Abstract
The master circadian pacemaker located in the suprachiasmatic nucleus (SCN) is entrained by light intensity–dependent signals transmitted via the retinohypothalamic tract (RHT). Short-term plasticity at glutamatergic RHT–SCN synapses was studied using stimulus frequencies that simulated the firing of light sensitive retinal ganglion cells. The evoked excitatory postsynaptic current (eEPSC) was recorded from SCN neurons located in hypothalamic brain slices. The eEPSC amplitude was stable during 0.08 Hz stimulation and exhibited frequency-dependent short-term synaptic depression (SD) during 0.5 to 100 Hz stimulus trains in 95 of 99 (96%) recorded neurons. During SD the steady-state eEPSC amplitude decreased, whereas the cumulative charge transfer increased in a frequency-dependent manner and saturated at 20 Hz. SD was similar during subjective day and night and decreased with increasing temperature. Paired-pulse stimulation (PPS) and voltage-dependent Ca2+ channel (VDCC) blockers were used to characterize a presynaptic release mechanism. Facilitation was present in 30% and depression in 70% of studied neurons during PPS. Synaptic transmission was reduced by blocking both N- and P/Q-type presynaptic VDCCs, but only the N-type channel blocker significantly relieved SD. Aniracetam inhibited AMPA receptor desensitization but did not alter SD. Thus we concluded that SD is the principal form of short-term plasticity at RHT synapses, which presynaptically and frequency-dependently attenuates light-induced glutamatergic RHT synaptic transmission protecting SCN neurons against excessive excitation.
INTRODUCTION
The master circadian oscillator located in the suprachiasmatic nucleus (SCN) is entrained by light. Intrinsically photosensitive retinal ganglion cells (ipRGCs) project axons to the SCN comprising the retinohypothalamic tract (RHT) (Berson et al. 2002; Warren et al. 2003). Depolarization of ipRGCs by light induces glutamate release from RHT axon terminals. The glutamate binds to N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which depolarize SCN neurons, causing a Ca2+ influx that activates intracellular signaling pathways and induces phase shifts of circadian clock during the night (Colwell 2001; Ding et al. 1998; Irwin and Allen 2007, 2009). The change in the action potential firing frequency of ipRGCs and SCN neurons and the magnitude of phase shifts in behavior are dependent on the light intensity (Meijer et al. 1998; Nelson and Takahashi 1991). Fos expression in SCN neurons also increased proportionately to increases in both irradiance and duration of light exposure (Dkhissi-Benyahya et al. 2000). Therefore the timing and the intensity of light stimuli are critical to maintain entrainment (Albers et al. 1982).
In spite of an abundance of studies demonstrating light effects on the circadian clock and activity of SCN neurons, the relationship between the action potential firing pattern in the optic nerve and the synaptic current in SCN neurons induced by glutamate released from RHT axon terminals remains unknown. Excitatory synapses in different preparations show frequency-dependent synaptic plasticity the characteristics of which are determined by the firing frequency in the presynaptic terminal (Abbott et al. 1997; Forsythe et al. 1998). The frequency and duration of ipRGCs action potential firing strongly depend on the light intensity and may define the different modes of synaptic plasticity at RHT synapses. Although optic chiasm and optic nerve stimulation has been used widely to study the light entrainment pathway, the stimulation parameters have been chosen empirically and how closely the stimulation simulates the firing pattern of ipRGCs is hard to estimate. Therefore we designed experiments to characterize the functional relationship between light-induced action potential firing and the release probability at RHT axon terminals. To study short-term plasticity at RHT synapses we recorded excitatory postsynaptic currents (EPSC) evoked in SCN neurons by optic nerve or optic chiasm stimulation over a range of stimulus frequencies that simulate light-induced action potential firing of ipRGCs (Berson et al. 2002; Tu et al. 2005; Warren et al. 2003). An additional goal of this study was to compare the effect of optic nerve and optic chiasm stimulation. Both types of stimulation are often used to study the modulatory effect of retinal input on the circadian pacemaker and it was important to determine whether they will produce similar or different effects to evaluate the relevance of their application. We found that synaptic depression, which is regulated presynaptically, is the main form of short-term synaptic plasticity in RHT–SCN synapses.
METHODS
Animal entrainment and preparation of brain slices
Male Sprague–Dawley rats (4–6 wk old; Charles River Laboratories, Wilmington, MA) were housed in an environmental chamber (Percival Scientific, Perry, IA), maintained at 20–21°C on a 12 h:12 h light (L):dark (D) cycle, with unrestricted access to food and water. Lights were turned on at 8:00 am (Zeitgeber Time [ZT]: 00:00) for LD entrained animals (day recordings) and turned off at 11:30 am (ZT: 12:00) for reverse (DL) cycle animals (night recordings). During the lights-on phase, rats were deeply anesthetized with isoflurane; their brains were removed and submerged in an ice-cold Krebs solution consisting of (in mM): NaCl 126, KCl 2.5, NaH2PO4 1.2, MgCl2 4.0, CaCl2 0.5, glucose 11, and NaHCO3 26, saturated with 95% O2-5% CO2 (pH 7.3–7.4, 301–303 mOsm). Coronal (250 μm thick) or horizontal (∼500 μm thick) slices of the hypothalamus containing the SCN were cut with a vibrating-blade microtome (VT 1000 S; Leica Biosystems, Nussloch, Germany). The Institutional Animal Care and Use Committee of Oregon Health and Science University approved all experimental procedures involving animals and all efforts were made to minimize pain and the number of animals used.
Whole cell patch clamp recording
Recordings were made at 28°C using the whole cell patch clamp technique from 1.5 to 8 h after slice preparation. The superfusion solution was warmed with a heater (Model SH-27B inline heater; Warner Instruments, Hamden, CT) just before the solution entered the recording chamber. The bath temperature in the recording chamber was monitored continuously with a thermistor probe, which provided feedback to a dual automatic temperature controller (TC-344B; Warner Instruments). The recording solution was (in mM): NaCl 132.5, KCl 2.5, NaH2PO4 1.2, CaCl2, 2.4, MgCl2 1.2, glucose 11, and NaHCO3 22, saturated with 95% O2-5% CO2 (pH 7.3–7.4, 300–305 mOsm). Microelectrodes with resistances of 7–9 MΩ were pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) and filled with a solution containing (in mM): CH3O3SCs 102, CsCl 20, CaCl2 1, HEPES 10, EGTA 11, CsOH 28, MgATP 3, Tris-GTP 0.3, and QX-314 5. Lidocaine N-ethyl chloride (QX-314) was included in the patch pipette solution to block voltage-dependent Na+ currents. Cs+ was used to block postsynaptic K+ channels, including γ-aminobutyric acid type B (GABAB)–activated K+ channels (Jiang et al. 1995). To prevent activation of GABAA receptors, the GABAA receptor antagonist picrotoxin (50 μM) was added to the external solution in all experiments. Individual SCN neurons were visualized with infrared illumination and differential interference contrast optics using a DMLFS microscope (Leica Biosystems) with video camera and display (Sony, Tokyo). On-line data collection and analysis were performed using an EPC-7 patch clamp amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany), a Macintosh G3 computer, and Pulse and PulseFit (HEKA Elektronik). The records were filtered at 3 kHz and digitized at 10 kHz.
To allow equilibration between the pipette solution and the cell cytoplasm, whole cell patch clamp recording started about 10 min after rupturing the membrane. A small voltage step (2 mV, 5 ms) was applied prior to optic chiasm or optic nerve stimulation to monitor the series resistance. SCN neurons were voltage-clamped at −60 mV. During the 1–2 h whole cell recording the series resistance remained stable and only recordings with series resistance changes of <10% were included in the data analysis (Moldavan et al. 2006).
Optic nerve and optic chiasm stimulation
EPSC was evoked by electrical stimulation of the optic chiasm or optic nerve with a Grass S88 stimulator (Grass Medical Instruments, Quincy, MA). The optic nerve was stimulated with a suction electrode in horizontal brain slices with both optic nerves attached. A chlorided silver wire located inside the plastic tubing of the suction electrode was used for optic nerve stimulation. The reference electrode consisted of a chlorided silver wire wrapped around the outer wall of the plastic tubing. An approximately 5 mm section of the optic nerve was sucked into the plastic tubing, which had an internal diameter that closely matched the optic nerve diameter. Stimulation of the optic nerve avoided possible activation of other glutamatergic synaptic inputs, such as those projecting to the SCN from the anterior paraventricular thalamus (Alamilla and Aguilar-Roblero 2010). The evoked EPSC (eEPSC) was recorded from neurons located in the ipsilateral SCN (Jiang et al. 1995).
The optic chiasm was stimulated in the coronal brain slice with a concentric bipolar tungsten electrode (outer pole diameter: 0.125 μm; Cat. No. CBASC75, FHC, Bowdoinham, ME) connected to a stimulus isolation unit (Model SIU5B; Grass Medical Instruments). The stimulating electrode was placed in the middle part of the optic chiasm as far as possible from the SCN.
The stimulus pulse duration was 0.13–0.17 ms and the stimulation intensity was set 1.5- to twofold higher than that needed to evoke a threshold response and usually varied between 8 and 40 V. eEPSCs were elicited by trains of 5 or 25 stimuli (square pulses) at 0.08–200 Hz separated by 40 s intervals or by PPS at 2–200 Hz with a 3 s interval between each pair of stimuli. The duration of repetitive stimulation was too short and applied frequencies too low to induce loosening of paranodal myelin in the optic nerve fibers (Moran and Mateu 1983). Both the inclusion of ion channel blockers in the internal solution and voltage clamping at −60 mV prevented the activation of voltage-dependent ionic currents in SCN neurons.
Criteria for monosynaptic transmission
The following criteria were used to distinguish mono- from polysynaptic transmission. The monosynaptic eEPSC was characterized by a constant onset latency during repetitive stimulation, the ability to follow high-frequency stimulation, and persistence of the response in a high extracellular Ca2+ solution (40 mM) (Berry and Pentreath 1976; Jiang et al. 1995). The high Ca2+ concentration increases the spike threshold, which prevents action potential generation in the intercalated neuron, with consequent loss of the postsynaptic response; a direct connection will be unaffected (Berry and Pentreath 1976). The high Ca2+ solution contained (in mM): NaCl 89.7, KCl 2.5, CaCl2 40.0, MgCl2 1.2, Na-HEPES 10.0, and glucose 11, saturated with 95% O2-5% CO2 (pH 7.4, 308 mOsm). eEPSC amplitude was measured during the second minute of high Ca2+ solution application. After 2–5 min in high Ca2+ artificial cerebrospinal fluid (ACSF), the eEPSC amplitude in the majority of recorded neurons gradually declined and eEPSC was frequently completely blocked. The decrease of eEPSC could be explained by activation of a Ca2+-dependent K+ conductance that effectively decreased axonal excitability (Callewaert et al. 1996; Lev-Ram and Grinvald 1986).
Test agent application
All test agents were bath applied by perfusion in ACSF containing the final concentration of the compound. Chamber volume was about 400 μl. A complete change of the external solution took <30 s at a flow rate of 1.5–2 ml/min. The substances used were: picrotoxin, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), d-(−)-2-amino-5-phosphonopentanoic acid (d-AP5), N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium chloride (QX-314, Sigma, St Louis, MO); 1-(4-methoxybenzoyl)-2-pyrrolidinone (aniracetam; Tocris Cookson, Ellisville, MO); and ω-agatoxin TK and ω-conotoxin GVIA (Alomone Labs, Jerusalem, Israel). The toxins were dissolved in ACSF and applied for 20–40 min by perfusion through a micropipette (Moldavan et al. 2006). The micropipette was placed close to the slice surface upstream of the SCN and the ACSF containing the toxins flowed out of the perfusion micropipette in the same direction as the flow of ACSF in the recording chamber and completely covered the SCN. Appropriate stocks were made and diluted with ACSF just before application. Aniracetam was dissolved in DMSO and picrotoxin was dissolved in ethanol and then diluted into the ACSF.
Statistical analysis
eEPSC amplitude was measured as the difference between the peak eEPSC current and the baseline level before the stimulus artifact. Even at high stimulus frequencies (25 Hz) the baseline remained stable [SE was in the range 1–5.5% (mean 2.75%), n = 8]. To compare synaptic depression under different conditions and between different neurons the amplitude of each subsequent eEPSC (eEPSCn) during repetitive stimulation was normalized (in %) to the amplitude of the first eEPSC (eEPSC1) in the stimulus train: ratio eEPSCn/eEPSC1. To take into account the variability of the eEPSC amplitude the mean amplitude of the first eEPSC was calculated from two to three stimulus trains at each stimulus frequency. Synaptic depression caused the eEPSC amplitude to decrease to a steady state (plateau) during application of stimulus trains. The number of stimuli needed to reach the steady-state amplitude was lower at low frequencies than that at high frequencies, but did not exceed 15 stimuli during a 25 stimuli train. Therefore to estimate the steady-state eEPSC amplitude the amplitudes of the last 10 eEPSCs in the train were averaged. The normalized steady-state eEPSC amplitude represented the magnitude of synaptic depression at each stimulus frequency. The estimated steady-state amplitude was averaged across all recorded neurons (n), presented as the mean ± SE and plotted against stimulus frequency (Hz).
The time constant (τ) that characterized the time course of the decreasing phase of the eEPSC amplitude to steady state during optic chiasm stimulation was estimated using the equation: I(t) = I0 + A exp[−(t − t0)/τ], where I(t) is the eEPSC amplitude at any given time t; I0 is a constant (steady-state eEPSC amplitude), A is a constant, t is the given time, t0 is the initial time (t = 0), and τ (tau) is the time constant.
The extra sum of squares F-test was used to compare the data sets recorded under different conditions. Paired and unpaired two-tailed t-tests were used to compare the control and test data for each data set (for each stimulus frequency). For an unpaired t-test equal variances were assumed. A confidence level of 95% was used to determine statistical significance. Synaptic charge transfer (Q) represented the area enclosed by the eEPSC. The area between two cursors was integrated using IGOR Pro software (WaveMetrics, Lake Oswego, OR). Charge transfer was estimated for eEPSC with a short onset latency (∼5 ms), where the duration of the fast component of eEPSC was <10–15 ms and the recorded current returned to the baseline in <40 ms (that corresponded to the interstimulus interval at 25 Hz stimulus train). The first cursor indicated the crossing of eEPSC onset with baseline. The second cursor was placed 30 ms after the stimulus artifact. The cumulative charge transfer (Qcum) was defined as the sum of the charge transfer of all eEPSCs during the first second of stimulation. Qcum was determined for each stimulus frequency (1–25 Hz) and normalized to the Qcum at 1 Hz. Data were averaged across all recorded neurons (n) and reported as mean ± SE. The paired-pulse ratio (PPR) was calculated as the ratio (mean eEPSC2/mean eEPSC1) of the mean peak amplitude of the second eEPSC (eEPSC2) to the mean peak amplitude of the first eEPSC (eEPSC1) evoked during PPS (Kim and Alger 2001). IGOR Pro (version 5.0, WaveMetrics), KaleidaGraph (version 3.6, Synergy Software, Reading, PA), and Excel 11.1.1 (Microsoft, Redmond, WA) were used for curve fitting, data analysis, and graphic presentation.
RESULTS
Synaptic depression in RHT–SCN synapses
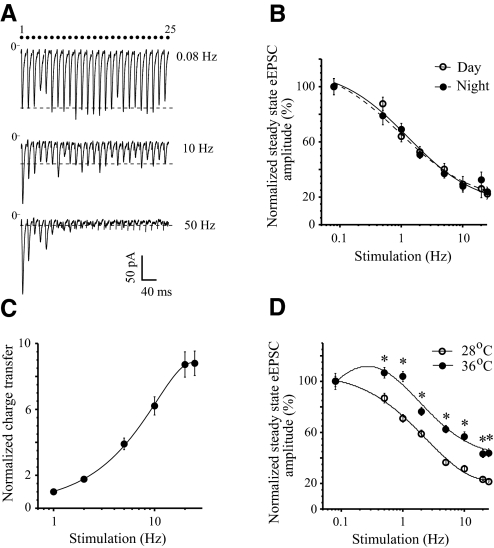
To examine the functional properties of RHT synapses we stimulated the optic chiasm or optic nerve with trains of 25 stimuli that simulated the firing frequency of ipRGCs activated by light. Synaptic depression was not detected during 0.08 Hz stimulation of the optic chiasm. At frequencies >0.5 Hz a frequency-dependent synaptic depression was observed and the amplitude of successive eEPSCs showed a progressive decline that reached a new steady-state level (Fig. 1, A, B, and D). Even though the peak amplitude progressively decreased, each stimulation of the optic chiasm reliably evoked an EPSC up to 50 Hz (steady state: 21 ± 3.9% of control; control is the amplitude of the first eEPSC in the train, range 11–43%, n = 7, Fig. 1A). The eEPSC followed 100 Hz stimulation in some cells with fast onset eEPSC (steady state: 21 ± 5.6% of control; range: 6–51%, n = 7) but did not follow 200 Hz stimulation. The time required for the eEPSC amplitude to reach steady state was shorter at higher stimulation frequencies and was characterized by a specific time constant (τ) (see Statistical analysis in methods). For example, the τ was 329 ± 53 ms at 2 Hz (n = 30), 220 ± 24 ms at 5 Hz (n = 31), 83 ± 6 ms at 25 Hz (n = 31), 49 ± 5 ms at 50 Hz (n = 7), and 28 ± 3 ms at 100 Hz (n = 7). Although the plateau was reached faster at higher stimulus frequencies, more stimulus pulses were required to reach the steady state: 3.7 ± 0.6 stimuli at 2 Hz (steady state: 55.3 ± 3.0% of control), 5.5 ± 0.5 stimuli at 5 Hz (steady state: 37.3 ± 3.3% of control), and 7.6 ± 0.8 stimuli at 25 Hz (steady state: 22.3 ± 2.8% of control, n = 24). The eEPSC amplitude recovered to control values during about 40 s after completion of the stimulus train.
Fig. 1.
Frequency dependence of synaptic depression during repetitive stimulation of the optic chiasm. A: evoked excitatory postsynaptic currents (eEPSCs) were recorded from the same neuron during trains of 25 stimuli at 0.08, 10, and 50 Hz (the onset latency of eEPSC: 4.72 ± 0.05 ms). Each eEPSC recording is an average of (n) trials: 0.08 Hz (n = 3), 10 Hz (n = 7), 50 Hz (n = 10). Note: these records are not shown on a timescale (the dots show the stimuli number). Dashed line is the steady-state eEPSC amplitude (mean of last 10 eEPSCs in the train). B: the frequency dependence of the steady-state eEPSC amplitude during the subjective day (Zeitgeber Time [ZT]: 6.5–10.0; n = 10) and night (ZT: 13.5–17.0; n = 7). C: frequency dependence of the cumulative charge transfer estimated during the first second of 1–25 Hz stimulation. Data were normalized to the charge transfer at 1 Hz and averaged across the recorded neurons: means ± SE (n = 5). D: temperature dependence of synaptic depression. eEPSCs were recorded from the same neurons at 28 and 36°C. *P < 0.001, n = 4 (paired t-test, 2-tailed). B and D: the amplitude of each eEPSC was normalized (%) to the amplitude of the first eEPSC in the stimulus train, after which the steady-state eEPSC amplitude was calculated. In some cases the error bars were sometimes smaller than the symbols.
The time course of the synaptic depression during the subjective day (ZT: 6.5–10.0, n = 10) and night (ZT: 13.5–17.0, n = 7) was compared. The frequency dependence of steady-state eEPSC amplitude was similar in both conditions [F-test: F(3,10) = 0.36, P = 0.78, Fig. 1B]. For all applied stimulus frequencies P ≥ 0.31 (unpaired t-test, two-tailed). During optic chiasm stimulation the charge transfer and the peak amplitude of each individual eEPSC in the train decreased simultaneously. Despite the development of synaptic depression the cumulative charge transfer during the first second of stimulation increased in a frequency-dependent manner and saturated at 20 Hz (Fig. 1C).
The temperature dependence of synaptic depression was studied. The steady-state EPSC amplitude was recorded in the same neurons at 28°C and after the temperature of ACSF in the recording chamber was increased to 36°C over the 7–18 min period (mean 11.3 ± 2.4 min; n = 4) required for the recording chamber temperature to stabilize. Increasing the temperature from 28 to 36°C increased the mean steady-state eEPSC amplitude at 0.08 Hz from 216.3 ± 14.5 to 252.0 ± 20 pA (ratio 1.16), at 5 Hz from 105.0 ± 7.8 to 193.3 ± 14.0 pA (ratio 1.84), and at 25 Hz from 66.9 ± 4.0 to 142.2 ± 9.6 pA (ratio 2.12; n = 4). The amplitude of each eEPSC was normalized to the first eEPSC in the train and the estimated steady-state amplitude at each temperature was compared (Fig. 1D). Normalized steady-state eEPSC amplitudes indicated a reduction of synaptic depression at a physiological temperature compared with 28°C in the range 0.5–25 Hz [steady state at 25 Hz was 21.7 ± 1.1% (28°C) vs. 43.8 ± 3.2% (36°C) of control; F-test: F(3,10) = 19.55, P < 0.00017].
Synaptic depression was observed in 95 of 99 neurons (96%) studied during 0.5–100 Hz repetitive stimulation of the optic chiasm. However, in 4 neurons (4%) synaptic depression was observed only during 0.5–5 Hz stimulation and a progressive increase of the steady-state eEPSC amplitude was revealed during 10–25 Hz (≤160% at 25 Hz). The increase of steady-state eEPSC amplitude did not result from an increase in the series resistance. The SE of the series resistance for recorded neurons was in the range 0.9–10.7% (mean 4.5%, n = 4).
In neurons that demonstrated synaptic depression during 0.5–100 Hz stimulus trains the ratio of the amplitude of the second eEPSC to the first one (eEPSC2/eEPSC1) was used to estimate the initial release probability. Initial facilitation (ratio >1) appeared in 5% (2 of 40 neurons) and in 14% (3 of 21 neurons) during 2 or 25 Hz stimulation of the optic chiasm and optic nerve, respectively. The remaining neurons revealed an initial depression (ratio <1) of synaptic transmission.
Synaptic depression confirmed during monosynaptic RHT–SCN transmission (optic nerve stimulation)
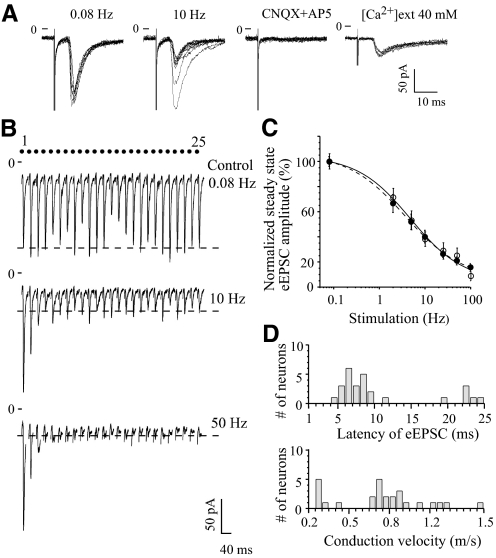
Additional experiments were performed to demonstrate that the observed synaptic depression was a form of synaptic plasticity that characterized the neurotransmitter release from RHT axon terminals and not an artifact of configuration of the slice preparation or the stimulation procedure. Horizontal brain slices, containing the SCN with optic nerves attached, were prepared and the optic nerve was stimulated with a suction electrode. The eEPSC was recorded in the ipsilateral SCN because in previous studies a higher rate of responding neurons was observed in the ipsilateral compared with the contralateral SCN (Jiang et al. 1995). To determine that the SCN neurons received a direct retinal projection the eEPSC was tested using the criteria for monosynaptic transmission (see Criteria for monosynaptic transmission in methods). At the end of each experiment CNQX (10 or 20 μM) and d-AP5 (50 μM) were applied to confirm the glutamatergic transmission.
Strong frequency-dependent synaptic depression of the eEPSC was observed in the group of neurons (n = 9) that satisfied all the criteria for monosynaptic neurotransmission (Fig. 2). During 0.08 Hz stimulation with a 25 stimuli train, the onset latency and peak eEPSC amplitude were stable, showing small variations around the mean (Fig. 2, A and B). The eEPSC followed 10 Hz stimulation without failures and the onset latency of eEPSC remained stable while the amplitude decreased to a new steady state. Coapplication of CNQX and d-AP5 completely blocked synaptic transmission. In 40 mM extracellular Ca2+ solution EPSCs were evoked without failure. During 10 and 50 Hz stimulation the eEPSC amplitude progressively decreased to the steady state (Fig. 2B). Frequency dependence of the steady-state eEPSC amplitude was similar for neurons tested for monosynaptic transmission (n = 9) and other neurons (n = 12) recorded during optic nerve stimulation [F-test: F(3,8) = 0.39, P = 0.76; Fig. 2C]. The maximal depression was reached at 50 Hz (steady state: 22.9 ± 3.3% of control; range: 9.7–47.0%, n = 14) and at 100 Hz (steady state: 13.6 ± 2.7% of control; range: 4.8–26.0%, n = 7) during optic nerve stimulation.
Fig. 2.
Monosynaptic glutamatergic transmission in retinohypothalamic (RHT) synapses revealed depression during repetitive stimulation of the optic nerve. A: EPSC evoked by optic nerve stimulation in the same neuron. From left to right: superposition of 10 recordings during 0.08 Hz (control) and 10 Hz stimulation (the recording demonstrates the constant latency and persistence of the response during high-frequency stimulation), block of eEPSC during 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μM) and d-(−)-2-amino-5-phosphonopentanoic acid (d-AP5, 50 μM) coapplication, persistence of eEPSC in 40 mM extracellular Ca2+ (0.08 Hz stimulation). B: EPSCs evoked in the same neuron by 0.08, 10, and 50 Hz stimulation of the optic nerve with trains of 25 stimuli. Each eEPSC recording is an average of 3 trials. Note: these records are not shown on a timescale (the dots show the stimuli number). Dashed line: the mean eEPSC amplitude during steady state. C: frequency dependence of steady-state eEPSC amplitude during optic nerve stimulation. The amplitude of eEPSC was normalized (see Fig. 1). Open circles: neurons satisfying all the criteria for monosynaptic transmission (n = 9); black circles: other recorded neurons that were not tested for monosynaptic transmission (n = 12). P ≥ 0.28 for all applied stimulus frequencies (unpaired t-test, 2-tailed). D: the distribution of eEPSCs onset latency (top) and the conduction velocity of the optic nerve fibers (bottom) (n = 27 neurons).
The eEPSC onset latency was measured from the onset of the stimulus artifact to the onset of the eEPSC and was in the range 4.0–24.3 ms (n = 27 neurons, 19 preparations) (Fig. 2D). The conduction velocity for the optic nerve fibers projecting to the SCN was estimated, taking into consideration the distance between the stimulated end of the optic nerve and the recorded neuron, which was 5.5–6 mm in different preparations. The recorded neurons were divided into two groups with different eEPSC onset latencies. The onset latencies in the first group were in the range of 4.0–11.4 ms [mean 6.9 ± 0.4 ms, n = 21 (78%) of 27 studied neurons] and estimated conduction velocity was in the range of 0.44–1.50 m/s (mean 0.90 ± 0.05 m/s). The onset latencies in the second group ranged from 19.4 to 24.3 ms [mean 22.1 ± 0.7 ms, n = 6 (22%) neurons] and the conduction velocity was calculated to be 0.27–0.31 m/s (mean 0.27 ± 0.01 m/s). These data were consistent with previous estimates (Jiang et al. 1995; Kim and Dudek 1991). The different conduction velocities recorded for RHT fibers may reflect the morphological heterogeneity of ipRGCs (Baver et al. 2008). In a given neuron the eEPSC onset latency was relatively constant. The mean average absolute deviation (AAD) of onset latency of the eEPSC estimated for each neuron was 0.28 ms (range: 0.07–0.66 ms, n = 27 neurons). In 70% (n = 19) of recorded neurons AAD of onset latency had a narrower range of 0.07–0.3 ms (mean ADD 0.21 ms).
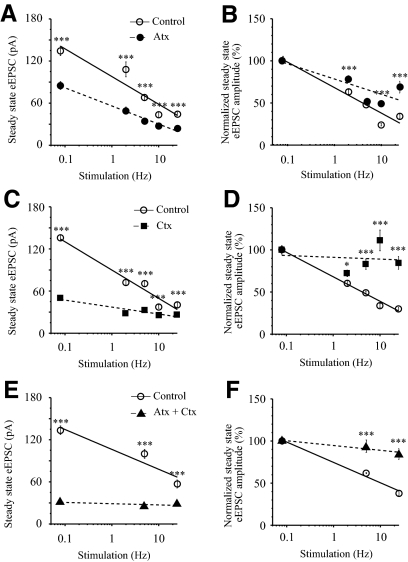
Blocking of presynaptic VDCCs attenuated the eEPSC amplitude and relieved synaptic depression
Reducing the presynaptic Ca2+ influx at higher rates of synaptic transmission decreases neurotransmitter release and relieves synaptic depression (Brenowitz and Trussell 2001; Forsythe et al. 1998; Zucker and Regehr 2002). Therefore we predicted that blocking presynaptic voltage-dependent Ca2+ channels (VDCCs) should reduce transmitter release from RHT axon terminals and relieve synaptic depression. ω-Conotoxin GVIA (1 μM) and ω-agatoxin TK (500 nM) alone or together significantly reduced the steady-state eEPSC amplitude (pA) during 0.08–25 Hz repetitive stimulation (Fig. 3, A, C, and E). During 0.08 Hz stimulation ω-agatoxin (n = 6) and ω-conotoxin (n = 7) reduced eEPSC amplitude to 63.4 and 40.0% of control, respectively and, when applied together, to 23.8% of control (n = 3). The toxins significantly decreased the steady-state eEPSC amplitude over the entire range of applied frequencies [F-test: F(3,4) = 47.9, P < 0.0014 for ω-agatoxin; F(3,4) = 8.7, P < 0.031 for ω-conotoxin]. The slope of the frequency-dependence curve was steeper for ω-conotoxin (Fig. 3C) than that for ω-agatoxin (Fig. 3A). The effect of VDCC blockers on synaptic depression was compared by normalizing the amplitude of each EPSC in the stimulus train to the amplitude of the first eEPSC, which was set to 100% in each condition (Fig. 3, B, D, and F). The curves of normalized steady-state eEPSC amplitude were compared at each condition. The blocker of P/Q-type VDCCs ω-agatoxin did not significantly alter frequency dependence of steady-state eEPSC amplitude (Fig. 3B) [extra sum of squares F-test: F(3,4) = 1.88, P = 0.27], whereas the N-type VDCC blocker ω-conotoxin (Fig. 3D) significantly relieved synaptic depression decreasing the slope of the curve [F-test: F(3,4) = 6.34, P < 0.05] as well as the coapplication of toxins (Fig. 3F).
Fig. 3.
Inhibition of presynaptic voltage-dependent Ca2+ channels decreased synaptic transmission and relieved synaptic depression. A, C, and E: frequency-dependent decrease of steady-state eEPSC amplitude (pA) during 0.08–25 Hz optic chiasm stimulation with trains of 25 stimuli in control and following application of N- and P/Q-type voltage-dependent Ca2+ channel blockers: ω-conotoxin GVIA (1 μM) and ω-agatoxin TK (500 nM), respectively. A: ω-agatoxin (Atx, n = 6). C: ω-conotoxin (Ctx, n = 7). E: coapplication of Atx and Ctx (n = 3). B, D, and F: the same data as in A, C, and E normalized (%) to the amplitude of the first eEPSC in the stimulus train at each condition (see Fig. 1). *P < 0.05, ***P < 0.001.
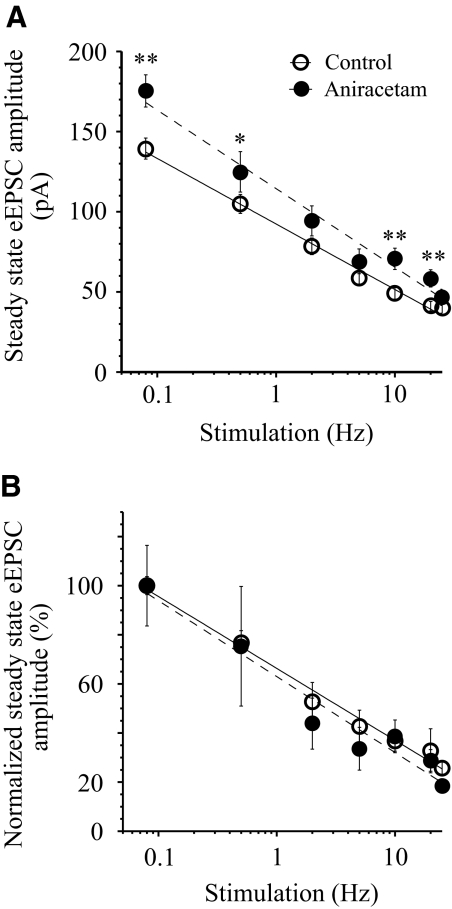
Contribution of AMPA receptor desensitization to synaptic depression
The role of AMPA receptor desensitization in the development of synaptic depression was investigated using aniracetam (5 mM), a compound that blocks AMPA receptor desensitization. To estimate the relative changes of eEPSC amplitude induced by aniracetam, the steady-state amplitude (pA) was compared before (control) and after aniracetam application at each stimulus frequency (Fig. 4A). Aniracetam increased the steady-state eEPSC amplitude 1.2- to 1.4-fold in the range of applied stimulus frequencies [F-test: F(3,8) = 4.19, P < 0.047]. To estimate the contribution of AMPA receptor desensitization to the synaptic depression the amplitude of each EPSC evoked by stimulus train was normalized (%) of the amplitude of the first eEPSC in the train at each condition: before (control) and after aniracetam application. The normalized steady-state eEPSC amplitudes at each condition were compared (Fig. 4B). The curves demonstrating frequency-dependent changes of steady-state eEPSC amplitude in control and after aniracetam application were not significantly different in the range 0.5–25 Hz [F-test: F(3,8) = 0.11, P = 0.95]. For all applied frequencies P ≥ 0.15 (paired t-test, two-tailed).
Fig. 4.
Contribution of AMPA receptor desensitization to synaptic depression. A: aniracetam (5 mM) inhibition of AMPA receptor desensitization increased (1.2–1.4 times) the steady-state eEPSC amplitude (pA) during repetitive stimulation (0.08–25 Hz). *P < 0.05, **P < 0.01, n = 4. Recordings in control and during aniracetam application were made in the same neurons. B: the frequency dependence of the steady-state eEPSC amplitude in control and after aniracetam application. eEPSC amplitude of each eEPSC was normalized to the first eEPSC in the stimulus train (see Fig. 1) and steady-state amplitudes before and after aniracetam application were compared. NMDA receptors were not activated during glutamate release from RHT axon terminals due to the configuration of our experiments (1.2 mM extracellular Mg2+, Vh −60 mV). AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA, N-methyl-d-aspartate; Vh, holding potential.
Short-term synaptic plasticity in RHT synapses during optic nerve and optic chiasm paired-pulse stimulation
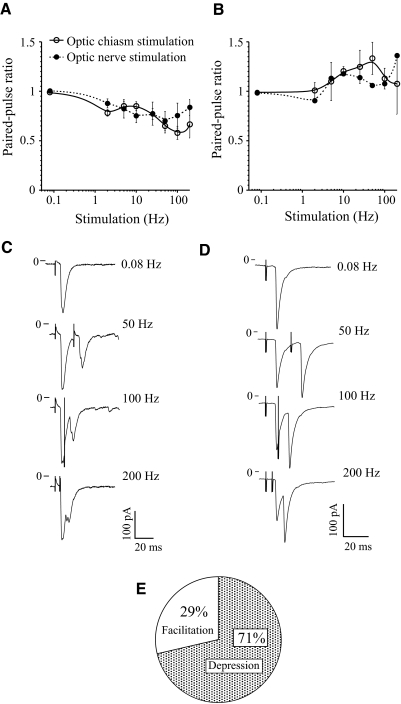
Initial facilitation (eEPSC2/eEPSC1 ratio >1) followed by short-term synaptic depression was recorded in 5–14% of SCN neurons during optic chiasm and optic nerve stimulation, with stimulus trains at low and high stimulus frequencies. We predicted that these changes in initial release probability have a presynaptic origin. PPS is widely used as an assay to study presynaptic mechanisms and the PPR reflects use-dependent changes in the release probability (Zucker 1989). We thus stimulated the optic nerve and optic chasm with paired pulses to define the frequency-dependent changes of synaptic current in RHT–SCN synapses. The PPR was calculated as a ratio of the mean amplitude of the second eEPSC to the mean amplitude of the first eEPSC (Kim and Alger 2001) (see Statistical analysis). During 2–200 Hz PPS of the optic chiasm synaptic depression was observed in 8 of 12 neurons (67%) and facilitation in the remaining 4 neurons (33%) (Fig. 5, A and B). Similarly, synaptic depression was recorded in 7 of 9 neurons (78%), whereas facilitation was present in 2 of the SCN neurons (22%) during PPS of the optic nerve. All together, during PPS of the optic chiasm and the optic nerve 15 of 21 neurons (71%) demonstrated depression (ZT: 4.5–11.5), whereas 6 neurons (29%) showed facilitation (ZT: 6.0–9.0) (Fig. 5E). Thus both modes of synaptic plasticity (depression and facilitation) were recorded in the subjective day. The frequency dependence of negative (synaptic depression) or positive (facilitation) PPR was similar when either the optic nerve or optic chiasm was stimulated [F-test: F(3,10) = 0.81, P = 0.52 for depression; F(3,10) = 0.039, P = 0.99 for facilitation]. The maximal paired-pulse depression (PPR of 0.6–0.7 of control; Fig. 5, A and C) and facilitation (PPR about 1.4 of control; Fig. 5, B and D) were reached during 50–200 Hz PPS. The first eEPSC was fully separated from the second eEPSC up to 25–50 Hz PPS. The paired eEPSCs were partially (Fig. 5D) or completely (Fig. 5C) merged during 100–200 Hz stimulation.
Fig. 5.
Synaptic depression and facilitation during paired-pulse stimulation (PPS) of optic nerve and optic chiasm. A: synaptic depression during optic nerve (n = 7) and optic chiasm (n = 8) PPS. B: facilitation during optic nerve (n = 2) and optic chiasm (n = 4) PPS. A and B: paired-pulse ratio (PPR) estimated as a ratio of the mean amplitude of the second EPSC to the mean amplitude of the first EPSC (mean EPSC2/mean EPSC1). For all applied stimulus frequencies P ≥ 0.11 for depression and P ≥ 0.33 for facilitation (unpaired t-test, 2-tailed). C and D: EPSC recordings in 2 neurons during 0.08 Hz (control) and 50 to 200 Hz PPS of the optic nerve. C: synaptic depression (mean of 5 sweeps). D: facilitation (mean of 35 sweeps). The stimulus artifact is shown attenuated. E: percentage of the neurons demonstrating depression or facilitation during PPS.
DISCUSSION
The circadian clock located in the SCN receives environmental light intensity information via glutamatergic input from ipRGCs. Light depolarizes the ipRGCs, which leads to action potential firing, at frequencies reaching 25 Hz, for the duration of a light stimulation (Berson et al. 2002; Tu et al. 2005; Warren et al. 2003). In our recordings, EPSCs reliably followed optic chiasm or optic nerve stimulation with stimulus trains ≤100 Hz. Thus our experiments indicate that glutamate released from RHT axon terminals can faithfully transmit light intensity information by following the frequency of light-induced action potentials fired by ipRGCs. We found that synaptic depression of monosynaptic transmission in RHT–SCN synapses could be defined as homosynaptic or Hebbian activity dependent (Bailey et al. 2000). The magnitude of synaptic depression was proportional to the stimulation frequency that simulated the action potential firing of ipRGCs (Berson et al. 2002; Callewaert et al. 1996; Forsythe et al. 1998; Tu et al. 2005; Warren et al. 2003). It is generally accepted that synaptic depression occurs during repetitive stimulation when the readily releasable pool of vesicles is depleted and the amount of transmitter released with each presynaptic action potential is reduced (Curtis and Eccles 1960; Zucker and Regehr 2002). During stimulation the eEPSC amplitude reaches a plateau (steady-state level) when the rate of transmitter release is matched by the rate of vesicle replenishment (Brenowitz et al. 1998). Synaptic depression was not detected during 0.08 Hz stimulation (one stimulus every 12 s) of the optic chiasm, a frequency that correlated with the estimated synaptic vesicle recycling time (time constant ∼10 s) in hippocampal neurons (Stevens and Tsujimoto 1995). During optic chiasm or optic nerve stimulation the steady-state eEPSC amplitude decreased with increasing stimulus frequency and the maximal value of synaptic depression (14–23% of control) was reached at 50–100 Hz. Although the eEPSC amplitude was decreased by synaptic depression, the cumulative charge transfer increased in a frequency-dependent manner and saturated at 20 Hz. Similarly, somatic and dendritic Ca2+ concentration in SCN neurons increased during repetitive optic chiasm stimulation and saturated between 10 and 20 Hz (Irwin and Allen 2007).
Synaptic depression is a predominant form of short-term plasticity at synapses with high release probability; thus RHT synapses on SCN neurons could belong to this category of synapses (Zucker and Regehr 2002). Several mechanisms including vesicle depletion, inactivation of the presynaptic Ca2+ current, and activation of Ca2+-dependent K+ channels may contribute to presynaptic regulation of synaptic depression (Callewaert et al. 1996; Forsythe et al. 1998; Lev-Ram and Grinvald 1986; Xu and Wu 2005; Zucker and Regehr 2002). The reduction of external [Ca2+] decreases synaptic transmission and relieves synaptic depression (Brenowitz and Trussell 2001; Brenowitz et al. 1998; Forsythe et al. 1998; Lev-Tov and Pinco 1992; Zucker and Regehr 2002). Consistent with this model, N- and P/Q-type VDCC blockers reduced Ca2+ influx in RHT axon terminals and the synaptic current (Moldavan et al. 2006). Block of presynaptic P/Q-type Ca2+ channels did not significantly relieve synaptic depression, whereas blocking of N-type Ca2+ channels, which control >60% of synaptic current, almost completely eliminated synaptic depression in our experiments.
Postsynaptic mechanisms, such as AMPA receptor desensitization and saturation of NMDA receptors, also strongly contribute to synaptic depression at glutamatergic synapses (Brenowitz and Trussell 1998, 2001; Trussell et al. 1993). In the SCN, aniracetam facilitated AMPA receptor-mediated currents elicited by either local AMPA application or optic chiasm stimulation (Moriya et al. 2003). Aniracetam potentiates the behavioral phase delay induced by low-intensity but not high-intensity light applied early in the subjective night (Moriya et al. 2003). Consistent with this observation, aniracetam increased the steady-state eEPSC amplitude 1.2- to 1.4-fold in the range of applied stimulus frequencies. Decreasing AMPA receptor desensitization with aniracetam enhanced the synaptic current but did not alter synaptic depression. Thus our data show that AMPA receptor desensitization was not the main determinant of depression in RHT synapses. Under our recording conditions (1.2 mM extracellular Mg2+ and holding potential at −60 mV) NMDA receptors did not contribute to the recorded eEPSC. The collected data indicated that presynaptic mechanisms play the major role in synaptic depression in RHT synapses.
Synaptic depression was reduced after increasing the temperature from 28°C to a physiological value (36°C), which is consistent with the vesicle depletion model. Reduction of the rate of vesicle pool depletion and an increase of the release probability by accelerated vesicle recruitment, such as occurs when the temperature is increased to physiological values, reduce synaptic depression and increase the total recycling vesicle pool (Brenowitz et al. 1998; Kushmerick et al. 2006).
Glutamate transporter and the amount of neurotransmitter in mouse brain synaptic vesicles are increased during the subjective day (Darna et al. 2009). Conversely, neither expression levels of the vesicular glutamate transporters nor numbers or morphometric features of glutamatergic terminals showed nycthemeral variations in the rat SCN (Girardet et al. 2010). Thus the stable conditions of glutamatergic inputs to the SCN provided a similar magnitude of synaptic depression in RHT synapses during subjective day and night.
During stimulus trains a small population (4%) of studied neurons demonstrated synaptic depression at frequencies <5 Hz and prolonged facilitation at higher frequencies (≤25 Hz). Because the stimulation protocol was not changed during this set of experiments and the series resistance was stable, the differences between neurons responding by depression or facilitation possibly reflect functional peculiarities of RHT axon terminals that may belong to different types of ipRGCs (Baver et al. 2008). The function of RHT synapses showing facilitation is not clear. The glutamate released from facilitating synapses may activate a subset of GABAergic SCN neurons.
Our finding, that synaptic depression was much stronger during stimulus trains than during PPS, are in a good accordance with observations that the circadian pacemaker and the correspondent photoreceptive system are more sensitive to the irradiance of longer-duration stimuli than to irradiance of briefer stimuli (Nelson and Takahashi 1991). Synaptic depression acts as a low-pass filter reducing synaptic transmission at high-frequency stimulation and does not alter it at low frequencies (Bertram 2001; Fortune and Rose 2001). The suppression of glutamate release from RHT axon terminals during high-frequency firing in ipRGCs induced by strong accidental light signals should protect SCN neurons against excessive excitation and allow them to adjust the circadian clock to slow changes of the light intensity during the 24 h light–dark cycle.
In 30% of SCN neurons, PPS induced facilitation, demonstrating that synaptic depression was not the only form of short-term plasticity in RHT synapses. Similarly, in 5–14% of SCN neurons, which showed synaptic depression during stimulus trains, initial facilitation was recorded. The facilitation is defined by the mechanisms regulating Ca2+ influx. The activity-dependent facilitation demonstrated Ca2+ dependence and was induced by Ca2+ influx through P/Q- but not N-type Ca2+ channels in the calyx of Held nerve terminals (Cuttle et al. 1998; Ishikawa et al. 2005). Ca2+ current facilitation in these synapses contributes to roughly 40% of the paired-pulse facilitation of transmitter release, whereas the remaining component was independent of Ca2+ current facilitation but was strongly dependent on dynamic changes of the residual free [Ca2+]i (Muller et al. 2008). Also, exogenous expression of Cavβ4b subunits at the presynaptic terminal favors facilitation during PPS (Xie et al. 2007). In spite of reported initial transient facilitation, synaptic depression dominated in RHT synapses during PPS and stimulus trains and increased proportional to the frequency and duration of stimulation.
ipRGCs continuously fire action potentials in the presence of a light signal (Berson et al. 2002; Tu et al. 2005; Warren et al. 2003). Therefore during the subjective day, SCN neurons receive a constant flow of glutamatergic synaptic signals from the RHT that have the potential to overwhelm weaker synaptic inputs. Synaptic depression down-regulates excitatory synaptic inputs while allowing the postsynaptic neuron to remain sensitive to changes in the frequency of that excitatory input (Abbott et al. 1997; Bertram 2001; Rothman et al. 2009). Also, during the subjective day, depression in RHT synapses would allow SCN neurons to process other biologically important input signals such as those mediated by the intergeniculate leaflet, raphe nuclei, and anterior paraventricular thalamus while being sensitive to changes in the frequency of action potentials arriving via the RHT (Alamilla and Aguilar-Roblero 2010; Hay-Schmidt et al. 2003; Pickard et al. 1987). Synaptic depression, as a low-pass filter, should be less efficient at dusk when the light intensity and the action potential firing frequency of ipRGCs decrease. Synaptic depression also alters the balance between excitatory and inhibitory synaptic inputs and, as such, has significant consequences for the function of neuronal networks (Abbott et al. 1997). This may be important in the SCN, where the neural network synchronizes and stabilizes the rhythms of individual SCN neurons (Liu et al. 2007).
Conclusion
Optic chiasm and optic nerve stimulation with paired pulses and stimulus trains that simulate the discharge frequencies of ipRGCs revealed frequency-dependent depression in the overwhelming majority of studied SCN neurons. We predict that light-induced activation of ipRGCs will similarly produce synaptic depression, attenuating glutamate release in RHT axon terminals to maintain sensitivity of SCN neurons to other biologically important signaling pathways.
The optic chiasm and optic nerve stimulation induced similar changes of eEPSC, suggesting that in both cases the same retinal inputs to the SCN neurons were activated. Our data defined the range of stimulus frequencies that could be most effective for inducing the phase shift in behavior or for affecting the neuronal network in the SCN in studies in which stimulation of the optic nerve (optic chiasm) will be applied.
GRANTS
The work was supported by National Institute of Mental Health Grant MH-70922.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science 275: 220–224, 1997. [DOI] [PubMed] [Google Scholar]
- Alamilla J, Aguilar-Roblero R. Glutamate and GABA neurotransmission from the paraventricular thalamus to the suprachiasmatic nuclei in the rat. J Biol Rhythms 25: 28–36, 2010. [DOI] [PubMed] [Google Scholar]
- Albers HE, Lydic R, Moore-Ede MC. Entrainment and masking of circadian drinking rhythms in primates: influence of light intensity. Physiol Behav 28: 205–211, 1982. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci 1: 11–20, 2000. [DOI] [PubMed] [Google Scholar]
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci 27: 1763–1770, 2008. [DOI] [PubMed] [Google Scholar]
- Berry MS, Pentreath VW. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res 105: 1–20, 1976. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295: 1070–1073, 2002. [DOI] [PubMed] [Google Scholar]
- Bertram R. Differential filtering of two presynaptic depression mechanisms. Neural Comput 13: 69–85, 2001. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, David J, Trussell LO. Enhancement of synaptic efficacy by presynaptic GABAB receptors. Neuron 20: 135–141, 1998. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Minimizing synaptic depression by control of release probability. J Neurosci 21: 1857–1867, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G, Eilers J, Konnerth A. Axonal calcium entry during fast “sodium” action potentials in rat cerebellar Purkinje neurones. J Physiol 495: 641–647, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. NMDA-evoked calcium transients and currents in the suprachiasmatic nucleus: gating by the circadian system. Eur J Neurosci 13: 1420–1428, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Eccles JC. Synaptic action during and after repetitive stimulation. J Physiol 150: 374–398, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T. Facilitation of the presynaptic calcium current at an auditory synapse in rat brainstem. J Physiol 512: 723–729, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darna M, Schmutz I, Richter K, Yelamanchili SV, Pendyala G, Holtje M, Albrecht U, Ahnert-Hilger G. Time of day-dependent sorting of the vesicular glutamate transporter to the plasma membrane. J Biol Chem 284: 4300–4307, 2009. [DOI] [PubMed] [Google Scholar]
- Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, McPherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature 394: 381–384, 1998. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Sicard B, Cooper HM. Effects of irradiance and stimulus duration on early gene expression (Fos) in the suprachiasmatic nucleus: temporal summation and reciprocity. J Neurosci 20: 7790–7797, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron 20: 797–807, 1998. [DOI] [PubMed] [Google Scholar]
- Fortune ES, Rose GJ. Short-term synaptic plasticity as a temporal filter. Trends Neurosci 24: 381–385, 2001. [DOI] [PubMed] [Google Scholar]
- Girardet C, Blanchard MP, Ferracci G, Leveque C, Moreno M, Francois-Bellan AM, Becquet D, Bosler O. Daily changes in synaptic innervation of VIP neurons in the rat suprachiasmatic nucleus: contribution of glutamatergic afferents. Eur J Neurosci 31: 359–370, 2010. [DOI] [PubMed] [Google Scholar]
- Hay-Schmidt A, Vrang N, Larsen PJ, Mikkelsen JD. Projections from the raphe nuclei to the suprachiasmatic nucleus of the rat. J Chem Neuroanat 25: 293–310, 2003. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J Neurosci 27: 11748–11757, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur J Neurosci 30: 1462–1475, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Kaneko M, Shin HS, Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J Physiol 568: 199–209, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z-G, Allen CN, North RA. Presynaptic inhibition by baclofen of retinohypothalamic excitatory synaptic transmission in rat suprachiasmatic nucleus. Neuroscience 64: 813–819, 1995. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci 21: 9608–9618, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Dudek FE. Intracellular electrophysiological study of the suprachiasmatic nucleus neurons in rodents: excitatory synaptic mechanisms. J Physiol 444: 269–287, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick C, Renden R, von Gersdorff H. Physiological temperatures reduce the rate of vesicle pool depletion and short-term depression via an acceleration of vesicle recruitment. J Neurosci 26: 1366–1377, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ram V, Grinvald A. Ca2+- and K+-dependent communication between central nervous system myelinated axons and oligodendrocytes revealed by voltage-sensitive dyes. Proc Natl Acad Sci USA 83: 6651–6655, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Tov A, Pinco M. In vitro studies of prolonged synaptic depression in the neonatal rat spinal cord. J Physiol 447: 149–169, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, 3rd, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129: 605–616, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Détári L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J Neurosci 18: 9078–9087, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldavan MG, Irwin RP, Allen CN. Presynaptic GABAB receptors regulate retinohypothalamic tract synaptic transmission by inhibiting voltage-gated Ca2+ channels. J Neurophysiol 95: 3727–3741, 2006. [DOI] [PubMed] [Google Scholar]
- Moran O, Mateu L. Loosening of paranodal myelin by repetitive propagation of action potentials. Nature 304: 344–345, 1983. [DOI] [PubMed] [Google Scholar]
- Moriya T, Ikeda M, Teshima K, Hara R, Kuriyama K, Yoshioka T, Allen CN, Shibata S. Facilitation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor transmission in the suprachiasmatic nucleus by aniracetam enhances photic responses of the biological clock in rodents. J Neurochem 85: 978–987, 2003. [DOI] [PubMed] [Google Scholar]
- Muller M, Felmy F, Schneggenburger R. A limited contribution of Ca2+ current facilitation to paired-pulse facilitation of transmitter release at the rat calyx of Held. J Physiol 586: 5503–5520, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J Physiol 439: 115–145, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard GE, Ralph MR, Menaker M. The intergeniculate leaflet partially mediates effects of light on circadian rhythms. J Biol Rhythm 2: 35–56, 1987. [DOI] [PubMed] [Google Scholar]
- Rothman JS, Cathala L, Steuber V, Silver RA. Synaptic depression enables neuronal gain control. Nature 457: 1015–1018, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc Natl Acad Sci USA 92: 846–849, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron 10: 1185–1196, 1993. [DOI] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron 48: 987–999, 2005. [DOI] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci 17: 1727–1735, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Li X, Han J, Vogt DL, Wittemann S, Mark MD, Herlitze S. Facilitation versus depression in cultured hippocampal neurons determined by targeting of Ca2+ channel Cavbeta4 versus Cavbeta2 subunits to synaptic terminals. J Cell Biol 178: 489–502, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron 46: 633–645, 2005. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci 12: 13–31, 1989. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002. [DOI] [PubMed] [Google Scholar]