high-CF regions have short dendrites that likely are electrotonically compact. This raises the possibility that conductance from proximal synaptic contacts could influence temporal filtering in the somatic compartment. The effect of excitatory synaptic conductance was tested by using an analog dynamic-clamp amplifier to inject artificial conductances via the somatic recording electrode (Reyes et al. 1996; Robinson and Kawai 1993; Sharp et al. 1993). Rather than injecting broadband noise current, the same stimulus waveform was applied as excitatory conductance (mean = 20 nS, SD = 10 nS, Erev = 0 mV). Figure 9A plots the STA current (gray, n = 12) or conductance (black, n = 5) versus time relative to each isolated spike in a population of middle-CF neurons. The STA had a faster time course with conductance noise than with current noise. The corresponding gain functions (Fig. 9C) derived from conductance had a significantly higher average peak frequency (438 ± 19 Hz, 42% greater) compared with those derived from current injection (308 ± 6 Hz) (P < 0.001). The gain half-width during conductance noise (706 ± 32 Hz) was not significantly different from the half-width during current noise (635 ± 12 Hz). The different units of current and conductance do not allow a direct comparison of peak gain. These results show that excitatory synaptic conductance shifts the relative intrinsic tuning to higher frequencies.
Fig. 9.
Artificial excitatory and inhibitory synaptic conductances increase the peak frequency of intrinsic gain. A: the average STA current or conductance vs. time relative to each isolated spike in a population of middle-CF neurons. The neurons were stimulated with either current noise (gray, n = 12) or excitatory conductance noise (black, n = 5), applied with a dynamic-clamp amplifier. B: the STA current vs. time relative to each isolated spike in response to current noise alone (gray, n = 12) or current noise plus steady-state inhibition (black, n = 5), applied with a dynamic-clamp amplifier. C: the relative gain of spike output vs. frequency in middle-CF neurons in control (gray) and under the influence of excitatory (solid black) or inhibitory (dashed black) conductance. The inset shows the same data on logarithmic axes, over the entire frequency range represented in NL.
NL neurons receive inhibitory inputs at the soma from neurons in the superior olivary nucleus (Burger et al. 2005; Funabiki et al. 1998; Yang et al. 1999). The synaptic input is GABAergic and depolarizing from rest; the reversal potential (Erev = ECl) is around −36 mV, due to the high concentration of intracellular Cl− known to be present in these cells (Monsivais and Rubel 2001). The kinetics of the GABAergic inputs are slow relative to those of the excitatory inputs (Funabiki et al. 1998; Yang et al. 1999): the inhibitory synaptic conductances show pronounced temporal summation. Therefore inhibitory synaptic conductance was approximated by applying a constant conductance (20 nS, Erev = −36 mV) with a dynamic-clamp amplifier, in addition to the standard broadband noise current. Figure 9B shows the STA current versus time relative to each isolated spike in response to current noise alone (gray, n = 12) or current noise plus steady-state inhibition (black, n = 5). The temporal filtering in middle-CF neurons with inhibition was faster than that in control neurons. The gain (Fig. 9C) computed from the STA current under the effect of the inhibitory conductance had a significantly higher peak frequency (410 ± 9 Hz, 33% greater) than that in the control population (308 ± 6 Hz, P < 0.001). However, the gain half-width during artificial inhibitory conductance (662 ± 39 Hz) was not significantly different from the half-width during current noise alone (635 ± 12 Hz). The amplitudes of the peak gains were highly variable. Thus at present no conclusion can be drawn about the effect of constant inhibitory conductance on this measure.
Our data indicate that constant inhibitory conductance and noisy excitatory conductance of the same magnitude have similar effects on the peak frequency of the gain function. These results suggest that the mean level of synaptic conductance, rather than the conductance fluctuations or the reversal potential, most strongly affects frequency tuning in NL neurons. The effect of added conductance is consistent with a decrease in the membrane time constant.
Temperature affects intrinsic gain at low frequencies
The standard recording temperature in the experiments was 35°C, which is lower than chicken body temperature (40°C). Therefore experiments were conducted to estimate the intrinsic tuning of middle-CF neurons at physiological temperature. Spikes from middle-CF neurons at 40°C were very small (<10 mV), making their detection unreliable against the background of subthreshold voltage fluctuations caused by the noisy current stimulus. To circumvent this limitation, the frequency tuning of middle-CF neurons was measured at several temperatures and then extrapolated to 40°C. Figure 10A shows the average STA current in a population of middle-CF neurons, with bath temperatures of 25°C (dashed black, n = 11), 30°C (gray, n = 11), and 35°C (solid black, n = 12). In general, STAs became larger and faster with increasing temperature. The corresponding gain functions also changed with temperature (Fig. 10B). The average peak frequency increased significantly with temperature: for 25, 30, and 35°C, peak frequency = 167 ± 6, 254 ± 10, and 308 ± 6 Hz, respectively. The gain half-width also increased significantly (255 ± 7, 420 ± 23, and 635 ± 12 Hz). The peak gain decreased significantly at higher temperatures (959 ± 113, 596 ± 83, and 361 ± 64 Hz/nA).
The peak frequency, peak gain, and gain half-width at physiological temperature were estimated by extrapolation. The peak frequency versus temperature (Fig. 10C, left) was fit with a quadratic [peak frequency = −16.6(T − 25)2 + 104 (T − 25) + 169], which predicted a value of 330 Hz at 40°C, 7% greater than that at 35°C (see methods). The peak gain versus temperature (Fig. 10C, middle) was fit with a line [peak gain = −298(T − 25) + 937] and predicted a value of 43 Hz/nA at 40°C. The gain half-width versus temperature (Fig. 10C, right) was fit with a line [gain half-width = 38(T − 25) + 247] and predicted a value of 817 Hz at 40°C.
The changes caused by varying the bath temperature affected gain only at low frequencies (Fig. 9B). For example, at 1,000 Hz the gain functions were not significantly different. Although the mechanisms by which temperature affects neuronal responses are complex and incompletely understood, this result suggests that the primary effect of increased temperature may be mediated by higher conductance, which reduces membrane impedance at low frequencies (below the corner frequency of 1/(2πtm)) but not at much higher frequencies. Consistent with this interpretation, increased temperature is known to increase the single channel conductance of some ion channels (Hille 2001).
Correction for temperature and synaptic conductance resolves the discrepancy between estimated characteristic frequency and model frequency preference
We created an altered middle-CF model adjusted for the effects of physiological temperature and synaptic conductance. We altered the uEPSC (synaptic filter) time course based on the temperature dependence of the decay rate measured in NM neurons (Zhang and Trussell 1994). This study reported a Q10 of 2.2, which reduced the middle-CF uEPSC half-width from 0.54 to 0.33 ms (Fig. 11A, left). We adjusted the uEPSC amplitude based on the temperature dependence of EPSC amplitudes measured in mammalian MNTB (medial nucleus of the trapezoid body) neurons (Postlethwaite et al. 2007). From this study we estimated a Q10 of 1.3, which increased the uEPSC amplitude from 192 to 225 pA (Fig. 11A, left).
Changes in the intrinsic filter were less obvious than those in the uEPSC. Excitatory conductance, inhibitory conductance, and temperature increased the peak frequency by 42, 33, and 7%, respectively (Figs. 9 and 10). The assumption that these factors are additive gives an 82% increase in peak frequency, whereas a multiplicative assumption gives a 102% increase. For simplicity, we increased the peak frequency of the middle-CF intrinsic filter by 100%. This was done by resampling the middle-CF intrinsic filter at every other time point, resulting in a compression in the time domain (Fig. 11A, middle). Finally, we set the model threshold at a level to match the gain of the unadjusted middle-CF model at frequencies ≥1,400 Hz (Fig. 11A, right). This adjustment was based on the experimental result that temperature had no significant effect on gain at high frequencies (Fig. 10). The combined effect of adjustment of the two filters is shown in Fig. 11B along with the unadjusted middle-CF gain for the combined synaptic and intrinsic filters.
The adjusted middle-CF model was driven with NM spike trains phase-locked to 500, 1,600, and 2,500 Hz (Fig. 11C). At 500 Hz sound frequency the model showed strong rate modulation with IPD (>200 Hz) only at the lowest simulated sound intensity (Fig. 11, C, left and D, dashed line). However, at higher sound intensities the rate modulation decreased dramatically, reaching zero at the highest intensity. At 1,600 Hz, the model had the greatest amount of rate modulation with IPD at the four highest intensities (Fig. 11, C, middle and bold line and D, solid black line). At 2,500 Hz, the adjusted middle-CF model responded with low rate modulation at all sound intensities (Fig. 11, C, right and D, gray line).
The responses of the adjusted middle-CF model to simulated NM input appear well matched to the physiological frequency range of these neurons. These results resolve the apparent twofold discrepancy between the CF range of NL neurons and the sound frequencies at which our data obtained at 35°C with no added synaptic conductance predicted that IPD encoding would be best. Correction for these factors suggests that the general result shown in Fig. 8 may hold across the whole tonotopic axis. That is, tonotopic tuning of the synaptic and intrinsic filters specializes NL neurons to encode IPD at each sound frequency. Well-tuned filtering allows these cells to respond to the input component at the sound frequency, while attenuating the higher harmonics that naturally occur for out-of-phase input.
DISCUSSION
Tonotopic variation of the input to NL
An analysis of binaural input to NL (Fig. 1) shows that the mean (zero-frequency) input is invariant with phase delay; input amplitude varies monotonically as a function of IPD only in a narrow band around the sound frequency and varies nonmonotonically at higher harmonics (Fig. 1). This analysis suggested that NL neurons must respond to the input component at the sound frequency and filter components at other frequencies and thus require tonotopic tuning of synaptic and/or intrinsic filtering. We measured synaptic tuning by recording uEPSCs and measured intrinsic tuning by calculating frequency-dependent gain in response to noise.
Tonotopic gradient of synaptic tuning
Our results revealed a tonotopic gradient of uEPSC amplitude across NL and confirmed a reported gradient of EPSC time course (Kuba et al. 2005); uEPSCs were smallest and slowest in low-CF neurons and became larger and faster toward the high-frequency pole of the nucleus. The gradient of unitary EPSC amplitude has not been reported in previous studies, given that the present study is the first to use minimal stimulation to record unitary EPSCs in NL. The measured gradient of synaptic time course is consistent with the prediction that EPSC duration is optimized to provide the maximal change in input current fluctuation as a function of ITD.
Several mechanisms may form the uEPSC gradient, including the tonotopic gradient of dendritic length. The dendrites of NL neurons are <75 μm long in the middle- to high-frequency regions, but reach nearly 300 μm in the low-frequency region. In dendritic neurons, voltage clamp of the soma achieves voltage control of only the soma and the most proximal portion of the dendrites (Williams and Mitchell 2008) so the time course and amplitude of the uEPSCs will be influenced by unclamped distal synaptic inputs. Thus cable theory predicts that passive dendritic filtering will slow the uEPSC measured at the soma and attenuate its amplitude (Rall 1962), producing smaller, slower uEPSCs in low-CF neurons. Under conditions in which there is an incomplete space clamp both passive and active dendritic processing can affect the uEPSC. However, the time courses reported here were measured with Cs+ and QX314+ in the patch pipette to block K+ and Na+ channels, yet are similar to those measured without blockers (Kuba et al. 2005). Thus active dendritic processing appears unlikely to be the dominant mechanism producing the uEPSC gradient.
Other possible causes of the amplitude gradient include differences in release probability, multivesicular release, and postsynaptic receptor density; differences in receptor kinetics might affect the uEPSC time course. Future studies are required to investigate these mechanisms.
Tonotopic gradient of intrinsic tuning
Intrinsic filtering in NL was measured by the frequency-dependent gain. The peak frequency was highest in the middle-CF region, lower in the high-CF region, and lowest in the low-CF region. The overall gain—the product of the synaptic and intrinsic gains—was greatest in the high-CF region and decreased monotonically toward the low-CF region.
Several mechanisms may give rise to the shape and variation of intrinsic tuning. The measured gain of NL neurons approached zero for DC, consistent with previous observations that these cells will not fire to steady current alone, a function known as “DC filtering.” However, DC can change the firing rate of an NL neuron when high-frequency stimulus components are present. Increasing DC amplitude while injecting a constant level of noise produces an n-shaped relation between firing rate and DC (Higgs et al. 2006). Therefore the near-zero gain for DC observed in the present study likely resulted from operating near the peak of the firing rate–DC relation. Nonetheless, these neurons consistently show band-pass filtering. In NM neurons, DC filtering depends on GKlt. Blocking this conductance in NL neurons did not produce a significant gain for DC with the noise stimuli applied, but did increase gain at low frequencies (Supplemental Fig. S2). Low sodium conductance can also contribute to DC filtering. Evidence suggests that some NL and MSO (medial superior olive) neurons have electrically distal, axonal spike generation and few or no somatic sodium channels and that this promotes band-pass filtering (Kuba et al. 2006; Scott et al. 2007; Svirskis et al. 2004).
Our data suggest that gain at low frequencies is reduced by membrane conductance. Artificial conductance (Fig. 9) and higher temperature (Fig. 10), which may increase single channel conductance (Hille 2001), reduced low-frequency gain, whereas blocking GKlt with dendrotoxin (Supplemental Fig. S2) increased it. These manipulations did not significantly affect high-frequency gain.
The results of previous studies are consistent with the hypothesis that gain at high frequencies is governed primarily by membrane capacitance, similar to the impedance of a resistor–capacitor circuit (Ashida et al. 2007). We found that high-CF NL neurons had the largest high-frequency gain, followed by middle- and low-CF neurons. This result is consistent with direct measurements of the opposite gradient of membrane capacitance (Kuba et al. 2005). One mechanism of the capacitance gradient is the tonotopic gradient of dendritic length (Smith and Rubel 1979). High-CF NL neurons have shorter dendrites, which reduces their membrane capacitance. In addition, the gradient of distance to the axonal spike-initiation zone may effectively lower the capacitance felt by the spike-generating mechanism of higher-frequency neurons (Kuba et al. 2006) and amplify their response to high-frequency inputs (Ashida et al. 2007).
Tonotopic tuning in NL may improve sound localization
We examined intrinsic tuning in NL neurons using a simple linear–nonlinear model. In contrast to a detailed biophysical model, this approach provides an intuitive, reduced description of the filtering properties of the neuron, while avoiding the multiple free parameters and assumptions required by a biophysical model. Such a model constructed from noise-based measurements predicted IPD tuning in response to simulated binaural EPSC trains injected into the same neuron (Fig. 6). Thus a simple filter waveform captured much of the functional specialization of an NL neuron. Based on this result, the average synaptic and intrinsic filters measured in each frequency region were combined to model a typical NL neuron in each region. These models were driven with simulated NM spike trains at different frequencies, IPDs, and intensities. The responses demonstrated that tonotopic differences help to encode IPD at different sound frequencies.
At low sound frequency (e.g., 300 Hz), NM spikes are distributed across relatively few cycles (making the input to an NL neuron on each cycle large) and the monotonic changes in input amplitude occur at a low frequency. Thus NL neurons with relatively slow filtering and low gain encode IPD best. Faster neurons with higher gain would show response saturation to the in-phase input and would respond to components of the out-of-phase input that appear at higher harmonics of the sound frequency.
At higher sound frequencies, the input is distributed across more cycles and amplitude changes with IPD occur at higher frequencies. Therefore faster filtering and greater gain are needed. The measured gradients serve both functions.
Our results suggest that differences in synaptic and intrinsic filtering provide tonotopic specialization in NL, enhancing IPD encoding in each frequency region. This is in contrast to the previous interpretation that the fastest intrinsic kinetics found in the middle-CF region make it the most acute with respect to IPD encoding (Kuba et al. 2005). This previous conclusion may derive from the use of less physiological stimuli to assess ITD sensitivity. In the previous study, ITD sensitivity in NL was measured by single-shock stimulation of ipsi- and contralateral NM fibers at varied time delays. This procedure does not reproduce the ongoing temporal pattern of NM input that is thought to occur in vivo. When this temporal pattern of NM input is taken into account, we find that a model of each CF region encodes IPD most accurately when the measured intrinsic and synaptic properties from that region are used.
Additional factors that influence tonotopic tuning
In the simulations of Fig. 8, the frequency range over which each CF model functioned best was lower than the CF range measured in vivo. Furthermore, the weak rate modulation of the models at 1,200 Hz sound frequency suggested that the high- and middle-CF regions of NL cannot encode IPD across the full range of frequencies (∼1–3.3 kHz). By estimating possible contributions of physiological temperature and synaptic conductance, we found that the frequency range of maximal IPD sensitivity in middle-CF neurons could be shifted into the physiological range (Fig. 11).
Development may also influence the tuning of NL neurons and synapses. Previous investigations showed that the synaptic and intrinsic kinetics of chicken auditory brain stem neurons accelerate with age (Brenowitz and Trussell 2001; Howard et al. 2007). EPSCs in NM neurons of older animals have larger amplitudes, faster kinetics, and reduced depression (Brenowitz and Trussell 2001). These changes will increase synaptic gain at high frequencies. A study in NL found that GKlt is larger in older animals (Kuba et al. 2002a), which will reduce intrinsic gain for low frequencies. Finally, increased myelination in older animals may reduce capacitance at the axonal spike initiation zone, thus increasing gain at high frequencies.
The firing rate modulation for high-frequency input could also be increased by synaptic depression and inhibition. The depression found in this study and in previous studies (Cook et al. 2003; Kuba et al. 2002b) will reduce the strength of synaptic inputs and lower firing rates at all phase delays, but is predicted to reduce firing rate most for out-of-phase input (Cook et al. 2003). Inhibition (Monsivais and Rubel 2001), by changing spike threshold, may have a similar effect (Grande et al. 2004; Pena et al. 1996). Recent evidence suggests that inhibition enhances IPD tuning in low-CF NL neurons in the chicken (Nishino et al. 2008).
Concluding remarks
Recent in vivo evidence demonstrates that NL neurons in the chicken encode IPD over the entire tonotopic axis (Koppl and Carr 2008; Nishino et al. 2008). We find that gradients of synaptic and/or intrinsic properties are required to maintain the IPD responses observed in vivo. The experimentally measured gradient of uEPSCs agrees with a theoretical prediction, maximizing the change in synaptic current fluctuation as a function of IPD. A model combining measured synaptic and intrinsic filtering showed that the gradient of temporal filtering allows for good ITD encoding across the full range of CFs that occur in NL. Our results provide testable predictions for in vivo experiments. For example, we predict that IPD tuning will be less sharp when the sound frequency differs from the CF (and is presented at a higher intensity to match firing rates). Future studies are needed to provide a critical test of this prediction.
GRANTS
This work was supported by a Veterans Administration Merit Review to W. J. Spain, a National Institutes of Health training grant to S. J. Slee, and a Burroughs-Welcome Careers at the Scientific Interface award and Sloan Research Fellowship to A. L. Fairhall.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank F. Rieke and P. Detwiler for helpful discussions, A. Simons for help with experimental procedures, and C. Robbins and S. Usher for technical assistance.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Aguera y Arcas B, Fairhall AL, Bialek W. Computation in a single neuron: Hodgkin and Huxley revisited. Neural Comput 15: 1715–1749, 2003. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Rose JE, Hind JE, Brugge JF. Temporal position of discharges in single auditory nerve fibers within the cycle of a sine-wave stimulus: frequency and intensity effects. J Acoust Soc Am 49, Suppl. 2: 1131–1139, 1971. [DOI] [PubMed] [Google Scholar]
- Ashida G, Abe K, Funabiki K, Konishi M. Passive soma facilitates submillisecond coincidence detection in the owl's auditory system. J Neurophysiol 97: 2267–2282, 2007. [DOI] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature 417: 543–547, 2002. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. J Neurosci 21: 9487–9498, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger RM, Cramer KS, Pfeiffer JD, Rubel EW. Avian superior olivary nucleus provides divergent inhibitory input to parallel auditory pathways. J Comp Neurol 481: 6–18, 2005. [DOI] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci 10: 3227–3246, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DL, Schwindt PC, Grande LA, Spain WJ. Synaptic depression in the localization of sound. Nature 421: 66–70, 2003. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol 61: 809–834, 1999. [DOI] [PubMed] [Google Scholar]
- Fukui I, Sato T, Ohmori H. Improvement of phase information at low sound frequency in nucleus magnocellularis of the chicken. J Neurophysiol 96: 633–641, 2006. [DOI] [PubMed] [Google Scholar]
- Funabiki K, Koyano K, Ohmori H. The role of GABAergic inputs for coincidence detection in the neurones of nucleus laminaris of the chick. J Physiol 508: 851–869, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32: 613–636, 1969. [DOI] [PubMed] [Google Scholar]
- Grande LA, Kinney GA, Miracle GL, Spain WJ. Dynamic influences on coincidence detection in neocortical pyramidal neurons. J Neurosci 24: 1839–1851, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs MH, Slee SJ, Spain WJ. Diversity of gain modulation by noise in neocortical neurons: regulation by the slow afterhyperpolarization conductance. J Neurosci 26: 8787–8799, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001. [Google Scholar]
- Howard MA, Burger RM, Rubel EW. A developmental switch to GABAergic inhibition dependent on increases in Kv1-type K+ currents. J Neurosci 27: 2112–2123, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffress L. A place theory of sound localization. J Comp Physiol Psychol 61: 468–486, 1948. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Responses to amplitude-modulated tones in the auditory nerve of the cat. J Acoust Soc Am 91: 215–232, 1992. [DOI] [PubMed] [Google Scholar]
- Keat J, Reinagel P, Reid RC, Meister M. Predicting every spike: a model for the responses of visual neurons. Neuron 30: 803–817, 2001. [DOI] [PubMed] [Google Scholar]
- Koppl C, Carr CE. Maps of interaural time difference in the chicken's brainstem nucleus laminaris. Biol Cybern 98: 541–559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature 444: 1069–1072, 2006. [DOI] [PubMed] [Google Scholar]
- Kuba H, Koyano K, Ohmori H. Development of membrane conductance improves coincidence detection in the nucleus laminaris of the chicken. J Physiol 540: 529–542, 2002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Koyano K, Ohmori H. Synaptic depression improves coincidence detection in the nucleus laminaris in brainstem slices of the chick embryo. Eur J Neurosci 15: 984–990, 2002b. [DOI] [PubMed] [Google Scholar]
- Kuba H, Yamada R, Fukui I, Ohmori H. Tonotopic specialization of auditory coincidence detection in nucleus laminaris of the chick. J Neurosci 25: 1924–1934, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova MS, Higgs MH, Spain WJ. Adaptation of firing rate and spike-timing precision in the avian cochlear nucleus. J Neurosci 28: 11906–11915, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Berry MJ., 2nd The neural code of the retina. Neuron 22: 435–450, 1999. [DOI] [PubMed] [Google Scholar]
- Mills AW. On the minimum audible angle. J Acoust Soc Am 30: 237–246, 1958. [Google Scholar]
- Monsivais P, Rubel EW. Accommodation enhances depolarizing inhibition in central neurons. J Neurosci 21: 7823–7830, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino E, Yamada R, Kuba H, Hioki H, Furuta T, Kaneko T, Ohmori H. Sound-intensity-dependent compensation for the small interaural time difference cue for sound source localization. J Neurosci 28: 7153–7164, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JL, Viete S, Albeck Y, Konishi M. Tolerance to sound intensity of binaural coincidence detection in the nucleus laminaris of the owl. J Neurosci 16: 7046–7054, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite M, Hennig MH, Steinert JR, Graham BP, Forsythe ID. Acceleration of AMPA receptor kinetics underlies temperature-dependent changes in synaptic strength at the rat calyx of Held. J Physiol 579: 69–84, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Theory of physiological properties of dendrites. Ann NY Acad Sci 96: 1071–1092, 1962. [DOI] [PubMed] [Google Scholar]
- Rayleigh L. On our perception of sound direction. Philos Mag 13: 214–232, 1907. [Google Scholar]
- Reyes AD, Rubel EW, Spain WJ. In vitro analysis of optimal stimuli for phase-locking and time-delayed modulation of firing in avian nucleus laminaris neurons. J Neurosci 16: 993–1007, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson HP, Kawai N. Injection of digitally synthesized synaptic conductance transients to measure the integrative properties of neurons. J Neurosci Methods 49: 157–165, 1993. [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev 81: 1305–1352, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Parks TN. Organization and development of brain stem auditory nuclei of the chicken: tonotopic organization of n. magnocellularis and n. laminaris. J Comp Neurol 164: 411–433, 1975. [DOI] [PubMed] [Google Scholar]
- Scott LL, Hage TA, Golding NL. Weak action potential backpropagation is associated with high-frequency axonal firing capability in principal neurons of the gerbil medial superior olive. J Physiol 583: 647–661, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AA, O'Neil MB, Abbott LF, Marder E. Dynamic clamp: computer-generated conductances in real neurons. J Neurophysiol 69: 992–995, 1993. [DOI] [PubMed] [Google Scholar]
- Simoncelli EP, Pillow JW, Paninski L, Schwartz O. Characterization of neural responses with stochiastic stimuli. In: The Cognitive Neurosciences (3rd ed.), edited by Gazzaniga M. Cambridge, MA: MIT Press, 2004, p. 327–338 [Google Scholar]
- Slee SJ, Higgs MH, Fairhall AL, Spain WJ. Two-dimensional time coding in the auditory brainstem. J Neurosci 25: 9978–9988, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Rubel EW. Organization and development of brain stem auditory nuclei of the chicken: dendritic gradients in nucleus laminaris. J Comp Neurol 186: 213–239, 1979. [DOI] [PubMed] [Google Scholar]
- Strohmann B, Schwarz DW, Puil E. Electrical resonances in central auditory neurons. Acta Otolaryngol 115: 168–172, 1995. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Kotak V, Sanes DH, Rinzel J. Sodium along with low-threshold potassium currents enhance coincidence detection of subthreshold noisy signals in MSO neurons. J Neurophysiol 91: 2465–2473, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Dallos P. Neural coding in the chick cochlear nucleus. J Comp Physiol A Sens Neural Behav Physiol 166: 721–734, 1990. [DOI] [PubMed] [Google Scholar]
- Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11: 790–798, 2008. [DOI] [PubMed] [Google Scholar]
- Yang L, Monsivais P, Rubel EW. The superior olivary nucleus and its influence on nucleus laminaris: a source of inhibitory feedback for coincidence detection in the avian auditory brainstem. J Neurosci 19: 2313–2325, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol 64: 465–488, 1990. [DOI] [PubMed] [Google Scholar]
- Zhang S, Trussell LO. Voltage clamp analysis of excitatory synaptic transmission in the avian nucleus magnocellularis. J Physiol 480: 123–136, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.