Abstract
Animals depend on learned rules to guide their actions. Prefrontal (PFC) and premotor (PMC) cortex of primates have been reported to display rule-related neural activity, but it is unclear how signals encoded here are utilized to enforce the decision to act. The supplementary eye field (SEF) is a candidate for enforcing rule-guided ocular decisions because the activity of neurons here is correlated with the rule in an ocular decision-making task and because this area is anatomically more proximal to movement structures than PFC and PMC and receives inputs from them. However, in the previous work, the rule encoding and ocular outcome were confounded, leaving open the question of whether SEF activity is related to the rule or the behavior. In the present study, we attempted to discriminate between these alternatives by increasing task difficulty and forcing errors, thereby putting the stimulus and the behavior at odds. Single SEF neurons were recorded while monkeys performed the task in which the rule is to pursue a moving target if it intersects a visible square and maintain fixation if it does not. A delay period was imposed to monitor neural activity while the target approached the square. Two complementary populations of go and nogo neurons were found. When task difficultly was increased, the monkeys made more errors, and the neurons took longer to encode the rule. However, in error trials, most neurons continued to reflect the rule rather the monkey's ocular decision in both the delay period and after square intersection (movement period). This was the case for both directionally tuned and nondirectional SEF neurons. The results suggest that SEF neurons encode the ocular decision rule but that the decision itself likely occurs in a different structure that sums rule information from the SEF with information from other areas.
INTRODUCTION
Primates rely on learned rules to guide their decisions. Previous research has suggested that the prefrontal cortex (PFC) plays a critical role in this process (Miller 1999; Passingham 1985, 1993; Wallis et al. 2001; Wise et al. 1996). Neural activity in the PFC reflects perceptual categories better than motor choices specified by rules (Muhammad et al. 2006; Wallis and Miller 2003) and differentially encodes spatial or nonspatial rule-related visual cues (White and Wise 1999). The premotor cortex (PMC) is also involved in processing rule-related information (Muhammad et al. 2006; Wallis and Miller 2003). Neural activity in different parts of PMC reflects better perceptual features and motor decision, respectively (Hoshii and Tanji 2006; Wallis and Miller 2003). The different characteristics of neural activity in these two structures, and the fact that the PFC sends output to the PMC (Barbas and Pandya 1987, 1989; Pandya and Yeterian 1990), suggest a transformation of rule encoding in this complex with the PFC encoding perceptual categories and the PMC specifying the alternative response choices, likely based on input from the PFC.
Currently it is not clear how the rule-encoding neural signal in the PFC and PMC is further utilized downstream to enforce a motor decision. The supplementary eye field (SEF) is a likely region where this process is carried out for ocular decision. The SEF receives inputs from the PFC and PMC (Carmichael and Price 1995; Wang et al. 2005). It also receives information from visual structures such as MT and MST where perceptual decisions are encoded (Britten et al. 1996; Huerta and Kaas 1990; Newsome et al. 1989). Furthermore, it projects to the FEF (Luppino et al. 2003) and SC (Fries 1985; Shook et al. 1990) where neural activity reflects an ocular decision (FEF: Kim and Shadlen 1999; SC: Horwitz and Newsome 2001). The unique anatomical position of the SEF suggests a role for this structure in enforcing ocular decision. Our recent work suggests that the SEF is involved in rule-based decision making in a go/nogo ocular task. Human SEF is more active when making the decision than it is for either the same visual stimulation or smooth pursuit behavior when no decision is required (Heinen et al. 2006) and separate populations of neurons in monkey SEF signal go or nogo, respectively (Kim et al. 2005). However, a problem arises with interpretation of the previous results because in those experiments, the behavior and the stimulus were confounded, i.e., most responses that were made were consistent with the rule. In other words, SEF activity in these experiments may have merely signaled the rule and hence was not necessarily related to the ocular decision that was made. Therefore a claim that activity here was related to the ocular decision is premature.
The present study investigated whether the SEF encodes the rule or enforces the ocular decision. To achieve this, monkeys performed a delayed go/nogo ocular decision task while single-unit activity was recorded from the SEF. If SEF neurons encode the rule rather than enforce the ocular decision, one would expect their activity to veridically predict the rule and continue to do so even when a decision error is committed. Conversely, if SEF neurons participate in enforcing the ocular decision, their activity should signal the behavior regardless if the decision is correct or not.
METHODS
Subjects and surgical procedures
Three juvenile male Macaque monkeys (VC, ED, and LE) participated in the experiments. Two of them (VC and ED) were also involved in an earlier study (Yang et al. 2008). Surgeries were performed on the monkeys under aseptic conditions to implant a recording chamber, head holder, and a search coil to measure eye movements. With the monkey under isoflurane gas anesthesia, a 2 cm craniotomy was trephined in the skull. The chambers implanted on the monkeys were centered 24 mm anterior in Horsley-Clark stereotaxic coordinates. A stainless steel recording chamber was positioned over the craniotomy. The coil was constructed from Teflon-coated stainless-steel wire and was implanted under the conjunctiva of one eye (Judge et al. 1980). The head holder was positioned on the midline. After surgery, the monkey was returned to its cage and was allowed to recover fully before experiments began. Antibiotics and analgesics were administered under the direction of a veterinarian during the postoperative period. All procedures were approved by the Institutional Animal Care and Use Committee and were in compliance with the guidelines set forth in the United States Public Health Service Guide for the Care and Use of Laboratory Animals.
Testing procedure and the ocular go/nogo task
At the beginning of each recording session, the monkey was seated in a primate chair with its head fixed by a post. The eyes were 50 cm from the screen and the line of sight perpendicular to it. Eye position was calibrated while the monkeys made saccades to visual targets that were composed of a single dot with 0.5° angular extent and 10 vertical or horizontal eccentricities. An 85–100 mm tungsten electrode that usually had 1.0–2.0 MΩ impedance was lowered into the chamber via a stainless steel tube to a predetermined site through a Crist grid (http://www.cristinstrument.com/). The recording session typically lasted 2 h.
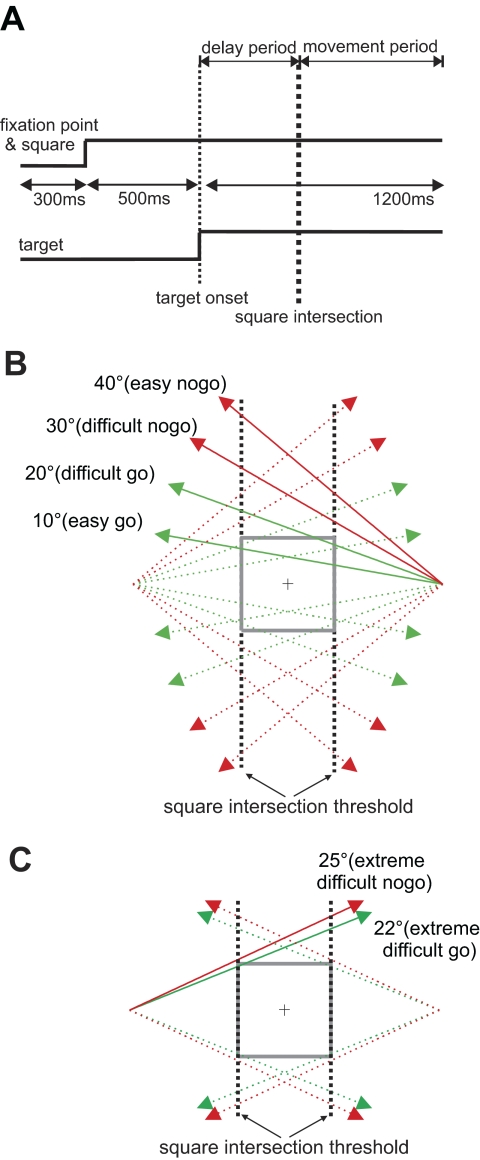
Monkeys were initially trained to successfully perform an easy version of an ocular go/nogo task as described in Kim et al. (2005). For the experiments described here, a modified version of the task was used where task difficulty was manipulated. Figure 1A shows the temporal schematic of the task. Each trial began with the appearance of a 0.5° white spot at the center of the screen, surrounded by a visible 8 × 8° square. To trigger the appearance of a moving target, also a 0.5° white spot, the monkey had to maintain its gaze within a 4 × 4° invisible electronic square window centered at the fixation point for 500 ms. At the end of the fixation period, the target appeared 20° to the left or right and moved toward the visible square at a constant velocity (30°/s) and angle relative to horizontal (easy: 10 and 40°; difficult: 20 and 30°) for 1,200 ms. The fixation point and the square remained visible throughout the trial. Figure 1B shows the possible trajectories in relation to the visible square and fixation point and the defined point of square intersection. Because of the difference in the trajectory angle, the timing of square intersection varies as indicated in Fig. 1A. In some recording sessions when time allowed, additional blocks of trials were conducted in which the target trajectory angle was either 22° (extremely difficult go trial) or 25° (extremely difficult nogo trial). Again the angle and direction of target motion was randomly determined and balanced within a block of trials. Figure 1C shows the trajectories for extremely difficult trials with higher constant velocity of 40°/s.
Fig. 1.
The delayed ocular go/nogo paradigm. A: temporal schematic. The square and fixation point stayed on for the duration of the trial. The target appeared 500 ms after fixation acquisition and moved at a constant angle and velocity. The time period before square intersection is defined as the delay period and the time period after that the movement period. B: spatial schematics of the target-square relationship. In each trial, a target appeared either left or right 20° in the periphery and moved from the periphery with 1 of the 4 possible angles relative to horizontal meridian. The target moved in 40° (easy nogo) and 30° (difficult nogo) and missed the square, and in others, it moved in 10° (easy go) and 20° (difficult go) and intersected the square. C: spatial schematic for extremely difficult trials. The target moved in higher radial velocity (40°/s) and in 22° (extremely difficult go) and 25° angle (extremely difficult nogo).
In the delay period, defined as the time between target onset and square intersection, the monkey must maintain fixation regardless of whether the target eventually intersects the square. Targets moving at 10, 20, and 22° angles intersect the square (go trials), and those moving at 25, 30, and 40° angles do not (nogo trials). In go trials, the monkey must initiate a pursuit movement to acquire the target within 300 ms of square intersection and maintain gaze within 3° of distance to the target until the end of the trial. In nogo trials, the monkey must maintain fixation throughout the trial. Because the angle and direction of target movement was randomized, the monkey had to evaluate the target-square relationship on each trial to determine whether it was a go or nogo condition in the delay period (before square intersection) and enforce the ocular decision in the movement period (after intersection). Liquid reward was given at the end of a trial only when the monkey successfully executed the task; the same amount of liquid was given for trials of different difficulties. The intertrial interval was set at 300 ms.
Data analysis
PURSUIT EYE MOVEMENTS.
Vertical and horizontal eye position signals were digitized (1 kHz) and stored for off-line analysis. Eye velocity was obtained by digital differentiation of eye position, and movement initiation time was detected using two criteria modified from a previous study (Badler and Heinen 2006): either the pursuit movement had a minimum velocity of 10°/s for ≥250 ms or it surpassed the saccade threshold of 40°/s for 80 ms and was followed by a pursuit movement. Matlab and its Signal Processing and Statistical Toolboxes (The Mathworks 2006) were used to conduct signal processing and data analysis.
BEHAVIORAL CHOICES.
Four types of responses were defined in the present study: go success, go error, nogo success, and nogo errors. Go success trials had to satisfy two criteria: the animal had to maintain fixation within a 4° fixation window during the delay period and the target had to be acquired and continuously pursued within the 3° window in the movement period. Nogo success trials were defined as continuous fixation within the 4° fixation window for the duration of the trial. Go errors were defined as maintaining fixation throughout a go trial, whereas in nogo errors a pursuit movement was initiated after square intersection.
TASK-RELATED ACTIVITY.
To characterize task-related activity as a continuous function, the neural spike rate recorded in each trial was convolved with 30 ms Gaussian. To determine if and when a neuron signaled a rule state in the task, the difference in spike rate for corresponding 20 ms time intervals in go and nogo trials for each neuron was statistically tested (paired 2-tail t-test). A sliding window moved at 5 ms steps, and the test was repeated for each step. Separation time was defined as the beginning of the first step of the first 20 consecutive steps in the delay period that were significantly different, with a familywise α ≤ 0.05. When a neuron initially displayed a higher spiking rate in go trials than in nogo trials, it was classified as a go neuron; when activity was higher in nogo trials, it was classified as a nogo neuron.
DIRECTIONALITY INDEX.
To determine whether SEF activity reflected the rule or was signaling the direction of target trajectory or planned pursuit movement, a directionality index (DI) was calculated for each neuron:
Here DI is for an individual neuron. The mean spike rate for the preferred direction, or μpref, was derived from the pair of trajectories that specify the same rule and have the highest pooled mean spike rate compared with other eight pairs of trajectories; the mean spike rate for the two opposite trajectories was noted as μopp. It also removes any rule-related influence on the spike rate, as the opposite pairs always have the same rule state. The resultant DI value is between 0 and 1. The activity of a neuron is spatially tuned if the DI value is significantly different from zero. DI values were calculated for the delay and movement periods respectively for each neuron.
CHOICE PROBABILITY.
To determine whether the activity of a task-related neuron predicted the eventual ocular decision, we calculated the choice probability (CP) of its activity at different time intervals in the delay period. CP is adopted from the signal detection theory and is used to specify the degree to which single neurons reflect the animal's decision (Britten et al. 1996). CP was derived by first compiling a distribution of neuronal activity for each of the choices that the animal made. A receiver operating characteristic (ROC) curve was then computed from the two distributions, and the area under the curve was integrated to yield the CP. CP is therefore an estimate of the probability that an observer would correctly predict whether the monkey pursued or fixated on a given trial from the neuron's firing rate. Note, however, that because different target trajectories were used to specify go or nogo trials, activity of a go or nogo neuron could also reflect the trajectory in addition to the rule. Although the neural activity was sorted by the behavioral choice but not the trajectory, the CP could still partially reflect target trajectory itself.
NORMALIZED MEAN SPIKE RATE.
To compare neural activity in trials with different behavioral choices (go success, nogo success, and go error), the mean spike rate for each neuron in go error trials in the delay period was first computed separately and then normalized against that for success go and nogo trials respectively using the following equation
Here Ze is the normalized mean spike rate for decision error trials. The mean spike rate for go success, nogo success, and go error trials are μs, μb, and μe, respectively. The normalized mean spike rate is −1 when the mean spike rate in go error trials is the same as that in nogo success trials and 1 when it is the same as that in go success trials. A value >1 indicates the firing rate in go error trials was higher than that in success go trials. This was not done for nogo error trials because they were infrequent.
Histology
After completing the experiments, the monkeys were perfused, and the brains were fixed with 10% formalin. A block of cortical tissue encompassing the probed area was taken from monkey VC, and 5 μm thick coronal slices were obtained from the SEF tissue block and stained (Hematoxylin-and-eosin, or HandE, and Perl) to highlight the electrode tracks. In addition, before perfusing monkey VC, two electrical lesions were made with DC current located 2 mm laterally from the center of the chamber and 4.5 mm in depth. After perfusion, the reference lesions were revealed using cresyl violet staining. The recorded site depth and locations in experimental sessions were then aligned to these reference tracks.
RESULTS
Behavioral choice
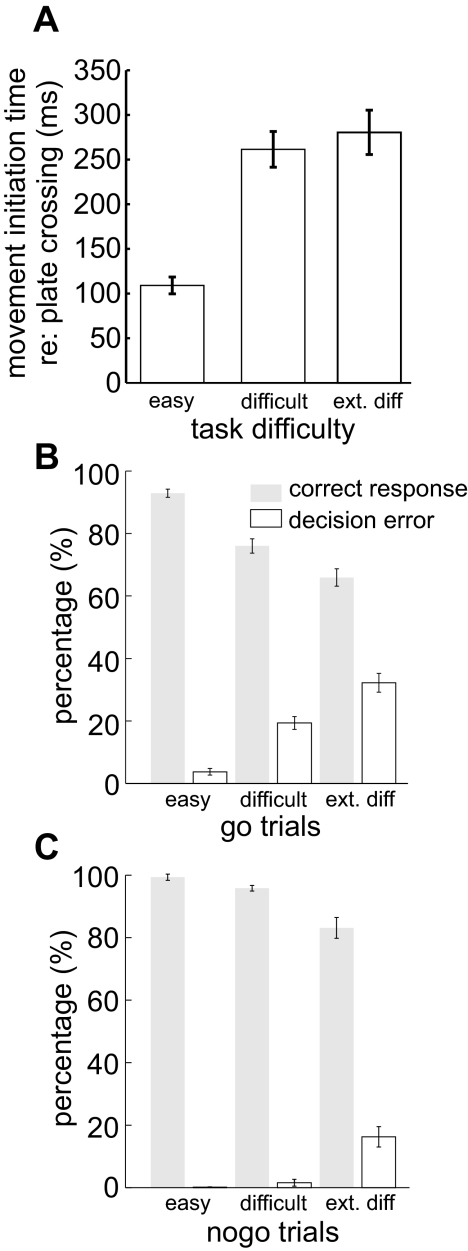
Four types of responses were defined according to the go/nogo rule: go success, go error, nogo success, and nogo error (see methods). Response errors due to premature eye movements or failure to achieve initial fixation were excluded from the analysis (2.8% of trials). Figure 2 shows typical eye traces from a single block of trials in which easy and difficult trials were randomly interleaved, and another block of extremely difficult trials. Shown in Fig. 2A are Cartesian eye position traces for easy (top), difficult (middle), and extremely difficult go trials. In easy trials, the monkey usually acquired the target close to where it intersected the square, and go errors were rare. In difficult trials, the monkey at times acquired the target further away from the intersection point, and go errors were more frequent. In extremely difficult go trials, the target was acquired consistently further away from the intersection point, and more errors occurred. Figure 2B shows radial velocity traces for the same trials, aligned to the time of square intersection. Here it can be seen that movement initiation time was closer to that of square intersection in easy trials but was later in difficult and extremely difficult trials. Figure 2C shows eye position traces from easy, difficult, and extremely difficult nogo trials. The monkey successfully maintained fixation in all easy trials and made few errors in difficult trials. More errors were made in extremely difficult trials. Figure 2D shows radial eye velocity for the nogo trials. Eye movements in nogo error trials appear similar to those in successful difficult and extremely difficult go trials in their timing and initial intersection location.
Fig. 2.
Eye movements recorded from example blocks of ocular go/nogo trials. Blue traces indicate success trials and red traces decision errors. A: eye position traces in easy, difficult, and extremely difficult go trials. Catch-up saccades were removed from the velocity traces. B: corresponding radial eye velocity traces in easy, difficult, and extremely difficulty go trials shown above. The small spikes shown before square intersection are microsaccades that did not bring the gaze outside of the 4 × 4° fixation window. C: eye position traces for nogo trials. D: radial eye velocity traces in nogo trials.
Figure 3 summarizes behavioral results from all recording sessions for the three monkeys. Figure 3A shows that overall the mean pursuit latency relative to square intersection was longer in difficult go trials than in easy trials but was not longer in extremely difficult go trials than in difficult trials. Here only successful go trials were included. Figure 3B shows the percentages of decision success and error trials and the corresponding SEs. Not shown here are the percentages of other errors unrelated to decision making, such as failure to acquire the fixation points or premature eye movements, that can be inferred based on the percentages shown here. It can be seen that the frequency of go errors increased with task difficulty level (for all 3 difficulty levels: χ2 = 3452, P < 0.001), and there were also more nogo errors for more difficult trials (χ2 = 312, P < 0.001). In addition, there were more errors in difficult go than in difficult nogo trials (χ2 = 623, P < 0.0001), and more errors in extremely difficult go than extremely difficult nogo (χ2 = 1067, P < 0.0001). Therefore our manipulation successfully heightened task difficulty and resulted in more decision errors.
Fig. 3.
Behavioral outcomes in ocular no/nogo obtained from all 3 monkeys. A: movement initiation time relative to square intersection in all go success trials. Error bars indicate SEs. B: percentages of successes and errors in go and nogo trials and their SEs. Not shown here are the percentages of trials where the monkey failed to acquire the fixation point or initiated the eye movement prematurely.
Effects of task difficulty on SEF activity
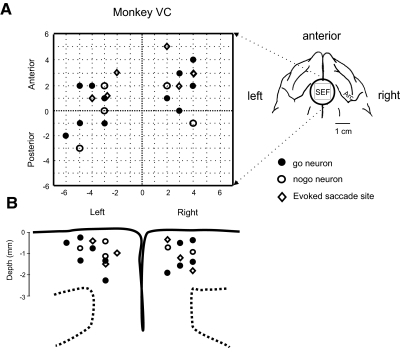
Of 149 neurons recorded from the SEF of the three monkeys, 44 were classified as rule-related because they displayed differential activity between easy go and nogo trials in the delay period (see methods). Of these, 34 displayed higher activity in go trials (go neurons), whereas 10 did so in nogo trials (nogo neurons). Figure 4 summarizes the location of task-related neurons and evoked saccade sites obtained from monkey VC from which a large portion of neurons were recorded. Figure 4A shows the topographic location of neurons and sites where eye movements were evoked at low currents (50–75 μA) plotted relative to the center of the recording chamber (see histology analysis in methods). The inset shows the location of the recording chamber. Most task-related neurons were located 2–5 mm from the midline and 21–28 mm AP in Horsley-Clarke stereotaxic coordinates. Figure 4B shows the depth of the same sites. Most sites were within 2.5 mm of the cortical surface. Therefore the recorded neurons were mostly located in the SEF.
Fig. 4.
Locations and depths of rule-related neurons and evoked saccade sites. The symbols indicate sites from which go (●) and nogo (○) neurons were recorded.  , evoked saccade sites were located with 50–75 μA current. A: topographic map of recorded sites from monkey VC. The center of the map corresponds to the chamber center as indicated in the inset in the upright corner, which was centered 24 mm anterior and on the midline. Symbols are offset slightly if they were recorded from the same electrode track. Positive x and y axis values indicate anterior and right locations, and negative values indicate posterior and left. B: horizontal positions and measured depths of recording sites obtained from monkey VC. The coronal schematic of probed sites is based on a reference histological slice close to the center of the supplementary eye field (SEF) chamber.
, evoked saccade sites were located with 50–75 μA current. A: topographic map of recorded sites from monkey VC. The center of the map corresponds to the chamber center as indicated in the inset in the upright corner, which was centered 24 mm anterior and on the midline. Symbols are offset slightly if they were recorded from the same electrode track. Positive x and y axis values indicate anterior and right locations, and negative values indicate posterior and left. B: horizontal positions and measured depths of recording sites obtained from monkey VC. The coronal schematic of probed sites is based on a reference histological slice close to the center of the supplementary eye field (SEF) chamber.
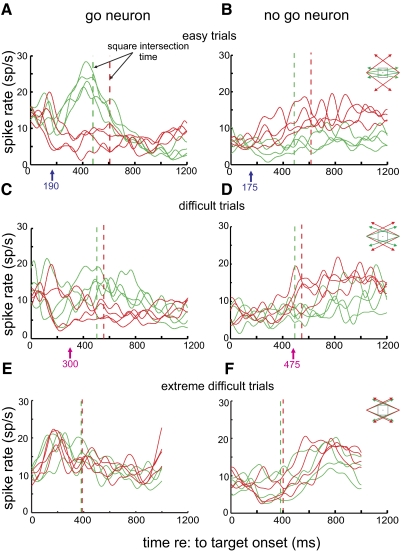
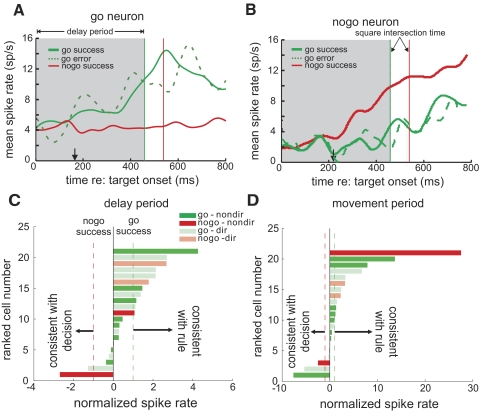
Figure 5 shows the activity of typical go and nogo neurons in easy, difficult, and extremely difficult trials with correct behavioral responses. The mean spike rate of the cells for each target trajectory in the delay and movement periods is displayed separately. Figure 5A shows activity from a go neuron in easy trials. Shortly after target onset, go trial activity rose above nogo trial activity, peaking just before square intersection. Note that the traces segregate to reflect whether the trial type is go or nogo rather than for a set of trajectories that have the same general motion directions. In difficult go trials (Fig. 5C), neural activity appears to rise more slowly and the difference between it and activity in nogo trials appears less than that observed in easy trials. Furthermore, the separation time, the time that the difference in activity level between go and nogo trials first reached significance (see methods), was later for difficult than for easy trials (easy, 190 ms; difficult, 300 ms). Figure 5, B and D, shows a typical nogo neuron exhibiting the necessary criterion of greater activity in nogo trials than in go trials. For this cell, the separation time was also later in difficult trials than in easy ones (easy, 175 ms; difficult, 470 ms). Figure 5, E and F, shows results from a block of extremely difficult trials from other go and nogo neurons. It can be seen that there is little separation between go and nogo trials, and no preintersection separation in mean spike rate was observed.
Fig. 5.
Neural activity recorded from example go and nogo neurons in easy, difficult, and extremely difficult trials. Each curve represents the mean spike rate for targets of a specific motion trajectory (green: go; red: nogo). Same-color dashed lines indicate corresponding square intersection times. The arrows below the panels indicate the separation time for all go and nogo trials with the mean spike rate of all trajectories pooled. A: mean spike rates of a typical go neuron in easy go and nogo trials. B: mean spike rates of a typical nogo neuron in easy trials. C: mean spike rates of the same go neuron in difficult trials. D: mean spike rates of the same nogo neuron in difficult trials. E: mean spike rates of a go neuron in extremely difficult trials. F: mean spike rates of a nogo neuron in extremely difficult trials.
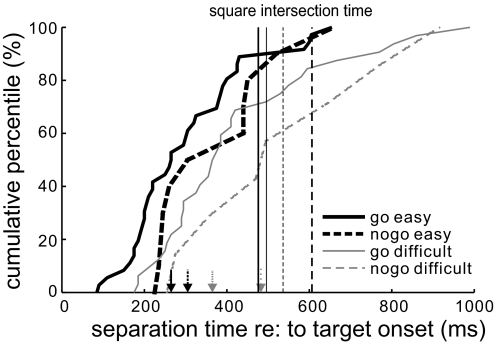
In Fig. 6 we summarize the separation times for all rule-related neurons by plotting their cumulative percentile functions for easy go (thick solid), easy nogo (dashed), difficult go (thin solid), and difficult nogo trials (thin dashed), respectively. Results from extremely difficult trials were not plotted here because of a lack of separation in the delay period for all but one neuron. Median separation times are indicated with arrows. The figure shows separation times in difficult trials were later than those in easy ones as shown by the results of Mann-Whitney test (go neurons: W = 612, P < 0.0001; nogo neurons: W = 52, P = 0.0058). Note that several neurons, which had a separation in mean spike rate in go and nogo trials, did not have significantly different activity at any time in the delay period in difficult go and nogo trials (go neuron separations: easy = 39, difficult = 27; nogo neuron separations: easy = 14, difficult = 11). The later separation times observed in difficult trials are consistent with the later pursuit onsets and more decision errors observed in the behavior in these trials (see Figs. 2 and 3).
Fig. 6.
Cumulative curves of separation times for all neurons in easy and difficult trials (thick solid: go neurons in easy trial; thick dashed: nogo neurons in easy trials; thin solid: go neurons in difficult trials; thin dashed: nogo neurons in difficult trials). The corresponding vertical lines indicate the timing of square intersection. Arrows at bottom indicate the median separation times for corresponding conditions. Note that most neurons encoded go/nogo activity before square intersection even in difficult trials. Results from extremely difficult trials are not shown here because all but 1 neuron had a separation before square intersection.
To examine whether these go/nogo-related neurons are movement and fixation neurons and have differential involvement in the delay and movement periods, the directionality of their activity in these two periods was analyzed. A neuron is more likely to be involved in enforcing the ocular decision if it had greater spatial selectivity in the movement period than in delay period. To achieve this, the mean spike rate for each trajectory in these two periods was computed for success trials. Figure 7 A, left, shows results from an example go neuron for which all go trajectories had a similarly higher spike rate than nogo trajectories in the delay period. The pair of trajectories having the highest spike rate was marked as the “pref” trajectory, and the opposite ones the “opp” trajectory; the mean spike rate for all trajectories was normalized against the trajectory with the highest mean spike rate (green: go trials; red: nogo trials). The arrows indicate the motion direction. Figure 7A, right, shows the mean spike rate in the movement period for the same neuron. There was consistently higher activity for some go trajectories compared with all nogo trajectories. Figure 7B, left, shows the mean spike rates in the delay period for another example, a nogo neuron, for which there was significantly higher activity for leftward and downward nogo trajectories than other nogo trajectories in the delay period. However, activity for most nogo trajectories was still higher than that for adjacent go trajectories. In the movement period, as shown in Fig. 7B, right, a similar directional selectivity and rule-related difference is shown, although one of the opp trajectories now had a higher mean spike rate.
Fig. 7.
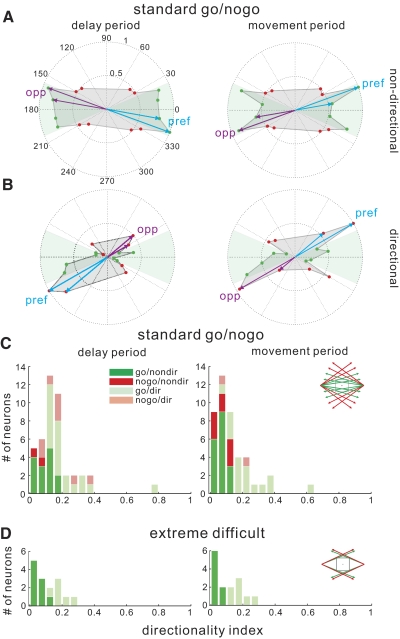
Directionality of SEF activity. A: mean spike rates for all target trajectories in the delay period (left) and in the movement period (right) for an example go neurons that were not directionally tuned. B: mean spike rates for all trajectories for a directionally tuned nogo neuron. C: directionality index (DI) values for all neurons in standard go/nogo trials the delay (left) and movement periods (right). D: DI values for all neurons in the extremely difficult go/nogo trials.
To assess the spatial selectivity of SEF neurons as a whole in the delay and movement period, in Fig. 7, C and D, we plot histograms of the DI for individual neurons (see methods). Figure 7C summarizes the DI value in standard go/nogo trials (easy and difficult) for all 44 task-related neurons. Neurons having a DI value significantly >0.0 as determined by the 95% confidence interval of the DI value, are considered directionally selective and marked by lighter symbols. During the delay period, as shown in the left panel, a good number of task-related SEF neurons (14 of 34 go neurons; 2 of 10 nogo neurons) are not directionally tuned in the delay period. For those that were, 48% of them displayed higher contralateral spike rate (12 of 20 for go neurons, 1 of 8 for nogo neurons); four neurons showed a higher ipsilateral spike rate in the delay period (3 go neurons and 1 no go neuron). During the movement period, as shown in the right panel, a greater portion of go (18/34) and nogo (8/10) neurons were not directionally tuned. Figure 7D shows results for extremely difficult go/nogo trials. The portion of directionally tuned neurons was <50% (6/15) in the delay period (left), and only increased slightly (7/15) in the movement period (right).
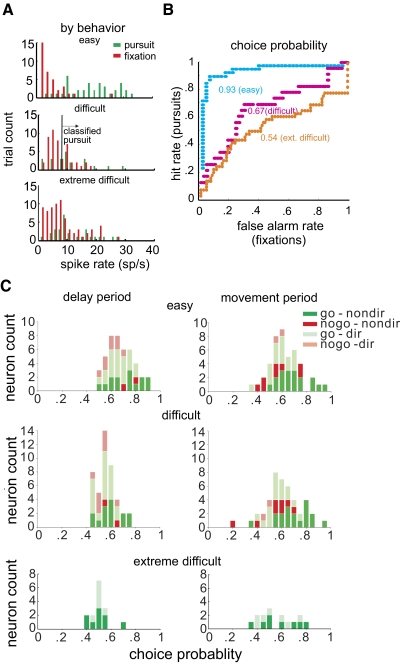
To assess whether the differential activity for rule-related neurons in go and nogo trials predicted the actual behavioral choices, we computed the CP for individual neurons as has been done previously with neurons implicated in perceptual decision making (Purushothaman and Bradley 2005). If a neuron has a CP of 1.0, it perfectly signals the animal's behavioral decision. Note that although the CP value mainly reflects the ocular decision, it is also likely affected by other factors such as target trajectory (see methods). Figure 8 shows the quantitative derivation of CP for an example go neuron, and the population results of CP over the delay period. In Fig. 8A the frequency distributions of mean spike rate in a 25 ms interval for individual trials in a single block of trials are displayed, sorted by the subsequent behavioral choice. The pursuit and fixation distributions are analogous to “signal+noise” and “noise” distributions in the signal detection theory from which this analysis is derived (Green and Swets 1966). When a decision criterion (vertical line) is set at a given spike rate, the proportion of pursuits to the right of the criterion spike rate is correctly predicted by the spike rate. Shifting the criterion further left results in an increasing number of fixations classified incorrectly as pursuits. The ROC functions (Fig. 8B) are derived by plotting the proportion of correct pursuits against the proportion of incorrect fixations predicted by different criterion spike rates, and the area under each curve yields the CP. For this example neuron, CP had a relatively high value of 0.93 for easy trials. For difficult trials, however, its CP decreased (W = 721, n = 29, P < 0.001).
Fig. 8.
Choice probability for SEF neurons. A: the frequency distributions of mean spike rate in a 25 ms interval for individual trials obtained from a go neuron, sorted by the subsequent behavioral choice. Results from easy, difficulty and extremely difficult trials were plotted separately. B: the receiver operating characteristics (ROC) functions derived by plotting the proportion of correct pursuits against the proportion of incorrect pursuits predicted by different criterion spike rates, and the area under each curve yields the choice probability (CP). C: CP in easy, difficult, and extremely difficult trials in the delay period. D: CP results in the movement period.
Figure 8C, left, are the CP values in the delay period for all neurons in easy, difficult and extremely difficult trials. Directional neurons were marked with light-colored symbols. Here it can be seen that the CP values in the delay period decreased when task difficulty was increased (easy: 0.70; difficult: 0.59, P < 0.05). Directionally tuned (0.67) and nondirectional neurons (0.75). In the right panel are the CP values for the movement period. The change in CP value in relation to task difficulty was not apparent (easy = 0.65; difficult = 0.64), and there is even higher CP values for directional neurons (0.67) than for nondirectional ones (0.58, P < 0.01). In addition, for easy trials, the CP value was higher in the delay period (0.68) than in the movement period (0.65, P < 0.05). That CP, an indicator of whether a neuron agrees with the animal's ocular decision, was higher in the delay period and decreased in the difficult trials, suggests that these cells are not directly involved in enforcing the ocular decision. The lack of a relationship between directionality and CP value further refute a direct role of these SEF neurons in specifying the ocular response.
SEF activity and decision making
To directly test whether these SEF neurons were veridically encoding the rule state or enforcing the decision, we compared their activity in go error trials (failure to pursue in go trials) to that in go success trials. Because there were very few go errors in easy trials and only few neurons with extremely difficult results, only results from difficult trials were analyzed. Nogo errors were not analyzed also because of their low frequency. Figure 9A shows the mean activity of a typical go neuron in go success, nogo success, and go decision error trials recorded in a block of difficult trials. Here the activity in go error trials had a similar profile to that observed in go success trials. Figure 9B shows the result obtained from a nogo neuron. The activity in go error trials was similar to that in go success trials as well. Figure 9C shows the spike rate of individual go and nogo neurons in go error trials normalized relative to go and nogo successful trials during the delay period in difficult trials (see methods). The direction of the bars indicates whether the activity in error trials is better predicted by the rule or the behavioral choice. A value of −1 or 1 indicates the mean spike rate is the same as that of success nogo and go trials, respectively. Only neurons with a minimum of 10 error trials in difficult trials are shown. Here it can be seen that the activity of most neurons reflect the rule better than the behavior. Figure 9D shows the results in the movement period, revealing a similar tendency of veridically signaling the rule rather than the ocular decision.
Fig. 9.
Neural activity in success and decision error trials. A: spike rate in go error (dashed green), go success (green), and nogo success (red) trials recorded from a go neuron in difficult trials. The vertical line indicates the corresponding square intersection times for go and nogo trials, and the black arrow indicates the separation time for go decision error and nogo success trials. The shaded area indicates the delay period with which the mean spike rate of these types of trials is compared in Fig. 8C. B: spike rate in success and go errors trials recorded from a nogo neuron in difficult trials. C: the mean spike rate of go and nogo neurons in error trials in the delay period that was normalized against that in go-success and nogo-success trials in the delay period (see methods). Bars pointing to the right indicate that the spike rate is more similar to that in go success trials, thus consistent with the rule; left-pointing bars indicate that the activity is consistent with the behavioral choice. The dashed vertical lines indicate the normalized go rate for success go (green) and nogo (red) trials. D: the normalized mean spike rate of go and nogo neurons in error trials in the movement period.
DISCUSSION
The present study investigated whether the SEF is involved in encoding the rule of an ocular go/nogo task or in enforcing the ocular decision. We found that the activity of individual SEF neurons reflected pursuit latency and the difficulty of the ocular decision task. Furthermore, in both the delay and movement periods, the activity reflected whether a given trajectory specified go or nogo but not the decision of the animal, i.e., to fixate or pursue. Many of these go and nogo neurons lacked spatial selectivity in both the delay and movement periods. The spatially selective neurons were no more predictive of the ocular decision than were the nonselective ones. Therefore the SEF appears to play a similar role to the PMC in signaling a rule-conforming response and does not enforce the ocular decision itself.
Given that PMC and SEF activity both suggest the rule-conforming choice, what different function could the SEF perform relative to the PMC? Because the SEF receives inputs from the PMC, the rule-encoding signal from the PMC might be further transformed in the SEF to suggest a response more specific to the oculomotor system. Previous research has shown that microstimulation of the PMC evokes forelimb movements as well eye movements (Fujii et al. 1998, 2002), whereas SEF stimulation only affects saccades and pursuit (Park et al. 2006; Tehovnik 1995; Tian and Lynch 1995). Other studies have demonstrated that the PMC is involved in planning movement sequences (Nakayama et al. 2008), whereas the SEF is more active in specifying a single movement when a movement sequence is performed (Isoda and Tanji 2002, 2003). Therefore the PMC could help specify a complex multimodal movement and/or sequential responses, whereas the signal in the SEF might be specific to a single, pending oculomotor response.
If the SEF does not make the ocular decision, where is the decision made? The FEF is certainly a candidate structure. This region is involved in initiating voluntary saccadic and pursuit eye movements (Gottlieb et al. 1993; Schall 1991a), and lesions here impair saccade and pursuit initiation (Guitton et al. 1985; Keating 1991; Lynch 1987; Morrow and Sharpe 1995). Furthermore, the FEF has been shown to encode the behavioral choice in another decision task (Kim and Shadlen 1999) and has extensive reciprocal connections with the SEF (Huerta and Kaas 1990; Luppino et al. 2003). The SEF and FEF are both active in many ocular decision tasks, such as antisaccade (Amador et al. 2004; Schlag-Rey et al. 1997) and go/nogo tasks (Brown et al. 2008; Hanes et al. 1998; Stuphorn et al. 2000, Stuphorn and Schall 2006). Finally, a functional magnetic resonance imaging (fMRI) study showed that human FEF is significantly activated in our ocular go/nogo task but not for the same stimuli or smooth pursuit movements when the decision is not required (Heinen et al. 2006). If the FEF does make the decision, it likely sums SEF input with that from other structures, such as those involved in attention, e.g., cingulate cortex (Stanton et al. 2005; Wang et al. 2004).
We do not believe that SEF neurons recorded in our study were simply movement or fixation neurons for several reasons. For one, a large portion of the go neurons did not display spatial selectivity as would be required for a neuron to be involved directly in movement planning. Furthermore, the directionality of the go neurons was not higher in the movement period than in the delay period, evidence against these neurons merely being involved in sustaining pursuit movements. A similar portion of nogo neurons displayed spatial selectivity, a property that would seem unnecessary to merely maintain fixation. Finally, the majority of the neurons continued to reflect the rule even when it was inconsistent with the behavior on error trials, and directionally tuned neurons were equally likely to reflect the rule rather than the decision as nondirectional neurons.
We think that rule-encoding SEF neurons in the present study are more likely to be preparatory neurons previously observed in the SMA and SEF (Schall 1991a,b). When these studies utilized a go/nogo task to elucidate SEF activity in the waiting period, a subpopulation of neurons in the SMA and SEF maintained a higher firing rate until before the go or nogo cue was given, similar to the behavior of our neurons in the delay period. However, because the visual cue in these studies was not given until after the neurons exhibited their preparatory activity, whether these preparatory neurons were go or nogo neurons remained ambiguous. Our task allows us to discriminate go and nogo neurons within the population of movement preparation neurons because the state of the rule (go or nogo) evolves during the delay period. Therefore in the present study, rule-encoding SEF neurons could serve to hold a motor choice (go or nogo) based on the rule temporarily and signal to a downstream structure which choice should be taken, so that that structure could execute (or not) the movement. In this framework, the involvement of the SEF would be minimal after the waiting period when downstream areas have received the rule information, which might be why the activity of SEF and the CP values decrease in the movement period.
Our findings are in contrast with the results of earlier studies, in which SEF activity was observed to reflect the location of a selected target in ocular decision tasks (Chen and Wise 1995, 1996). These authors demonstrated that SEF neurons signaled the target location of an intended saccade based on a nonspatial cue, e.g., a rightward saccade cued by a red square and a leftward saccade cued by a blue circle, even when the cue was displayed at a location inconsistent with the corresponding target location for saccade. Also at odds with our results, Tremblay et al. (2002) found that SEF activity did not reflect the rule; rather the activity was tuned to the position of the selected target relative to that of the other target, but not its position in the visual field. These results suggest that SEF neurons can specify the target location of the intended saccade relative to competing distractors rather than simply indicating the location of a visual cue. Unlike in these previous studies, the current results and that of an earlier study (Kim et al. 2005) demonstrated that the spatial selectivity of SEF neurons did not predict the specifics of motor responses in a task where the rule does not specify a relationship between perceptual categories (intersect or miss the square) and a specific motor response (pursue a leftward or rightward moving target).
We do not believe that the contrasting observations of SEF spatial selectivity during rule-based ocular decisions suggest a fundamental difference in SEF function. Instead they may reflect that different types of SEF neurons were recorded from, and/or the different tasks were performed in these studies. In the earlier work, each potential target had a specific location relative to other competing targets, which might have recruited only SEF neurons capable of spatial tuning. In contrast, in our task a go response is not specified by a target's location in space, rather it is guided by the abstract rule that the target must intersect a square for the response to be required. Therefore populations of spatially selective and nonselective SEF neurons may be both recruited to signal go and nogo rule-conforming responses.
To conclude, in the cascading process of oculomotor decision making, the SEF appears to be a stage where rule-conforming go-nogo ocular responses are specified; however, it is unlikely directly involved in making the ocular decision. Other downstream areas, that likely include the FEF, might act on the SEF signal and decide on the ocular response instead. Different areas displaying rule-encoding activity therefore might serve as functionally distinctive stages to transform abstract rules into executable motor commands. The current results highlight a way forward in understanding these processes.
GRANTS
The studies described here are supported by National Eye Institute Grant EY-117720 to S. Heinen and by a R. C. Atkinson Fellowship at Smith-Kettlewell Eye Research Institute.
REFERENCES
- Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol 91: 1672–1689, 2004. [DOI] [PubMed] [Google Scholar]
- Badler JB, Heinen SJ. Anticipatory movement timing using prediction and external cues. J Neurosci 26: 4519–4525, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol 256: 211–228, 1987. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 286: 353–375, 1989. [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996. [DOI] [PubMed] [Google Scholar]
- Brown JW, Hanes DP, Schall JD, Stuphorn V. Relation of frontal eye field activity to saccade initiation during a countermanding task. Exp Brain Res 190: 135–151, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 363: 642–664, 1995. [DOI] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Supplementary eye field contrasted with the frontal eye field during acquisition of conditional oculomotor associations. J Neurophysiol 73: 1122–1134, 1995. [DOI] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Evolution of directional preferences in the supplementary eye field during acquisition of conditional oculomotor associations. J Neurosci 16: 3067–3081, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries W. Inputs from motor and premotor cortex to the superior colliculus of the macaque monkey. Behav Brain Res 18: 95–105, 1985. [DOI] [PubMed] [Google Scholar]
- Fujii N, Mushiake H, Tanji J. An oculomotor representation area within the ventral premotor cortex. Proc Natl Acad Sci USA 95: 12034–12037, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, Mushiake H, Tanji J. Distribution of eye- and arm-movement-related neuronal activity in the SEF and in the SMA and pre-SMA of monkeys. J Neurophysiol 87: 2158–2166, 2002. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol 69: 786–799, 1993. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966. [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res 58: 455–472, 1985. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, 2nd, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol 79: 817–834, 1998. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Rowland J, Lee BT, Wade AR. An oculomotor decision process revealed by functional magnetic resonance imaging. J Neurosci 26: 13515–13522, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol 86: 2543–2558, 2001. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol 95: 3596–3616, 2006. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Kaas JH. Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol 293: 299–330, 1990. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol 265: 332–361, 1987. [DOI] [PubMed] [Google Scholar]
- Isoda M, Tanji J. Cellular activity in the supplementary eye field during sequential performance of multiple saccades. J Neurophysiol 88: 3541–3545, 2002. [DOI] [PubMed] [Google Scholar]
- Isoda M, Tanji J. Contrasting neuronal activity in the supplementary and frontal eye fields during temporal organization of multiple saccades. J Neurophysiol 90: 3054–3065, 2003. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980. [DOI] [PubMed] [Google Scholar]
- Keating EG. Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp Brain Res 86: 311–323, 1991. [DOI] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci 2: 176–185, 1999. [DOI] [PubMed] [Google Scholar]
- Kim YG, Badler JB, Heinen SJ. Trajectory interpretation by supplementary eye field neurons during ocular basenogo. J Neurophysiol 94: 1385–1391, 2005. [DOI] [PubMed] [Google Scholar]
- Leigh R, Zee D. The Neurology of Eye Movements. New York: Oxford, 2007. [Google Scholar]
- Luppino G, Rozzi S, Calzavara R, Matelli M. Prefrontal and agranular cingulate projections to the dorsal premotor areas F2 and F7 in the macaque monkey. Eur J Neurosci 17: 559–578, 2003. [DOI] [PubMed] [Google Scholar]
- Lynch JC. Frontal eye field lesions in monkeys disrupt visual pursuit. Exp Brain Res 68: 437–441, 1987. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex: Complex neural properties for complex behavior. Neuron 22: 15–17, 1999. [DOI] [PubMed] [Google Scholar]
- Miller EK, Freedman DJ, Wallis JD. The prefrontal cortex: categories, concepts and cognition. Philos Trans R Soc Lond B Biol Sci 357: 1123–1136, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Nieder A, Freedman DJ, Wallis JD. Neural correlates of categories and concepts. Curr Opin Neurobiol 13: 198–203, 2003. [DOI] [PubMed] [Google Scholar]
- Morrow MJ, Sharpe JA. Deficits of smooth-pursuit eye movement after unilateral frontal lobe lesions. Ann Neurol 37: 443–451, 1995. [DOI] [PubMed] [Google Scholar]
- Muhammad R, Wallis JD, Miller EK. A comparison of abstract rules in the prefrontal cortex, premotor cortex, inferior temporal cortex, and striatum. J Cogn Neurosci 18: 974–989, 2006. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Fujii N, Tanji J. Microstimulation of the lateral wall of the intraparietal sulcus compared with the frontal eye field during oculomotor tasks. J Neurophysiol 81: 1443–1448, 1999. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Yamagata T, Tanji J, Hoshi E. Transformation of a virtual action plan into a motor plan in the premotor cortex. J Neurosci 28: 10287–10297, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature 341: 52–54, 1989. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Prefrontal cortex in relation to other cortical areas in rhesus monkey: architecture and connections. Prog Brain Res 85: 63–94, 1990. [DOI] [PubMed] [Google Scholar]
- Park J, Schlag-Rey M, Schlag J. Frames of reference for saccadic command tested by saccade collision in the supplementary eye field. J Neurophysiol 95: 159–170, 2006. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Cortical mechanisms and cues for action. Philos Trans R Soc Lond B Biol Sci 308: 101–111, 1985. [DOI] [PubMed] [Google Scholar]
- Passingham RE. The Frontal Lobes and Voluntary Action. Oxford Psychology Series. Oxford, UK: Oxford, 1993, vol. 21 [Google Scholar]
- Purushothaman G, Bradley DC. Neural population code for fine perceptual decisions in area MT. Nat Neurosci 8: 99–106, 2005. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res 12: 1795–1808, 1972. [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Effect of eye position within the orbit on electrically elicited saccadic eye movements: a comparison of the macaque monkey's frontal and supplementary eye fields. J Neurophysiol 69: 800–818, 1993. [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Neurons in the supplementary eye field of rhesus monkeys code visual targets and saccadic eye movements in an oculocentric coordinate system. J Neurophysiol 76: 825–848, 1996. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, Kaas JH. Topography of supplementary eye field afferents to frontal eye field in macaque: implications for mapping between saccade coordinate systems. Vis Neurosci 10: 385–393, 1993. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. J Neurophysiol 66: 559–579, 1991a. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys. J Neurophysiol 66: 530–558, 1991b. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol 57: 179–200, 1987. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature 390: 398–401, 1997. [DOI] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field. I. Comparative aspects of mesencephalic and pontine connections. J Comp Neurol 301: 618–642, 1990. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Friedman HR, Dias EC, Bruce CJ. Cortical afferents to the smooth-pursuit region of the macaque monkey's frontal eye field. Exp Brain Res 165: 179–192, 2005. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci 9: 925–931, 2006. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature 408: 857–860, 2000. [DOI] [PubMed] [Google Scholar]
- Tanji J, Mushiake H. Comparison of neuronal activity in the supplementary motor area and primary motor cortex. Brain Res Cogn Brain Res 3: 143–150, 1996. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ. The dorsomedial frontal cortex: Eye and forelimb fields. Behav Brain Res 67: 147–163, 1995. [DOI] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Slow and saccadic eye movements evoked by microstimulation in the supplementary eye field of the cebus monkey. J Neurophysiol 74: 2204–2210, 1995. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Gettner SN, Olson CR. Neurons with object-centered spatial selectivity in macaque SEF: do they represent locations or rules? J Neurophysiol 87: 333–350, 2002. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature 411: 953–956, 2001. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol 90: 1790–1806, 2003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Isoda M, Matsuzaka Y, Shima K, Tanji J. Prefrontal cortical cells projecting to the supplementary eye field and presupplementary motor area in the monkey. Neurosci Res 53: 1–7, 2005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Matsuzaka Y, Shima K, Tanji J. Cingulate cortical cells projecting to monkey frontal eye field and primary motor cortex. Neuroreport 15: 1559–1563, 2004. [DOI] [PubMed] [Google Scholar]
- White IM, Wise SP. Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res 126: 315–335, 1999. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Crit Rev Neurobiol 10: 317–356, 1996. [DOI] [PubMed] [Google Scholar]
- Yang SN, Heinen SJ, Missal M. The effects of microstimulation of the dorsomedial frontal cortex on saccade latency. J Neurophysiol 99: 1857–1870, 2008. [DOI] [PubMed] [Google Scholar]