Abstract
The connectivity of large neurons of the nucleus reticularis gigantocellularis (NRGc) in the medullary reticular formation potentially allows both for the integration of stimuli, in several modalities, that would demand immediate action, and for coordinated activation of cortical and motoric activity. We have simultaneously recorded cortical local field potentials, neck muscle electromyograph (EMG), and the neural activity of medullary NRGc neurons in unrestrained, unanesthetized rats to determine whether the activity of the NRGc is consistent with the modulation of general arousal. We observed excitatory responses of individual NRGc neurons to all modalities tested: tactile, visual, auditory, vestibular, and olfactory. Excitation was directly linked to increases in neck muscle EMG amplitude and corresponded with increases in the power of fast oscillations (30 to 80 Hz) of cortical activity and decreases in the power of slow oscillations (2 to 8 Hz). Because these reticular formation neurons can respond to broad ranges of stimuli with increased firing rates associated with the initiation of behavioral responses, we infer that they are part of an elementary “first responder” CNS arousal mechanism.
INTRODUCTION
Large neurons in the medullary reticular formation may have considerable powers of integrative actions. Their sensory inputs and the sensory inputs to neighboring cells include those that would often demand immediate action. These include sexual stimuli (Hubscher and Johnson 1996; Kow and Pfaff 1982; Marson and McKenna 1996; McKenna 1999; Pfaff 1980), painful stimuli (Fort et al. 1994; Wall et al. 1999; Willis Jr 1985), vestibular stimuli (Ladpli and Brodal 1968; Peterson and Abzug 1975), visceral stimuli (Brown et al. 2002; Curtis et al. 2002; Hubscher et al. 2004; Kaddami and Hubscher 2006; Lovick 1997; Mayne et al. 1998), thermal stimuli (Farham and Douglas 1985), baroreceptor stimuli (Guilbaud et al. 1973; Haxhiu and Loewy 1996), and, from the ventral medullary surface, chemosensation of arterial Paco2 (Andreatta-van Leyen et al. 1990; Guyenet et al. 2005). The extensive distribution of reticulospinal axons to several levels of the spinal cord, in some cases bilaterally, has been demonstrated (Peterson et al. 1975, 1979), indicating clearly that such neurons may be important for activating many behaviors (Mason 2005). All of these properties suggest a role for these medullary reticular neurons in CNS arousal.
Carrying on from the early work of Morison and Dempsey (1942) and Moruzzi and Magoun (1949), a concept of generalized CNS arousal has been defined (Pfaff 2005) and given a quantitative assay in mouse behavior (Shelley et al. 2007). A more aroused animal is defined as one that is more responsive to all sensory stimuli, emits more voluntary motor activity, and is more reactive emotionally; the assay is described in Shelley et al. (2007). If these large reticular neurons are importantly involved in elevating generalized CNS arousal, we would expect: 1) that they could respond to several sensory modalities, and, for tactile stimuli, with broad receptive fields; 2) that their responses would habituate; 3) that their excitation would be correlated with higher frequency activity in the cortex; 4) that their excitation would be correlated with higher amplitude neck muscle electromyograph (EMG); and 5) that their excitation would be correlated with the initiation of behavioral responses. The experiments reported here used electrophysiological recordings from medullary nucleus reticularis gigantocellularis (NRGc) in unanesthetized rats to test these hypotheses.
METHODS
Surgical preparation
All procedures were performed using female Wistar rats (200–250 g) in accordance with national, state, and institutional guidelines according to AALAC regulations and approved by the Rockefeller University IACUC. Under the assumption that the elementary properties of the medullary reticular neurons recorded are not under strong ovarian hormone control, recordings were made without reference to estrus cycle. Surgery was performed under a surgical plane of anesthesia (Nembutal, 50 mg/kg, administered intraperitoneally [ip]), titrated with bolus injections (Nembutal, 5 mg/kg, ip) to eliminate reflexes without causing undue cardiac suppression. The animal's head was restrained in a Kopf Instruments stereotaxic device, a 3.0 cm incision was cut along the midline of the skull, and the skin was retracted to expose the parietal and interparietal plates. The connective tissue attached to the exposed surface was removed and the area was cleaned with hydrogen peroxide (3%). Access to the right NRGc was achieved by drilling a 2.0 × 2.0 mm hole, centered at intraaural −8.0 mm and midline +2.8 mm, in the animal's skull. Extracellular activity was recorded using a movable electrode (Kralik et al. 2001), substituting the original design's electrode bundle with a single, 50 μm diameter, Teflon-coated nickel–chromium microwire (1.0–1.5 MΩ). The single microwire projected from 1.0 to 2.0 mm beyond the 30 gauge, 8.0 mm long, stainless steel guide cannula. After implantation, the electrode could be lowered via the 0–80 screw component of the electrode. Given this design, all electrodes could be lowered ≥2.0 mm from the initial implantation site. A second microwire was soldered to the guide cannula, allowing the guide cannula to serve as the reference electrode. The movable electrode was lowered (depth = 8.0 mm at ∠18°; 200 μm/min) into the right NRGc and electrode placement was confirmed by high signal-to-noise action potentials evoked by gentle stroking of the animal's leg, back, and tail with a cotton-tipped applicator. The isolated neurons tended to have very low (<0.1 Hz) spontaneous activity while the animal was in the anesthetized state. Access to the frontoparietal cortex was achieved by drilling a 1.0 × 1.0 mm hole, centered at intraaural +2.1 mm and midline +1.2 mm, in the skull. An uninsulated stainless steel insect pin (Fine Science Tools) was lowered (depth = 1.0 mm) into the frontoparietal cortex to record cortical local field potentials (LFPs). LFP recordings theoretically measure the sum of inhibitory and excitatory synaptic events at the site of the electrode (reviewed in Mitzdorf 1985).
To establish EMG recordings, the skin and connective tissue over the neck muscle were retracted and two braided stainless steel wires (Fine Science Tools), which were stripped of 1.0 mm of insulation at the point of contact between the electrode and the muscle, were sutured bilaterally into the neck muscles. Three cranial screws and one ground electrode were implanted in the skull, with one screw implanted in each of the parietal plates and one screw implanted in the interparietal plate. All electrodes were attached to a miniature connector and then anchored to the cranial screws using dental acrylic. During recovery, animals were given aspirin (100 mg/kg, oral) for 3 days postsurgery, starting at 6 h postsurgery.
Recording and stimulus presentation
Recordings were collected from a total of 10 animals and included 14 single-unit and 5 multiunit recordings (see Analysis and statistics). All animals were handled for ≥15 min per day for 3 consecutive days prior to surgery. Experiments were conducted starting at either 8 h after the animal was removed from the stereotaxic frame (14/19 recordings) or 3 to 6 days postsurgery (5/19 recordings). The shorter recovery period (8 h) was necessitated by the poor recording stability of some of the electrodes in the medulla (see discussion). All recordings took place during the light portion of an animal's 12 h:12 h light:dark cycle.
An experiment was initiated by attaching the rat's headplug to the electrophysical apparatus via a lightweight, counterbalanced cable leading to a commutator mounted into the chamber's ceiling. The animal was then acclimatized to both the recording chamber and the cable connector for ≥1 h prior to presentation of any stimuli. If there were multiple recordings made from a single animal, the electrode was lowered ≥250 μm from the previous recording site. To minimize bias, all potential recording sites were observed for ≥5 min. After lowering the electrode, the experimenter waited ≥30 min prior to the initiation of a new recording session. During the recording sessions, extracellular unit, cortical LFP, and EMG activity were simultaneously recorded (20 kHz, PowerLab) while the animal was freely behaving in a 20 × 20 × 40 inch wooden chamber. In 3 of 19 recording sessions, cortical LFP and EMG activity were not recorded.
The chamber was lit by a 10 W, 12 V bulb located at 35 inch height. Electrical flow to the bulb was under software control via a 5 VDC micro-relay (RadioShack). A 92 dB piezoelectric buzzer (RadioShack) was attached to the cage at 28 inch height. A fan, located at 30 inch height, provided chamber ventilation and white noise. Prior to the start of each recording session, the base of the chamber was lined with a deotized animal cage board (DACB) board.
During recording, the animal was presented with each of five sensory stimuli: 1) tactile: the experimenter gently stroked (1.0 to 3.0 mm; ∼500 ms) the animal's leg (4 trials/recording session) or back (4 trials/recording session) with a cotton-tipped applicator; 2) vestibular: the experimenter horizontally shifted (2.0 to 3.0 mm; ∼500 ms) the DACB board base of the recording chamber; it was returned to its original position after 10 s; 3) olfactory: the experimenter brought a benzaldehyde-soaked cotton-tipped applicator to within 2.0 to 3.0 mm of the animal's nose, taking care to avoid the animal's vibrissae, and then removed the applicator after 1 s; although it unlikely that the applicator touched the animal, it is possible that this stimulus had auditory or visual components (see Technical considerations in discussion); benzaldehyde was chosen because it emits a strong odor with a minimum of trigeminal stimulation; 4) visual: a 5 V pulse (200 ms) was delivered to the 5 V DC relay via the PowerLab system, turning off the light for 200 ms (in a circadian framework, lights-off would be associated with the initiation of activity); and 5) auditory: an 8 V pulse (200 ms) was delivered via the PowerLab system, turning on the buzzer at about 80 dB SPL (depending on the animal's position) for 200 ms. The auditory buzzer was turned on at a rate of either 0.5 Hz (4/19 recordings) or 1.0 Hz (12/19 recordings) for eight repetitions per trial to test for habituation; in the remaining 3/19 recordings, the auditory buzzer was turned on for only one repetition per trial. The difference between trial number and repetition number is illustrated later in Fig. 10A.
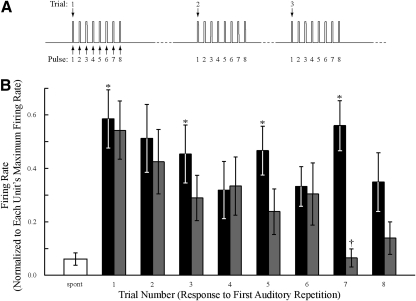
Fig. 10.
Mean firing rate (±SE) as a function of increasing trial number. A: schematic of the difference between trial number and repetition number. B: both the onset response (black; 0.0 to 0.2 s) and the sustained response (gray; 0.2 to 1.0 s) trend toward decreased activity. Asterisks (*) mark trials that significantly differed from spontaneous activity; daggers (†) mark trials that significantly differed from the activity evoked during repetition 1 (P < 0.050, after Bonferroni correction).
To decrease the variability of the behavioral state of the animal at the time of stimulus presentation, the animal was required to be motionless for 60 s prior to the presentation of each trial of a stimulus. Motionlessness is not a unitary state and can correspond to a state of rest or fear. Although there was some variance across trials, the EMG waveform was predominantly of low amplitude in the 60 s prior to stimulus presentation, suggesting that the animal was in a resting state.
Each sensory stimulus was presented for eight consecutive trials prior to advancing to the next stimulus. The order of the stimulus presentation was: tactile, auditory, olfactory, vestibular, and visual. A full recording session lasted from 3.0 to 3.5 h. The time stamp of stimulus onset was manually triggered for the tactile, vestibular, and olfactory stimuli and is thus approximate. In contrast, the time-stamping of the onset of the visual and auditory stimuli was under software control.
Analyses and statistics
The goals of our analyses included the determination of whether neurons of the medullary NRGc show multimodal responses and whether these responses are associated with increased motoric and cortical arousal. Specifically, we quantified the changes in the mean firing rate (spikes/s), the amplitude of the neck muscle EMG, and the power of behaviorally relevant oscillatory frequencies in cortical LFP.
The spontaneous activity analysis window was defined as −10.0 to −5.0 s (all times are given relative to the time-stamping of stimulus onset). Onset activity is the window from 0.0 to 0.2 s and sustained activity is the window from 0.2 to 5.0 s.
Spike rate, EMG amplitude, and the power of behaviorally relevant oscillation frequencies were calculated using in-house Matlab functions. Single unit and multiunit recordings were spike-sorted off-line using a hierarchical clustering algorithm that is based on spike shape and the statistics of spike arrival times (Fee et al. 1996), available at chronux.org. The data for single units and multiunits are combined for analysis in Figs. 7–10. The results in Fig. 9 were calculated from 69 spike pairs (including 12 single unit recordings from 7 rats and 4 multiunit recordings from 4 rats). Each spike pair is defined as two consecutive action potentials, from the same unit, that have an interspike interval (ISI) of <5 ms. The instantaneous firing frequency (Figs. 2–6) was calculated as described in Gabbiani and Krapp (2006). Briefly, at each spike time t instantaneous firing frequency is the reciprocal of the mean of the preceding and following ISIs. The EMG waveform was low-pass filtered (10 Hz, Butterworth filter) and full-wave rectified. To find bouts of high EMG, the mean and SD of the EMG were calculated from −10.0 to +10.0 s relative to the time-stamping of stimulus presentation for each unit. A bout was defined as suprathreshold EMG that lasted for ≥1.0 s; bouts that were within 1.0 s of each were combined (Foo and Mason 2005). Cortical activation is measured as the power in low (2–8 Hz) and high (30–80 Hz) frequency bands. Power was determined using Matlab function pmtm.m, with the time–bandwidth product set to 4 and the length of the discrete Fourier transform (nfft) set to 2e14. Power is presented as the magnitude of the power summed within a frequency band relative to the total power from 0 to 100 Hz.
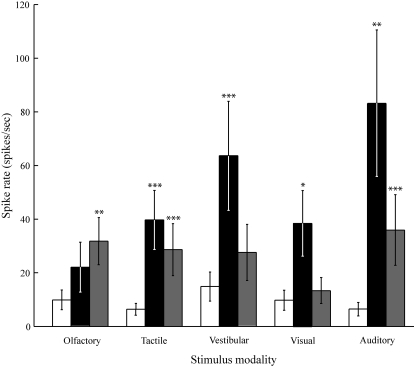
Fig. 7.
Responses to the 5 stimulus modalities. The bar graphs show mean firing rate (±SE), averaged across all units. The white bars show spontaneous activity (−10.0 to −5.0 s); the black bars show the mean spike rate from 0.0 to 0.2 s; the light gray bars show mean spike rate from 0.2 to 5.0 s. With respect to the 0.0 to 0.2 s window, we note that the timing is absolutely accurate for computer controlled stimuli (auditory and visual), but approximate for the other 3 stimulus modalities. The NRGc showed excitatory responses to each stimulus modality. *P < 0.050; **P < 0.010; ***P < 0.001.
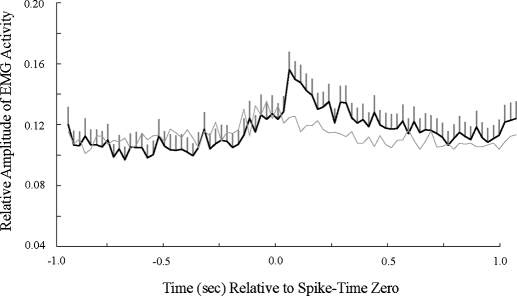
Fig. 9.
Neck muscle EMG amplitude (±SE) relative to the timing of spike firing. Burst firing in the NRGc is followed by increased motoric activation. Average of the EMG amplitude before and after spike pairs with interspike intervals of <5 ms. The mean EMG amplitude of the spike-time shuffled control is shown in light gray. Time 0 represents the time when an action potential occurred.
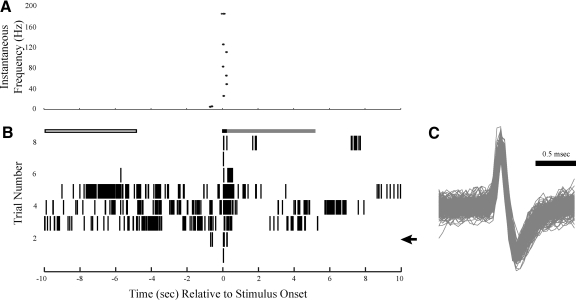
Fig. 2.
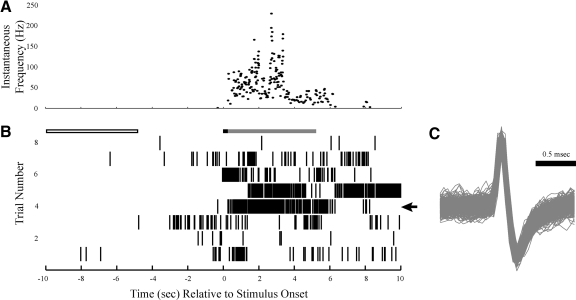
Example of a single unit's (Unit A) responses to olfactory stimulus presentations. A: the instantaneous firing frequency is shown for trial 4. B: timing of action potential firing relative to stimulus onset. Each hash mark represents an action potential. The arrow in the right margin marks the trial shown in A. Time zero is the approximate time at which the benzaldehyde-scented cotton swab was brought to the animal's nose. Prior to the presentation of the stimulus, there was low spontaneous activity. Presentation of the stimulus led to a long-duration increase in firing rate. The open, black, and gray boxes above the raster plot represent the time windows, respectively, for spontaneous firing, onset firing, and sustained firing measurements. C: the overlaid waveforms for all action potentials in B.
Fig. 6.
Examples of unit responses to auditory stimulus presentations. A: the instantaneous firing frequency for trial 3. B: single unit (Unit A) responses to 8 auditory repetitions (illustrated as 8 black boxes above the figure) at a rate of 0.5 Hz. C: the overlaid waveforms for all of unit A's action potentials. D: the instantaneous firing frequency for trial 1. E: multiunit responses to 8 auditory pulses at a rate of 1.0 Hz. At increased repetition numbers, the phasic response becomes difficult to discriminate from the sustained excitation. F: the overlaid waveforms for all action potentials from D.
The variances in firing rate between spontaneous and evoked activity (Figs. 7 and 10) were unequal and required nonparametric testing for significance. Whether there existed an effect of stimulus modality (Fig. 7) or trial number (Fig. 10) on spike activity was first determined using the nonparametric Kruskal–Wallace test for paired data (kruskal.test; S-Plus, TIBCO Software, Palo Alto, CA). If there was a significant effect, post hoc significance testing was determined using the nonparametric paired Wilcoxon test (wilcox.test; S-Plus) with Bonferroni correction for multiple comparisons. In contrast to the firing rate data, the variances in EMG amplitude (Fig. 9) were normally distributed and approximately equal among the conditions. As such, whether there existed an effect of stimulus repetition number was tested using one-way ANOVA (aov; S-Plus). If there was a significant effect, post hoc significance testing was determined using the two-sided paired Student's t-test (t.test; S-Plus) with Bonferroni correction for multiple comparisons. The relationship between firing rate and either oscillatory power or EMG amplitude (Fig. 8) was tested with the Pearson's χ2 test (chisq.test; S-Plus).
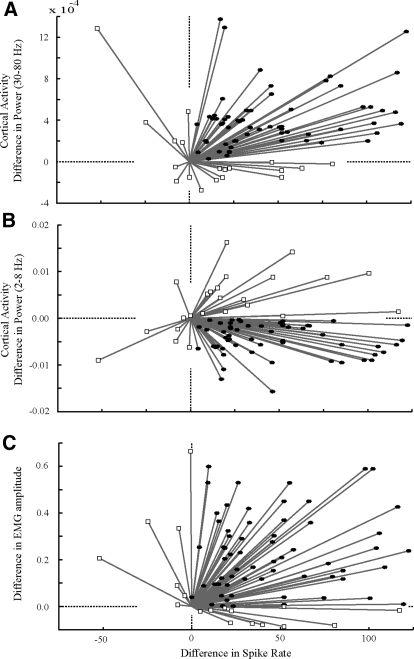
Fig. 8.
Changes in the power of cortical oscillations and electromyographic (EMG) amplitude as a function of changes in spike rate. Data points show evoked activity (0.0 to 5.0 s after stimulus onset) minus spontaneous activity (10.0 to 5.0 s prior to stimulus onset) for (A, B, and C, x-axis) mean firing rate and (A, y-axis) high-frequency oscillation power (B, y-axis) low-frequency oscillation power, or (C, y-axis) EMG amplitude. Increases in spike rate were associated with (A) increased high-frequency activity, (B) decreased low frequency power, and (C) increased EMG amplitude. Filled circles show instances in which the spike rate and the electrophysiological measure of cortical or motoric activation both increased. Statistics are reported in the text.
The presence of a statistically significant increase in excitatory activity (Table 1) was determined by considering the spontaneous activity as a Poisson process. The spontaneous mean firing rate λ was calculated from −10 to −5 s for each trial, yielding 40 s of data for each stimulus and unit pairing. The number of spikes x, which represented a significant increase in activity, was determined at P(x) < 0.010, as expressed in the following equation
| (1) |
If the total number of spikes, for a given modality, summed across all trials from 0.0 to 5.0 s was greater than x at P(x) < 0.010, then the response was considered significant. The relationship between motoric activity and neuronal firing (Table 1) was determined by comparing the number of spikes during bouts of high EMG (see preceding text) to the expected number of spikes given a Poisson distribution using Eq. 1 at P(x) < 0.010.
Table 1.
Units (A–S) increase firing after stimulus presentation and during motor bouts
| Stimulus Modality | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olfactory | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |||
| Tactile | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| Vestibular | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |||
| Visual | * | * | * | * | * | * | * | * | * | * | * | * | * | ||||||
| Auditory | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Motor | * | – | – | * | – | * | * | * | * | ||||||||||
| Single | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
Asterisks (*) mark units that showed significant excitation (P < 0.01), assuming a Poisson distribution, to a given stimulus modality or during bouts of high EMG. Dashes (–) mark units for which EMG waveforms were not recorded. Plus signs (+) mark recordings that are single units.
Histology
The locus of each recording site was reconstructed after histological analysis. At the end of all recording sessions for an animal, the animal was overdosed with Nembutal (150 mg/kg, ip), a lesion was made at the medullary electrode (80 μA DC, 15.0 s), and the animal was perfused with 0.1 M PBS followed by 4% paraformaldehyde. The brain was sliced to obtain 40 μm sections and stained with cresyl violet. The location of each recording site was reconstructed (Fig. 1) and the brain stem area was determined from local anatomy (Paxinos and Watson 1982).
Fig. 1.
Locations of all recording sites. The unit whose responses are illustrated in Figs. 2 to 6 is represented by the letter A. There were no apparent trends in firing pattern based on the locations of the recording sites. NRGc, gigantocellular reticular nucleus; py, pyramidal tract; icp, inferior cerebellar peduncle; SPIV, spinal vestibular nucleus; VII, facial nucleus.
RESULTS
This study observed extracellular unit activity in the medullary NRGc of freely behaving rats presented with five sensory stimuli and related unit activity to both muscle activation and to changes in behaviorally relevant oscillations in cortical LFPs. We had hypothesized that neurons in the NRGc can integrate multiple inputs and that the neurons' activity can activate multiple arousal systems. The expectations were that neurons in the NRGc would show increases in firing rate to all sensory stimuli and that the increase in firing rate would be associated with an increase in EMG amplitude (representing muscle contraction), an increase in the power of high-frequency oscillations, and a decrease in the power of low-frequency oscillations (representing cortical activation).
Responses to sensory stimuli
An illustration of a single unit's (location in Fig. 1, unit “A”) response to the olfactory stimulus is shown in Fig. 2. Prior to stimulation (during which time the animal was motionless) there was low spontaneous activity (<2.0 Hz), which was typical of 5 of the 14 single units and 4 of the 5 multiunits. On presentation of the olfactory stimulus, there was an increase in firing rate (Fig. 2, A and B), which occasionally preceded the time-stamping (e.g., Fig. 2B, trial 3). The unit showed a long-duration increase in firing rate, with activity persisting ≤10.0 s or longer after stimulus presentation, which was typical of the majority (11/19) of NRGc units. There was variability among both the firing rate and the firing pattern among trials, exemplified in Fig. 2B by strong, burst firing in trial 5 and by sparse, tonic firing in trial 8. Variability across trials was typical of the majority of the units' responses to the olfactory stimulus; in approximately a third of the units, the variability could be connected to qualitatively observed behavior, with low activity associated with no responsiveness in the animal and high activity associated with orientation, postural shifting, or the initiation of other movements. Two of the 19 units, including one multiunit, showed a complex pattern of firing, with a fluctuation between excitation and inhibition (data not shown). No purely inhibitory responses were observed.
Examples of unit A's responses to the tactile stimuli are illustrated in Fig. 3. Compared with the olfactory stimulus, the activity was less variable across trials despite the stimulus being applied to both the leg (4 trials) and the back (4 trials) during each experiment. The unit showed low spontaneous activity (<2.0 Hz), which was typical of 9 of the 19 units. On presentation of the stimuli there was an increase in firing rate that could persist beyond 10 s after stimulus presentation. The sustained increases in firing rate tended to show complex patterns of instantaneous firing frequency as a function of time (Fig. 3A). In some instances, the firing rate increased prior to time-stamping (Fig. 3, A and B), which is likely explained by the inaccuracy of manual time-stamping. All neurons that responded to the tactile stimuli (Table 1) showed increased firing to both the leg and back stimuli, which is indicative of these neurons' broad tactile receptive fields. The majority of the units (9/14 single units, 2/5 multiunits) showed sustained excitatory responses, ≤10.0 s, whereas 5 of 14 single units and 2 of 5 multiunits showed strong onset responses followed by weak or absent sustained responses. As with the olfactory stimulus, there was a qualitative observation that increased excitation was associated with the initiation of movement.
Fig. 3.
Example of a single unit's (Unit A) responses to tactile stimulus presentations. A: the instantaneous firing frequency is shown for trial 3. B: prior to the presentation of the stimulus, there was low spontaneous activity. Presentation of the stimulus evoked a sustained increase in firing rate. C: the overlaid waveforms for all action potentials in B.
Examples of unit A's responses to the vestibular stimulus are illustrated in Fig. 4. The unit showed low spontaneous activity and strong excitation at the onset of stimulus presentation. The unit's sustained firing tended to have a duration of 2.0 to 5.0 s, which was typical of 4 of 19 units, including one multiunit; another 4 single units showed longer duration firing, lasting ≤10.0 s, with 2 of those units showing complex responses of excitation fluctuating with inhibition. Seven of the units, including 3 multiunits, responded to the vestibular stimulus with short-duration onset responses followed by weak or absent sustained responses. Both the onset and sustained response patterns occasionally showed correspondence with the initiation of movement.
Fig. 4.
Example of a single unit's (Unit A) responses to vestibular stimulus presentations. A: the instantaneous firing frequency is shown for trial 8. B: the unit showed low spontaneous activity prior to stimulus presentation. Poststimulus, there was a sustained (2.0 to 8.0 s) excitatory response. C: the overlaid waveforms for all action potentials in B.
Examples of unit A's responses to the visual stimulus are shown in Fig. 5. When presented with the visual stimulus, this unit, typical of all units, responded primarily with onset responses and weak or absent sustained responses. When the neuron was excited by the visual stimulus, the average minimum latency was 24.7 ms, with a range of 5.2 to 43.0 ms (n = 11; SE = 3.9 ms). The visual stimulus rarely roused the animal from rest.
Fig. 5.
Example of a single unit's (Unit A) responses to visual stimulus presentations. A: the instantaneous firing frequency is shown for trial 2. B: the unit showed 3 trials of high spontaneous activity, despite the animal's being motionless. The remaining trials showed low spontaneous activity. On presentation of the stimulus, the unit showed, relative to spontaneous activity, primarily a short-duration onset response. C: the overlaid waveforms for all action potentials in B.
Figure 6 shows two examples of unit responses to the repeated (0.5 or 1.0 Hz) auditory stimuli. Unit A (Fig. 6, A and B) shows onset responses that are time-locked to the onset of the auditory stimulus for the first three repetitions. After the third repetition, the responses tended to decrease in the number of action potentials. This general pattern was consistent with 5 of the 16 units, including 2 multiunits. The second example (Fig. 6, D and E) shows multiunit onset responses time-locked to the onset of the first repetition of the auditory stimulus, but with the responses' firing patterns becoming less ordered with increasing repetition number. At higher repetition numbers, stimulus-locked responses could not be discriminated from the increases in sustained response. This general pattern was consistent with 7 of the 16 units, including the one multiunit shown in Fig. 6. The remaining units showed less impressive, but significant, increases in firing rate that did not have clear fluctuations with repetition number. The average minimum latency for units to the first repetition of the auditory stimulus was 11.6 ms (n = 19; SE = 3.36 ms), with a range from 8.8 to 72.1 ms.
The mean firing rates (averaged across all units) evoked by the sensory stimuli are shown in Fig. 7. Activity is shown in three time windows: spontaneous (−10.0 to −5.0 s), onset (0.0 to 0.2 s), and sustained (0.2 to 5.0 s). After Bonferroni correction for five comparisons, the units showed a significant increase in onset activity, relative to spontaneous activity, for the tactile (P < 0.010), vestibular (P < 0.010), visual (P < 0.050), and auditory (P < 0.010) modalities. There was significant excitation in the sustained activity window for the olfactory (P < 0.005), tactile (P < 0.001), and auditory (P < 0.001) modalities. The stimuli that evoked the strongest behavioral responses, which were the auditory and vestibular stimuli, showed the strongest onset responses among the five stimuli.
Assuming a Poisson distribution, 8 of the 19 units showed significant excitation to all five of the sensory stimuli (Table 1). Nine of the units showed significant excitation to four of the five sensory stimuli, with the visual stimulus being the least likely to evoke a significant response. Two of the units showed significant excitation to only three of the five stimuli. All units showed significant excitation to the auditory stimulus.
Motoric and cortical activation
The timing and rate of spiking activity were related to qualitative observations of motor activity and to electrophysiological measures of arousal. Seven of the 19 units had both high signal to noise action potentials and relatively low spontaneous activity. This allowed for qualitative association of neural activity with behavior during the experimental session. Among these units, action potentials were observed to be associated with an animal's behavior in 6 of the 7 units, with the units showing increased excitation before the initiation of movement. Increases in firing rate were also observed while the freely behaving animal was exploring the cage environment (Supplemental Video S1) and, in instances where the animal was motionless for <60 s, an increase in firing rate sometimes preceded the animal's movement by about 2.0 to 5.0 s.1
The electrophysiological measures of cortical and motoric activation significantly increased from pre- to poststimulus presentation, in concert with NRGc activation. Figure 8 shows the changes in neuronal spike rate and the changes in either the power of cortical LFP oscillations or EMG amplitude for all stimulus/unit pairs (5 stimuli; 16 units). Firing rate increased in 91% of the samples (Fig. 8, A–C); power in the high-frequency band of cortical oscillations (Fig. 8A) increased in 83% of the samples from the spontaneous (−10.0 to −5.0 s) to poststimulus (0.0 to 5.0 s) analysis windows; power in the low-frequency band of cortical oscillations (Fig. 8B) decreased in 85% of the samples; and EMG amplitude (Fig. 8C) increased in 76% of the samples. Overwhelmingly, the increase in firing rate was concurrent (within a 5.0 s window) with 1) an increase in power in the high-frequency band (76% of the samples, P < 0.001); 2) a decrease in power in the low-frequency band (70%, P < 0.001); and 3) an increase in the EMG amplitude (76%, P < 0.001). Note that the magnitude of the difference between pre- and poststimulus spike rate and the difference between pre- and poststimulus cortical or muscular electrophysiological measures were not directly proportional—this is visualized as the divergence of data points from the x = y axis (Fig. 8, A–C).
To determine whether there was a direct relationship between spike activity and EMG amplitude, the spike-triggered average of the EMG waveform, which is the average of the EMG waveforms that temporally flank (±1.0 s) each occurrence of a spike (de Ruyter van Steveninck and Bialek 1988), was determined for all spikes within ±10.0 s of each stimulus onset. Looking at all spikes, there was a nonsignificant increase in the average EMG amplitude within ±250 ms of spiking compared with 1.0 to 0.5 s prior to spiking (data not shown). To determine whether bursts of spikes were associated with changes in EMG amplitude, the spike-triggered average of the EMG waveform was determined for all spike pairs with ISIs of <1, 5, 10, or 50 ms. Compared with the period from 1.0 to 0.5 s prior to spiking, there was a statistically significant increase (P < 0.001) in the average EMG amplitude, within ±250 ms of a spike pair, following pairs of spikes (69 pairs of spikes from 11 rats; see methods) that had <5 ms ISIs (Fig. 9). The peak increase was at approximately +40 ms. The 10 ms ISI yielded similar results (data not shown).
The relationship between spike activity and motoric activation was further tested by determining whether any units had statistically significant increases in spike activity during motor bouts. Motor bouts are defined as continuous periods of high EMG amplitude (see methods). Six of the 16 units showed a statistically significant increase in spike activity during motor bouts (Table 1). No units showed significant decreases in spike rate during the motor bouts.
To determine whether there was a direct relationship between spike activity and changes in the power of low- and high-frequency oscillations of the cortical LFP, the spike-triggered average of the power spectrum was calculated for the 5 s window, in 1 s intervals, preceding and following each spike within ±10 s of each stimulus onset. Looking at all spikes, there was no significant change in power at any frequency. This was also true for all ISIs tested (data not shown).
Habituation
In response to the presentation of repeated auditory stimuli, the units tended to show habituation, followed by recovery, with increasing stimulus repetition number (Supplemental Fig. S1). On average, evoked responses were initially associated with a decrease in activity from repetitions 1 to 5, followed by an increase in firing rate from repetitions 5 to 8. The increase in firing rate during the last three repetitions was primarily the result of increases in persistent, sustained activity temporally overlapping into the onset window (Fig. 6, D and E).
Stimuli from a given modality were presented as a block of consecutive trials (for auditory stimuli, consecutive trials of eight stimulus repetitions each) before switching to the next modality. Therefore it was possible to observe whether the responses evoked by the auditory stimulus habituated with increasing trial number (as opposed to increasing repetition number). The mean firing rate in the onset window (0.0–0.2 s) was significantly increased, relative to spontaneous activity, in trials 1 (P < 0.050), 3 (P < 0.050), 5 (P < 0.050), and 7 (P < 0.050) (Fig. 10) and approached significance (P < 0.050) in trial 6. The mean firing rate in the sustained window (0.2 to 1.0 s) approached significant excitation to the first auditory stimulus (P = 0.068) and showed a strong trend of decreasing firing rate, with trial 6 being significantly different from the first response (P < 0.050).
DISCUSSION
This study showed that neurons in the medullary NRGc are responsive to a broad array of sensory stimuli and that their firing is associated with increases in physiological arousal. Our findings can be compared with those of Wu et al. (1988) and Olivéras et al. (1989, 1990, 1991) who described multimodal (somatic, noxious, visual, and/or auditory) and movement-related activity in the brain stems of awake cats and awake rats, respectively. Exploring a basis for acoustic startle in the reticular nucleus, Wu et al. (1988) showed a subpopulation (11%) of multimodal, movement-related neurons in the pontomedullary junction that were subject to prepulse inhibition, but did not directly relate these attributes to neurons in the NRGc. Olivéras and colleagues described medullary NRGc neurons, primarily located adjacent to the midline, that showed either multimodal activity or exclusively movement-related activity. In contrast to these studies we have explicitly shown that: 1) individual neurons can be both multimodal and have firing rate increases that correspond to both increases in EMG amplitude and with the activation of behavior; 2) medullary NRGc activity is related to changes in physiological markers of cortical arousal; 3) NRGc neurons can habituate to an innocuous stimulus with >60 s between each trial; and 4) these neurons exist throughout the mNRGc. We infer that the NRGc may be an important part of an elementary “first responder” CNS arousal mechanism.
Multimodal responsiveness
Neurons in the NRGc are in a position to integrate salient information across sensory modalities (Humphries et al. 2007; Pfaff 2005). In the anesthetized or decerebrated cat, the majority of NRGc neurons respond with short-duration (<100 ms) excitation to stimuli from multiple modalities (Bowsher et al. 1968; Saadé et al. 1983) and, within a modality, the neurons have broad receptive fields (Bowsher et al. 1968; Pearl and Anderson 1978).
We have shown the presence of excitatory responses to stimuli from several modalities in both multiunit and single-unit recordings (Figs. 2–6). The mean response, averaged across all units, evoked by stimuli from each modality was a significant increase in firing rate (Fig. 7). Within the tactile modality, the neurons' receptive fields included both the animals' leg and back regions during both the anesthetized and awake states. On a unit-by-unit basis, all of the units showed significant excitation to at least three of the five modalities presented (Table 1). The stimulus that evoked the least excitation was the visual stimulus; this is not surprising, in that the animal model used, Wistar rats, have poor visual acuity because of the absence of eye pigmentation. Additionally, lights-out signals a change in external condition more so than it signals an external event such as the arrival of a predator or territorial rival. It may be for this reason that the visual stimulus was the stimulus to which the lowest proportion of units, albeit still a majority, responded. It would be interesting to explicitly determine whether cells respond to an ethologically relevant stimulus such as looming, as suggested by the early firing during olfactory stimulation (see Technical considerations). In awake cats with intact brains, Casey (1971) previously showed that NRGc neurons respond to nociceptive stimuli with sustained responses while reporting that they were unresponsive to visual or auditory stimuli and did not fire during active or passive movements. The discrepancy between our data and those of Casey (1971) may be explained by differences in the state of the animal, species differences, or the characteristics of the presented stimuli.
One surprising characteristic of the NRGc neurons was their long-duration (>10 s) responses to brief (∼200 ms) stimuli (Figs. 2, 3, and 7). Long-duration activity to brief stimuli has been demonstrated in the raphe magnus of the awake rat (Olivéras et al. 1991). Interestingly, Olivéras and colleagues (1991) demonstrated that Nembutal anesthesia dramatically reduced the duration of the stimulus-evoked responses. Likewise in our study, when implanting the electrode, NRGc responses to the tactile search stimuli were of short duration. Two possible interpretations, not mutually exclusive, are that the pattern of NRGc neurons' responses to sensory stimuli may be determined by the state of the animal and vice versa.
Cortical activation
The ascending axons of NRGc neurons project to regions involved in arousal, including the pontine central gray, midbrain central gray, mesencephalic reticular field, and the mesopontine tegmental anesthesia area (Jones and Yang 1985; Klemm and Vertes 1990; Vertes et al. 1986). Membrane depolarization at the upstream targets of NRGc neurons results in the disruption of the slow delta rhythm (Steriade et al. 1997) and stimulation of NRGc neurons predominantly evokes short-latency excitatory postsynaptic potentials in midbrain regions (Mancia et al. 1974). Medullary NRGc neurons are most active during waking and rapid eye movement sleep (Siegel et al. 1979; Steriade et al. 1984), states that are partially defined by high cortical arousal. Experimental stimulation of the NRGc has been associated with increased cortical arousal (Ledebur and Tissot 1966).
Our data support the suggestion that excitation of neurons in the NRGc is associated with increased cortical arousal. Cortical arousal was measured by recording slow and fast oscillations in cortical LFP. Local field potential recordings theoretically measure the sum of inhibitory and excitatory synaptic events at the site of the electrode (reviewed in Mitzdorf 1985) and are thought to be the predominant signal measured by EEG (reviewed in Berens et al. 2008). After stimulus presentation, animals showed increases in the power of fast oscillations, which are associated with attentive behavior in rats (Destexhe et al. 1999; Maloney 1997), and decreases in the power of slow oscillations that are indicative of a resting state (Destexhe et al. 1999). In sum, this represented an increase in cortical activation (reviewed in Jones 2003). These changes corresponded with increases in the mean firing rate of NRGc neurons (Fig. 8).
Motoric activation
The descending axons of NRGc neurons predominantly project to the intermediate zone and ventral horn of the spinal cord (Jones 1995; Martin et al. 1985), which are regions that are involved in the execution and modulation of motor activity. One body of evidence suggests that the NRGc is involved in motor inhibition. Electrical stimulation of the NRGc is followed by decreases in muscle tone in unanesthetized cats (Ito and McCarley 1987). Likewise, lesions of the NRGc or the NRGc reticulospinal tract lead to an increase in neck and limb movements during slow-wave sleep and paradoxical sleep (cat: Holmes and Jones 1994; Webster et al. 1986).
In contrast, infusion of N-methyl-d-aspartate in the NRGc has resulted in increased muscle tone and locomotion (cat: Lai et al. 1999), although it should be stated that the study reported by Lai and colleagues did not differentiate between the pontine and medullary NRGc. Stimulation of the NRGc in monkeys evoked ipsilateral movement of the head in what could be described as an orientation response (Quessy and Freedman 2004) and stimulation of the NRGc in the rat evoked short-latency increases in axial motor activity (anesthetized: Femano et al. 1984; unanesthetized: Winson 1981).
Our data show that spike activity in the NRGc of the awake, freely behaving rat can be associated with increased muscular activation (Table 1 and Figs. 8 and 9). Quantitatively, we showed that bouts of high-amplitude EMG were associated with significant increases in firing for 6 of 16 neurons (Table 1). Anecdotally, increases in firing rate were concurrent with certain postures and exploratory behaviors (Supplemental Video S1), which has been previously described in neurons of the medullary reticular formation of the awake cat (Siegel et al. 1979).
Habituation and sensitization
Presentation of repeated stimuli—with parameters similar to those used in our study—primarily results in habituation of reticular formation activity in both the anesthetized animal (Carlson and Willott 1998; Peterson et al. 1976) and the awake animal (Fox and Wolstencroft 1976; Miyazato et al. 1999). Although the rate and amplitude of the repeated auditory stimuli in our study were appropriate for demonstrating habituation (Peterson et al. 1976; Sharpless and Jasper 1956) or the acoustic startle response (Koch 1999), the habituation was of lower magnitude (Supplemental Fig. S1) than what has been documented in other brain stem regions (Miyazato et al. 1999; Peterson et al. 1976). In agreement with plate-recordings of brain stem activity (Sharpless and Jasper 1956), we have shown that the neurons in the NRGc habituate with increasing trial number (Fig. 10).
The above-cited data and references are consistent with the idea that the NRGc exemplifies what control system engineers call a “bow-tie” control structure (Csete and Doyle 2002, 2004). This design potentially allows strong state control over an animal's behavior due to its ability to integrate information widely across systems (and would probably necessitate a strong inhibitory input to ignore external arousing stimuli when necessary) and its broad projections facilitate the quick activation of multiple arousal systems, as would be necessary in a fight or flight situation. Furthermore, the redundancy of neurons at the core of the bow-tie makes it resilient to damage (Humphries et al. 2007) and to a decrease in the potential of failed detections.
Technical considerations
It is possible that the neurons recorded are not directly associated with the excitation of neck musculature, with the increase in EMG amplitude having occurred at a peak of about 25 to 50 ms after spiking without showing apparent inhibition prior to spiking. Hajnik et al. (2000) reported that the latency to inhibition of neck musculature with electrical stimulation of the medulla is 11 ms. The difference in latencies is likely attributable either to the measurement of the peak response rather than a minimum response time or to a delay of excitation because of an initial inhibition of the neck motor system.
It is critical to know whether the increase in NRG unit activity is what triggers movement or whether movement itself is what triggers, or sustains, the increases in unit activity. It is unlikely that movement alone is a trigger for unit activity because units at the recording location (whereas the animal is under pentobarbital anesthesia and nonreflexive) are responsive to tactual stimuli. Qualitative inspection of the responses to auditory stimuli, where the onset time is precisely defined, show that unit responses to the stimulus almost always precede the increases in EMG. Likewise, Fig. 9 shows that the increases in EMG follow increases in unit bursting activity. Although motoric activity could theoretically increase NRG activity, we have no direct evidence of this in our data.
Due to the difficulty of obtaining long duration, stable recordings, 80% of the recordings were initiated at 8 h postsurgery while the animal was being medicated with aspirin. Such difficulty may have been caused by local damage at the recording site, possibly a consequence of local brain movements at the site of the electrode. The 8 h duration between surgery and recording presents three possible confounds: 1) the animal may still have been feeling some surgical pain; 2) the aspirin may have affected neural activity; and 3) the animal's circadian rhythm may have been disrupted. The first two points are particularly important in that the NRGc is thought to be involved in nociception (Nagata et al. 2003). Nevertheless, no recordings were made until ≥2 h after the animal had eaten, restored its normal gait and posture, had begun grooming, and showed no signs of distress (e.g., vocalizations on touch, piloerection). Furthermore, the recordings made at 3 to 5 days postsurgery, a point where pain should be minimized, did not significantly differ from the data collected on the day of surgery. Possible changes in the animal's circadian rhythm were not controlled for and we do not know what confounds it may have introduced. Despite this, the criterion that the animal be motionless for 60 s prior to stimulus presentation likely provided a consistent resting brain state. We also recognize that changes in hormones during the animals' estrous cycles, which were not measured, may have contributed to changes in behavioral arousal.
The figures show occasions during which some increased neuronal activity preceded the time-stamping for the stimulus, particularly before the olfactory stimulus. This was potentially caused by: 1) the stimulus reaching the animal prior to the manual time-stamping or 2) the animal responding to something other than the intended stimulus. In the case of the olfactory stimulus, it is the authors' opinion that early responses were due to the looming of the experimenter's arm en route to delivering the stimulus; it is unlikely that the early responses arose from inaccuracies in time-stamping. Conditions under which the experimenter does not apply the olfactory, tactual, or vestibular stimuli may show a different time course of neuronal response. The responses to visual stimuli (Fig. 5) show a high level of activity, prior to the stimulus onset, in three of the illustrated trials. The responses did not appear to be associated with any external stimulus and likely reflected changes that were intrinsic to the animal. It should be noted that the stimuli were presented strictly according to 60 s of motionlessness, without regard to the level of background spike activity.
A higher percentage of neurons were multimodal than had been previously reported in the awake cat (Wu et al. 1988). The larger percentage of multimodal units in our study was potentially caused by a sensitization of the responses due to the temporal proximity to time of surgery (see preceding text). Although 5 of the 19 recordings were multiunit, which could bias them toward increased responsiveness to multiple modalities, we did not observe significant differences between the single unit and multiunit responses. Finally, our study may have been biased toward responsive neurons by the use of a tactile search stimulus. Unit selection based only on the presence of spontaneous action potentials may have reduced the percentage of multimodal units.
Inference
We show in the unanesthetized animal that individual neurons, or small clusters of neurons, can potentially integrate information from multiple modalities, that these same neurons were associated with increases EMG amplitude, and that they may be associated with cortical activation. From the new results reported here, combined with the references herein, we infer that the medullary NRGc neurons recorded participate in a “first responder” CNS arousal mechanism (Pfaff 2005), with likely implications for CNS state regulation.
GRANTS
This work was supported by National Institutes of Health Grants HD-05751 and MH-38273.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Andreatta-van Leyen S, Averill DB, Guertzenstein PG. Cardiorespiratory effects induced by acetazolamide on the ventromedullary surface of the cat. J Physiol 421: 171–184, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P, Keliris GA, Ecker AS, Logogthetis NK, Tolias AS. Feature selectivity of the gamma-band of the local field potential in primary primate visual cortex. Front Neurosci 2: 199–207, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher D, Mallart A, Pitt D, Albe-Fessard D. A bulbar relay to the centre median. J Neurophysiol 31: 288–300, 1968. [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci 22: 8850–8859, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S, Willott JF. Caudal pontine reticular formation of C57BL/6J mice: Responses to startle stimuli, inhibition by tones, and plasticity. J Neurophysiol 79: 2603–2614, 1998. [DOI] [PubMed] [Google Scholar]
- Casey KL. Somatosensory responses of bulboreticular units in awake cats: Relation to escape-producing stimuli. Science 173: 77–80, 1971. [DOI] [PubMed] [Google Scholar]
- Csete ME, Doyle JC. Reverse engineering of biological complexity. Science 295: 1664–1669, 2002. [DOI] [PubMed] [Google Scholar]
- Csete ME, Doyle JC. Bow ties, metabolism and disease. Trends Biotechnol 22: 446–450, 2004. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Krause EG, Contreras RJ. Fos expression in non-catecholaminergic neurons in medullary and pontine nuclei after volume depletion induced by polyethylene glycol. Brain Res 948: 149–154, 2002. [DOI] [PubMed] [Google Scholar]
- de Ruyter van Steveninck R, Bialek W. Real-time performance of a movement sensitive neuron in the blowfly visual system: coding and information transmission in short spike sequences. Proc R Soc Lond B Biol Sci 234: 379–414, 1988. [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Cortically-induced coherence of a thalamic-generated oscillation. Neuroscience 92: 427–443, 1999. [DOI] [PubMed] [Google Scholar]
- Farham CJ, Douglas RJ. The response of neurons of the medial pontomedullary reticular-formation of rats to peripheral thermal stimuli. Brain Res 336: 107–115, 1985. [DOI] [PubMed] [Google Scholar]
- Fee MS, Mitra PP, Kleinfeld D. Automatic sorting of multiple unit neuronal signals in the presence of anisotropic and non-Gaussian variability. J Neurosci Methods 69: 175–188, 1996. [DOI] [PubMed] [Google Scholar]
- Femano PA, Schwartz-Giblin S, Pfaff DW. Brain stem reticular influences on lumbar axial muscle activity. I. Effective sites. Am J Physiol Regul Integr Comp Physiol 246: R389–R395, 1984. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Movement related discharge of ventromedial medullary neurons. J Neurophysiol 93: 873–883, 2005. [DOI] [PubMed] [Google Scholar]
- Fort P, Luppi PH, Jouvet M. Afferents to the nucleus reticularis parvicellularis of the cat medulla oblongata: a tract-tracing study with cholera toxin B subunit. J Comp Neurol 342: 603–618, 1994. [DOI] [PubMed] [Google Scholar]
- Fox JE, Wolstencroft JH. The reduced responsiveness of neurones in nucleus reticularis gigantocellularis following their excitation by peripheral nerve stimulation. J Physiol 258: 687–704, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG. Spike-frequency adaptation and intrinsic properties of an identified, looming-sensitive neuron. J Neurophysiol 96: 2951–2962, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbaud G, Besson JM, Olivéras J-L, Wyonmail MC. Modifications of firing rate of bulbar reticular units (nucleus gigantocellularis) after intra-arterial injection of bradykinin into limbs. Brain Res 63: 131–140, 1973. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8938–8947, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnik T, Lai YY, Siegel JM. Atonia-related regions in the rodent pons and medulla. J Neurophysiol 84: 1942–1948, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhiu MA, Loewy AD. Central connections of the motor and sensory vagal systems innervating the trachea. J Auton Nerv Syst 57: 49–56, 1996. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience 62: 1179–1200, 1994. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Responses of medullary reticular formation neurons to input from the male genitalia. J Neurophysiol 76: 2474–2482, 1996. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Kaddumi EG, Johnson RD. Brain stem convergence of pelvic viscerosomatic inputs via spinal and vagal afferents. Neuroreport 15: 1299–1302, 2004. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Gurney K, Prescott TJ. Is there a brainstem substrate for action selection? Philos Trans R Soc Lond B Biol Sci 362: 1627–1639, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, McCarley RW. Physiological studies of brainstem reticular connectivity. I. Responses of mPRF neurons to stimulation of bulbar reticular formation. Brain Res 409: 97–110, 1987. [DOI] [PubMed] [Google Scholar]
- Jones BE. Reticular formation: cytoarchitecture, transmitters, and projections. In: The Rat Nervous System (2nd ed.), edited by Paxinos G. San Diego, CA: Academic Press, 1995, p. 155–171 [Google Scholar]
- Jones BE. Arousal systems. Front Biosci 8: S438–S451, 2003. [DOI] [PubMed] [Google Scholar]
- Jones BE, Yang I-Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol 242: 56–92, 1985. [DOI] [PubMed] [Google Scholar]
- Kaddami EG, Hubscher CH. Convergence of multiple pelvic organ inputs in the rat rostral medulla. J Physiol 572: 393–405, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm WR, Vertes RP. Brainstem Mechanisms of Behavior. New York: Wiley, 1990. [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol 59: 107–128, 1999. [DOI] [PubMed] [Google Scholar]
- Kow L-M, Pfaff DW. Responses of medullary reticulospinal and other reticular neurons to somatosensory and brainstem stimulation in anesthetized or freely-moving ovariectomized rats with or without estrogen treatment. Exp Brain Res 47: 191–202, 1982. [DOI] [PubMed] [Google Scholar]
- Kralik JD, Dimitrov DF, Krupa DJ, Katz DB, Cohen D, Nicolelis MAL. Techniques for long-term multisite neuronal ensemble recordings in behaving animals. Methods 25: 121–150, 2001. [DOI] [PubMed] [Google Scholar]
- Ladpli R, Brodal A. Experimental studies of commissural and reticular formation projections from the vestibular nuclei in the cat. Brain Res 8: 65–96, 1968. [DOI] [PubMed] [Google Scholar]
- Lai Y-Y, Siegel JM. Physiological and anatomical link between parkinson-like disease and REM sleep behavior disorder. Mol Neurobiol 27: 137–151, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledebur IX, Tissot R. Modification de l'activité électrique cérébrale du lapin sous l'effet de microinjections de précurseurs des monoamines dans les structures somnogènes bulbaires et pontiques [Modification of the cerebral electrical activity of the rabbit by micro-injections of monoamine precursors into bulbar and pontine sleep-producing structures]. Electroencephalogr Clin Neurophysiol 20: 370–381, 1966. [DOI] [PubMed] [Google Scholar]
- Lovick TA. The medullary raphe nuclei: a system for integration and gain control in autonomic and somatomotor responsiveness? Exp Physiol 82: 31–41, 1997. [DOI] [PubMed] [Google Scholar]
- Maloney KJ. High-frequency γ electroencephalogram activity in association with sleep-wake states and spontaneous behavior in rat. Neuroscience 76: 541–555, 1997. [DOI] [PubMed] [Google Scholar]
- Mancia M, Mariotti M, Spreafico R. Caudo-rostral brain stem reciprocal influences in the cat. Brain Res 80: 41–51, 1974. [DOI] [PubMed] [Google Scholar]
- Marson L, McKenna KE. CNS cell groups involved in the control of the ischiocavernosus and bulbospongiosus muscles: a transneuronal tracing study using pseudorabies virus. J Comp Neurol 374: 161–179, 1996. [DOI] [PubMed] [Google Scholar]
- Martin GF, Vertes RP, Waltzer R. Spinal projections of the gigantocellular reticular formation in rat. Evidence for projections from different areas to laminae I and II and lamina IX. Exp Brain Res 58: 154–162, 1985. [DOI] [PubMed] [Google Scholar]
- Mason P. Ventromedial medulla: pain modulation and beyond. J Comp Neurol 493: 2–8, 2005. [DOI] [PubMed] [Google Scholar]
- Mayne RG, Armstrong WE, Crowley WR, Bealer SL. Cytoarchitectonic analysis of Fos-immunoreactivity in brainstem neurones following visceral stimuli in conscious rats. J Neuroendocrinol 10: 839–847, 1998. [DOI] [PubMed] [Google Scholar]
- McKenna KE. Central nervous system pathways involved in the control of penile erection. Annu Rev Sex Res 10: 157–183, 1999. [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral-cortex: investigation of evoked-potentials and EEG phenomena. Physiol Rev 65: 37–100, 1985. [DOI] [PubMed] [Google Scholar]
- Miyazato H, Skinner RD, Garcia-Rill E. Sensory gating of the P13 midlatency auditory potential and the startle response in the rat. Brain Res 822: 60–71, 1999. [DOI] [PubMed] [Google Scholar]
- Morison RS, Dempsey EW. A study of thalamo-cortical relations. Am J Physiol 135: 281–292, 1942. [Google Scholar]
- Moruzzi G, Magoun HW. Brainstem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1: 455–473, 1949. [PubMed] [Google Scholar]
- Nagata T, Suzuki H, Zhang RH, Ozaki M, Kawakami Y. Mechanical stimulation activates small fiber mediated nociceptive responses in the nucleus gigantocellularis. Exp Brain Res 149: 505–511, 2003. [DOI] [PubMed] [Google Scholar]
- Olivéras J-L, Martin G, Montagne J, Vos B. Single-unit activity at ventrolmedial medulla level in the awake, freely moving rat: effects of noxious heat and light touch tactile stimuli onto convergent neurons. Brain Res 506: 19–30, 1990. [DOI] [PubMed] [Google Scholar]
- Olivéras J-L, Montague-Clavel J, Martin G. Drastic changes of ventromedial medulla neuronal properties induced by barbiturate anesthesia. I. Comparison of the single-unit types in the same awake and pentobarbital-treated rats. Brain Res 563: 241–250, 1991. [DOI] [PubMed] [Google Scholar]
- Olivéras J-L, Vos B, Martin G, Montagne J. Electrophysiological properties of ventromedial medulla neurons in response to noxious and non-noxious stimuli in the awake, freely moving rat: a single unit study. Brain Res 486: 1–14, 1989. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press, 1982. [Google Scholar]
- Pearl GS, Anderson KV. Response patterns of cells in the feline caudal nucleus reticularis gigantocellularis after noxious trigeminal and spinal stimulation. Exp Neurol 58: 231–241, 1978. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Abzug C. Properties of projections from vestibular nuclei to medial reticular formation in the cat. J Neurophysiol 38: 1421–1435, 1975. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Franck JI, Pitts NG, Daunto NG. Changes in responses of medial pontomedullary reticular neurons during repetitive cutaneous, vestibular, cortical, and tectal stimulation. J Neurophysiol 39: 564–581, 1976. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and branching of reticulospinal neurons. Exp Brain Res 24: 333–351, 1975. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res 36: 1–20, 1979. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Estrogens and Brain Function: Neural Analysis of a Hormone Controlled Mammalian Reproductive Behavior New York: Springer Verlag, 1980. [Google Scholar]
- Pfaff DW. Brain Arousal and Information Theory: Neural and Genetic Mechanisms Boston, MA: Harvard Univ. Press, 2005. [Google Scholar]
- Quessy S, Freedman EG. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis. 1. Characteristics of evoked head movements. Exp Brain Res 156: 342–356, 2004. [DOI] [PubMed] [Google Scholar]
- Saadé NE, Salibi NA, Banna NR, Towe AL, Jabbur SJ. Spinal input pathways affecting the medullary gigantocellular reticular nucleus. Exp Neurol 80: 582–600, 1983. [DOI] [PubMed] [Google Scholar]
- Sharpless S, Jasper H. Habituation of the arousal reaction. Brain 79: 655–681, 1956. [DOI] [PubMed] [Google Scholar]
- Shelley DN, Dwyer E, Johnson C, Wittkowski KM, Pfaff DW. Interactions between estrogen effects and hunger effects in ovariectomized female mice. I. Measures of arousal. Horm Behav 52: 546–553, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Wheeler RL, McGinty D. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res 179: 49–60, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA. Thalamus: Organization and Function, Vol 1 Oxford, UK: Elsevier, 1997. [Google Scholar]
- Steriade M, Sakai K, Jouvet M. Bulbo-thalamic neurons related to thalamocortical activation processes during paradoxical sleep. Exp Brain Res 54: 463–475, 1984. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Martin GF, Waltzer R. An autoradiographic analysis of ascending projections from the medullar reticular-formation in the rat. Neuroscience 19: 873–898, 1986. [DOI] [PubMed] [Google Scholar]
- Wall PD, Lidierth M, Hillman P. Brief and prolonged effects of Lissauer tract stimulation on dorsal horn cells. Pain 83: 579–589, 1999. [DOI] [PubMed] [Google Scholar]
- Webster HH, Friedman L, Jones BE. Modification of paradoxical sleep following transections of the reticular formation at the pontomedullary junction. Sleep 9: 1–23, 1986. [DOI] [PubMed] [Google Scholar]
- Willis WD., Jr The pain system. The neural basis of nociceptive transmission in the mammalian nervous system. Pain Headache 8: 1–346, 1985. [PubMed] [Google Scholar]
- Winson J. Reticular formation influence on neuronal transmission from perforant pathway through dentate gyrus. Brain Res 225: 37–49, 1981. [DOI] [PubMed] [Google Scholar]
- Wu M-F, Suzuki SS, Siegel JM. Anatomical distribution and response pattern of reticular neurons active in relation to acoustic startle. Brain Res 457: 399–406, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.