Abstract
We studied the circuitry that underlies the behavior of the local edge detector (LED) retinal ganglion cell in rabbit by measuring the spatial and temporal properties of excitatory and inhibitory currents under whole cell voltage clamp. Previous work showed that LED excitation is suppressed by activity in the surround. However, the contributions of outer and inner retina to this characteristic and the neurotransmitters used are currently unknown. Blockage of retinal inhibitory pathways (GABAA, GABAC, and glycine) eliminated edge selectivity. Inverting gratings in the surround with 50-μm stripe sizes did not stimulate horizontal cells, but suppressed on and off excitation by roughly 60%, indicating inhibition of bipolar terminals (feedback inhibition). On pharmacologic blockage, we showed that feedback inhibition used both GABAA and GABAC receptors, but not glycine. Glycinergic inhibition suppressed GABAergic feedback inhibition in the center, enabling larger excitatory currents in response to luminance changes. Excitation, feedback inhibition, and direct (feedforward) inhibition responded to luminance-neutral flipping gratings of 20- to 50-μm widths, showing they are driven by independent subunits within their receptive fields, which confers sensitivity to borders between areas of texture and nontexture. Feedforward inhibition was glycinergic, its rise time was faster than decay time, and did not function to delay spiking at the onset of a stimulus. Both the on and off phases could be triggered by luminance shifts as short in duration as 33 ms and could be triggered during scenes that already produced a high baseline level of feedforward inhibition. Our results show how LED circuitry can use subreceptive field sensitivity to detect visual edges via the interaction between excitation and feedback inhibition and also respond to rapid luminance shifts within a rapidly changing scene by producing feedforward inhibition.
INTRODUCTION
The local edge detector (LED) was first described by Levick (1967) who characterized its response as sluggish, with a narrow receptive field center and a strong antagonistic surround. He found that a stimulus consisting of drifting gratings confined to the receptive field center elicited vigorous spiking, but spiking was strongly suppressed when the drifting stimulus was expanded to include the surround. This property was noted as the LED's “trigger feature.” Roska et al. (2001, 2006) showed that these cells responded with sustained spiking to extended edges, suggesting that a static inhibition was elicited by illumination of the receptive field surround, which limited the region of response. This type of antagonistic surround is crucial for performing a type of edge detection proposed by Marr and Hildreth (1980) and the LED was suggested in a recent study (Zeck et al. 2005) to be a candidate for delineating “zero crossings” of contrast (a point in space that straddles a large differential in luminance). Behaviorally, signals that encode such edges play a crucial role in locating prey (Cuthill et al. 2005) and the various camouflaging methods used by prey species seem to purposely aggravate these signals (Stevens and Cuthill 2006).
The dendrites of the LED in rabbits span about 100 to 200 μm (the smallest of any ganglion cell) and overlap extensively with each other, suggesting a spacing of about 30 μm near the visual streak (van Wyk et al. 2006). This implies that the function of the LED is performed at high visual resolution. Morphology resembling the LED is also found in several mammalian species (Berson et al. 1998; Xu et al. 2005; Zeck et al. 2005), including macaque fovea (Calkins and Sterling 2007), further implying a generalized high-acuity function.
The complex center–surround interaction originally discovered by Levick (1967) was further characterized in a recent work by van Wyk et al. (2006). They found that the surround antagonism was a result of suppression of excitation, as opposed to direct inhibition onto the cell (feedforward inhibition). Their study, however, did not design stimuli to specifically separate the effect of horizontal cells from inhibitory neurons that reside in the inner retina (amacrine cells; see Supplemental Fig. S1 for retinal structures and terminology)1 and they concluded that further work was needed to do so. Such an investigation would require answering an additional question that remained open: which neurotransmitter systems are involved in building LED circuitry? Their conclusions about the temporal properties of feedforward inhibition also required further investigation. Although the LED does not respond to high-frequency stimuli, transient spiking is produced at the initial onset of such stimuli, suggesting that feedforward inhibition might not play a role in creating the LED's sluggish response property.
In this study, we defined more of the details of the neural circuitry that lead to the edge encoding and temporal response properties of the LED. We pharmacologically dissected the excitatory and inhibitory pathways in the center and surround of the receptive field, using spatial stimuli designed to separate the contributions of the inner plexiform layer (IPL, driven by inhibitory amacrine cells) and the outer plexiform layer (OPL, driven by horizontal cells). We show that γ-aminobutyric acid (GABA) inhibits bipolar cells that excite the LED. This feedback inhibition is driven by stimuli in the surround, uses both GABAA and GABAC receptors, and is itself inhibited by glycine. Furthermore, we show that the trigger feature of the LED first found by Levick (1967) was the result of excitation and feedback inhibition being capable of responding to features smaller than their receptive fields. Feedforward inhibition also exhibited this same subreceptive field spatial tuning. We also characterized the temporal properties of feedforward inhibition and suggest that it plays a role in blocking spikes during rapid shifts in luminance, as an alternative to the spike-delaying function proposed by van Wyk et al. (2006). We conclude that these components combine to form a circuit that is capable of encoding spatial edges and shifts in luminance.
METHODS
All procedures involving live animals were approved by the University of California Berkeley Animal Care and Use Committee and performed in accordance with institutional guidelines. Rabbits were killed and their eyes were removed and hemisected as described previously (Roska and Werblin 2001; Roska et al. 2006). Segments of the visual streak along with their associated sclera were stored in oxygenated Ames medium in the dark. Individual segments were removed from the sclera and flat-mounted on Millipore paper containing a 4-mm center hole. The mounts were perfused with Ames solution at 32°C. The solution was saturated with a 95% O2-5% CO2 mixture and pH-buffered with NaHCO3 to a pH of 7.4.
Whole cell voltage clamp
Local edge detectors, identified as having a sustained on–off spiking pattern with loose patch, as indicated by van Wyk et al. (2006), were whole cell patch-clamped with glass electrodes, with a resistance between 5 and 10 MΩ. The electrodes were filled with a cesium-based intracellular solution (in mM): 113 CsMeSO4 (Sigma, St. Louis, MO); 1 MgSO4 (Fisher Scientific, Pittsburgh, PA); 7.8 × 10−3 CaCl2 (Fisher Scientific); 0.5 BAPTA [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; Fisher Scientific]; 10 HEPES [4-(2-hyrdoxyethyl)-1-piperazineethanesulfonic acid; Sigma]; 4 ATP-Na2 (Sigma); 0.5 GTP-Na3 (Sigma); 5 QX-314 chloride (Tocris Bioscience); and 7.5 Neurobiotin chloride (Vector Laboratories, Burlingame, CA), pH 7.2.
Excitatory currents were measured by voltage clamping the cell at the calculated reversal potential for chloride (−60 mV). Feedforward inhibitory currents were recorded by voltage clamping the cell at the cation reversal potential (0 mV). The chloride reversal potential was confirmed by inhibitory synaptic noise, which reversed polarity at −60 mV. In most cases, excitation was recorded first, followed by inhibition. Recordings were digitized and sampled at 10 kHz. Signals were filtered and down-sampled to a 60-Hz sample rate, the same as the update rate of the stimulus. For spike recordings, no down-sampling was performed prior to spike sorting.
Stimulus paradigms
For each of the clamp states, several stimuli were presented against a gray background, at an irradiance of 19.02 μW/cm2, using a standard DLP (digital light processing) projector, projected onto a diffuser, and focused onto the photoreceptor layer via a condenser at the start of each experiment. Rabbit rods are saturated at 0.38 μW/cm2, above which no responses can be measured (Dacheux and Raviola 1986). Our background level is roughly 50-fold this amount. Since our stimuli are all gray-scaled bitmaps, their spectral distribution can be approximated with that of white light. According to Stephan's law, 19.02 μW/cm2 of white light produces a photon flux of 7.7 × 105 photons·s−1·μm−2. The absorption coefficient of rabbit rods for white light is 27.6% (calculated from Nakatani et al. 1991; Warrant and Nilsson 1998). Rabbit rods have an inner segment area of 0.5 μm2 (Nakatani et al. 1991) and a quantum efficiency of 67% (Rodiek 1998), giving an effective rod capture rate of 71,000 photons/s. This is about 140-fold the saturation catch rate of mammalian rods for steady light (Baylor et al.,1984; Nakatani et al. 1991). Irradiance measurements and photon capture estimates show that our stimuli lie in the photopic range and responses do not include the rod pathway.
All −100% (off), +100% (on), and +300% (on) luminance values had irradiance values of 1.08, 41.29, and 72.58 μW/cm2, respectively. Special care was taken to make striped stimuli luminance-neutral, meaning decrements in irradiance by off bars were offset by increments in irradiance by adjacent on bars. This ensured that the image would produce no net change in luminance versus background at the diffuser.
As an added measure of safety, ultrafine grating stimuli (5.7-μm stripes, presumably below the detection level of bipolars) were presented to the cell under whole cell voltage clamp, thus ensuring that no response was elicited. Gratings were also used as a static background when possible. When stripe luminance was inverted, a duty cycle of 50% was used to ensure a constant luminance level (Dhingra et al. 2003). For center–surround recording, instances of center only (spot) followed by center and surround (spot plus inverting gratings) were alternated three times and averaged together before analysis to ensure against rundown.
Responses to dark edges were recorded under voltage clamp by sequentially displaying 1-s squares flashed at 21 spatial locations, spaced 30 μm apart, with a 5-s interstimulus rest time. For drifting grating squares, the square was displayed for 2 s, during which time it was drifted across the display a distance of 100 μm (one full stripe cycle), reset, and drifted again. No square was visible during the interstimulus interval. Some stimuli were run backwards or run as a limited stimuli set with a 10-s interstimulus interval to confirm that effects were not due to rundown.
Pharmacology
Experiments were repeated in the presence of pharmacologic blockers of excitation and inhibition. To block ionotropic GABAA or GABAC receptors, 5 μM SR95531 [6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide; Sigma] or 100 μM TPMPA [(1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid; Tocris Bioscience] was added to the Ames medium and perfused across the preparation. Recordings were performed a third time, with only the Ames medium as a control when it was possible to confirm washout. To block ionotropic glycine receptors, 1 μM strychnine (Sigma) was added to the Ames medium and perfused across the preparation. The wash step was repeated, but we found that strychnine was very slow to wash out and so was rarely reversible. In most instances, rundown of excitation was observed between control and drug perfusion. To compensate for this, all pharmacologic data were expressed in terms of an internal control stimulus response, often a single flashed spot.
Commonly used GABA receptor antagonists, including SR95531, also can antagonize glycine receptors (GlyRs) (Wang and Slaughter 2005). However, the concentration of SR95531 used in this study was much smaller (5 μM) than that required to appreciably affect GlyRs (20 μM). TPMPA does not affect GlyRs (Wang and Slaughter 2005).
Data analysis
All analyses were performed with Matlab R2007b (The MathWorks, Natick, MA). To derive numbers corresponding to the response of a cell to a single stimulus event, the average current over the duration of the stimulus was subtracted from the recording's baseline (the statistical mode of the entire recording), to yield a measure of current change.
To obtain an edge selectivity ratio, the average current was calculated for the 1-s duration of each flash and subtracted from the baseline current to obtain a current change value for each. For each cell, the ratio of distal (300 μm inside the edge) versus proximal (60 μm inside the edge) currents were calculated.
For measurements in which currents were expressed as a single value (as opposed to showing a current recording), currents were first measured in response to stimuli as indicated earlier. The average current was calculated by subtracting recorded values at each time from the baseline and averaging them. One value per stimulus condition per cell was generated this way. For multiple cells, average current values were averaged together and, when appropriate, statistical analyses were performed of their distribution. For normalized values, means were expressed as the proportion of the maximum average current change in a single stimulus sequence. Some cells produced peak values at different input parameters and thus average normalized peak values were sometimes <1.0.
Since feedback inhibition acts at bipolar axon terminals, it can be measured only by inference and not by DC measurements (as in the case with feedforward inhibition). We used a center-only stimulus to measure a baseline level of excitation, followed by a center–surround (the same center spot plus stripes in the surround) and record excitation to measure how much it was reduced by surround stimulation. To calculate the strength of feedback inhibition, we divided the average current recorded over the duration of the center–surround stimulus by the center-only stimulus, which gives the amount of current remaining when surround stimulus is included. We then subtracted this from 1.0 and expressed as a percentage to show the current reduction. See Supplemental Fig. S2 for an illustration of how feedback inhibition is measured and calculated.
All error bars indicate an interval corresponding to the SE. Statistical tests comparing means of populations were performed with a paired t-test. Statistical tests comparing a population mean to a specific number were performed with a one-sample t-test. The threshold for statistical significance was P < 0.05.
RESULTS
Inhibition enhances representation of luminance edges
The LED shows strong response to a flashed edge (Roska and Werblin 2001; Roska et al. 2006). This effect could be generated by horizontal cell-mediated surround antagonism, amacrine cell surround feedback inhibition, or both. What retinal circuitry mediates this edge enhancement? To answer this, we used a series of dark squares flashed for 1 s at 21 positions across the receptive field of the LED (Fig. 1A), while recording excitatory currents.
Fig. 1.
Feedback inhibition enhances the excitatory current profile representing an edge. A: dark gray square represents the edge of a 600 × 600 μm dark (−100% luminance) square against a gray background as it is flashed at different positions with respect to the cell dendritic field shown in the dashed circle. Stimulus position is shown at 5 key locations with respect to the dendritic field. Traces show the average excitatory currents for each position under control (n = 29) and with all inhibition blocked (γ-aminobutyric acid types A and C [GABAA and GABAC, respectively] and glycine, n = 5). Gray bar in each recording indicates the 1 s duration of the flash. Location intervals are spaced at 30 μm. The ratio of currents for 2 indicated stimulus positions is calculated. B: profile excitatory current measurements for 21 stimulus positions under control and with all inhibition blocked. Offset of square (relative to perfect centering at 0 μm) is indicated on the x-axis. Large gray square indicates locations where the dendritic field of the cell is located inside the square.
The strong edge selectivity of excitation seen in Roska et al. (2001, 2006) was reproduced (Fig. 1B), with locations on the inside of the edge producing a vigorous response and locations distal from the edge being highly attenuated. On blockage of all common forms of retinal inhibitory receptors (GABAA with SR95531, GABAC with TPMPA, and glycine with strychnine), edge enhancement was suppressed, resulting instead in a stronger response over a broader area (Fig. 1B). We took the ratio of average currents for areas located 300 μm inside the edge (offset value of 0 μm) versus 60 μm inside the edge (offset value of 240 μm), to yield a measure of “edge selectivity” (lower numbers indicate higher selectivity; 1.0 would indicate no selectivity). Under control conditions, the LED had an average edge selectivity of 0.33 ± 0.04 (n = 29), whereas when inhibition was blocked, edge selectivity was 0.71 ± 0.07 (n = 5), showing that all or some of the inhibitory systems produce a twofold increase in edge selectivity (0.71/0.33 = 2.15), which is significant (P < 0.01).
Feedback inhibition originates from the inner plexiform layer and has a broad spatial tuning
Figure 1 suggests that the majority of surround antagonism in the LED circuit originates from interneurons that release either GABA or glycine. However, the stimulus used in Fig. 1 cannot separate inner plexiform versus outer plexiform effects due to the fact that the large luminance change could generate responses in both amacrine cells (IPL) and horizontal cells (OPL) (see Supplemental Fig. S1). To address this problem, we designed a series of stimuli and pharmacologic conditions that show the activation of horizontal cells components individually.
Figure 2A shows two spot sizes used in Fig. 2B: a 150-μm followed by a 2,000-μm dark spot of −100% luminance. The 150-μm spot covers only the excitatory portion of the LED receptive field (Supplemental Fig. S3A), whereas the 2,000-μm spot stimulates the excitatory portion plus any antagonistic surround components. Figure 2B shows average current recordings in response to these spots under control conditions and under blockage of GABAA, GABAC, and glycine receptors. The 2,000-μm spot induces a significant reduction in excitatory currents versus the 150-μm spot, under control conditions (Fig. 2C, white circle, P < 0.01), due to its ability to recruit OPL circuitry (plus any putative IPL inhibitory circuitry). Since the action of horizontal cells on the photoreceptor terminal is known not to be GABAergic or glycinergic (Cook and McReynolds 1998; Hare and Owen 1996; McMahon et al. 2004; Verweij et al. 1996, 2003), but is instead mediated by a non-neurotransmitter-mediated mechanism (Hirasawa and Kaneko 2003; Kamermans et al. 2001), the significant reduction in excitation seen under blockage of inhibition (Fig. 2C, black circle, P < 0.01) must be due to horizontal cells alone. Thus this pharmacologic condition, stripped of any IPL-originated surround inhibition, allows us to assess the antagonistic effect of horizontal cells on excitation in response to a range of stripe sizes presented and inverted in the surround, using a 150-μm center spot to drive excitation (Fig. 2D).
Fig. 2.
Spatial tuning of surround antagonism under control and inhibitory block. A: stimuli used for B and C. Left: 150-μm off (−100% luminance) spot used to stimulate only the excitatory center. Right: 2,000-μm off spot used to stimulate both center excitation and antagonistic surround. B: average excitatory current recordings in response to large and small spot under control (n = 10) and with all inhibitory receptors blocked in (GABAA, GABAC, and strychnine, n = 10). C: normalized average currents for each pharmacologic condition. Values are derived by dividing the 2,000-μm average spot currents by 150-μm average spot currents. Error bars = SE, n = 10 for both conditions. D: grating stimuli used for E. All consist of luminance-neutral stripes, of widths from 50 to 800 μm. All contain a 150-μm off spot in the center to drive excitation. Stripe polarity is inverted at 2 Hz for 3 s. E: normalized average currents recorded in response to stimuli in D. Values are derived by dividing the average currents in response to stripe-containing stimuli by a 150-μm off spot flashed for the same duration. Error bars = SE, n = 10 for both pharmacologic conditions.
Figure 2E shows the average excitatory currents measured in response to each stripe size presented to the surround of the LED, normalized against a center spot stimulus. The control condition represents the contribution of both inner- and outer plexiform circuitry, whereas the “inhibition blocked” condition eliminates inner plexiform interactions, leaving only horizontal antagonisms intact. At stripe sizes of 100–400 μm, the reduction in current is significant (P < 0.05), suggesting that rabbit horizontal cells respond optimally to this range of strip widths. However, at 50 μm and 600–800 μm, no reduction in current is significant (P = 0.27, 0.43, 0.35, respectively). This horizontal cell tuning curve allows us to select a stripe size of 50 μm for subsequent experiments that can exclude horizontal cell effects. This also shows that the spatial tuning for feedback inhibition is broad and responds to a wide range of feature sizes.
Feedback inhibition extends to 750 μm and uses both GABAA and GABAC receptors, but not glycine
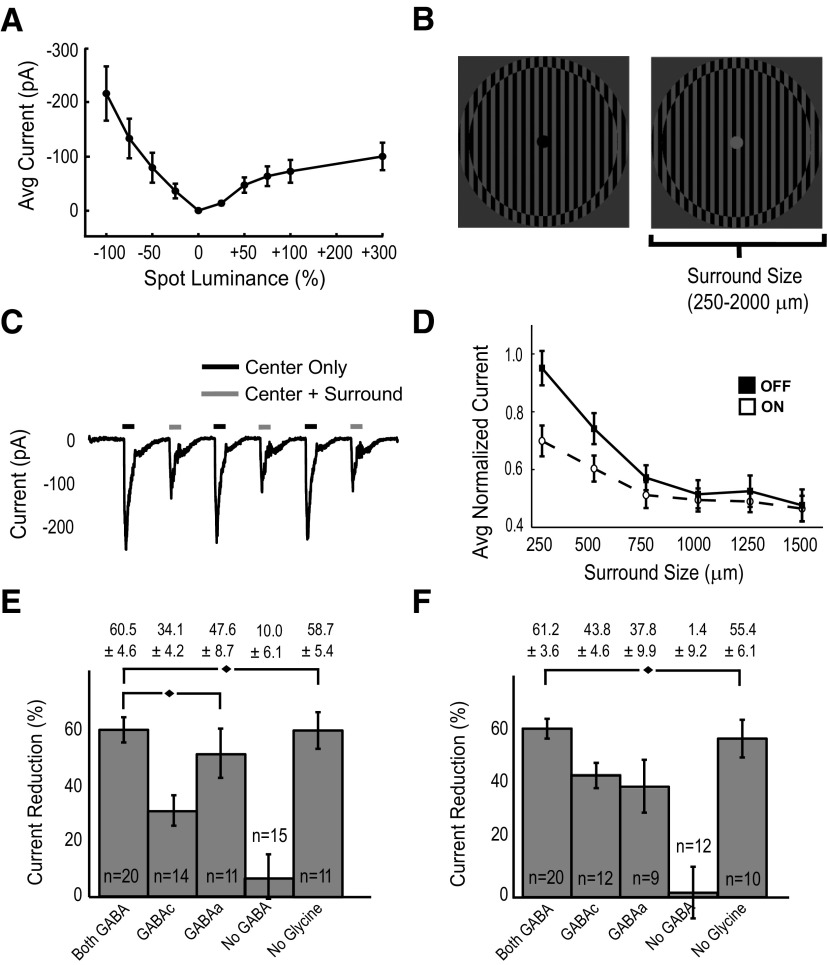
We stimulated the center 150 μm of the LED's receptive field with spots varying in luminance from −100 to +300% (Fig. 3A). Within the range of luminance values tested, the LED is off-dominant: a 100% reduction in luminance elicited more than twice the excitatory current magnitude as a 100% increment in luminance. Since +300% appeared not to saturate the response, we used this luminance level in the center to evoke meaningful on excitatory currents. We used −100% center luminance to evoke off excitatory currents.
Fig. 3.
Luminance response, inhibitory receptive field size, and pharmacology of presynaptic inhibition. A: average current recordings in response to 200-μm spots of varying luminance levels from −100% to +300%. Error bars = SE, n = 10. B: stimuli used in C–F. The center 150 μm was either solid on (+300% luminance) or off (−100% luminance). All stripes were 50 μm and inverted luminance polarity at 2 Hz for 3 s. The area that was inverted could be varied (from 250 to 1,500 μm for D, or the entire 2,000 μm for C, E, and F). C: an example excitatory current trace (for an off center spot) in response to center only (spot) stimulation vs. center plus surround (spot plus inverting gratings) stimulation. D: average normalized excitatory current of off and on center spot stimulation for increasing areas of inverting gratings in the surround. All current levels were normalized against a solid 150-μm center spot. Error bars = SE, n = 17 for the off system, n = 20 for the on system. E and F: average excitatory current reduction of center spot stimulation when full-field inverting gratings are used in the surround for the off spot (E) or for the on spot (F). Bars represent the percentage of this reduction under various pharmacologic conditions, labeled by which receptor system(s) remains intact after pharmacologic blockage. Error bars = SE, n values as indicated for each condition. All condition values that are nonsignificant relative to control are indicated with a ♦.
We tested properties of feedback inhibition by using an inverting grating with a stripe width of 50 μm (Fig. 3B), a dimension we found to be below the feature sensitivity of horizontal cells in rabbit (Fig. 2), as well as shown by others in several other species (Dacey et al. 2000; Demb et al. 1999, 2001a,b; Passaglia et al. 2001; Werblin 1972). The ensemble of stripes was luminance-neutral, thus eliciting no response in horizontal cells. Example current traces for center-only (spot) and center plus inverting grating surround stimulation (as described earlier) are shown in Fig. 3C. All stimuli were presented in this alternating pattern to ensure that there was no bias due to current rundown. Center plus surround currents were divided by center-only currents and subtracted from 1.0 to obtain a measure of the percentage reduction in current. This functioned as an internal control for any baseline current or gain changes that might occur under various pharmacologic conditions.
We also used a version of this same grating stimulus in which the area of inverting grating was varied progressively from 200 to 1,500 μm, activating different amounts of surround area. Data in Fig. 3D show that excitatory currents attenuated monotonically up to 750 μm. Specifically, attenuation of 52.8 ± 4.3% (n = 20, P < 0.01) occurred for the off system and attenuation of 58.8 ± 4.5% (n = 17, P < 0.01) occurred for the on system, beyond which further reductions in current seem apparent but are no longer statistically significant. Thus for off and on systems, 750 μm is the spatial extent of effective feedback inhibition, which is approximately fivefold the size of the receptive field center.
To determine the neurotransmitter systems used by feedback inhibition, we presented center-only and center–surround (full 2,000-μm size) under various combinations of inhibitory receptor blockers. We blocked GABAA with SR95531 alone, GABAC with TPMPA alone, or with both, in addition to strychnine alone. This produced conditions under which only GABAC, GABAA, or neither GABA receptor system was operational, respectively. For off currents (Fig. 3E), we observed an average reduction of 60.5 ± 4.6% (n = 20, P < 0.01) due to feedback inhibition under control conditions, with both GABAA and GABAC pathways intact. The GABAC pathway alone produced a reduction of only 34.1 ± 4.2% (n = 14, P < 0.01); the GABAA pathway alone produced a reduction of 47.6 ± 8.7% (n = 11, P < 0.01). These current reductions were also of greater magnitude than the condition when both GABAA and GABAC were blocked (10.0 ± 6.1%; n = 15, P < 0.01).
The on system (Fig. 3F) showed a reduction under control conditions of 61.2 ± 3.6% (n = 20, P < 0.01). The GABAC pathway alone produced a reduction of 43.8 ± 4.6% (n = 12, P < 0.01) and the GABAA pathway alone resulted in a reduction of 37.8 ± 9.9% (n = 9, P < 0.01). As with the off system, these current reductions were also of significantly greater magnitude than the condition under which both GABAA and GABAC were blocked (1.4 ± 9.2%; n = 12, P < 0.01).
Differences in current reductions produced by each individual drug condition compared with control allowed us to suggest the pharmacology of the off and on systems. For the off system, the GABAC pathway alone significantly reduced currents (P < 0.01), but not as much as the control (P < 0.01), and the GABAA pathway alone reduced currents the same as if both receptors were operational (P = 0.16). For the on system, both GABAA and GABAC receptor systems significantly reduced currents (P < 0.01), but also not as much as the control (P < 0.01). This suggests that feedback inhibition to the off system was mediated more by GABAA receptors, with a smaller role by the GABAA system. Feedback inhibition to the on system was mediated by both GABAC and GABAA receptor systems.
Stimulation of the entire surround caused a roughly 60% suppression of excitatory currents. This suppression was eliminated by pharmacologic blockage of both GABAA and GABAC receptors. Blockage of glycine using strychnine (1 μM) did not show a significant difference compared with control conditions in the off (n = 11, P =0.81) or on (n = 10, P = 0.40) systems. This shows that feedback inhibition is mediated by GABA and not glycine.
Center excitation is enhanced by glycine
Bipolars cells that synapse onto the LED receive strong feedback inhibition, most likely provided by a network of amacrine cells. One consequence of this structure is that even stimuli that are limited to the center only could still elicit enough feedback inhibition to cancel out a large amount of excitation, even though the surround is not being activated. Since this would result in a loss of edge selectivity, it is possible that inhibition of GABA feedback inhibition could counteract this.
To test this, we flashed off and on spots in the center of the LED receptive field. Figure 4A shows average recordings used for calculations in Fig. 4B. For both the off (Fig. 4B) and on (Fig. 4B) systems, eliminating GABA via blockage with SR95531 and TPMPA showed no change in average current elicited from a single off or on spot restricted to the center for off (n = 10, P = 0.76) or on (n = 10, P = 0.31) spots.
Fig. 4.
Effect of pharmacologic blockage on excitatory currents elicited by spots in the center. A: average excitatory recordings for off (−100% luminance) and on (+300% luminance) spots (200 μm) under control (n = 11) and GABA receptor blockage (n = 10). B: average current magnitudes for off spots and on spots under control and GABA receptor blockage. Error bars = SE, n as indicated. Significant differences are indicated with an asterisk (*). C: average excitatory currents for off and on spots (200 μm) under control (n = 19), strychnine (n = 13), and all inhibition blocked (n = 16). D: average current magnitudes for off and on spots (200 μm) under control, strychnine, and all inhibition blocked. Error bars = SE, n as indicated. Significant differences are indicated with *.
When strychnine alone was perfused (average recordings shown in Fig. 4C), we observed a large decrease in current level compared with its control (Fig. 4D; n = 13, P < 0.01 for both off and on). This decrease in current was subsequently rescued via blockage of GABA to restore current levels to control for off (Fig. 4D; n = 16, P = 0.99). GABA blockage did not return the on system to control current levels (Fig. 4D, n = 16, P < 0.01). However, current levels were significantly greater than those under strychnine-only blockage (P < 0.01), suggesting a partial rescue. These data suggest the presence of serial inhibition at the receptive field center. Specifically, glycinergic inhibition of GABAergic feedback inhibition. Addition of strychnine causes an up-regulation of GABAergic inhibition, suppressing excitatory currents. Subsequent blockage of GABA receptors rescues currents by eliminating this high level of feedback inhibition.
Inhibition and excitation are generated by receptive field subunits
The stimuli illustrated in Fig. 3 used 50-μm gratings to drive feedback inhibition. This shows that feedback inhibition, in addition to being responsive to luminance changes, is also sensitive to fine detail, most likely driven by the population of individual bipolar cells within the receptive field surround of the ganglion cell, which constitute functional “subunits” of the receptive field surround. We were interested to know whether excitation and feedforward inhibition also had similar spatial tuning characteristics. Inward currents were elicited at both increments and decrements in luminance (Roska and Werblin 2001; Roska et al. 2006; van Wyk et al. 2006), indicating a nonlinear bipolar cell input to the ganglion cell in response luminance (Fig. 3A). This raises the possibility that the LED could sense detail much smaller than its receptive field, assuming bipolar cells are responding independently. To test the ability of the LED to respond to subunits, we designed a luminance-neutral inverting grating stimulus (Fig. 5A) that was restricted to the center of the LED's receptive field (150 μm; Supplemental Fig. S3A). Stripe widths varied from 10 to 70 μm to measure the spatial tuning properties of excitation. We also investigated the spatial tuning of feedforward inhibition by holding the membrane at 0 mV (the reversal potential for cations) to accentuate inhibition and eliminate excitatory currents. Figure 5 shows that excitation and feedforward inhibition responded to detail sizes as small as 20 μm (Fig. 5, B and C, P < 0.05). Response magnitude increased until reaching 40 μm. At this stripe size and above, excitation produced an average change in current for the duration of the stimulus (vs. baseline) of −87.1 ± 9.0 pA (n = 8, P < 0.01). Feedforward inhibition produced an average current change of 110.72 ± 16.07 pA (n = 10, P < 0.01). This observed 20-μm threshold size is approximately that of a typical bipolar cell dendritic field in the rabbit retina (MacNeil et al. 2004; Mills and Massey 1992). These data show that LED excitation at the receptive field center responded to features as small as one eighth the size (20 μm) of its already small receptive field (150 μm) and its response saturates to features one fourth the size (40 μm).
Fig. 5.
Local edge detector (LED) receptive field subunits. A: example of the stimulus presented. The stripe width varied from 10 to 70 μm and each was inverted at 2 Hz for 3 s against a gray background, followed by a 10-s pause. B and C: an example (B) excitatory current recording and (C) feedforward inhibitory recording in response to the series of stimuli of increasing strip width (A). Response magnitude is indicated by the scale on the left of the traces and average current in response to each stripe width (overlayed gray curve, scale right). Error bars = SE, n = 10 for both B and C. D: average spike rate in response to stimuli presented in A, n = 8.
Since feedforward inhibition had subreceptive field tuning similar to that of excitation, this raised the possibility that the two might completely negate each other for features of the size range tested. To ensure that feedforward inhibition to the LED was not canceling excitation, we also made spike recordings to these same stimuli (Fig. 5D). We observed average peak spike rates of 25–30 Hz for all stripe sizes >40 μm and spiking was elicited as low as 20 μm, mirroring the results seen for excitation.
Feedback inhibition enhances representation of textured edges
As shown thus far, feedback inhibition can be activated by luminance-neutral fine detail (features that are sized similar to bipolar dendritic fields; Figs. 2 and 3) and can also be activated in proportion to the amount of surround area stimulated (Fig. 3D). LEDs should then respond differently to areas of active texture, depending on their distance from an edge (a border delineating an area of texture and nontexture). Presumably, cells located close to a textured edge would receive less feedback inhibition than those located more distally.
Figure 6A shows a series of stimuli consisting of a luminance-neutral flashed square containing 50-μm stripes drifted at 1 Hz for 2 s. Excitatory response (Fig. 6B) to this stimulus is similar to that in Fig. 1B, although with a weaker edge selectivity (0.62). Spiking also shows a strong response selectively near the edge, nearly identical to excitation (0.63), indicating that excitatory input to the LED is enhanced at boundaries of texture, and this edge response is transmitted via spiking.
Fig. 6.
Excitatory response to a drifting striped edge. A: illustration of stimulus position (600 × 600-μm luminance-neutral striped square against a gray background containing 50-μm stripes, drifted vertically at 2 Hz) relative to LED dendritic field (dashed circle) for 5 key horizontal locations. Location intervals are spaced at 30 μm. The ratio of currents for 2 indicated stimulus positions is calculated below. B: profile of excitatory current and spike count measurements for 21 stimulus positions. Offset of square (relative to perfect centering at 0 μm) is indicated on the x-axis. Gray square indicates locations where the dendritic field of the cell is located inside the drifted square. n = 15 for both excitation and spiking.
Feedforward inhibition is glycinergic, has differences in rise time and decay time, and does not delay spiking
We investigated the pharmacology of feedforward inhibition. A strong on–off response was elicited by both off and on 200-μm spots (Fig. 7A). These inhibitory responses were eliminated on perfusion of 1 μM strychnine. Excitatory recordings were taken in conjunction with these stimuli to ensure cell viability (not shown). These data show that feedforward inhibition is generated by a population of on and off glycinergic amacrine cells, with a narrow receptive field that is cospatial with excitation (Supplemental Fig. S3, A and B).
Fig. 7.
Kinetics of glycinergic feedforward inhibition. A: pharmacology of feedforward inhibition. off (−100% luminance) and on (+300% luminance) spot (200 μm) recordings under control and 1 μM strychnine. B: average traces and time to half-maximum and half-life decay times for off and on feedforward inhibition. Time to half-maximum was calculated by first finding the half-maximum current value by subtracting the peak current from the baseline current and dividing by 2. The time to half-maximum was the earliest time the current crossed above this value in relation to the stimulus onset. Decay half-life was the earliest time the current crossed below this value in relation to the peak time; n = 6. C: average spiking, excitatory, and inhibitory responses to an off (−100% luminance, left) or on (+300% luminance) spot. Region represents 150 ms before stimulus onset to 250 ms after stimulus onset. Spike trace is an average of spike recordings; n = 15 for all recordings. D and E: average feedforward inhibitory currents in response to 33-, 67-, or 133-ms off (C) or on (D) flashes; n = 5.
Additionally, we measured the time course of feedforward inhibition to the same 200-μm stimulus. As a measure of the speed by which inhibition rises and falls, we calculated the amount of time elapsed from baseline to half the peak current (time to half-maximum), and the time elapsed from the peak back to half the peak current (decay half-life) (Fig. 7B). For off currents, the rise time (113 ms) was faster than the decay time (520 ms). Rise time (90 ms) was also faster than decay time (373 ms) for on currents. This allows the possibility of both on and off inhibition being active simultaneously if a shift from on to off (or vice versa) occurs quickly.
When overlaying spiking, excitation, and feedforward inhibition in response to off and on spots (Fig. 7C), their onsets are all delayed by about 100 ms and respond at coincident times, suggesting that feedforward inhibition is not interacting with excitation to delay spiking.
Feedforward inhibition can signal rapid luminance shifts
Because we measured a quick rise time of feedforward inhibition, we investigated whether brief stimulus durations could elicit an inhibitory response. When showing a 200-μm dark spot for 33, 67, and 133 ms, we found that in all cases an inhibitory current was generated (Fig. 7D). When showing a bright spot for the same durations, inhibition was similarly generated, although weaker (Fig. 7E). These results show that feedforward inhibition can be triggered by rapid and brief changes in the visual scene.
We then investigated whether such shifts could produce additional inhibition under scene conditions in which feedforward inhibition was already activated. A 50-μm drifting grating in the 150-μm center drifted at 1 Hz produced a sustained level of feedforward inhibition and enough excitation to overcome it, resulting in a sustained spike train (Fig. 8A). We superimposed rapid luminance shifts with a single 33-, 67-, or 133-ms flash of either a narrow (150 μm) or wide (1,000 μm), off (−100% luminance) or on (+100% luminance) spot during the drifting grating (Fig. 8B), as indicated by gray bars (Fig. 8, C–F).
Fig. 8.
Excitation, feedforward inhibition, and spiking in response to rapid luminance shifts. A: excitation, feedforward inhibition, and spiking, in response to a drifting grating containing 50-μm stripes in the center 150 μm of the receptive field. Duration of the drift is indicated with gray bar. B: stimuli used for C–F. Drifting grating is presented for the duration of the gray bar. One of the 4 indicated images is presented at the time represented by the dark or white bar. Presentation durations were 33, 67, or 133 ms. Line legend is shown for C–F. C–F: feedforward inhibition in response to stimuli indicated in B. Substantial inhibitory waves are indicated (*); n = 5 for narrow conditions, n = 7 for wide conditions.
For both wide off and on flashes (Fig. 8, D and F), feedforward inhibition was barely affected for all shift durations. For narrow off or on flashes (Fig. 8, C and E), an additional wave of feedforward inhibition was produced on top of the baseline elicited by the drifting grating for the longer dark shift times (67 and 133 ms). Bright shifts required the longest shift time to elicit a substantial inhibitory wave. This shows that even in dynamic visual environments supporting sustained inhibition, feedforward inhibitory circuitry is still responsive to narrow-field rapid luminance shifts.
DISCUSSION
Isolating interactions at the inner plexiform layer
One of the primary goals of our study was to separate and characterize the interactions at the inner plexiform layer as distinct from outer plexiform activity mediated by horizontal cells. We isolated horizontal cell activity by measuring surround antagonism in the presence of glycine and GABA blockers, leaving only horizontal cell antagonism of the excitatory pathway intact. Figure 2E shows the spatial tuning curve for horizontal cells, as seen at the photoreceptor–bipolar cell synapse, after GABA and glycine inhibition were blocked. Horizontal cells do not significantly suppress excitation when stimulated with small (50 μm) stripes because the broad tuning of horizontal cells cannot resolve this size and synaptic input to the horizontal cells is relatively linear (Demb et al. 2001a; Zaghloul et al. 2007). We then used this stripe size for subsequent experiments to measure interactions at the IPL.
However, our experimental conditions may have blocked the possible GABA release by horizontal cells. Some studies show positive staining for GABA receptors at the dendrites of mammalian bipolar cells (Billups and Attwell 2002; Vardi and Sterling 1994) and horizontal cells (Varela et al. 2005), suggesting GABAergic feedforward inhibition. GABA does not seem to be used as an inhibitory neurotransmitter in horizontal cells to inhibit cones (Kamermans et al. 2001; Verweij et al. 2003) or to bipolar cells (Cook and McReynolds 1998; Hare and Owen 1996). However, this does not matter for our purposes; the non-GABA-mediated action of horizontal cells remained intact under inhibitory blockage (Fig. 2, B and C), so even if horizontal cells release GABA, either fed back to photoreceptors or forward to bipolar cells, the grating width of 50 μm, to which horizontal cells do not respond, will not induce a measurable excitatory current reduction in our experiments.
GABA's modulatory roles in the outer retina could possibly confound our measurements. GABA has been shown to modulate horizontal surround inhibition (Kamermans et al. 2002). However, in our study only blockage of GABA receptors was performed, which is known to enhance horizontal cell feedback (Kamermans et al. 2002). The presence of an artificially high level of horizontal feedback is irrelevant to our conclusions, since no suppression of excitation was observed with 50-μm stripes in any event. Only an artificial reduction of horizontal cell feedback would lead to the false conclusion that horizontal cells exert no suppressive effect on excitation at this stripe size.
GABA receptor blockers could also act on photoreceptors, causing a gain change by shifting resting potentials. However, Tatsukawa et al. (2005) showed that the GABAA antagonist SR95531 does not change cone resting currents. The evidence for GABAC receptors on cones is unclear. Thus one cannot rule out the possibility of a TPMPA-induced gain change, although this seems unlikely due to the stability of absolute current values in response to a test stimulus when both GABAA and GABAC antagonists are applied (Fig. 4, A and B).
Contributions of GABA and glycine to edge enhancement
GABAA and GABAC pathways contribute to feedback inhibition to bipolar cells that drive the LED, but with different relative effectiveness in the off and on pathways. The off system is suppressed most effectively by the GABAA pathway, whereas the on system seems to use both pathways (Fig. 3, E and F). This suggests that both systems act additively to achieve the full 60% suppression of excitatory currents, but perhaps with varying degrees of synaptic efficacy at amacrine cell–bipolar cell inhibitory synapses.
The efficacy of synapses is largely dependent on the gain at the presynaptic terminal (as defined by the relationship between depolarization and vesicle release). Differences in gain would be a necessary feature of this circuit, since presumably bipolar cells that drive feedback-generating amacrine cells also receive feedback inhibition. If synaptic gain from amacrine cells to bipolar cell terminals is low compared with the bipolar cell–ganglion cell synapse, then this would allow release from a bipolar cell to still occur even if a large amount of neighboring bipolar cells were also stimulated and feeding back onto it.
Glycine inhibition of GABA inhibition may enhance edge representation
Strong feedback inhibition creates the spatial pattern of excitatory response seen in Fig. 1, which suggests that the LED is performing classic luminance edge detection. Even though the effectiveness of edge selectivity is reduced by blockage of inhibition, no shift in the peak of excitation (located at 60 μm inside the edge) is seen because horizontal cells are still performing some edge detection. This location should be where the LED receives the greatest amount of excitation and the least amount of surround antagonism, regardless of the source. The addition of feedback inhibition merely increases the relative amount of antagonism seen at locations more interior to the edge.
According to classic works (Enroth-Cugell and Robson 1966; Rodieck 1965), ganglion cell receptive fields can be thought of as performing a Laplacian operation (also known as a difference-of-Gaussian), which is well suited for edge detection. This function requires the presence of a strong antagonistic surround. However, sufficiently strong excitation is also necessary and a strong local feedback inhibition could attenuate center excitation. The LED circuitry appears to reduce this attenuation by suppressing GABA feedback at the receptive field center with a fed-back glycinergic inhibition (Fig. 3). Thus glycinergic and GABAergic inhibition may interact to approximate an edge-detection function by creating a strong center combined with a locally strong donut-shaped antagonistic surround. Examples of such serial inhibition have been previously reported (Jiang and Shen 2009; Zhang et al. 1997).
The possibility of disinhibition of GABA by glycine is supported by the increase in excitatory currents by GABA receptor blockers in the presence of strychnine (Fig. 4D). Although current levels are completely restored for off excitation and partially (but significantly) restored for on excitation, their waveforms become more transient. A possible explanation for this could lie in the relative kinetics of inhibition: GABA could function early after the stimulus onset to keep excitatory current magnitudes <250 pA (see the difference between heavy and dashed traces in Fig. 4A). Later after the stimulus onset (>500 ms), glycine could then turn off the GABAergic inhibition, allowing its waveform to follow a course uninfluenced by inhibition (Fig. 4A, heavy line). Such relative timings of inhibitory currents have been observed before in the shaping of excitatory currents (Eggers and Lukasiewicz 2006; Eggers et al. 2007) and, in conjunction with our data, raise the possibility that inhibition itself is capable of being fine-tuned by other inhibitory components.
Sensitivity to subreceptive field feature sensitivity at center and surround encodes textured edges
Both increments and decrements in luminance induce net inward currents (Fig. 3A). This nonlinearlity suggests that each bipolar cell system can contribute to excitation in a rectified manner without being canceled by outward currents from the opposing system. This would suggest that the excitatory receptive field of the LED is capable of responding to subreceptive field detail (i.e., features that are smaller than the 150-μm excitatory receptive field). This characteristic was in fact found not only for excitation (Fig. 5B) and consequent spiking output (Fig. 5D), but also for feedforward (Fig. 5C) and feedback inhibition (Figs. 2E and 3, C–F).
Nonlinearities and subreceptive field resolution have also been measured in the Y (alpha) cell (Demb et al. 2001a; Zaghloul et al. 2007), enabling the cell to represent textured areas. This feature has also been shown in the primate analogue for the Y cell (Crook et al. 2008), suggesting that subreceptive field resolution is present in human vision. In the case of the rabbit LED, the addition of a similarly texture sensitive feedback inhibition allows cell populations to encode luminance variance contained in its receptive field surround in addition to the absolute luminance level. This feature allows the border of the textured area to be represented as a kind of spatial edge (Fig. 6B).
Conventional role of feedforward inhibition
We found on–off feedforward inhibition to be glycinergic, responsive to subreceptive field features, and to have a receptive field peak of 150 μm, all features similar to those of excitation. The temporal properties of feedforward inhibition differ from those of excitation.
Van Wyk et al. (2006) observed that feedforward inhibition is more transient than excitation and functioned to delay the onset of spiking. Yet they observed transient spiking at the onset of even high-frequency stimuli, an unexpected observation in light of the high temporal sensitivity of feedforward inhibition. Our measurements show that inhibition is unlikely to delay spiking, due to the temporal coincidence of the onset of excitation, feedforward inhibition (Fig. 7C). Here we suggest an alternative function for feedforward inhibition.
An alternative function for feedforward inhibition
Figure 7, D and E illustrates that a large inhibitory wave can be triggered by a luminance shift as short as 33 ms for both on and off phases. Figure 8, C and E shows that this can also work within a changing scene: an additional inhibitory wave was generated on top of a steady level of inhibition during an instantaneous shift in narrow-field luminance. This can be accomplished using receptive field subunit responses (Fig. 5C). During a drifting grating, on or off feedforward inhibitory subunits are either active or inactive (depending on the luminance of the bar stimulating them). At the onset of shift to dark, for example (Fig. 8C), all off subunits respond to the luminance decrement. Because the rise time of feedforward inhibition is faster than the decay time (Fig. 7B), both on and off feedforward inhibition are active at this instant (off inhibition is rising and on inhibition is falling). These add together to produce a wave of inhibition on top of the baseline. The same mechanism applies for a shift to a bright spot (Fig. 8E).
A narrow-field drifting grating will elicit a steady level of feedforward inhibition. Spiking still persists, however (Fig. 8A), suggesting that feedforward inhibition does not block spiking under conditions of moderate continuous stimulation. Nonetheless, luminance shifts can generate additional inhibition that could suppress spiking.
Summary of our results
We have shown that the LED circuitry serves to endow the cell with sensitivity to fine detail and to allow surround activity to strongly suppress center activity. Figure 9A outlines the pharmacologic components of the circuitry derived from our measurements. Figure 9B illustrates our primary finding: the receptive field of excitatory bipolar cell input is shaped by feedback inhibition. Bipolar cells receive antagonism from horizontal cells in the OPL, creating a difference-of-Gaussian spatial profile of the receptive field (orange). Feedback inhibition has a wider Gaussian profile (blue), with the added characteristic of having no effect on the center due to glycinergic inhibition (Figs. 4D and 9A, parts l and m). This glycinergic inhibition of GABA feedback inhibition is likely restricted to the receptive field center. If glycinergic inhibition extended into the surround we would expect a greater reduction in excitation when glycine receptors were blocked and the full GABA-mediated surround inhibition was activated (see Fig. 3, E and F, “no glycine” condition), due to disinhibition of GABAergic inhibition. When the receptive fields of excitation and feedback inhibition are added together (black), center excitation is unattenuated and the immediate surround becomes highly antagonistic. This creates strong edge selectivity that can approximate the spatial location of “zero crossings” in luminance (Fig. 1; Enroth-Cugell and Robson 1966; Rodieck 1965; Zeck et al. 2005). Likewise, locations where there is a transition from texture to nontexture can be signaled via the same feedback pathway if it responds to subreceptive field details (Fig. 6). This surround property seems to be present in many ganglion cell types (Caldwell and Daw 1978a,b; Enroth-Cugell and Jakiela 1980; Solomon et al. 2006), including the alpha (Y) cell (Zaghloul 2007). This suggests that the retina may be using the diversity of ganglion cell receptive field radii to detect complex edges over a wide range of resolutions.
Fig. 9.
LED circuit diagram. A: pathways mediating LED activity. a and b: on and off bipolar cells. c and d: on and off high resolution excitatory pathways to LED dendrites. e and f: high resolution on and off glycinergic feedforward pathways. g and h: high resolution, GABAA and GABAC feedback pathways to on and off bipolar cells. j and k: high resolution excitatory on and off inputs to on and off glycinergic narrow field amacrine cells. l and m: center glycinergic suppression of GABA feedback. Glutamatergic pathways: orange arrows; GABAergic pathways: blue lines; glycinergic pathways: green lines. B: model of summation of excitatory and inhibitory receptive fields. Excitatory bipolar input to the LED (orange) has a standard difference-of-Gaussian shape, reflecting horizontal cell antagonism of excitation. Feedback inhibition likely has a Gaussian shape, but with a “blank” region due to glycinergic inhibition in the center. Excitation and inhibition add together (black) to form the excitatory receptive field of the LED: a Gaussian with strong inhibitory lobes immediately outside of the excitatory center.
GRANTS
This work was supported by National Eye Institute Grant EY-015512.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Siegert for helpful comments in preparation of this manuscript.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise, and spectral sensitivity of rods of the monkey Macaca fasicularis. J Physiol 357: 575–607, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Pu M, Famiglietti EV. The zeta cell: a new ganglion cell type in cat retina. J Comp Neurol 399: 269–288, 1998. [DOI] [PubMed] [Google Scholar]
- Billups D, Attwell D. Control of intracellular chloride concentration and GABA response polarity in rat retinal ON bipolar cells. J Physiol 545: 183–198, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW. New properties of rabbit retinal ganglion cells. J Physiol 276: 257–276, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW, Hyatt HJ. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interaction for cells with more complex receptive fields. J Physiol 276: 277–298, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ, Sterling P. Microcircuitry for two types of achromatic ganglion cell in primate fovea. J Neurosci 27: 2646–2653, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PB, Lukasiewicz PD, McReynolds JS. Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina. J Neurosci 18: 2301–2308, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Peterson BB, Packer OS, Robinson FR, Troy JB, Dacey DM. Y-cell receptive field and collicular projection of parasol ganglion cells in macaque monkey retina. J Neurosci 28: 11277–11291, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthill IC, Stevens M, Sheppard J, Maddocks T, Párraga CA, Troscianko TS. Disruptive coloration and background pattern matching. Nature 434: 72–74, 2005. [DOI] [PubMed] [Google Scholar]
- Dacey D, Packer OS, Diller L, Brainard D, Peterson B, Lee B. Center surround receptive field structure of cone bipolar cells in primate retina. Vision Res 40: 1801–1811, 2000. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci 6: 331–345, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Haarsma L, Freed MA, Sterling P. Functional circuitry of the retinal ganglion cell's nonlinear receptive field. J Neurosci 19: 9756–9767, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Haarsma L, Sterling P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J Neurosci 21: 7447–7454, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Sterling P. Cellular basis for the response to second-order motion cues in Y retinal ganglion cells. Neuron 32: 711–721, 2001. [DOI] [PubMed] [Google Scholar]
- Dhingra NK, Kao Y, Sterling P, Smith RG. Contrast threshold of a brisk-transient ganglion cell in vitro. J Neurophysiol 89: 2360–2369, 2003. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 568–582, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Jakiela HG. Suppression of cat retinal ganglion cell responses by moving patterns. J Physiol 302: 49–72, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol 187: 517–552, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare WA, Owen G. Receptive field of the retinal bipolar cell: a pharmacological study in the tiger salamander. J Neurophysiol 76: 2005–2019, 1996. [DOI] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol 122: 657–671, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Shen W. Role of neurotransmitter receptors in mediating light-evoked responses of retinal interplexiform cells. J Neurophysiol 103: 924–933, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science 292: 1178–1180, 2001. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Sjoerdsma T. GABAergic modulation of ephaptic feedback in the outer retina (Abstract). Invest Ophthalmol Vis Sci 43: E-2920, 2002. [Google Scholar]
- Levick WR. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol 188: 285–307, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The population of bipolar cells in the rabbit retina. J Comp Neurol 472: 73–86, 2004. [DOI] [PubMed] [Google Scholar]
- Marr D, Hildreth E. Theory of edge detection. Proc R Soc Lond B Biol Sci 207: 187–217, 1980. [DOI] [PubMed] [Google Scholar]
- McMahon MJ, Packer OS, Dacey DM. The classical receptive field surround of primate parasol ganglion cells is mediated primarily by a non-GABAergic pathway. J Neurosci 24: 3736–3745, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Morphology of bipolar cells labeled by DAPI in the rabbit retina. J Comp Neurol 321: 133–149, 1992. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Tamura T, Yau K. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol 97: 413–435, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaglia CL, Enroth-Cugell C, Troy JB. Effects of remote stimulation on the mean firing rate of cat retinal ganglion cells. J Neurosci 21: 5794–5803, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res 5: 583–601, 1965. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. The First Steps in Seeing. Sunderland, MA: Sinauer, 1998, p. 143–145, 471,–481, 508. [Google Scholar]
- Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J Neurophysiol 95: 3810–3822, 2006. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature 410: 583–587, 2001. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Lee BB, Sun H. Suppressive surrounds and contrast gain in magnocellular-pathway retinal ganglion cells of macaque. J Neurosci 26: 8715–8726, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Cuthill IC. Disruptive coloration, crypsis and edge detection in early visual processing. Proc Biol Sci 273: 2141–2147, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa T, Hirasawa H, Kaneko A, Kaneda M. GABA-mediated component in the feedback response of turtle retinal cones. Vis Neurosci 22: 317–324, 2005. [DOI] [PubMed] [Google Scholar]
- van Wyk M, Taylor WR, Vaney DI. Local edge detectors: a substrate for fine spatial vision at low temporal frequencies in rabbit retina. J Neurosci 26: 13250–13263, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Res 34: 1235–1246, 1994. [DOI] [PubMed] [Google Scholar]
- Varela C, Rivera L, Blanco R, Villa PD. Depolarizing effect of GABA in horizontal cells of the rabbit retina. Neurosci Res 53: 257–264, 2005. [DOI] [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci 23: 10249–10257, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res 36: 3943–3953, 1996. [DOI] [PubMed] [Google Scholar]
- Wang P, Slaughter MM. Effects of GABA receptor antagonists on retinal glycine receptors and on homomeric glycine receptor alpha subunits. J Neurophysiol 93: 3120–3126, 2005. [DOI] [PubMed] [Google Scholar]
- Warrant EJ, Nilsson D. Absorption of white light in photoreceptors. Vision Res 38: 195–207, 1998. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science 175: 1008–1010, 1972. [DOI] [PubMed] [Google Scholar]
- Xu Y, Dhingra NK, Smith RG, Sterling P. Sluggish and brisk ganglion cells detect contrast with similar sensitivity. J Neurophysiol 93: 2388–2395, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Manookin MB, Borghuis BG, Boahen K, Demb JB. Functional circuitry for peripheral suppression in mammalian Y-type retinal ganglion cells. J Neurophysiol 97: 4327–4340, 2007. [DOI] [PubMed] [Google Scholar]
- Zeck GM, Xiao Q, Masland RH. The spatial filtering properties of local edge detectors and brisk-sustained retinal ganglion cells. Eur J Neurosci 22: 2016–2026, 2005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci 14: 553–563, 1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.