Abstract
In the cochlea, afferent transmission between inner hair cells and auditory neurons is mediated by glutamate receptors. Glutamate transporters located near the synapse and in spiral ganglion neurons are thought to maintain low synaptic levels of glutamate. We analyzed three glutamate transporter blockers for their ability to alter the effects of glutamate, exogenously applied to the synapse via perfusion of the scala tympani of the mouse, and compared that action to their ability to alter the effects of intense acoustic stimulation. Threo-beta-benzyloxyaspartate (TBOA) is a broad-spectrum glutamate transporter antagonist, affecting all three transporters [glutamate/aspartate transporter (GLAST), glutamate transporter-1 (GLT1), and excitatory amino acid carrier 1 (EAAC1)]. l-serine-O-sulfate (SOS) blocks both GLAST and EAAC1 without effect on GLT1. Dihydrokainate (DHK) is selective for GLT1. Infusion of glutamate (10 μM for 220 min), TBOA (200 μM for 220 min), or SOS (100 μM for 180 min) alone did not alter auditory neural thresholds. When infused together with glutamate, TBOA and SOS produced significant neural threshold shifts, leaving otoacoustic emissions intact. In addition, both TBOA and SOS exacerbated noise-induced hearing loss by producing larger neural threshold shifts and delaying recovery. DHK did not alter glutamate- or noise-induced hearing loss. The evidence points to a major role for GLAST, both in protecting the synapse from exposure to excess extracellular glutamate and in attenuating hearing loss due to acoustic overstimulation.
INTRODUCTION
In the cochlea, neurotransmission between the inner hair cells and their afferent neurons is mediated by glutamate receptors (reviewed in Glowatzki et al. 2008; Ruel et al. 2007). Glutamate must be maintained at levels low enough to ensure a high signal-to-noise ratio for afferent neurotransmission and to prevent excitotoxic damage to the afferent neurons. The rapid clearance of synaptic glutamate is accomplished by high-affinity glutamate transporters (Bridges and Esslinger 2005; Danbolt 2001; Seal and Amara 1999). Five subtypes of high-affinity, sodium-dependent glutamate transporters (EAAT1-5) have been identified of which the primary transporters involved in clearance of glutamate from the extracellular space are: glutamate/aspartate transporter (GLAST; EAAT1), glutamate transporter-1 (GLT1; EAAT2), and excitatory amino acid carrier 1 (EAAC1; EAAT3). EAAT4 and EAAT5 may have additional or alternate functions given that they can form a chloride channel (Arriza et al. 1997; Fairman et al. 1995; Gegelashvili and Schousboe 1997). Immunocytochemical and RT-PCR studies have demonstrated that GLAST is present in supporting cells bordering the IHC and in Schwann cells surrounding the auditory neurons in the spiral ganglion; GLT1 and EAAC1 are found in spiral ganglion neurons (Furness and Lawton 2003; Furness and Lehre 1997; Li et al. 1994; Rebillard et al. 2003).
Several lines of evidence suggest that in the cochlea GLAST plays a significant role in clearing glutamate from the region of the afferent synapse. GLAST is the major glutamate transporter found in the adult cochlea (Li et al. 1994; Rebillard et al. 2003). It is packed into supporting cells that surround the IHC (Furness and Lawton 2003; Rebillard et al. 2003). The distribution of GLAST along the length of the cochlea parallels the distribution of AMPA receptors in the guinea pig (Furness and Lawton 2003). Recordings from inner phalangeal cells demonstrate the functional presence of GLAST in those cells, and analysis of GLAST reporter transgenic mice indicated that the presence of GLAST in the organ of Corti is limited to cells surrounding the IHC (Glowatzki et al. 2006). In GLAST-deficient mice, threshold shift following intense acoustic stimulation is exacerbated (Hakuba et al. 2000), an effect interpreted to indicate damage due to a reduced clearance of glutamate from the synapse. The intracochlear perfusion of a broad-spectrum glutamate transporter inhibitor l-trans-pyrrolidine-2,4-dicarboxylic acid (PDC) has been shown to decrease the amplitude of the cochlear compound action potential (CAP) in the guinea pig (Rebillard et al. 2003). In GLAST-deficient mice, baseline auditory brain stem response (ABR) thresholds were slightly but significantly higher than thresholds of wild-type mice (Hakuba et al. 2000). Together these observations suggest that glutamate transporters play a role in modulating auditory function during physiological conditions or noise trauma.
There is, however, little direct description of the role these transporters play in cochlear physiology in vivo. We have undertaken a pharmacological approach to the analysis of the effects of glutamate transporter blockers on cochlear responses to acoustic stimulation, to acoustic overexposure, and to administration of exogenous glutamate in the wild-type mouse. We analyzed three glutamate transporter blockers for their ability to alter the effects of glutamate, exogenously applied to the synapse via perfusion of the scala tympani, and compared that action to their ability to alter the effects of intense acoustic stimulation. dl-threo-beta-benzyloxyaspartate (TBOA) is a broad-spectrum glutamate antagonist, affecting all three transporters (GLAST, GLT1, and EAAC1) (Shimamoto et al. 1998). l-serine-O-sulfate (SOS) blocks both GLAST and EAAC1 without effect on GLT1 (Arriza et al. 1994). Dihydrokainate (DHK) is selective for GLT1 (Wang et al. 1998). Our evidence points to a major role for GLAST, both in protecting the synapse from exposure to extracellular glutamate and in attenuating responses to acoustic overstimulation.
METHODS
Animals and surgical procedures
Young adult CBA/CaJ mice (4–8 wk) were anesthetized with ketamine (100 mg/kg ip) and xylazine (10 mg/kg ip) with boosters (1/3 to 1/2 the original dose) delivered intraperitoneally as needed. All animal procedures were approved by the Animal Care and Use Committee of the Massachusetts Eye and Ear Infirmary.
The surgical procedure was performed in the warmed chamber as described previously (Chen et al. 2006). Briefly, a skin incision was made longitudinally extending from the mandible to the clavicle in the paramedian ventral surface of the neck. The submandibular gland was visualized and retracted laterally to expose the digastric muscle. The muscle was then cut with a bipolar cautery to expose the underlying tympanic bulla and stapedial artery. The stapedial artery was cauterized at the entrance to the bulla. This procedure does not affect cochlear function (Chen et al. 2006). A cochleostomy was drilled ∼300 μm beneath the stapedial artery stump using a 175 μm drill bit (Drill Bit City, Prospect Heights, IL). Then the tip of a 10 cm length of fused silica tubing (OD = 144 μm, ID = 75 μm; World Precision Instruments) was inserted into the cochleostomy. A silver wire electrode for CAP recording was placed against the bulla adjacent to the cochleostomy. Dental cement was applied to seal the hole and to secure the tubing and CAP electrode to the bulla.
Intra-cochlear drug infusion
The control solution and drugs were infused into the cochlea using a delivery system as described previously (Chen et al. 2006). Briefly, an infusion/withdrawal syringe pump (Harvard Apparatus PHD 2000) was operated at an infusion flow rate of 1 μl/h. There was a three-way miniature manifold (Warner Instruments, now Harvard Apparatus, MM-2) in between the silica tubing and the pump to allow for changes of infusion solutions. The silica tubing was inserted into one input of the manifold. The inlet line from the infusion pump was placed in a second input to the manifold, and a drain line was placed into the third input. A three-way valve was placed between the pump and the manifold and an open/shut valve on another end of the drain line. When the drain open/shut valve was closed, the flow from the pump was directed into the cochlea. To change the injection solution, the drain valve was opened, and the new solution was loaded through the three-way into the line between the pump and manifold. The dead space of the 10 cm infusion tubing (ID = 75 μm) is 0.44 μl.
The control solution and vehicle for the drugs was artificial perilymph (AP) with a composition (in mM) of 120 NaCl, 3.5 KCl, 1.5 CaCl2, 5.5 glucose, and 20 HEPES, 20. Glutamate (10 μM, Sigma) and sodium salicylate (5 mM, Sigma) were prepared by dissolving these drugs in AP and adjusting the pH to 7.5. Three different inhibitors of glutamate transporters, TBOA (200 μM, Tocris Bioscience, Bristol, UK), SOS (100 μM, Sigma), and DHK (1 mM, Sigma) were brought into solution in AP and pH adjusted to 7.5. These concentrations were chosen following an initial series of experiments that surveyed the effects of a broad range of concentrations for each drug.
In all animals, the initial infusion was of AP (1 μl/h for 80 min). Drug solutions were then loaded and infused at 1 μl/h. Glutamate, TBOA, and SOS were infused alone (220 min for glutamate and TBOA, 180 min for SOS, n = 4 mice/group) to test the individual effects of these drugs on cochlear function. To test the effect of glutamate transporter inhibitors on glutamate-induced hearing loss, glutamate was infused for 80 min following the initial AP. Then each inhibitor (TBOA, SOS, or DHK) was infused with glutamate for 80–120 min. If no drug effect was seen, salicylate (5 mM), a drug with previously characterized effects (Chen et al. 2006) was infused to verify infusion system function and cochlear responsiveness.
Auditory function measurements
CAPs of the auditory nerve and distortion product otoacoustic emissions (DPOAEs) were recorded on the operated ear (right ear) as described previously (Chen et al. 2006). The mice were anesthetized as described in the preceding text, and a small slit was made in the external canal to visualize the tympanic membrane and to verify the normal appearance of the membrane and middle ear space beyond. The sound stimuli were created with 16-bit A/D, D/A boards (National Instruments) controlled in a LabView environment by a PC workstation and delivered to the external ear canal with a custom coupler. The coupler connects transducers (Tucker Davis EC1) and a Knowles EK3103 electret microphone to measure ear-canal sound pressure (for DPOAEs) via a probe tube concentric with the 2.54 mm (OD) sound delivery tube. Sensitivity versus frequency calibration curves were generated for the monitoring and probe microphones, respectively, enabling conversion from voltage to sound pressure level (SPL; in dB re: 20 μPa). The probe assembly was then placed at the animal's ear canal where “in-animal” calibration sweeps were accomplished and used to determine the actual SPLs generated at the entrance to the bony canal.
CAPs were detected with a silver wire electrode near the cochleostomy (the round window membrane is not exposed by this approach) with a subcutaneous electrode at vertex serving as reference, and one in the hindleg as ground. Responses were elicited by tone pip stimuli (5.6–45.2 kHz; 0.5-ms duration, 0.5-ms rise-fall; cos2 onset envelope; 16/s; 0–80 dB SPL in 5 dB steps), amplified (10,000 times), filtered (300 Hz to 3 kHz passband), and averaged (32 samples). The waveforms of the responses were stored. CAP threshold was defined as the lowest stimulus level at which the N1-P1 response peaks were clearly and reproducibly present. These visual detection threshold judgments were confirmed following termination of the experiment by off-line display and analysis of the stored waveforms.
The 2f1 − f2 DPOAEs were recorded as response amplitude versus primary level functions (L1 = 10–75 dB SPL; L2 = L1 − 10; primaries incremented together in 5 dB steps) with the frequency range f2 = 5.6–45.2 kHz (f2/f1 = 1.2). Ear-canal sound pressure was amplified, digitally sampled, and averaged (20 discrete spectra at each frequency-level combination) and fast Fourier transforms were computed from the averaged pressures. DPOAE level at 2f1 − f2 and surrounding noise floor values (±50 Hz of 2f1 − f2) were extracted. Iso-response contours (L2 levels required to generate a DPOAE amplitude of 0 dB SPL) were constructed from amplitude versus sound level data to facilitate comparison with CAPs.
The CAP and DPOAE responses were recorded as a postsurgical baseline, 10 min after each change of perfusate, and every 30–40 min during the drug infusion. As we have previously demonstrated (Chen et al. 2006), this procedure produced minimal (<5 dB) changes in thresholds following a cochleostomy and infusion of artificial perilymph.
Pure tone noise exposure and continuous CAP recording
Following the initial AP infusion (1 μl/h, 30 min), a subset of animals received a pure tone overexposure (22.63 kHz, 102 dB SPL for 10 min). CAPs at 32 kHz were measured immediately after the noise and every 30–40 s for ∼10 min. To test the effect of glutamate transporter inhibitors on the noise-induced threshold shifts, TBOA, SOS, or DHK was loaded and infused (1 μl/h) for 1 h after the initial AP infusion. Then the pure tone noise was presented for 10 min, and CAPs were measured following the noise every 30–40 s for ∼30 min with the drugs continuously infused. Salicylate was used as a positive control for an intact infusion system if no change in the auditory function was observed in response to drug infusion.
RESULTS
Glutamate transporters prevent access of exogenous glutamate to the afferent synapse
A number of studies have demonstrated that perfusion of the scala tympani requires relatively high (millimolar) concentrations of glutamate to affect afferent transmission. One hypothesis for this is that glutamate transporters within cochlear tissue protect the synapse from extracellular glutamate. We tested that hypothesis by blocking glutamate transporters while perfusing the cochlea with low concentrations of exogenous glutamate.
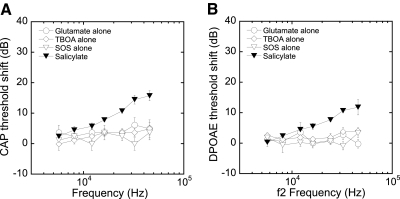
We first determined if low (10 μM) concentrations of glutamate alone or if any of the three glutamate transporter antagonists alone could alter cochlear potentials by infusing each into the cochlea at pharmacologically active concentrations. Previously we have demonstrated stability of auditory function during extended AP infusion (1 μl/h for 7 h) (Chen et al. 2006). Consistent with those results, CAP and DPOAE thresholds were stable (within 5 dB) during the 80 min predrug infusion. Infusion of a relatively low concentration of glutamate (10 μM, 1 μl/h) for 220 min also did not elevate CAP or DPOAE thresholds (changes were <5 dB; see Fig. 1). Similarly, there was little effect when the cochlea was infused with any of the glutamate transporter inhibitors alone. Neither TBOA (200 μM, 220 min) nor SOS (100 μM, 180 min) produced any consistent change in response thresholds (Fig. 1). In each of these cases, infusion of glutamate or a transport inhibitor was followed by infusion of salicylate, which served as a positive control to verify that perfused agents were actually getting to the hair cell region. The infusion of salicylate (5 mM) produced the expected ∼10–15 dB threshold shifts in both CAPs and DPOAEs at high frequencies with less effect on lower frequencies (Chen et al. 2006).
Fig. 1.
Infusion of glutamate (10 μM, 220 min), threo-beta-benzyloxyaspartate (TBOA; 200 μM, 220 min), or l-serine-O-sulfate (SOS 100 μM, 180 min) alone produced very little threshold shift (<5 dB) in compound action potentials (CAPs; A) or distortion product otoacoustic emissions (DPOAEs; B). A subsequent infusion of salicylate (5 mM, 10 min) served as a positive control, indicating drugs were getting to the organ of Corti. Salicylate produced 10–15 dB threshold shifts at high frequencies.
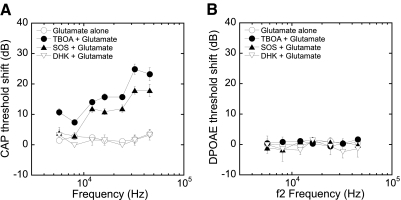
To determine whether glutamate transporters are protecting the synapse from excess glutamate in perilymph, we assessed the interactions of glutamate transport blockers with glutamate applied exogenously at a concentration (10 μM) shown in the preceding text to be ineffective. Neither an initial infusion of AP (80 min) nor glutamate (10 μM, 80 min) altered the CAP or DPOAE thresholds (Fig. 2). When this was immediately followed by an infusion of glutamate with TBOA (200 μM, 80 min) or SOS (100 μM, 120 min), we observed 15–25 dB CAP threshold shifts at high frequencies. Shifts were less at lower frequencies, consistent with the basal location of the cochlear infusion inlet. Even at the lowest frequencies tested (5.6 and 8 kHz), there were ∼10 dB CAP threshold shifts after the infusion of TBOA together with glutamate. In contrast, the specific GLT1 transporter inhibitor DHK (1 mM) did not alter CAP thresholds when infused with glutamate for 120 min (Fig. 2A). There was no change in DPOAE thresholds (Fig. 2B) with any of the drug combinations, consistent with the lack of effect of glutamate, even at high concentrations (data not shown), on the outer hair cell-based DPOAEs.
Fig. 2.
Infusion of glutamate (10 μM, 80 min) did not produce change in CAP (A) or DPOAE (B) thresholds. The infusion afterward of glutamate with TBOA or SOS resulted in 15–25 dB CAP threshold shifts at high frequencies with smaller shifts in low frequencies, leaving DPOAE intact. Infusion with the glutamate transporter-1 (GLT1) blocker, dihydrokainate (DHK), with 10 μM glutamate had no effect on either CAP or DPOAE thresholds.
Glutamate transporter inhibitors delay recovery following acoustic overstimulation
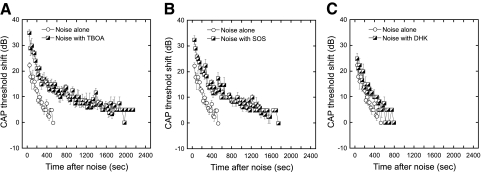
To determine the role of glutamate transporters in controlling the responses to high level acoustic stimulation, anesthetized mice were exposed to a pure tone (22.63 kHz at 102 dB for 10 min) followed by measurement of CAP thresholds at 32 kHz, the most affected characteristic frequency region for this stimulus. The CAP threshold shift 30 s after noise averaged ∼23 dB and recovered gradually to prenoise level by ∼10 min after the noise exposure (Fig. 3, A and B). We then determined the effects of glutamate transporter blockers on the recovery of thresholds from this intense acoustic stimulation. The scala tympani was initially infused with AP (60 min) followed by either TBOA (200 μM, 60 min) or SOS (100 μM, 60 min). Neither drug alone produced any change in CAP or DPOAE thresholds (data not shown). However, following exposure to the 22.63 kHz pure tone described in the preceding text, CAP threshold shifts were greater and recovered more slowly in the presence of TBOA or SOS than in AP controls. The GLT1 blocker, DHK (1 mM) had little effect on either the magnitude of the threshold shifts or the time course of their recoveries following the noise exposure (Fig. 3C).
Fig. 3.
Mice were exposed to a pure tone (22.3 kHz, 102 dB for 10 min) and CAPs recorded at 32 kHz and plotted (means ± SE). With infusion only of artificial perilymph (AP), CAP thresholds were shifted 23 dB at ∼30 s after termination of the noise exposure. (Data following noise exposure during infusion of artificial perilymph is replotted in each of the 3 panels.) Thresholds recovered gradually and returned to preexposure levels at ∼10 min. The infusion of TBOA (200 μM; A) or SOS (100 μM; B) during the noise exposure produced ∼33 dB CAP threshold shift at ∼30 s after noise. The CAP thresholds recovered ∼15 dB in the 1st 300 s after noise and then recovered more gradually to eventually reach near the preexposure level. The infusion of DHK (1 mM, GLT1 specific inhibitor; C) did not produce much change in the noise-induced reversible CAP threshold shifts. To quantify the changes associated with drug infusion, regression lines were fit to the data during the 1st 500 s following exposure, and slopes (recovery rates) and intercepts (initial shift) determined for each mouse (n = 6 each for AP, SOS, and TBOA; n = 4 for DHK). Recovery rates over the 1st 500 s were similar in all conditions. Changes in intercepts (initial shift) were significantly higher (P < .0025, Student's t-test) with TBOA and SOS compared with AP. Changes in intercepts with DHK were not significantly different from AP (P = 0.6).
In the presence of TBOA or SOS, CAP thresholds recovered with two different rate constants (please see Fig. 3, A and B). The initial rate of recovery (over the first few hundred seconds) was similar to that observed with AP alone. A slower rate of recovery was observed after 400-500 s, which was not observed in the absence of these drugs.
DISCUSSION
Our analysis demonstrates two functions of glutamate transporters in the cochlea. The first is that the transporters are needed to clear neurotransmitter from the synapse after intense acoustic stimulation, suggesting that a component of the rate of return of thresholds after intense stimulation is dependent on clearance of glutamate from the synapse. The second is that glutamate transporters protect the cochlea from exposure to glutamate present in the extracellular space.
Pharmacology
The three major glutamate transporters are EAAT1 (GLAST), EAAT2 (GLT1), and EAAT3 (EAAC1). Two of these, GLAST and GLT1, dominate glutamate transport in the CNS (Gadea and Lopez-Colome 2001). Drugs are available with specificities that can distinguish GLT1 from GLAST and EAAC1 (Namura et al. 2002; Wang et al. 1998). DHK is a specific GLT1 inhibitor with little affinity for GLAST and EAAC1. SOS has little affinity for GLT1. We found no effect of DHK on responses to intense acoustic stimulation or on responses to exogenously applied glutamate. Conversely, SOS influenced both processes. These pharmacological results lead us to consider it unlikely that GLT1 is playing a role in cochlear afferent synaptic transmission. It is plausible that the presence of GLT1 in ganglion cells indicates a role in the central processes of these neurons.
Both TBOA and SOS altered the response to intense acoustic stimulation and to exogenously applied glutamate. TBOA is a broad-spectrum glutamate transporter blocker (Shimamoto et al. 1998). SOS is selective for GLAST and EAAC1 (Arriza et al. 1994). We are unable to distinguish between GLAST and EAAC1 with the currently available pharmacological tools. Of GLAST and EAAC1, GLAST is the most likely candidate to mediate these effects. GLAST is densely packed in the supporting cells that surround the IHC (Furness and Lawton 2003; Rebillard et al. 2003). Several other studies looking at various aspects of synaptic transmission also conclude that GLAST is the major transporter in cochlear afferent transmission (Furness and Lawton 2003; Glowatzki et al. 2006; Hakuba et al. 2000; Ottersen et al. 1998; Rebillard et al. 2003).
TBOA and SOS produced similar effects on auditory function even though these drugs block glutamate transporters by very different mechanisms. Many glutamate transporter inhibitors, like SOS and PDC are substrate inhibitors (e.g., they are themselves substrates for uptake by the transporter, thus inhibiting uptake of glutamate), while TBOA binds to the transporter without becoming a substrate. Substrate inhibitors exhibit the phenomenon of heteroexchange whereby synaptic glutamate concentrations are elevated via reverse transport of endogenous glutamate from the intracellular to the extracellular (synaptic) space (Kanner and Bendahan 1982; Volterra et al. 1996). Thus heteroexchange can raise synaptic levels of glutamate independent of the release of neurotransmitter from the hair cell. Our observation of similar effects on auditory function with SOS and TBOA indicates the actions we observed were not due simply due to accumulation of synaptic glutamate via heteroexchange of glutamate.
Rebillard and colleagues (2003) observed dose-dependent reductions in CAP amplitudes when perfusing the scala tympani with relatively high concentrations of PDC, a broad-spectrum, substrate-based, glutamate transporter inhibitor (Griffiths et al. 1994). Their careful pharmacological analysis demonstrated that the effects they observed were consistent with activation of AMPA receptors. However, they acknowledged the difficulty in determining whether the glutamate accumulation was due to block of neurotransmitter uptake or to hetero-exchange of glutamate. We did not see an alteration in CAP thresholds with infusion of TBOA or SOS. The concentrations of these drugs (200 and 100 μM, respectively) were high enough to produce significant effects on physiological responses to high-level sound or exogenous glutamate. PDC, SOS, and TBOA have similar affinities for three glutamate transporters with Km's ranging between 10−5 and 10−4 M measured in a broad variety of tissues and organisms (Arriza et al. 1994; Shimamoto et al. 1998) and should have acted at similar concentrations. However, much higher (EC50 of 1.33 mM) concentrations of PDC were needed to produce a “resting excitotoxicity.” Thus our results suggest that the glutamate accumulation observed by Rebillard and colleagues (2003) may indeed have been due to hetero-exchange.
Effects on uptake of neurotransmitter released during stimulation
Noise-induced temporary threshold shifts were larger and more prolonged in ears treated with glutamate transport blockers. We observed changes in auditory nerve responses with glutamate transporter blockers when a relatively loud sound was administered. The primary observation was a prolonged period of temporary threshold shift. This response to intense acoustic stimulation is similar to that observed in the GLAST-deficient mouse. Transport kinetics of the glutamate transporters are relatively slow. Thus it seems plausible that some part of the phenomenon of temporary threshold shift may be due to accumulation of neurotransmitter in the synaptic cleft. Other factors likely also influence the time course of recovery. The auditory neuron has the means of reducing sensitivity to transmitter by trafficking AMPA receptors out of the postsynaptic membrane (Chen et al. 2007). And transmitter release from the hair cell may also be altered following intense acoustic stimulation (Schwid and Geisler 1982).
We observed no effect on ABR thresholds from infusing any of the transporter blockers through the scala tympani. We only observed effects of the drugs when we presented challenges to the transporters, either by infusing glutamate into the scala tympani or by exposure to intense acoustic stimulation. These observations indicate that glutamate transmitters play a role in clearing transmitter from the synapse after intense stimulation but raise the question of whether the transporters are required to clear transmitter in the presence of low or moderate level acoustic stimulation or with normal perilymphatic levels of glutamate. This implies that GLAST may not play a critical role in auditory function under quiet or moderate acoustic level conditions. Other uptake systems, such as sodium-independent, low-affinity glutamate uptake system, may be sufficient under those circumstances (Danbolt et al. 1994). GLAST may be needed to remove synaptic neurotransmitter released under extreme conditions and to quickly restore responsiveness following intense acoustic stimulation. GLAST-deficient mice showed an exacerbation of hearing loss after acoustic overstimulation (Hakuba et al. 2000). In our study, similar effects were observed with infusion of TBOA. These results were consistent with the study on GLAST-deficient mice and demonstrated glutamate transporters play an important role in minimizing noise-induced hearing loss. Although we did not specifically examine the effects of these drugs on excitotoxicity, our findings are consistent with the idea that that GLAST activity may reduce the potential for such damage to auditory neurons (Furness and Lawton 2003; Hakuba et al. 2000; Rebillard et al. 2003).
In conclusion, the presumptive blockade of high-affinity glutamate transporters by a variety of transporter antagonists did not elevate auditory thresholds under physiological conditions over the short term, suggesting that other glutamate uptake systems may also function in the cochlea. However, both glutamate- and noise-induced hearing loss were exacerbated with the blockade of glutamate transporters. Therefore glutamate transporters, probably GLAST, play an important role in limiting the damage to the auditory function under acoustic overstimulation or with elevations of perilymphatic levels of glutamate.
GRANTS
This work was supported by National Institute of Deafness and Other Communication Disorders Grant DC-19097 to W. F. Sewell and DC-8577 to S. G. Kujawa.
DISCLOSURES
No conflicts of interest are declared by the author.
ACKNOWLEDGMENTS
We thank M. Peppi and C. Shera for helpful discussions.
REFERENCES
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA 94: 4155–4160, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci 14: 5559–5569, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Esslinger CS. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Therap 107: 271–285, 2005. [DOI] [PubMed] [Google Scholar]
- Chen Z, Kujawa SG, Sewell WF. Auditory sensitivity regulation via rapid changes in expression of surface AMPA receptors. Nat Neurosci 10: 1238–1240, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Mikulec AA, McKenna MJ, Sewell WF, Kujawa SG. A method for intracochlear drug delivery in the mouse. J Neurosci Methods 150: 67–73, 2006. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol 65: 1–105, 2001. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Storm-Mathisen J, Ottersen OP. Sodium/potassium-coupled glutamate transporters, a “new” family of eukaryotic proteins: do they have “new” physiological roles and could they be new targets for pharmacological intervention? Prog Brain Res 100: 53–60, 1994. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 375: 599–603, 1995. [DOI] [PubMed] [Google Scholar]
- Furness DN, Lawton DM. Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. J Neurosci 23: 11296–11304, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Lehre KP. Immunocytochemical localization of a high-affinity glutamate-aspartate transporter, GLAST, in the rat and guinea-pig cochlea. Eur J Neurosci 9: 1961–1969, 1997. [DOI] [PubMed] [Google Scholar]
- Gadea A, Lopez-Colome AM. Glial transporters for glutamate, glycine and GABA. I. Glutamate transporters. J Neurosci Res 63: 453–460, 2001. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol 52: 6–15, 1997. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Cheng N, Hiel H, Yi E, Tanaka K, Ellis-Davies GC, Rothstein JD, Bergles DE. The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea. J Neurosci 26: 7659–7664, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Grant L, Fuchs P. Hair cell afferent synapses. Curr Opin Neurobiol 18: 389–395, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Dunlop J, Gorman A, Senior J, Grieve A. L-trans-pyrrolidine-2,4-dicarboxylate and cis-1-aminocyclobutane-1,3-dicarboxylate behave as transportable, competitive inhibitors of the high-affinity glutamate transporters. Biochem Pharmacol 47: 267–274, 1994. [DOI] [PubMed] [Google Scholar]
- Hakuba N, Koga K, Gyo K, Usami SI, Tanaka K. Exacerbation of noise-induced hearing loss in mice lacking the glutamate transporter GLAST. J Neurosci 20: 8750–8753, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner BI, Bendahan A. Binding order of substrates to the sodium and potassium ion coupled L-glutamic acid transporter from rat brain. Biochemistry 21: 6327–6330, 1982. [DOI] [PubMed] [Google Scholar]
- Li HS, Niedzielski AS, Beisel KW, Hiel H, Wenthold RJ, Morley BJ. Identification of a glutamate/aspartate transporter in the rat cochlea. Hear Res 78: 235–242, 1994. [DOI] [PubMed] [Google Scholar]
- Namura S, Maeno H, Takami S, Jiang XF, Kamichi S, Wada K, Nagata I. Inhibition of glial glutamate transporter GLT-1 augments brain edema after transient focal cerebral ischemia in mice. Neurosci Lett 324: 117–120, 2002. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Takumi Y, Matsubara A, Landsend AS, Laake JH, Usami S. Molecular organization of a type of peripheral glutamate synapse: the afferent synapses of hair cells in the inner ear. Prog Neurobiol 54: 127–148, 1998. [DOI] [PubMed] [Google Scholar]
- Rebillard G, Ruel J, Nouvian R, Saleh H, Pujol R, Dehnes Y, Raymond J, Puel JL, Devau G. Glutamate transporters in the guinea-pig cochlea: partial mRNA sequences, cellular expression and functional implications. Eur J Neurosci 17: 83–92, 2003. [DOI] [PubMed] [Google Scholar]
- Ruel J, Wang J, Rebillard G, Eybalin M, Lloyd R, Pujol R, Puel JL. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear Res 227: 19–27, 2007. [DOI] [PubMed] [Google Scholar]
- Schwid HA, Geisler CD. Multiple reservoir model of neurotransmitter release by a cochlear inner hair cell. J Acoust Soc Am 72: 1435–1440, 1982. [DOI] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol 39: 431–456, 1999. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol 53: 195–201, 1998. [DOI] [PubMed] [Google Scholar]
- Volterra A, Bezzi P, Rizzini BL, Trotti D, Ullensvang K, Danbolt NC, Racagni G. The competitive transport inhibitor L-trans-pyrrolidine-2, 4-dicarboxylate triggers excitotoxicity in rat cortical neuron-astrocyte co-cultures via glutamate release rather than uptake inhibition. Eur J Neurosci 8: 2019–2028, 1996. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chung HJ, Schnuer J, Lea E, Robinson MB, Potthoff WK, Aizenman E, Rosenberg PA. Dihydrokainate-sensitive neuronal glutamate transport is required for protection of rat cortical neurons in culture against synaptically released glutamate. Eur J Neurosci 10: 2523–2531, 1998. [DOI] [PubMed] [Google Scholar]