Abstract
The role of Ca2+ in the induction of neural correlates of memory has frequently been described in binary terms despite the fact that many forms of memory are graded in their strength and/or persistence. We find that Ca2+ dynamics encode the magnitude of sensorimotor adaptation of the electromotor output in a weakly electric fish. The neural correlate of this memory is a synaptically induced Ca2+-dependent enhancement of intrinsic excitability of neurons responsible for setting the electromotor output. Changes in Ca2+ during induction accurately predict the magnitude of this graded memory over a wide range of stimuli. Thus despite operating over a range from seconds to tens of minutes, the encoding of graded memory can be mediated by a relatively simple cellular mechanism.
INTRODUCTION
Sensorimotor adaptation is the appropriate adjustment of a motor output to a persistent change in sensory inflow. To function correctly, it must be graded to match the magnitude of the sensory perturbation. Theoretically the magnitude of such a correction is independent of the time course during which it was induced, allowing animals to integrate gradual changes as well as rapid ones. This type of motor learning has been described in numerous systems (Baroni et al. 2001; Held and Freedman 1963; Houde and Jordan 1998; Kluzik et al. 2005; Miles and Eighmy 1980; Peterka and Loughlin 2004) and has proven to be useful in the rehabilitation of individuals who suffer cognitive, limb control, and postural deficits (Brewer et al. 2008; Granacher et al. 2006; Patton et al. 2006; Rossetti et al. 1998).
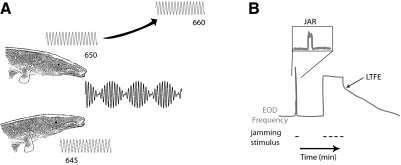
Despite the ubiquity of sensorimotor adaptation in normal function and its recent use in rehabilitation, mechanisms underlying sensorimotor adaptation remain less known. This has been complicated by the fact that it often occurs within distributed networks that employ several sensory and motor modalities. In conjunction with changes on the network level (Aksay et al. 2001, 2007; Paz et al. 2005; Wang et al. 2004), changes also occur within individual neurons within these networks (Masumura et al. 2007; Nelson et al. 2003; Smith et al. 2002). Here we have characterized an experimentally accessible form of sensorimotor adaptation in the electromotor output of a weakly electric brown ghost knifefish, Apteronotus leptorhynchus (Oestreich and Zakon 2002, 2005; Oestreich et al. 2006). These fish utilize distortions in the electric field they continually discharge from their electric organ to sense their environment. The electric organ discharge (EOD) frequency is different for each individual fish and is highly regular (coefficient of variation, CV = SD/mean period = 2.2 × 10-4) (Moortgat et al. 1998). However, when two fish with similar EOD frequencies are in proximity to one another, their electric fields will jam one another, confounding their ability to sense their environment (Fig. 1A). A fish exposed to electrosensory jamming will elevate its EOD frequency out of the jamming range. The EOD relaxes back to its original frequency when the jamming stimulus is removed. When this electrosensory jamming continues for periods longer than a few minutes, the fish will display a long-term frequency elevation (LTFE) that persists even once the jamming stimulus is removed (Fig. 1B). Similar to other forms of sensorimotor adaptation, the magnitude of LTFE depends on the intensity and duration of the sensory perturbation (Oestreich and Zakon 2002).
Fig. 1.
Long-term frequency elevation (LTFE) in a weakly electric fish Apteronotus leptorhynchus. A: when 2 Apteronotids with similar electric organ discharge (EOD, depicted as a sine wave next to the fish) frequencies are in close proximity to one another, their EODs will constructively and destructively interfere with one another, disrupting the fish's electrolocation. To avoid this, 1 of the fish will raise its EOD frequency out of the jamming range. B: an illustration of this jamming avoidance response (JAR) and LTFE in vivo. Changes in the EOD frequency over time are shown in gray, the jamming stimulus as a dashed black line. In cases where EOD jamming is brief (<2 min), the fish will perform a short jamming avoidance response (JAR, shown in expanded box), followed by a return to baseline EOD. After a prolonged JAR (>20 min), sensorimotor adapatation (LTFE) is revealed when the jamming stimulus is removed.
The neural circuitry underlying this adaptation has been well identified and is experimentally accessible. Neurons in the medullary pacemaker nucleus (PMn) directly control the EOD frequency via electrical coupling to spinal motor neurons the axons of which comprise the electric organ. As a result of this coupling, the firing frequency of PMn neurons is equal to the EOD frequency of the fish. Thus the activity in the PMn is a direct representation of the behavior in the fish. The PMn is the locus of the sensorimotor adaptation: LTFE in the isolated brain stem can be reproduced by stimulating afferent fibers from the pre-pacemaker nuclei that are activated by the jamming signal (Oestreich et al. 2006). Stimulating these glutamatergic fibers produces a transient elevation in the PMn firing frequency as result of their ionotropic synaptic input to PMn neurons. With sufficient activation, this transient elevation in PMn firing frequency is followed by a long-lasting elevation in the PMn neurons' firing frequency. This LTFE in the PMn neurons occurs at the same range of magnitudes as LTFE in the EOD and decays at the same rate. After induction, this LTFE persists even after synaptic activity has been blocked, meaning that the neural correlate for this sensorimotor adaptation is a synaptically induced graded persistent increase in the activity of the PMn neurons.
Previously, we have shown that N-methyl-d-aspartate (NMDA) receptors are necessary for the induction of LTFE and nonselective cation TRP channels are necessary for LTFE maintenance (Oestreich et al. 2006). However, it remains unclear how NMDA receptor activation recruits TRP channels. Several modeling studies have proposed that Ca2+ dynamics within individual neurons might underlie the maintenance of graded persistent increases in firing frequency that are similar to LTFE (Loewenstein and Sompolinsky 2003; Teramae and Fukai 2005).
In this paper, we sought to directly examine how Ca2+ dynamics translate synaptic inputs into this graded form of memory. Increases in Ca2+ might contribute to LTFE induction and might also be required for its long-lasting maintenance (Oestreich et al. 2006). Here we image Ca2+ in the PMn during LTFE induction and maintenance. We found that synaptically induced increases in Ca2+ signal rose concomitantly with LTFE induction across a broad range of stimuli but that LTFE was maintained well beyond these elevations. Interestingly, the cumulative Ca2+ signal during stimulation predicted the magnitude of the LTFE across a wide range of stimulus parameters, suggesting that Ca2+ levels during induction directly encode the magnitude of the sensorimotor adaptation.
METHODS
Animals
Wild-caught A. leptorhynchus were obtained from different vendors and then housed in community Plexiglas tanks in climate-controlled rooms with a circulating water system and a dark-light cycle of 12/12 h. The temperature in the rooms was held stable between ∼26 and 28°C, and the water conditions were relatively constant. Fish were fed with frozen brine shrimp every 2 days. Procedures and animal care are in accordance with guidelines from the animal care and use committee at University of Texas.
PMn slice preparation
The submerged PMn slice preparation was performed as previously described (Oestreich et al. 2006). Fish were anesthetized using 0.1% phenoxyethanol, and the dissections were performed on ice. The slice is composed of the ventral portion of the brain stem, spanning from a point in close proximity of the caudal aspect of the pacemaker nucleus to the pituitary fossa. Once secured to a coverslip with superglue, the slice was transferred to a recording chamber where it was continuously perfused with ACSF. Extracellular field recordings of the synchronized compound field potential of the PMn were acquired using a 1 M NaCl-filled patch electrode (1–3 MΩ), amplified using an Axopatch 200B, A/D-converted with the Digidata 1322, and digitally recorded using Clampex 9 (Molecular Devices, Sunnyvale, CA). The PMn firing frequency was monitored (20 kHz) during the experiment using Clampex on-line analysis of frequency count. More precise measurements of firing frequency were determined after the experiment using FastFourier analysis. LTFENMDA was induced by ejecting NMDA (3.3 mM, Tocris, Ellisville, MO) from a microelectrode pipette in close proximity to the pacemaker nucleus using a 1 s, low pressure (5 psi) pulse with a Picospritzer III. LTFEstim was induced by a bipolar stimulating tungsten electrode placed on the afferent fiber pathways rostral to the PMn.
Calcium imaging
Dye loading was performed using the multicell bolus loading (MCBL) technique (Stosiek et al. 2003). A stock solution (10 mM in DMSO and 20% pluronic acid) of Oregon Green bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid (BAPTA)-1-488-AM (OGB-1) or magnesium green (both purchased from Invitrogen, Carlsbad, CA) was diluted in artificial cerebrospinal fluid (ACSF) for a final concentration of 1 mM. Ca2+ signals were acquired with an Olympus Fluoview 300 confocal microscope (Olympus, Center Valley, PA). Excitation light was powered by an argon (488 nm) laser (max output: 1.2 W; Melles Griot, Carlsbad, CA). Images were acquired every 2.88 s, or for longer experiments, every 10 s to minimize bleaching with an Olympus ×40 water immersion lens (0.80 NA). Once the raw data were collected, each region was normalized to percent change [ΔF/F = (F − x)/x], where F was the fluorescence at a given time point, x was the average fluorescence of the initial 60 s in that region. The data were background-subtracted using a region of the pacemaker nucleus that is dye-loaded but that shows no response to NMDA application or stimulation as background. Individual pacemaker or relay neurons were distinguished from one another by their size as visualized by DIC optics.
Data analysis
Graphing and statistical analysis was performed using Excel (Microsoft, Redmond, WA), IGOR (Wavemetrics, Lake Oswego, OR), Adobe Illustrator (Adobe Systems, San Jose, CA), Origin (OriginLab, Northhampton, MA), and InStat (GraphPad, La Jolla, CA). Data are given as means ± SE. For statistical comparisons, normally distributed data were tested using either a student's t-test, or if across different stimulation paradigms, with an ANOVA followed by Bonferonni comparisons to test for significant differences between individual groups. For some multiple comparisons, the data were not normally distributed and thus a nonparametric ANOVA (Kruskal-Wallis) was used followed by a Dunn's to test for significant differences between individual groups.
RESULTS
Rises in intracellular Ca2+ are coincident with LTFE induction
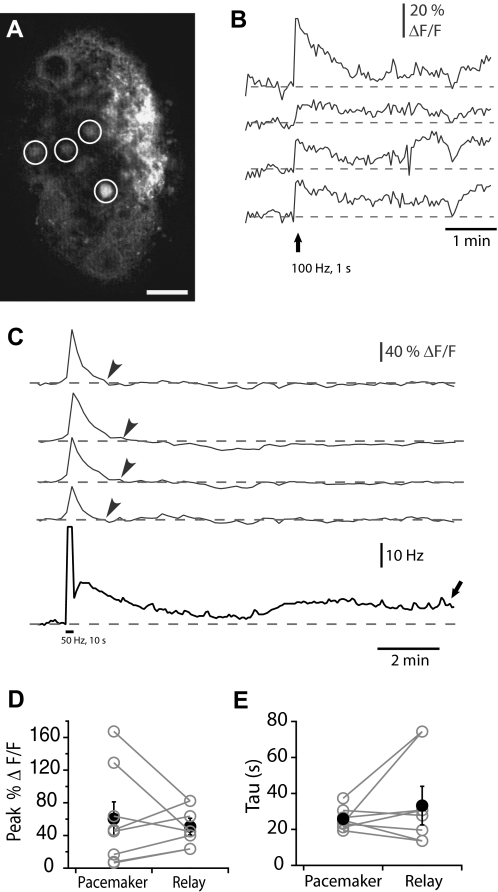
The neural correlate of LTFE is a synchronous synaptically induced elevation in the firing frequency of all the PMn neurons that is presumably accompanied by changes in intracellular Ca2+. To elucidate the magnitude and temporal characteristics of these changes in Ca2+, we imaged the Ca2+ signal in the PMn slice. An adapted multi-cell bolus loading technique established in zebrafish spinal cord was used to bulk load the calcium indicator dye Oregon Green BAPTA–AM (Fig. 2A) into the slice (Brustein et al. 2003). This membrane-permeable calcium dye allowed us to measure changes in Ca2+ in several neurons simultaneously during LTFE induction and maintenance. The dye was introduced with a microelectrode patch pipette (1–3 MΩ) placed at the surface or 50–100 μm within the pacemaker nucleus by pressure application (100 pulses of 50 ms at 5 – 10 psi). Ca2+ measurements were taken 30–60 min after dye application; at which time several pacemaker and/or relay PMn neurons are loaded.
Fig. 2.
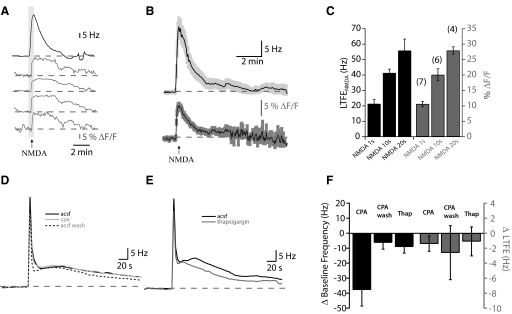
Imaging changes in Ca2+ in the pacemaker nucleus (PMn) using the multicell bolus loading (MCBL) technique. A: prior to dye loading, the PMn neurons exhibit a very little fluorescence. Forty-five minutes after dye ejection from the pipette, several cells are clearly labeled with OGB1-AM (circled in white). Scale bar is 100 μM. B: simultaneous recording of the increases in fluorescence of the Ca2+-binding dye OGB-1 (ΔF/F, in gray) in 4 different pacemaker neurons in response to synaptic stimulation (100 pulses delivered at 100 Hz, marked by arrow). C: long-term monitoring of the Ca2+ signal in the PMn. Simultaneous recording of the increase in the PMn firing frequency (Hz, in black) and the increases in fluorescence of the calcium-binding dye OGB-1 (ΔF/F, in gray) in 4 different pacemaker neurons in response to synaptic stimulation (500 pulses delivered at 50 Hz, marked by black bar). Note that minutes after the Ca2+ signal returns to baseline (gray arrowheads) LTFE persists. (black arrow). D: the peak of the Ca2+ signal (% ΔF/F) in relay neurons and pacemaker neurons imaged simultaneously in response to 500 pulses delivered at 50 Hz (n = 5). E: the decay of the Ca2+ signal in relay and pacemaker neurons imaged simultaneously after 500 pulses delivered at 50 Hz. Pairs of neurons are shown in gray, the average in black.
PMn neurons are synchronously active at a highly stable frequency (500-1,000 Hz, CV = 6.1 × 10−4, (Moortgat et al. 1998), and in the absence of synaptic activation, we observed no detectable changes in the basal Ca2+ signal. By contrast, stimulating the prepacemaker afferents briefly (100 pulses delivered at 100 Hz, Fig. 2B) triggered elevations in Ca2+ in several neurons. This Ca2+ signal was long-lasting with Ca2+ elevated for hundreds of seconds (180 s). Although the Ca2+ signal rises concurrently with the increase in firing frequency, these increases are not simply the result of higher PMn firing rates. When stimulating more robustly (500 pulses at 50 Hz), we sampled Ca2+ at a lower rate (0.1 Hz) to measure changes in the Ca2+ signal during LTFE maintenance (tens of minutes, Fig. 2C). The increase in the Ca2+ signal was greater with the stronger stimulation and returned to baseline more quickly (examined more thoroughly in the next section) but still was long-lasting (80 s). Notably LTFE persisted for several minutes after the Ca2+ signal had subsided. Thus the elevation of firing frequency itself during LTFE could not account for the elevation in the Ca2+ signal. Furthermore, changing the PMn firing frequency without synaptic activation by altering the bath temperature produced no concurrent change in the Ca2+ signal. Raising or lowering the bath temperature (±4°C) produced significant shifts in the PMn firing rate (+4°C = 14.24 ± 5.23 Hz, −4°C = −14.20 ± 3.10 Hz) with no corresponding changes in fluorescence [+4°C: ΔF/F = 0.61 ± 0.87%, −4°C: ΔF/F = 2.42 ± 0.56%, ANOVA for 3 temperatures: F(115,2) = 2.20, P = 0.115].
Elevations in Ca2+ occurred with a similar time course across multiple neurons, independent of whether they were pacemaker or relay neurons. The magnitude of the Ca2+ signals varied, but this difference did not correspond with neuron type. To examine this, we measured Ca2+ signals in pacemaker and relay neurons simultaneously (n = 5). The Ca2+ signal in the pacemaker neurons was on average slightly larger, but this relationship was not significant (paired t-test, P = 0.29, Fig. 2D). To test whether the time course of the Ca2+ signal was similar in pacemaker and relay neurons, we fit the decay of the Ca2+ signal to either a single or double exponential. In 3/12 cases (2 pacemaker neurons, 1 relay neuron) the decay was fit best by a double exponential, the slower exponent of which was similar to the single exponential fits. The decays of the Ca2+ signal did not depend on neuron type (single exponential tau, paired t-test, P = 0.1765, Fig. 2E).
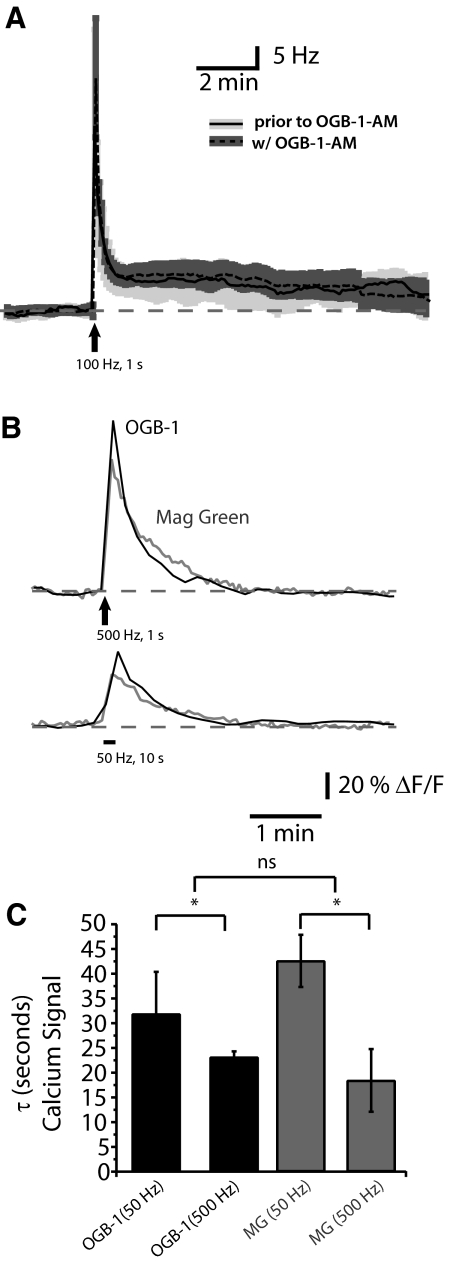
Our imaging technique did not appear to interfere with LTFE or the Ca2+ signals within PMn neurons during LTFE. Dye loading did not interfere with the PMn neurons' basal firing frequency (Δ −1.36 ± 0.28 Hz, paired t-test, P = 0.63; n = 20). Similarly dye loading did not interfere with LTFE (Fig. 3A), either the magnitude (Δ −0.35 ± 1.69 Hz; paired t-test P = 0.79, n = 6) or decay (Δ tau = −23 ± 81.76 s, paired t-test, P = 0.84, n = 6). To further confirm that the signal was not confounded by the high affinity of OGB-1 for Ca2+ (Kd = 165 nM), we utilized the low-affinity Ca2+ dye magnesium green (Kd = 6 μM). As was the case with OGB-1, the Ca2+ signal measured with magnesium green did not differ between neuron types (single exponential tau, paired t-test P = 0.3944). The Ca2+ signals of OGB-1 and magnesium green were similar across stimulation paradigms (Fig. 3B). The decays of these signals were not statistically different depending the dye used (at 50 Hz: t = 1.3, at 500 Hz: t = 0.6154, both P > 0.05, Fig. 3C) but did vary depending on the strength of the stimulus [ANOVA F(24,3) = 3.75, Bonferonni post hoc analysis t = 3.151, P < 0.05].
Fig. 3.
Dye loading does not alter PMn activity. A: LTFE (Hz) in response to afferent fiber stimulation (100 pulses at 100 Hz) prior to dye loading (average: solid black, SE: light gray) and with dye loaded into the PMn neurons (average: dashed black, SE, dark gray). B: changes in fluorescence in response to synaptic stimulation (500 pulses at 500 Hz or 500 pulses at 50 Hz) of high affinity calcium-binding dye (Oregon Green BAPTA-1, in black), the low affinity calcium-binding dye magnesium green (Kd = 6 μM; ΔF/F, in gray). C: decay kinetics of the Ca2+ signal when using OGB-1 (Kd ∼205 nM) vs. magnesium green. Decay kinetics (τ) were compared after synaptically stimulating the PMn with 500 pulses at 50 Hz and 500 Hz [ANOVA F(3,29) = 8.604, P < 0.01]. No significant difference between the decays of the Ca2+ signal using different dyes (Bonferonni's multiple comparisons), but significant difference was detected between 50 and 500 Hz stimulations (Bonferonni's multiple comparisons, P < 0.05).
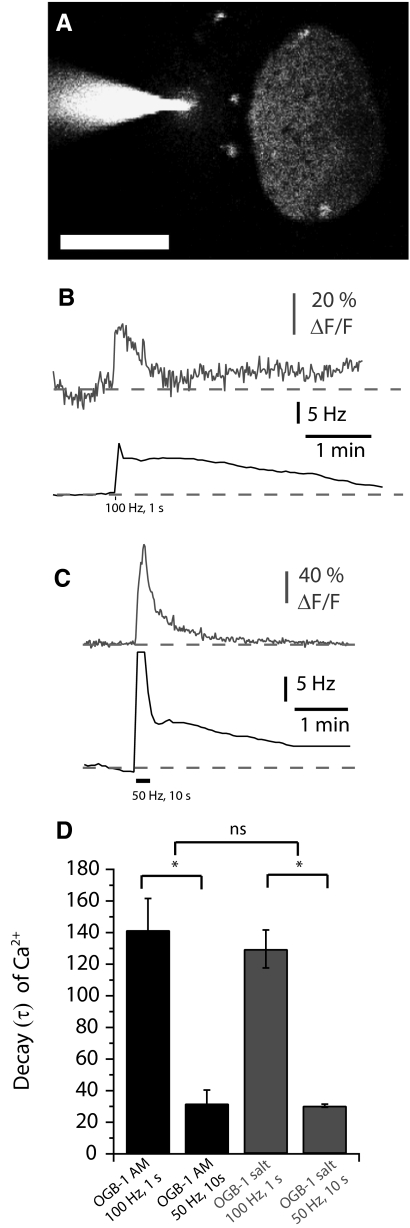
To compare the changes we observed with our bulk-loading technique to changes in Ca2+ signal at the single-cell level, we filled individual relay neurons using a low-resistance (5–8 MΩ) sharp electrode containing 100 μM membrane impermeant OGB-1 (Fig. 4). Ca2+ signals in individually loaded neurons were similar to those observed when bulk-loaded. No changes in Ca2+ were observed in the absence of synaptic stimulation. Synaptic stimulation for brief (100 Hz for 1 s, n = 3, Fig. 4B) or for more prolonged periods (50 Hz for 10 s, n = 4, C) elicited Ca2+ signals similar to bulk loaded neurons. The Ca2+ signals were similarly stimulus dependent (Dunn's multiple comparisons, P < 0.05) and consistently larger with individually loaded neurons across stimuli, presumably due to more localized image sampling (nonparametric ANOVA: KW = 16.868, P < 0.001, Dunn's multiple comparisons, P < 0.05). The Ca2+ signals also decayed in a stimulus-dependent manner [ANOVA F(18,3) = 20.493, Bonferonni post hoc analysis for OGB-salt: t = 4.455, for OGB-AM: t = 6.25, both P < 0.01] but not between loading techniques (at 50 Hz: t = 0.067, at 100 Hz: 0.6331, both P > 0.05, Fig. 4D). Thus Ca2+ signals at the single-cell level appeared to match our results from bulk loading. This suggested that our signal was indeed from the PMn neurons and not from prepacemaker afferent fibers that had been spuriously labeled.
Fig. 4.
Changes in Ca2+ signal within a relay neuron in response to synaptic stimulation. A: cell-impermeant OGB-1 was loaded via a low-resistance sharp electrode into a relay neuron (scale bar = 50 μM). B and C: simultaneous recording of the increases in the PMn firing frequency (Hz, in black) and the increases in fluorescence of the Ca2+-binding dye OGB-1 (ΔF/F, in gray) in a relay neuron in response to synaptic stimulation (B, 100 pulses delivered at 100 Hz; C, 500 pulses delivered at 50 Hz). D: decays of the Ca2+ signal from bulk loaded (in black) and single loaded (in gray) PMn neurons.
LTFE maintenance is not dependent on elevated Ca2+
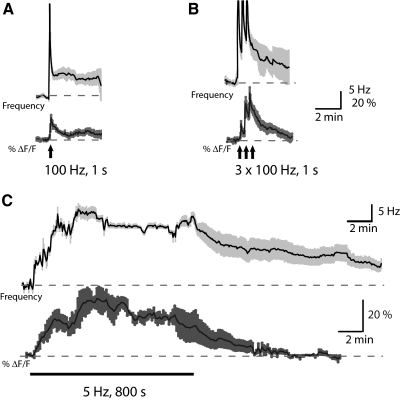
Having established the efficacy of our imaging technique, we sought to carefully examine how the Ca2+ signal rises and falls during LTFE induction and maintenance. LTFE in vivo is graded, depending on the intensity and duration of jamming stimulus the fish is exposed to. Although the firing frequency of the prepacemaker nuclei during a jamming stimulus remains unknown, stimulating the afferent fibers from these nuclei produces the same range of LTFE in vivo (Oestreich et al. 2006). LTFE output increases linearly with pulse count across a wide range of frequencies (1.5-1,000 Hz). We therefore sought to examine LTFE induction and maintenance across a wide range of stimulus parameters. Stimulating in a manner that mimics a mild LTFE induction in the fish (Fig. 5A: 100 pulses at 100 Hz) resulted in an increase in the Ca2+ signal (peak ΔF/F: 12.27 ± 1.55%, n = 9) and a modest firing frequency (LTFE = 6.17 ± 0.83 Hz; n = 9). The Ca2+ signal persisted well beyond the stimulation period and was best fit by a single exponential faster (τ = 141.64 ± 19.98 s) than the decay of the firing frequency (τ = 165.01 ± 44.71 s), although this difference was not statistically significant (Student's t-test, P = 0.18). Importantly, this was not true with more intense LTFE induction protocols. 3 × 100 pulses at 100 Hz, separated by 20 s produced LTFE of greater magnitude (10.35 ± 1.27 Hz, n = 7) and slower decay (τ = 245.62 ± 54.66 s; Fig. 5B). Concurrent with the increase in LTFE magnitude, we observed an increase in the Ca2+ signal during each stimulation (1st peak ΔF/F = 8.80 ± 1.62%, 2nd peak ΔF/F = 20.27 ± 3.62%, 3rd peak ΔF/F = 26.09 ± 3.19%, n = 7). However, with this more robust stimulus, the Ca2+ signal did not persist throughout the maintenance phase of LTFE. Although there was a twofold increase in the peak of Ca2+ signal by the third stimulation as compared with a single stimulation, the Ca2+ signal decay was still best fit by a single exponential (τ = 86.49 ± 26.29 s, Student's t-test, P < 0.05).
Fig. 5.
Changes in Ca2+ occur during LTFE induction but do not persist with LTFE maintenance. Averages of simultaneous recordings of the increases in the PMn firing frequency (Hz, in black) and the increases in fluorescence of the calcium-binding dye OGB-1 (ΔF/F, in gray) in response to frequency that pulse stimuli are delivered. Lightly shaded areas around the average represent SE and black bars below the trace denote afferent fiber stimulation. A: mild LTFE induction (100 pulses: 100 Hz, 1 s, n = 9). B: moderate LTFE induction (300 pulses: 3 × 100 Hz, 1 s each, n = 7). C: robust LTFE induction (4,000 pulses: 5 Hz for 800 s, n = 4).
We then examined changes in Ca2+ signal using a protocol that mimicked the maximal LTFE induction in the intact fish (Fig. 5C). To do this, we stimulated the PMn synaptically with 4,000 pulses delivered at a fixed rate (5 Hz) for 800 s. This stimulation protocol produced a near-saturating LTFE magnitude (16.43 ± 1.01 Hz, n = 3) that was maintained for a prolonged period (4.5 ± 1.6 Hz still above baseline at 30 min). During the induction of maximal LTFE, a steady increase in firing frequency was matched with an increase in the Ca2+ signal (peak ΔF/F = 41.53 ± 5.20%, n = 4). When synaptic stimulation terminated, both the elevation in firing frequency and the Ca2+ signal persisted well after the stimulus. However, the decay in the Ca2+ signal was best fit by a single exponential (τ = 198.6 ± 50.24 s) that was much more rapid than the decay of the firing frequency (τ = 1,538.82 ± 605.5 s). Thus with stronger stimulus paradigms, the Ca2+ signal increases become greater during LTFE induction but do not persist throughout LTFE maintenance.
Mechanisms underlying LTFE induction
Several lines of evidence suggest that Ca2+ influx into the PMn neurons through NMDA receptors is necessary for LTFE induction. NMDA receptors are found in the pacemaker and relay neurons within the PMn but not in the presynaptic terminals of afferent fibers nor in the glia within the PMn (Harvey-Girard et al. 2007; Spiro et al. 1994). NMDA-R antagonists block LTFE induction, whereas blocking metabotropic glutamate and kainate receptors does not (Oestreich et al. 2006). Furthermore, application of NMDA to the PMn mimics LTFE (Oestreich et al. 2006). This NMDA-induced LTFE (LTFENMDA) is blocked in Ca2+-free extracellular solution, suggesting that it is the Ca2+ influx that is necessary for LTFE not just depolarization. We examined whether direct NMDA application could mimic the Ca2+ signal observed during synaptic stimulation. Brief puffs of NMDA (5 μM, 10 psi, 1 s) caused an increase in Ca2+ in several neurons concurrent with the expected elevation in firing frequency (Fig. 6A). The Ca2+ signal was long-lasting, with a decay (τ = 60.67 ± 14.86 s) that was similar to the decay in firing frequency (τ = 59.79 ± 7.92 s, Student's t-test P = 0.96; Fig. 6B). Both LTFENMDA and Ca2+ signal were increased with more prolonged NMDA applications (Fig. 6C). These results indicate that NMDA receptor activation alone can result in Ca2+ influx into the PMn neurons and further implicate Ca2+ influx through NMDA receptors as the means by which LTFE induction occurs.
Fig. 6.
LTFE is mediated by Ca2+ influx through N-methyl-d-aspartate (NMDA) receptors, not intracellular Ca2+ stores. A: changes in Ca2+ signal during LTFENMDA. Simultaneous recordings of changes in PMn firing frequency (Hz, in black) and OGB-1 fluorescence in 4 cells (ΔF/F, in gray) with NMDA application (light gray bar). B: average traces (n = 25) of changes in PMn firing frequency and the Ca2+ signal during LTFENMDA. Lightly shaded areas around the average represent SE. C: with increasing durations of NMDA application (5 μM NMDA at 10 psi for 1, 10, and 20 s), both LTFE NMDA and the peak % ΔF/F increase. D: averages of LTFE (500 Hz, 1 s) before [artificial cerebrospinal fluid (ACSF), black) in cyclopiaozinic acid (CPA, gray) and CPA washout (Wash, dashed). There is no difference in LTFE with store depletion. E: average LTFE (500 Hz, 1 s) before (ACSF, black) and after thapsigargin (gray). F: changes in baseline frequency and LTFE as a result of store depletion using CPA (50 μM) and thapsigargin (2 μM).
Given the long-lasting nature of the Ca2+ signals with LTFE induction, we also examined whether Ca2+ stores might contribute to LTFE. Ca2+ influx through NMDA receptors has been shown to activate release from intracellular stores (Baba et al. 2003). To test whether Ca2+ stores might contribute to LTFE, we depleted calcium stores by applying cyclopiazonic acid (CPA) or thapsigargin (Fig. 6, D and E). Depleting Ca2+ stores lowered the PMn firing frequency (Student's t-test, P < 0.05, Fig. 6E), but neither of these compounds had an effect on LTFE magnitude [thapsigargin, 2 μM: paired t-test P = 0.314; CPA, 50 μM: ANOVA: F(15,2) = 0.91065, P = 0.42]. Thus although calcium stores may contribute to some aspect of setting the PMn firing frequency, these mechanisms do not appear to contribute to LTFE.
Pulse counting reflects the change in Ca2+ during stimulation
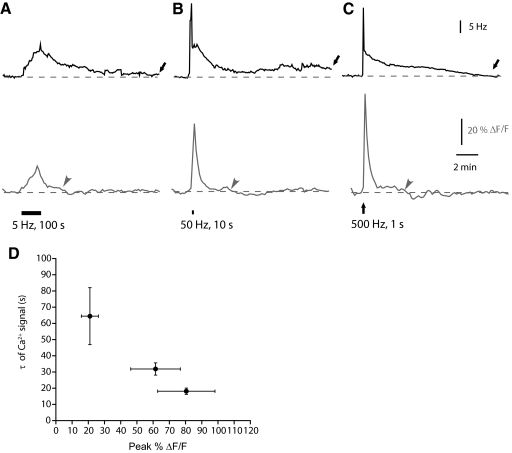
LTFE persists beyond the Ca2+ elevation (Figs. 2E and 5C), suggesting that continued elevation in Ca2+ is not necessary for LTFE maintenance. In contrast, the Ca2+ signal appeared to reflect LTFE induction more closely. We therefore examined whether Ca2+ contributes to the pulse-counting properties of LTFE induction. The LTFE pulse-counting mechanism follows two computational rules: it increases linearly with pulse count and it is largely insensitive to the frequency at which input pulses are delivered. If changes in Ca2+ contribute to the pulse-counting mechanism underlying LTFE, it is expected that they might also be constrained by these computational rules. As seen in Fig. 5, the Ca2+ elevation increases concomitantly with pulse count. However, for the Ca2+ signal to act as a component of the pulse-counting mechanism, it would also need to be insensitive to changes in the pulse frequency. To test for this, we compared changes in the Ca2+ signal during LTFE induction over a wide range of frequencies (5, 50, and 500 Hz) while keeping the pulse number constant (500; Fig. 7). Inducing LTFE with the same number of stimulus pulses over this wide temporal range (1, 10, and 100 s) yielded comparable magnitudes of LTFE [5 Hz = 11.83 ± 1.6 Hz, 50 Hz = 12.67 ± 0.83 Hz, and 500 Hz = 10 ± 1.45 Hz; ANOVA: F(13,2) = 1.054, P = 0.3765]. By contrast, changes in the Ca2+ signal were distinct from one another (Fig. 7). As stimulation frequency was increased, the peak of the Ca2+ signal increased [ANOVA F(13,2) = 10.34, P < 0.05]. In every case, LTFE persisted well after the Ca2+ signal had returned to baseline. In most cases the decays were best fit by a single exponential (5 Hz: 5/5; 50 Hz: 4/6; 500 Hz 7/9). The 5 Hz stimulus decayed slower than the signals from 50 and 500 Hz [ANOVA F(13,2) = 4.908, P < 0.05; the 500 Hz]. Although the stimulation protocols produced very different peak changes in the Ca2+ signal, they were similar in the total Ca2+ signal during LTFE induction. The cumulative Ca2+ signal during stimulation was similar despite the fact that the time course of LTFE induction varied over two orders of magnitude [5 Hz: ΔF/F = 103.55 ± 21.72%, 50 Hz: ΔF/F = 98.13 ± 28.68%, and 500 Hz: ΔF/F = 80.46 ± 17.68%, ANOVA F(13,2) = 1.019, P = 0.96]. Thus the cumulative Ca2+ during stimulation matched the amount of LTFE while the peak Ca2+ did not.
Fig. 7.
Changes in Ca2+ are stimulus frequency-independent. Average responses from simultaneous recordings of the increases in the PMn firing frequency (Hz, in black) and the increases in fluorescence of the calcium-binding dye OGB-1 (ΔF/F, in gray) in response to frequency that pulse stimuli are delivered. Error bars have been omitted for clarity. A: 500 pulses delivered at 5 Hz (n = 5). B: 500 pulses delivered at 50 Hz (n = 7). C: 500 pulses delivered at 500 Hz (n = 9). D: decreases in decay of the Ca2+ signal with increasing peak in fluorescence. Arrows represent when LTFE has returned to baseline, arrowheads when the Ca2+ signal has returned to baseline.
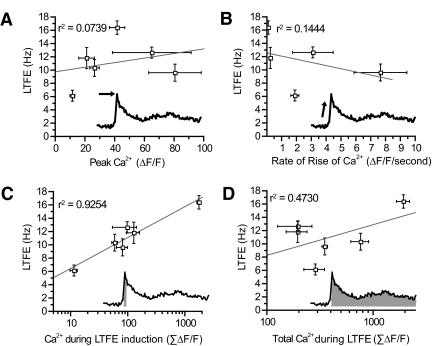
To determine whether Ca2+ participates in the pulse-counting mechanism in LTFE, we examined how LTFE magnitude correlated with the magnitude, rise time, and total change of the Ca2+ signal across all the different stimulus protocols used in this study (Fig. 8). Neither the magnitude nor rise time of the Ca2+ correlated with the magnitude of the LTFE (Fig. 8, A and B). In contrast, the cumulative sum of the [Ca2+]i signal during LTFE induction correlated strongly with LTFE magnitude (Fig. 8C; r2 = 0.9254, P < 0.005 linear regression). Therefore the change in Ca2+ during LTFE induction follows both computational rules of the LTFE pulse-counter, as it increases with pulse count and is independent of frequency. This was not true for the total change in Ca2+ during LTFE induction and maintenance (Fig. 8D), which is consistent with our previous finding that Ca2+ is not required for LTFE maintenance.
Fig. 8.
LTFE correlates with the total change in Ca2+ signal during stimulation across a wide range of stimulus protocols. LTFE magnitude and the Ca2+ signal varied depending on the number of pulses delivered (100, 300, 500, or 4,000) and the frequency at which they where delivered (5, 50, 100, 500 Hz). Summary plots of these data showing how changes in LTFE magnitude correlate with different parameters of the Ca signal. A: peak Ca2+ signal. B: rate of rise in Ca2+ signal with stimulation. C: the accumulated total Ca2+ signal during LTFE induction. D: the cumulative total of the Ca2+ signal.
DISCUSSION
In this study, we have taken advantage of an experimentally accessible form of sensorimotor adaptation to show how Ca2+ dynamics contribute to the encoding of a graded memory. LTFE in a weakly electric fish functions a pulse-counter, integrating changes in sensory input over a wide range of time scales to produce the appropriate compensatory electromotor output. By exploiting the computational rules that constrain how LTFE integrates synaptic inputs, we determined that the cumulative change in the Ca2+ during stimulation accurately predicts LTFE magnitude across a range of input stimuli.
Ca2+ signal in response to afferent fiber stimulation occurred with a similar time course in the PMn neurons. Although there was some variability in the magnitude of the changes in Ca2+ between neurons, Ca2+ fluctuations in a single intracellularly loaded relay neuron were very similar to fluctuations in several neurons imaged simultaneously. Of course, this does not preclude some variability in Ca2+ elevations between different neurons at faster time scales below the resolution of our Ca2+ imaging. Nonetheless, these findings suggest that at the time scale that LTFE occurs, Ca2+ dynamics in the PMn can be considered uniform among the neurons. Thus although pacemaker neurons are known to drive relay neurons, which in turn drive the electromotor neurons in the spinal cord (Elekes and Szabo 1985), both neuron types appear to participate in Ca2+ dynamics underlying LTFE. This is consistent with the fact that both neuron types receive glutamatergic input (Dye and Heiligenberg 1987; Heiligenberg et al. 1996), both express NMDA receptors (Harvey-Girard et al. 2007; Spiro et al. 1994) and the calcium-binding protein calbindin (Smith et al. 2000). Interestingly, a related species of electric fish (Eigenmannia viriscens) that generates a much longer-lasting LTFE (Oestreich and Zakon 2005), expresses more vitamin-D-sensitive calcium binding protein in relay neurons than pacemaker neurons (Maler et al. 1984). Future studies will have to determine whether protein differences in the two species might account for differences in the duration of their sensorimotor adaptation. The Ca2+ elevations in PMn neurons in response to afferent stimulation can be remarkably long-lasting (>100 s) but do not persist for the duration of LTFE maintenance (Figs. 2E, 5, A–C, and 7, A–C). Thus elevations in firing frequency do not themselves result in increased Ca2+ levels in PMn neurons. The fact that the Ca2+ signal does not persist for the duration of LTFE eliminates several mechanisms by which it could be maintained. Voltage-gated calcium channels, Ca2+-permeable cation channels and intracellular Ca2+ stores are unlikely to be directly involved during LTFE maintenance. This is in accord with several findings. The N-type Ca2+ channel blocker ω-conotoxin-GVIA applied after LTFE induction has no effect on LTFE (Oestreich et al 2006). Several nonspecific voltage-gated calcium channel blockers have little or no effect on PMn firing frequency (Smith and Zakon 2000). Depleting intracellular stores had no effect on LTFE (Fig. 6, D and E), consistent with the fact that Ca2+ elevations do not persist for the duration of LTFE. These results are consistent with our finding that LTFE is maintained by a calcium nonselective cation current (Oestreich et al. 2006). It is notable that the TRPM5 channel that we localized to neurons within the PMn (Oestreich et al. 2006) is one of the few TRP channels that is calcium-impermeable. However, unless TRPM5 deactivates on the time scale of hundreds of seconds, the TRPM5 recruitment would have to be more complex than Ca2+ directly gating TRPM5. The most obvious form this might take is Ca2+ activation of a secondary messenger phosphorylation cascade (i.e., CamKII or PKC activation) that in turn might alter the properties of TRPM5 within the PMn neurons.
The fact that the Ca2+ signals do not persist throughout LTFE maintenance suggests that several proposed models of graded persistent activity are insufficient to explain LTFE. Some of these models explicitly require elevations in Ca2+ throughout maintenance (Loewenstein and Sompolinsky 2003; Teramae and Fukai 2005) In contrast to these models in which Ca2+ signals encode the graded activity, our results favor the idea that transient Ca2+ signals result in the recruitment of a balance in kinase and phosphotase activity (Delord et al. 2007; Fransen et al. 2006). These types of changes in Ca2+ during LTFE are more reminiscent of the role of Ca2+ in synaptic plasticity, where Ca2+ dynamics during induction determine the magnitude of the plasticity (Bear 2003; Johnston et al. 2003; Sjostrom and Nelson 2002), but Ca2+ isn't persistently elevated during its expression. It will be of great interest to determine how Ca2+ contributes to the graded nature of other forms of Ca2+-dependent synaptically induced changes in intrinsic excitability (Nelson et al. 2003, 2005; Xu et al. 2005; Zhang et al. 2004).
It can be difficult to determine which parameters of a Ca2+ signal are relevant to its downstream effectors. We have taken advantage of the computation rules that constrain the LTFE pulse counting mechanism to address this. LTFE increases linearly with pulse count and is markedly insensitive (>2 orders of magnitude) to the frequency at which those pulses are delivered. By using a range of stimulations varying in pulse count and frequency, we determined that the change in the Ca2+ signal during stimulation is the most accurate parameter for predicting LTFE magnitude. Specifically, the log of the change in the Ca2+ signal during the stimulation correlated with LTFE magnitude. Remarkably, this relationship is reminiscent of an enzymatic reaction with a single binding site that saturates. It is possible that the relationship between Ca2+ and LTFE reflects a single binding site for Ca2+ onto its downstream targets. However, our findings suggest that Ca2+ handling in PMn neurons is more complex than this. There is a portion of the Ca2+ signal that persists once synaptic stimulation has passed, which does not appear to be salient to the LTFE mechanism. In cases where LTFE is not saturated, this extra Ca2+ does not trigger greater LTFE. It may be compartmentalized or buffered in some manner from the downstream effectors necessary for LTFE. In fact, the decay of the Ca2+ signal possesses a particularly interesting feature. The rate of decay in the Ca2+ signal appears to increase with larger peak Ca2+ elevations (Fig. 7B). This suggests that Ca2+ handling within the PMn neurons may itself be an active process. Active Ca2+ handling might contribute to the pulse-counting mechanism that is independent of the input pulse frequency by minimizing extraneous Ca2+ activation of downstream effectors.
The gradation of a memory is often assumed to be an emergent property of several processes occurring at many synapses and/or neurons. This is particularly relevant in the case of sensorimotor adaptation, which integrates sensory perturbation over a wide temporal range. Indeed for certain forms of sensorimotor adaptation, network activity appears to dominate the adaptation. For example, persistent firing in neurons within area I of the goldfish oculomotor integrator cannot be driven in vivo by simple somatic depolarization but rather appear dependent on network interactions (Askay et al. 2001). Nonetheless, sensorimotor adaptation is also likely to be mediated by Ca2+ dynamics altering individual neurons' intrinsic properties. Vestibular neurons that show adaptive changes in their firing rate responses correlating with VOR gain appear to be regulated by Ca2+-dependent processes (Nelson et al. 2003,. 2005; Smith et al. 2002; Straka et al. 2005). Similarly, other forms of memory that are similarly graded (e.g., working memory) may be mediated by Ca2+ driven single-cell mnemonic activity (Egorov et al. 2002, 2006; Fransen et al. 2006; Sidiropoulou et al. 2009). However, in all of these cases, it is difficult to relate Ca2+ signaling directly to the magnitude of behavioral changes in an animal. Here we have found that the Ca2+ signal during LTFE induction correlates with the magnitude of the memory whether it occurs in one second or over tens of minutes. Because the PMn neurons directly represent the EOD in a behaving fish, this suggests that a relatively simple cellular process can itself encode a graded memory across a wide dynamic range.
GRANTS
This work was supported by the National Institutes of Health Grants R01 MH-056535 to H. H. Zakon and R01 NS-044399 D. L. Pettit and the Ruth Kirchestein postdoctoral training fellowship F32 NS46949-02 to N. C. Dembrow.
ACKNOWLEDGMENTS
Thanks to R. Narayanan for helpful comments on the manuscript.
REFERENCES
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci 4: 184–193, 2001. [DOI] [PubMed] [Google Scholar]
- Aksay E, Olasagasti I, Mensh BD, Baker R, Goldman MS, Tank DW. Functional dissection of circuitry in a neural integrator. Nat Neurosci 10: 494–504, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba A, Yasui T, Fujisawa S, Yamada RX, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y. Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity. J Neurosci 23: 7737–7741, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni G, Pedrocchi A, Ferrigno G, Massion J, Pedotti A. Motor coordination in weightless conditions revealed by long-term microgravity adaptation. Acta Astronaut 49: 199–213, 2001. [DOI] [PubMed] [Google Scholar]
- Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci 358: 649–655, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer BR, Klatzky R, Matsuoka Y. Visual feedback distortion in a robotic environment for hand rehabilitation. Brain Res Bull 75: 804–813, 2008. [DOI] [PubMed] [Google Scholar]
- Brustein E, Marandi N, Kovalchuk Y, Drapeau P, Konnerth A. “In vivo” monitoring of neuronal network activity in zebrafish by two-photon Ca(2+) imaging. Pfluegers 446: 766–773, 2003. [DOI] [PubMed] [Google Scholar]
- Delord B, Berry H, Guigon E, Genet S. A new principle for information storage in an enzymatic pathway model. PLoS Computational Biology 3: e124, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye J, Heiligenberg W. Intracellular recording in the medullary pacemaker nucleus of the weakly electric fish, Apteronotus, during modulatory behaviors. J Comp Physiol 161: 187–200, 1987. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature 420: 173–178, 2002. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Unsicker K, von Bohlen und Halbach O. Muscarinic control of graded persistent activity in lateral amygdala neurons. European J Neurosci 24: 3183–3194, 2006. [DOI] [PubMed] [Google Scholar]
- Elekes K, Szabo T. Synaptology of the medullary command (pacemaker) nucleus of the weakly electric fish (Apteronotus leptorhynchus) with particular reference to comparative aspects. Exp Brain Res Exp Hirnforsch 60: 509–520, 1985. [DOI] [PubMed] [Google Scholar]
- Fransen E, Tahvildari B, Egorov AV, Hasselmo ME, Alonso AA. Mechanism of graded persistent cellular activity of entorhinal cortex layer v neurons. Neuron 49: 735–746, 2006. [DOI] [PubMed] [Google Scholar]
- Granacher U, Gollhofer A, Strass D. Training induced adaptations in characteristics of postural reflexes in elderly men. Gait Posture 24: 459–466, 2006. [DOI] [PubMed] [Google Scholar]
- Harvey-Girard E, Dunn RJ, Maler L. Regulated expression of N-methyl-d-aspartate receptors and associated proteins in teleost electrosensory system and telencephalon. J Comp Neurol 505: 644–668, 2007. [DOI] [PubMed] [Google Scholar]
- Heiligenberg W, Metzner W, Wong CJ, Keller CH. Motor control of the jamming avoidance response of Apteronotus leptorhynchus: evolutionary changes of a behavior and its neuronal substrates. J Comp Physiol 179: 653–674, 1996. [DOI] [PubMed] [Google Scholar]
- Held R, Freedman SJ. Plasticity in human sensorimotor control. Science 142: 455–462, 1963. [DOI] [PubMed] [Google Scholar]
- Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science 279: 1213–1216, 1998. [DOI] [PubMed] [Google Scholar]
- Johnston D, Christie BR, Frick A, Gray R, Hoffman DA, Schexnayder LK, Watanabe S, Yuan LL. Active dendrites, potassium channels and synaptic plasticity. Philos Trans R Soc Lond B Biol Sci 358: 667–674, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluzik J, Horak FB, Peterka RJ. Differences in preferred reference frames for postural orientation shown by after-effects of stance on an inclined surface. Exp Brain Res Exp Hirnforsch 162: 474–489, 2005. [DOI] [PubMed] [Google Scholar]
- Loewenstein Y, Sompolinsky H. Temporal integration by calcium dynamics in a model neuron. Nat Neurosci 6: 961–967, 2003. [DOI] [PubMed] [Google Scholar]
- Maler L, Jande S, Lawson EM. Localization of vitamin D-dependent calcium binding protein in the electrosensory and electromotor system of high frequency gymnotid fish. Brain Res 301: 166–170, 1984. [DOI] [PubMed] [Google Scholar]
- Masumura C, Horii A, Mitani K, Kitahara T, Uno A, Kubo T. Unilateral vestibular deafferentation-induced changes in calcium signaling-related molecules in the rat vestibular nuclear complex. Brain Res 1138: 129–135, 2007. [DOI] [PubMed] [Google Scholar]
- Miles FA, Eighmy BB. Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol 43: 1406–1425, 1980. [DOI] [PubMed] [Google Scholar]
- Moortgat KT, Keller CH, Bullock TH, Sejnowski TJ. Submicrosecond pacemaker precision is behaviorally modulated: the gymnotiform electromotor pathway. Proc Natl AcadSci USA 95: 4684–4689, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron 46: 623–631, 2005. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron 40: 609–620, 2003. [DOI] [PubMed] [Google Scholar]
- Oestreich J, Dembrow NC, George AA, Zakon HH. A “sample-and-hold” pulse-counting integrator as a mechanism for graded memory underlying sensorimotor adaptation. Neuron 49: 577–588, 2006. [DOI] [PubMed] [Google Scholar]
- Oestreich J, Zakon HH. The long-term resetting of a brain stem pacemaker nucleus by synaptic input: a model for sensorimotor adaptation. J Neurosci 22: 8287–8296, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich J, Zakon HH. Species-specific differences in sensorimotor adaptation are correlated with differences in social structure. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 845–856, 2005. [DOI] [PubMed] [Google Scholar]
- Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp Brain Res Exp Hirnforsch 168: 368–383, 2006. [DOI] [PubMed] [Google Scholar]
- Paz R, Natan C, Boraud T, Bergman H, Vaadia E. Emerging patterns of neuronal responses in supplementary and primary motor areas during sensorimotor adaptation. J Neurosci 25: 10941–10951, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol 91: 410–423, 2004. [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, Perenin MT. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 395: 166–169, 1998. [DOI] [PubMed] [Google Scholar]
- Sidiropoulou K, Lu FM, Fowler MA, Xiao R, Phillips C, Ozkan ED, Zhu MX, White FJ, Cooper DC. Dopamine modulates an mGluR5-mediated depolarization underlying prefrontal persistent activity. Nature Neurosci 12: 190–199, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Nelson SB. Spike timing, calcium signals and synaptic plasticity. Curr Opin Neurobiol 12: 305–314, 2002. [DOI] [PubMed] [Google Scholar]
- Smith GT, Lu Y, Zakon HH. Parvocells: a novel interneuron type in the pacemaker nucleus of a weakly electric fish. J Comp Neurol 423: 427–439, 2000. [PubMed] [Google Scholar]
- Smith GT, Zakon HH. Pharmacological characterization of ionic currents that regulate the pacemaker rhythm in a weakly electric fish. J Neurobiol 42: 270–286, 2000. [DOI] [PubMed] [Google Scholar]
- Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol 87: 2031–2042, 2002. [DOI] [PubMed] [Google Scholar]
- Spiro JE, Brose N, Heinemann SF, Heiligenberg W. Immunolocalization of NMDA receptors in the central nervous system of weakly electric fish: functional implications for the modulation of a neuronal oscillator. J Neurosci 14: 6289–6299, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol 76: 349–392, 2005. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA 100: 7319–7324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramae JN, Fukai T. A cellular mechanism for graded persistent activity in a model neuron and its implications in working memory. J Comput Neurosci 18: 105–121, 2005. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA 101: 1368–1373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci 25: 1750–1760, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shin JH, Linden DJ. Persistent changes in the intrinsic excitability of rat deep cerebellar nuclear neurones induced by EPSP or IPSP bursts. J Physiol 561: 703–719, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]