Abstract
Phase-dependent modulation of monosynaptic reflexes has been reported for several muscles of the lower limb of uninjured rats and humans. To assess whether this step-phase-dependent modulation can be mediated at the level of the human spinal cord, we compared the modulation of responses evoked simultaneously in multiple motor pools in clinically complete spinal cord injury (SCI) compared with noninjured (NI) individuals. We induced multisegmental responses of the soleus, medial gastrocnemius, tibialis anterior, medial hamstring, and vastus lateralis muscles in response to percutaneous spinal cord stimulation over the Th11–Th12 vertebrae during standing and stepping on a treadmill. Individuals with SCI stepped on a treadmill with partial body-weight support and manual assistance of leg movements. The NI group demonstrated phase-dependent modulation of evoked potentials in all recorded muscles with the modulation of the response amplitude corresponding with changes in EMG amplitude in the same muscle. The SCI group demonstrated more variation in the pattern of modulation across the step cycle and same individuals in the SCI group could display responses with a magnitude as great as that of modulation observed in the NI group. The relationship between modulation and EMG activity during the step cycle varied from noncorrelated to highly correlated patterns. These findings demonstrate that the human lumbosacral spinal cord can phase-dependently modulate motor neuron excitability in the absence of functional supraspinal influence, although with much less consistency than that in NI individuals.
INTRODUCTION
Activity-dependent plasticity of lumbosacral circuitry during locomotion in humans has been demonstrated with properties similar to those described in animal studies (Barbeau and Rossignol 1987; de Leon et al. 1998; Grillner et al. 1973; Lovely et al. 1987). These findings in humans have led to the wide use of a rehabilitative intervention, locomotor training (Barbeau et al. 2002; Behrman and Harkema 2000; Dietz et al. 1995; Nooijen et al. 2009; Wernig et al. 1995), which has been developed based on the observations that repetitive, task specific training can elicit recovery of hindlimb stepping in spinally transected animals (reviewed in Edgerton et al. 2008; van de Crommert 1998) and results in improvement in walking after human spinal cord injury (SCI; reviewed in Barbeau et al. 2002; Behrman and Harkema 2007; Dietz and Harkema 2004). Improvement of walking has not occurred in every spinal cord injured individual studied and the extent of recovery is highly varied, with little understanding of the mechanisms involved in the recovery. Understanding the neural mechanisms underlying the activity-dependent plasticity is essential to optimize translation to human therapies; however, neurophysiologic techniques allowable for use in humans with spinal cord injury are extremely limited.
Single electrical pulses applied percutaneously over the lumbosacral spinal cord have been shown to generate bilateral responses in leg muscles in noninjured (NI) humans lying supine (Hofstoetter et al. 2008; Maertens de Noordhout et al. 1988; Minassian et al. 2007; Troni et al. 1996) and during walking and running (Courtine et al. 2007; Dyhre-Poulsen et al. 2005). The responses were termed multisegmental monosynaptic responses (MMRs) because they display characteristics of reflexes in that they are suppressed by prior pulses and tendon vibration and are likely to reflect, at least in part, activation of large diameter dorsal root afferents over multiple segments of the spinal cord (Hultborn et al. 1996; Schieppati 1987). The onset of the MMR increases in muscles more distal to the site of stimulation (Courtine et al. 2007). In the soleus muscle, the latency of this response is approximately half that of soleus H-reflexes (verified in nine NI individuals). This latency is sufficient to account for synaptic delay at the neuromuscular junction and in the spinal cord and motor nerve transmission at a conduction velocity of 52.4 m/s (Shefner and Logigian 1994). The elicitation of MMRs is noninvasive, avoids movement artifacts that constitute a source of variability with peripheral reflexes, and can be used to assess how the nervous system modulates reflexes simultaneously at multiple segmental levels.
Phase-dependent modulation of the amplitude of percutaneous electrically evoked MMRs in extensors and flexors bilaterally in humans during walking and running demonstrated a functional connectivity in spinal sensorimotor pathways (Courtine et al. 2007). The phase-dependent modulation of the monosynaptic reflexes evoked by tibial nerve electrical stimulation at the popliteal fossa, soleus H-reflexes, is well established in humans (Capaday and Stein 1986; Crenna and Frigo 1987; Dyhre-Poulsen and Simonsen 2002; Simonsen and Dyhre-Poulsen 1999; Stein and Capaday 1988), rats (Chen et al. 2005), and cats (Akazawa et al. 1982; Duenas et al. 1990). However, after spinal cord injury, reduced or no phase-dependent modulation of soleus H-reflexes has been reported in cases of incomplete spinal injury during stepping (Fung and Barbeau 1994; Yang et al. 1991) and rhythmic pedaling (Boorman et al. 1992), suggesting supraspinal regulation in the transmission of monosynaptic afferents to the soleus muscle. The ability to regulate these reflexes may be essential for the recovery of walking and this capability may differentiate those individuals with severe SCI who recover with locomotor training from those who do not. Assessing multiple muscles simultaneously provides comprehensive and integrated information regarding the mechanisms of neural control of walking.
Although no individuals with clinically complete SCI have demonstrated improvements in overground walking related to locomotor training, plasticity of the lumbosacral circuitry in response to modulation of sensory information (Dietz et al. 1998; Harkema et al. 1997) and repetitive step training have been reported (Dietz et al. 1994, 1995). Adjunct therapies are required to improve standing and walking in these severely injured individuals and a promising approach is to access lumbosacral circuitry using epidural stimulation (Jilge et al. 2004; Minassian et al. 2004, 2007). A potential application of percutaneous electrical stimulation of the lumbosacral spinal cord in humans may be to modify the excitability of the spinal cord circuitry by repetitively stimulating during stepping in individuals with severely disrupted descending input from supraspinal centers.
In this study we assessed whether percutaneous stimulation of the lumbosacral spinal cord between the T11 and T12 spinous processes during stepping elicited short-latency multisegmental responses in leg muscles of individuals with clinically complete SCI similar to those observed in noninjured individuals. We investigated whether phase-dependent modulation of these responses can be regulated by the functionally isolated human spinal cord during stepping.
METHODS
Research participants
All individuals signed voluntary written consent approved by the Human Subject Protection Committee at the University of California, Los Angeles (UCLA) and conformed to the principles stated in the Declaration of Helsinki. Participants in the study were individuals with clinically complete SCI (n = 9) or without neurological injury (NI; n = 9). Individuals with clinically complete SCI (Table 1) were classified by the American Spinal Injury Association Impairment Scale as grade A (no clinically detectable motor or sensory function below the level of injury) and bilateral absence of cortical potentials in sensory evoked potential tests conducted by the UCLA clinical electrophysiology laboratory using routine clinical assessment methods. These individuals had varying levels of experience with exercise regimens involving increasing body weight load on the lower limbs. Two individuals (A22, A24) had experience with step training, two (A27, A28) had been trained in a unilateral standing regimen, and five individuals had no training experience.
Table 1.
Subject and experimental characteristics of individuals in the (A) noninjured (NI) and (B) spinal cord injured (SCI) groups
| ID | Age, yr | Sex, M/F | Time Postinjury, yr | Lowest Level Normal Function | BWS, % | Speed, mph | Intensity Stim, mA |
|---|---|---|---|---|---|---|---|
| A. NI individuals | |||||||
| N1 | 24 | F | — | — | 0 | 1.0 | 12.1 |
| N2 | 26 | F | — | — | 0 | 1.0 | 13.0 |
| N3 | 29 | M | — | — | 0 | 1.0 | 14.4 |
| N4 | 33 | M | — | — | 0 | 1.0 | 66.0 |
| N5 | 26 | F | — | — | 0 | 1.1 | 9.5 |
| N6 | 34 | M | — | — | 0 | 1.0 | 20.3 |
| N7 | 27 | M | — | — | 0 | 1.0 | 10.0 |
| N8 | 30 | M | — | — | 0 | 1.1 | 34.4 |
| N9 | 30 | M | — | — | 0 | 1.1 | 57.0 |
| B. SCI individuals | |||||||
| A21 | 30 | M | 7 | C5 | 60 | 1.1 | 30.2 |
| A22 | 30 | M | 2 | T7 | 44 | 1.1 | 30.6 |
| A24 | 43 | M | 4 | T3 | 47 | 1.1 | 44.0 |
| A27 | 50 | M | 31 | C6 | 60 | 1.2 | 27.8 |
| A28 | 22 | M | 2 | T6 | 47 | 1.1 | 24.7 |
| A29 | 23 | M | 1 | C5 | 48 | 1.1 | 30.8 |
| A30 | 34 | M | 1 | T6 | 61 | 1.1 | 41.9 |
| A31 | 35 | M | 6 | T3 | 66 | 1.2 | 27.8 |
| A32 | 26 | M | 4 | T4 | 70 | 1.1 | 83.0 |
ID, subject identification code; M, male; F, female; BWS, body weight support as a percentage of total body weight in standing and stepping. Speed: treadmill setting (in m/s). Intensity Stim: stimulator setting (in mA) during standing and stepping.
Experimental procedures
We measured leg electromyographic (EMG) activity during administration of percutaneous spinal cord electrical stimulation to participants lying prone, standing, and stepping. Muscle responses were recorded using bipolar (1.9 × 5.7 cm) surface electrodes with an interelectrode distance of 2 cm (Multi BioSensors, El Paso, TX) placed on the soleus, medial gastrocnemius, tibialis anterior, medial hamstring, and vastus lateralis muscles as described in previous studies (Courtine et al. 2007; Harkema et al. 1997). Data were acquired at 2,000 Hz using a 24-channel hard-wired A/D board and a customized LabVIEW software (National Instruments, Austin, TX) acquisition program. EMG amplifiers (Konigsberg Instruments, Pasadena, CA) were set to a gain of 500, with a bandwidth of 20–1,000 Hz. Three-dimensional joint angle and body segment position data were acquired using electromagnetic sensors (Skill Technology, Phoenix, AZ). The data were collected at 30 Hz and interpolated to 2,000 Hz to synchronize with EMG signals.
Percutaneous spinal cord stimulation was administered using a 0.25 cm round electrode (Lead-Lok, Sandpoint, ID) placed midline on the skin between the spinous processes of T11 and T12 as a cathode and two 5.0 × 10.2 cm electrodes (Ambu, Ballerup, Denmark) symmetrically on the skin over the iliac crests as anodes (Dyhre-Poulsen et al. 2005). Stimulation was delivered as single, 1 ms, monophasic, square-wave pulses. The placement of the stimulating electrode was determined when EMG activity could be observed in all measured muscles in the prone position. The intensity of stimulation was selected where evoked potentials were consistently observed in all measured muscles in standing. This was influenced by individual physique and required stimulus intensities ranging from 9.5 to 83 mA (Table 1).
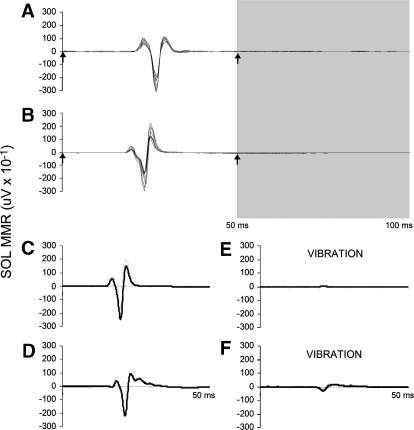
We first measured muscle responses evoked from 12 stimuli administered at 0.5 Hz in the prone position. Conditioning stimulation (i.e., a single pulse delivered 50 ms prior to each test pulse) (Fig. 1, A and B) and Achilles tendon vibration (Fig. 1, C and D), also administered in the prone position, inhibited the EMG amplitude, indicating characteristics consistent with short-latency, monosynaptic multisegmental responses (MMRs). Next, 12 stimuli were administered at 0.5 Hz while standing. Finally, stimulation was administered intermittently (0.25–0.33 Hz) during 12 min of stepping on a treadmill. The delivery of stimulation during stepping was controlled by a program that triggered single pulses with an interstimulus interval of ≥3 s, as monitored by footswitch sensors placed on the sole of the shoe (2.5 cm pressure sensors; Interlink Electronics, Camarillo, CA). Individual pulses were triggered randomly at one of 16 specified delays to disperse stimulation evenly throughout the step cycle. Delays were calculated by dividing the average step cycle duration by 16. Approximately 240 stimuli could be delivered in the 12 min period, allowing for 15 stimuli to occur at each of the 16 time bins. We required the step cycle duration to range between 0.9 and 1.4 s. This was a measure to prevent stimulation from occurring during irregular steps caused by stumbling or inaccurate placement of the foot on the treadmill belt.
Fig. 1.
A and B: overlay of 10 responses in the soleus muscle to double pulses of percutaneous spinal cord stimulation administered at 0 and 50 ms in a noninjured (NI) (A, N2) and a spinal cord injured (SCI) individual (B, A28) in the prone position. The time of the stimulus pulse is indicated by the up arrow and the time of the conditioned response is indicated in the area shaded gray. C–F: the mean response in the soleus muscle to percutaneous spinal cord stimulation in the absence (C and D) or the presence (E and F) of vibration of the Achilles tendon in NI (C and E) and SCI (D and F) individuals lying prone.
Body weight support and manual assistance of stepping allowed individuals in the SCI group to generate rhythmic stepping movements at speeds similar to those of NI stepping. The level of body weight support was the minimum amount necessary to enable the individual to stand with hip and knee joints fully extended and the shoe soles planted on the treadmill (Table 1). Manual assistance was provided by physical trainers positioned at each leg and another behind the hips, as described previously (Harkema et al. 1997). During standing, the trainers supported extension at the knee joints and stabilized the trunk. During stepping, the hip trainer stabilized the hips and trunk while the leg trainers assisted flexion and extension of the knee, plantar foot placement at the beginning of stance, and clearance of the toes over the treadmill belt at the beginning of swing.
Data analysis
Data were processed and synchronized using LabVIEW software customized by our laboratory. EMG data from nonstimulated steps were full-wave rectified and filtered using a fourth-order band-pass Butterworth filter (40–500 Hz). The peak-to-peak amplitude of each MMR was calculated and associated with the time in the step cycle (bin 1 to bin 16) in which the stimulus occurred. To find the response amplitude, we first calculated the latency of the response in each muscle as the time between the stimulus delivery and the onset. The onset of the MMR was later determined by visual assessment of deviation from EMG levels during quiescent standing 5 ms after a stimulus pulse to account for the stimulus artifact. The average of latencies of 12 MMRs evoked during standing served as the onset of a 30 ms window in which the maximum and minimum peaks were automatically detected during the step cycle. In proximal muscles, the combination of a larger stimulus artifact and shorter latency, relative to more distal muscles, created a situation in which the waveform could be obscured by the stimulus artifact. This occurred in the medial hamstring and vastus lateralis muscles of one individual (N4) and resulted in exclusion of these responses in subsequent analyses.
To calculate the time bin, we first defined the step cycle as the time elapsed between two successive contacts of the foot with the treadmill belt, as measured by the foot switch located under the heel of the foot. Second, the latency of each stimulus was calculated as the time from the beginning of the step cycle to the onset of the pulse. Third, the duration of the step was calculated as the time from the beginning to the end of the step cycle immediately preceding the stimulated step. Calculating bin assignment based on the step just prior to the stimulated step was based on the consideration that the prior step was the best representative of the length of the stimulated step had it not been stimulated, since the stimulation disrupts the step at the time it is delivered. Bins were assigned by rounding up to the integer value of 16 times the latency divided by the duration of the step. The peak-to-peak amplitudes of MMRs occurring within each bin were averaged. There was an approximately equal distribution of stimuli in each of 16 time bins with the maximal deviation of the actual response distribution from the expected response distribution being 1.5 responses for the SCI group and 2.7 responses for the NI group.
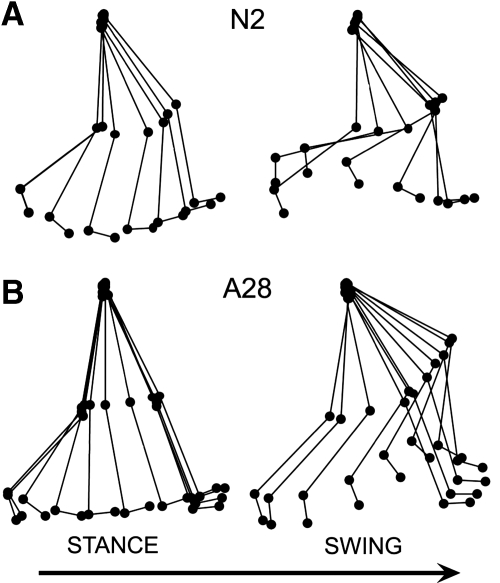
Ball and stick tracings of one exemplary step performed by an individual in each group were created by connecting the two-dimensional coordinates of the pelvis, hip, knee, and ankle during a step acquired using a 6D motion capture system (Skill Technologies, Phoenix, AZ). These tracings demonstrate differences in the limb kinematics between unassisted treadmill stepping performed by NI subjects (Fig. 2A, N2) and treadmill stepping with body weight support and manual assistance performed by SCI subjects (Fig. 2B, A28). Greater extension in the knee during stance, decreased flexion in the knee at the beginning of swing, and greater flexion in the knee at the end of swing were observed in the assisted compared with the unassisted step cycle. Average step cycle duration was estimated from 100 steps in each of the nine participants in each group. In general, step cycle duration was longer and stance shorter in the SCI group compared with NI group values (Table 2). We estimated the variability in step timing in NI and SCI subjects and found consistent step cycle duration in both the NI (mean standard deviation = 0.04 s) and SCI (mean standard deviation = 0.05 s) groups.
Fig. 2.
Ball and stick diagrams of right leg kinematics in the stance and swing phases of one exemplary step cycle in a NI individual (A) during stepping on a treadmill and in an SCI individual (B) during stepping on a treadmill with 47% bodyweight support and manual assistance. A 10 ms interval is between each stick trace. The arrow indicates the direction of stepping.
Table 2.
Variability in stepping was estimated in the (A) NI and (B) SCI groups, estimated from all right leg steps occurring prior to stimulated steps
| ID | Step Cycle Duration, s | Stance Duration, s |
|---|---|---|
| A. NI individuals | ||
| N1 | 1.11 ± 0.01 | 0.64 ± 0.02 |
| N2 | 1.10 ± 0.02 | 0.65 ± 0.02 |
| N3 | 1.15 ± 0.05 | 0.66 ± 0.04 |
| N4 | 1.10 ± 0.02 | 0.66 ± 0.02 |
| N5 | 1.11 ± 0.03 | 0.67 ± 0.02 |
| N6 | 1.22 ± 0.03 | 0.70 ± 0.02 |
| N7 | 1.16 ± 0.02 | 0.68 ± 0.01 |
| N8 | 1.05 ± 0.03 | 0.66 ± 0.02 |
| N9 | 1.09 ± 0.03 | 0.67 ± 0.04 |
| Mean | 1.12 | 0.66 |
| Median | 1.11 | 0.66 |
| SD | 0.05 | 0.02 |
| B. SCI individuals | ||
| A21 | 1.36 ± 0.06 | 0.63 ± 0.02 |
| A22 | 1.33 ± 0.07 | 0.65 ± 0.04 |
| A24 | 1.31 ± 0.05 | 0.74 ± 0.04 |
| A27 | 1.21 ± 0.07 | 0.58 ± 0.03 |
| A28 | 1.34 ± 0.10 | 0.76 ± 0.09 |
| A29 | 1.40 ± 0.10 | 0.60 ± 0.04 |
| A30 | 1.37 ± 0.05 | 0.60 ± 0.02 |
| A31 | 1.23 ± 0.06 | 0.57 ± 0.04 |
| A32 | 1.27 ± 0.09 | 0.48 ± 0.08 |
| Mean | 1.31 | 0.62 |
| Median | 1.33 | 0.60 |
| SD | 0.06 | 0.09 |
Step cycle duration (s): time (mean ± SD) from heel strike to next heel strike. Stance duration (s): time (mean ± SD) the right foot was in stance phase.
Assessment of MMRs evoked during standing
We assessed the relative amplitude of the MMRs evoked by percutaneous stimulation during standing in the two subject groups. The mean amplitudes of MMRs in each muscle were normalized to that of the soleus muscle because the placement of the percutaneous stimulation electrode was determined by the optimization of the soleus response. The significance of SCI and NI group differences in each muscle was assessed using bootstrapping methodologies. Specifically, we calculated the difference between two groups of nine data points that were sampled with replacement from the union of normalized NI and SCI values in each muscle. The bootstrapping procedure was repeated 5,000 times to create a distribution of resampled differences. Actual differences between NI and SCI groups were compared with the distribution and significance was determined at P < 0.012 after correction for multiple comparisons in four muscle groups.
Correlation of MMR modulation with step cycle EMG
We quantified the relationship between magnitude of underlying EMG and MMR amplitude throughout the step cycle. EMG levels were estimated from filtered (band-pass filter 40–500 Hz) and rectified signal beginning 25 ms before the onset of each stimulus pulse. MMR amplitudes were calculated for 16 bins across the step cycle. Cross-correlation analyses were used to determine the temporal relationship between MMR and EMG amplitudes was at the time of stimulus onset. This temporal relationship was used in subsequent comparisons with SCI group values. Correlations were significant (P < 0.05) for n = 16 at r > 0.5.
Quantification of the pattern of step cycle MMR modulation
To distinguish significant increases or decreases of MMRs across the step cycle, we compared the mean amplitude of MMRs in each of the 16 time bins with a distribution of amplitudes that could have occurred had MMRs been randomly modulated in the step cycle. The distribution was constructed by random reassignment (resampling without replacement) of individual MMR amplitudes into one of 16 bins, preserving the number of observations in the original bin assignment. In each bin, the mean value of the reassigned data were calculated. The resampling procedure was repeated to generate 5,000 estimates of the mean for each of the 16 bins. The upper and lower cutoff values of a 95% confidence interval (CI) around the means of the estimated values were calculated. We corrected for comparisons in the number of times the original mean MMR values exceeded the limits of the 95% CI and adjusted the width of the CI to the corrected P value. MMRs were categorized with respect to the corrected CI as those that were increased (above the upper cutoff of the CI), decreased (below the lower cutoff of the CI), and not modulated (within the CI).
Assessment of depth of modulation
We compared the magnitude of MMR modulation between groups using the modulation index, {[max (MR) − min (MR)]/max (MR)} × 100 (Yang et al. 1991), where MR was the mean amplitude of MMRs in a time bin. Statistically significant group differences between the mean group modulation index values were assessed. The difference between NI and SCI group mean values were compared with the 95th percentile cutoff of 5,000 estimated differences when values were randomly sampled with replacement between the two groups.
A profile of bins in which MMRs were increased, decreased, or not modulated across the step cycle was made for each muscle. To compare depth of modulation between SCI and NI patterns, maximum MMRs were selected only from the bins that were predominantly increased and minimum MMRs were selected only from the bins that were predominantly decreased across the step cycle in each muscle. Statistical analyses were repeated with modulation index values calculated under these temporally restricted conditions. Significant group differences in depth of modulation were determined after corrections for multiple comparisons in the restricted and the unrestricted calculations.
RESULTS
MMRs during standing
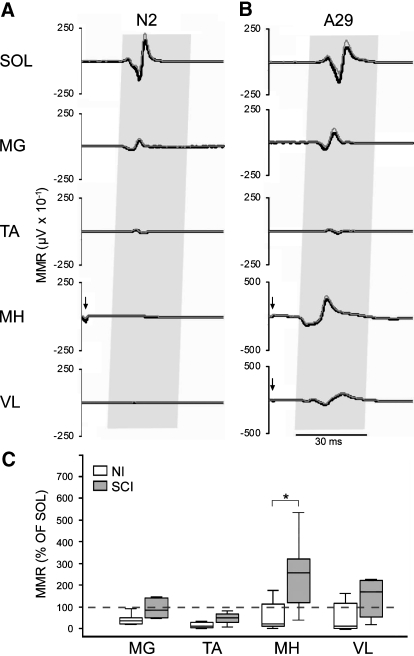
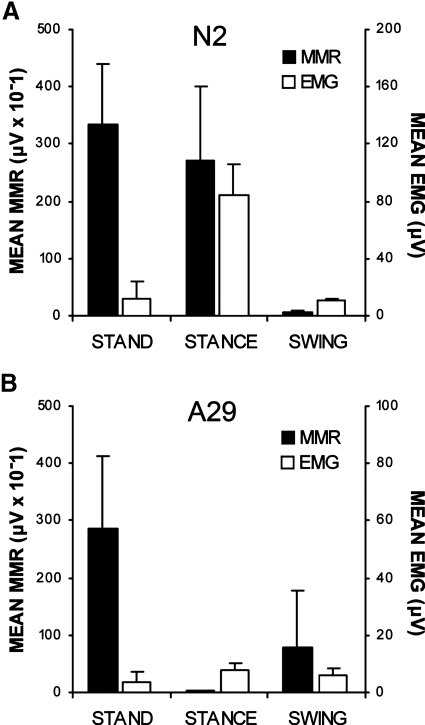
Eliciting multisegmental muscle responses by percutaneous stimulation over the Th11–Th12 spinal cord enabled us to assess the relative levels of excitability across motor pools. The relative amplitude of MMRs across motor pools evoked during standing differed between the two groups. In the NI group, MMRs were largest in the soleus muscle (Fig. 3A), whereas stimulation produced the largest responses in the medial hamstrings muscle in the SCI group (Fig. 3B). Group differences in the mean amplitude of MMRs in each muscle were compared as a percentage of the mean amplitude of the MMR in the soleus muscle (Fig. 3C). Medial gastrocnemius MMRs were 36% of soleus MMRs in the NI group compared with 84% in the SCI group. Tibialis anterior MMRs were 10% of soleus MMRs in the NI group compared with 50% in the SCI group. Medial hamstring MMRs were 20% of soleus MMRs in the NI group compared with 257% in the SCI group. Vastus lateralis MMRs were 10% of soleus muscle MMRs in the NI group compared with 169% in the SCI group. A significant group difference was observed in the medial hamstrings muscle (P < 0.012). Group differences were not significant in other muscles.
Fig. 3.
The average (dark trace) and SD (gray trace) of 12 multisegmental monosynaptic responses (MMRs) evoked during standing in the muscles of the right leg soleus (SOL), medial gastrocnemius (MG), tibialis anterior (TA), medial hamstrings (MH), and vastus lateralis (VL) muscles of exemplary (A) NI (N2) and (B) SCI (A29) individuals. Gray shading indicates expected time of MMRs. Arrows indicate stimulus artifact <0.5 ms after the stimulus pulse. C: group data of the average MMR in each muscle as a percentage of the average amplitude of the MMR in the soleus muscle during standing displayed as box and whisker plots showing the median (horizontal line in box), interquartile range (box height), and the range ≤1.5-fold the interquartile range (whiskers). Asterisk indicates significant differences between group median values at P < 0.05.
Correlation of MMRs to the step cycle EMG
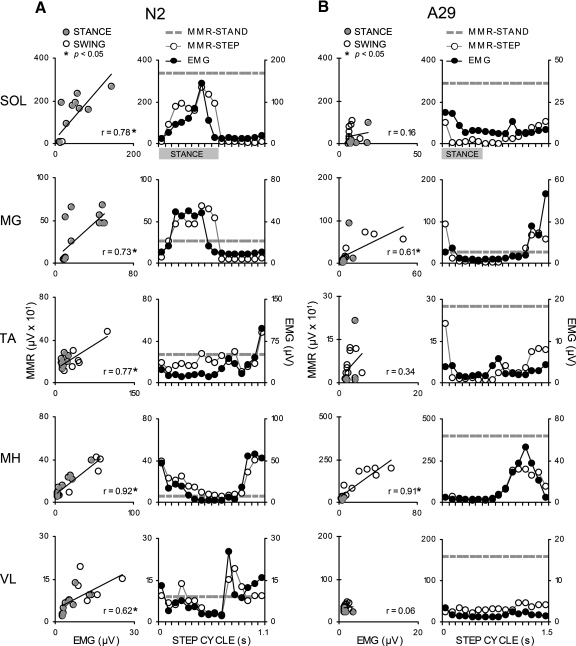
Linear correlations were used to quantify the strength of the relationship between the pattern of MMR modulation and EMG activity in each muscle as a function of the step cycle. In NI subjects, MMRs were correlated with EMG in the stance and swing phases of stepping in all muscles (Fig. 4A, left column). During stepping, soleus, and medial gastrocnemius MMRs and EMG varied throughout stance, but were always lowest during swing (Fig. 4A, right column). EMG in the tibialis anterior muscle was highest in early and late swing. MMRs in that muscle followed the pattern of EMG modulation during swing but varied more during stance (Fig. 4A, right column). EMG in the medial hamstrings and vastus lateralis muscles was lower as the leg was transitioning between stance and swing and higher in the transition from swing to stance. MMR modulation also generally followed the modulation of the EMG during the step cycle in those muscles (Fig. 4A, right column). Significant correlations (P < 0.05) between the amplitude of MMRs and EMG activity during stepping were observed in the soleus, medial gastrocnemius, and medial hamstrings muscles of all NI individuals (Table 3A). Correlations were significant in eight of nine individuals in the tibialis anterior muscle and in six of eight individuals in the vastus lateralis muscle (Table 3A).
Fig. 4.
Exemplary data in NI (A, N2) and SCI (B, A29) individuals showing modulation of electromyographic (EMG) activity compared with MMR amplitude across the step cycle. Left column graphs: the linear correlation between the amplitude of average EMG and MMRs was assessed across the step cycle (alpha 0.05 at r = 0.5) with identification of MMRs occurring in stance (filled circles) and swing (open circles). In all, 16 points were used to model the correlation, although similar values resulted in overlapping data points. Right column graphs: comparison of the amplitude of MMRs evoked during standing (dotted line), MMRs during stepping (line connecting open circles), and step cycle EMG activity (line connecting filled circles) as a function of time in the step cycle. Gray bars indicate time the leg was in stance.
Table 3.
Latency (mean ± SD) of MMR across each muscle was estimated (in ms) from 10 pulses of percutaneous spinal cord stimulation administered during standing in the (A) NI and (B) SCI groups
| ID | SOL | MG | TA | MH | VL |
|---|---|---|---|---|---|
| A. NI individuals | |||||
| N1 | 23.1 ± 0.3 | 21.1 ± 0.6 | 21.0 ± 2.0 | 15.1 ± 0.2 | 15.4 ± 0.6 |
| N2 | 19.5 ± 0.1 | 17.6 ± 0.3 | 19.7 ± 1.0 | 14.0 ± 0.9 | 13.6 ± 1.4 |
| N3 | 21.6 ± 0.5 | 19.7 ± 0.7 | 16.4 ± 0.9 | 13.5 ± 0.6 | 9.5 ± 0.3 |
| N4 | 22.7 ± 0.9 | 19.9 ± 0.3 | 13.5 ± 0.5 | — | — |
| N5 | 19.5 ± 0.0 | 17.4 ± 0.3 | 18.7 ± 0.6 | 13.3 ± 0.3 | 14.0 ± 1.7 |
| N6 | 25.7 ± 0.4 | 22.8 ± 0.3 | 23.1 ± 0.2 | 14.6 ± 0.2 | 10.0 ± 0.0 |
| N7 | 22.0 ± 0.3 | 20.5 ± 0.3 | 21.4 ± 0.4 | 14.0 ± 0.4 | 14.1 ± 1.5 |
| N8 | 22.5 ± 0.3 | 20.6 ± 0.2 | 15.7 ± 0.5 | 13.1 ± 0.2 | 9.6 ± 0.3 |
| N9 | 29.7 ± 0.4 | 26.8 ± 0.4 | 23.2 ± 0.3 | 16.0 ± 0.4 | 10.4 ± 0.3 |
| Mean | 22.9 | 20.7 | 19.2 | 14.2 | 12.1 |
| Median | 22.5 | 20.5 | 19.7 | 14.0 | 12.0 |
| SD | 3.16 | 2.84 | 3.40 | 0.99 | 2.43 |
| B. SCI individuals | |||||
| A21 | 26.9 ± 1.8 | 21.8 ± 2.4 | 16.5 ± 0.2 | 13.9 ± 0.3 | 10.5 ± 0.4 |
| A22 | 21.9 ± 0.3 | 21.5 ± 0.3 | 20.5 ± 0.1 | 14.3 ± 0.3 | 9.3 ± 0.3 |
| A24 | 29.7 ± 0.3 | 22.7 ± 0.3 | 19.3 ± 1.5 | 14.7 ± 0.2 | 15.5 ± 0.3 |
| A27 | 24.3 ± 1.0 | 22.3 ± 0.4 | 22.2 ± 0.5 | 15.8 ± 0.3 | 14.1 ± 0.3 |
| A28 | 20.4 ± 0.5 | 18.4 ± 0.2 | 18.7 ± 0.3 | 13.6 ± 0.4 | 13.1 ± 0.4 |
| A29 | 24.8 ± 0.3 | 20.7 ± 0.4 | 21.3 ± 1.2 | 14.0 ± 0.3 | 16.1 ± 0.3 |
| A30 | 25.1 ± 0.4 | 20.6 ± 0.3 | 22.0 ± 0.5 | 13.9 ± 0.4 | 12.9 ± 0.2 |
| A31 | 22.5 ± 0.4 | 22.2 ± 0.2 | 22.1 ± 0.4 | 13.7 ± 0.3 | 13.7 ± 0.5 |
| A32 | 26.3 ± 0.5 | 16.3 ± 0.3 | 17.0 ± 1.1 | 14.7 ± 0.5 | 8.8 ± 0.4 |
| Mean | 24.7 | 20.7 | 19.9 | 14.3 | 12.7 |
| Median | 24.8 | 21.5 | 20.5 | 14.0 | 13.1 |
| SD | 2.83 | 2.09 | 2.19 | 0.70 | 2.60 |
Group values are mean ± SD. Medial hamstring (MH) and vastus lateralis (VL) muscle responses for N4 were omitted because of an inability to measure MMRs.
In the SCI group, MMRs evoked during stepping were modulated relative to standing, as demonstrated in subject A29 (Fig. 4B). During stepping, significant correlations between MMR and EMG were observed, although not consistently across muscles or among individuals in the SCI group (Table 3B). Low correlations resulted when the pattern of MMR modulation did not follow the pattern of motor pool excitability. Weaker correlations were observed, particularly when EMG amplitudes were low throughout the step cycle, although modulation of MMRs was pronounced. Significant correlations could be observed even when the pattern of EMG step cycle activity differed substantially from that of the NI group (see medial gastrocnemius in Fig. 4B). Correlations were significant (P < 0.05, Table 3B) in the soleus of four of nine individuals (A21, A24, A30, A32); in the medial gastrocnemius of three of nine individuals (A27, A29, A30); in the tibialis anterior of three of nine individuals (A24, A30, A32); in the medial hamstrings of four of nine individuals (A28, A29, A31, A32); and in the vastus lateralis muscles of three of nine individuals (A21, A28, A30).
MMR modulation independent of EMG activity patterns was observed in both NI and SCI subjects. As demonstrated in subject N2, the largest MMRs were elicited during standing when the EMG amplitude was very low. EMG was higher but MMRs were lower during the stance phase of stepping compared with standing (Fig. 5A). In subject A29, MMR amplitudes changed from high during standing to lower in swing and even lower during stance, whereas EMG amplitudes remained low in all conditions (Fig. 5B).
Fig. 5.
Mean amplitudes of soleus MMRs and EMG from an individual in the NI (A, N2) and SCI (B, A29) groups during standing and the midstance (bin 7) and midswing (bin 14) of the step cycle.
Phase-dependent modulation of MMRs
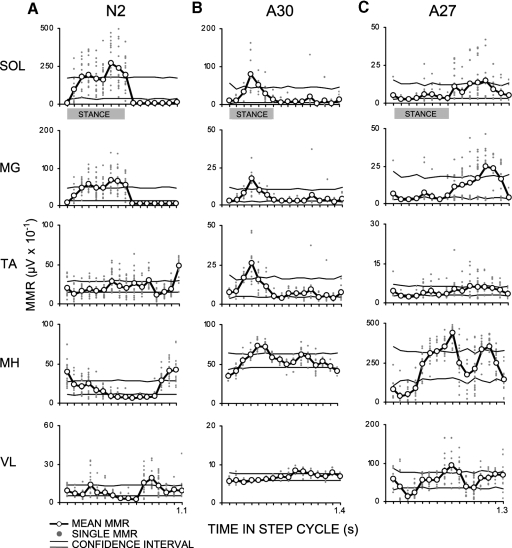
Patterns of MMR modulation across the step cycle differed between NI and SCI groups. Exemplary data from one individual in the NI group (N2, Fig. 6A) demonstrate soleus and medial gastrocnemius MMR amplitudes that were low in early stance, elevated at mid- and late stances, and low throughout swing. Tibialis anterior MMRs were elevated in late stance as well as early and late swing. Medial hamstring MMRs were elevated in early stance and late swing and reduced in late stance and early swing. Vastus lateralis MMRs were reduced in the middle of the step cycle and elevated in the middle of stance and the middle of swing (Fig. 6A).
Fig. 6.
A: NI (N2) data juxtaposed with SCI data to demonstrate modulation of soleus and medial gastrocnemius MMRs across the step cycle that was similar (B, A30) and dissimilar (C, A27) to the NI group pattern. Modulation of MMRs during stepping in all muscles was determined by comparing the average amplitude (black line connecting open circles) of single MMRs (gray dots) with a 95% confidence interval (CI) formed from 5,000 estimates of the mean amplitude of MMRs randomly resampled into 16 bins and corrected for multiple comparisons (upper and lower boundaries demarcated by horizontal lines). MMRs were classified by the location of the mean response in a bin being above, below, or within the CI. Gray bars indicate time the leg was in stance.
SCI subject A30 demonstrated near normal modulation of MMR in the soleus and medial gastrocnemius muscle (Fig. 6, A and B). The extent of similarity in the pattern of MMR modulation across the step cycle varied across motor pools and across subjects. The timing of modulation of MMRs of all muscles across the step cycle differed more widely in the SCI than in the NI subjects (Fig. 6, A vs. B and C).
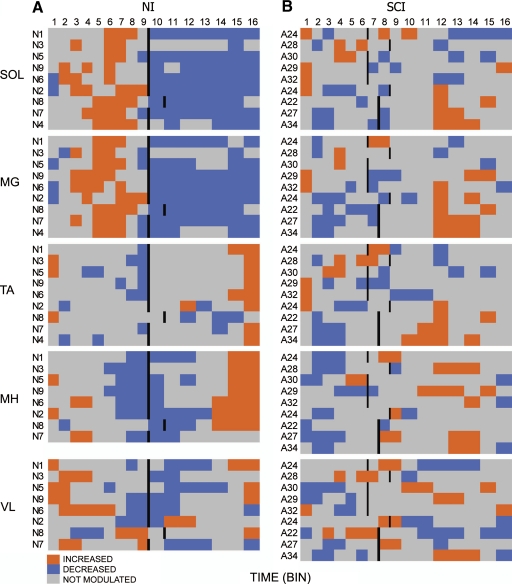
To visualize group trends in step cycle modulation, MMR values were transformed into a heat map representing significant increase (orange), decrease (blue), and no significant modulation (gray) in MMR amplitude (Fig. 7). In the NI group, the mean stance phase represented about 60% of the step cycle (Fig. 7A and Table 1). The stance phase began at bin 1 and ended between bins 9 and 11. Across the NI group, the soleus muscle was predominantly elevated in bins 2–8 and lowered in bins 1 and 10–16. Similar patterns were observed in the medial gastrocnemius muscle. In the tibialis anterior muscle, higher amplitude MMRs occurred predominantly in bins 1, 15, and 16 and lower amplitudes in bins 2–9 and 14. Medial hamstrings MMRs were higher in bins 1, 3, 4, and 14–16 and lower in bins 6–12. Vastus lateralis MMRs displayed a similar pattern of modulation as medial hamstring MMRs (Fig. 7A), although subject-to-subject variations were observed (N2, N6, N7, N8).
Fig. 7.
Heat map showing when during the step cycle MMRs were significantly increased (orange), decreased (blue), or not modulated (gray). The average step cycle was characterized by modulation in 16 time bins with the end of stance indicated by a vertical bar in the (A) NI and (B) SCI groups. Medial hamstring and vastus lateralis muscles for N4 were omitted because of an inability to measure MMRs in those muscles.
In the SCI group, the mean stance phase was roughly 45% of the step cycle and the step cycle duration was longer than that in NI individuals (Table 2, Fig. 6B). The stance phase began at bin 1 and ended between bins 6 and 9. Significant modulation of the MMR was observed during the step cycle in all muscles of all individuals of the SCI group (Fig. 6B). More subject-to-subject variation in the pattern of modulation was observed in the SCI compared with the NI group (Fig. 6B). Modulation in the soleus MMR closely resembled the NI pattern in two SCI individuals (A28, A30). The pattern of modulation of the soleus MMR in most subjects (A22, A24, A27, A29, A31) was the reverse of the NI pattern, (i.e., lower in bins 2–9 and elevated in bins 1 and 10–16). The two SCI individuals in whom soleus MMR modulation was similar to the NI pattern (A28 and A30) also displayed medial gastrocnemius MMR modulation that was similar to the NI pattern. The five SCI individuals in whom soleus MMR modulation was the reverse of the NI pattern (A22, A24, A27, A29, A31), displayed dissimilarities in the pattern of MMRs in the medial gastrocnemius muscle.
Plantarflexor and dorsiflexor MMR modulation during stepping was not reciprocally patterned and responses were often modulated in the same direction in all SCI subjects (Fig. 7). This suggested that an antagonistic relationship between plantarflexor and dorsiflexor MMRs was lacking. Although cross talk from plantarflexor to dorsiflexor electrodes cannot be totally excluded as a factor in all instances, as also noted in NI subjects (Courtine et al. 2007), this does not appear to have been a dominant factor in our observations.
In the more proximal muscles synchronous modulation between extensor and flexor pairs was more common in both NI and SCI groups. Medial hamstring and vastus lateralis MMRs tended to be modulated in the direction opposite to that observed in NI subjects, with low amplitudes occurring more often in the swing-to-stance transition and elevated responses occurring mostly in the swing and stance-to-swing transition.
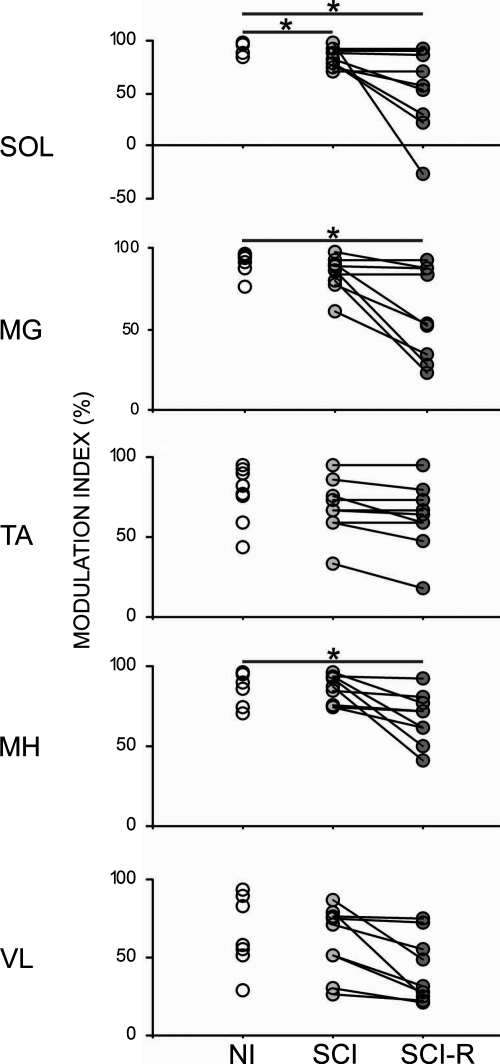
Depth of modulation of MMRs in stepping
The magnitude of increase and decrease of MMR amplitude, as assessed by the modulation index (Yang et al. 1991) (see methods for details on calculating the modulation index), was similar among the NI and SCI subjects for the medial gastrocnemius, tibialis anterior, medial hamstring, and vastus lateralis muscles (Fig. 8, NI vs. SCI). The modulation index in the soleus muscle of SCI subjects was significantly less than that of NI subjects (P < 0.025), although indices in four of nine SCI individuals were within range of the NI values (Fig. 8, NI vs. SCI). To identify temporal differences in MMR modulation we also calculated the modulation index with the restriction that the timing of the modulation had to be similar to that observed in NI subjects (Fig. 7A). Under this restriction, subjects in the SCI group still could demonstrate modulation index within the range of NI values in all muscles. Overall, mean modulation indexes in the SCI group were significantly lower than NI values in the soleus, medial gastrocnemius, and medial hamstring muscles (P < 0.025), whereas the tibialis anterior and vastus lateralis muscles were unchanged (Fig. 8, NI vs. SCI-R).
Fig. 8.
Greater depth of MMR modulation during stepping was represented by a higher modulation index in NI and SCI individuals. Modulation indexes were calculated by the equation {[max (MMR) − min (MMR)]/max (MMR)} × 100. An additional temporally restricted SCI group modulation index (SCI-R) was calculated by selecting maximum and minimum MMRs values only during times when MMRs were increased or decreased, respectively, in the NI step cycle. Asterisks indicate significant differences in group means in each muscle at P < 0.05.
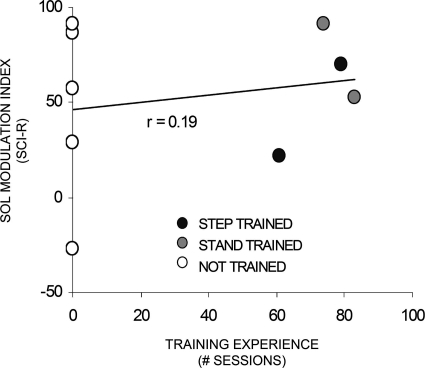
To determine whether the capacity for SCI subjects to modulate MMRs more similarly to NI was related to any previous training experience, we correlated training experience with the restricted modulation index for the soleus muscle (Fig. 9). Step training did not guarantee more normal levels, as demonstrated by one step trained individual who had a low modulation index (<0.5). The three individuals in whom the depth and pattern of modulation were most similar to NI values had no training experience or stand training.
Fig. 9.
Amount of training experience (number of 60 min sessions) was compared with the restricted modulation index shown in Fig. 8 (SCI-R) in the SCI group. Individuals could have experienced step-training (black circles), stand-training (gray circles), or not have any training experience (open circles).
DISCUSSION
The responses evoked by moderate-intensity percutaneous spinal cord stimulation over the T11–T12 spinous processes in humans have been attributed to activation of large diameter afferents in the dorsal roots of the lumbosacral spinal cord (Maertens de Noordhout et al. 1988; Minassian et al. 2007; Troni et al. 1996). The monosynaptic nature of these responses is implicated by their inhibition from tendon vibration and prior pulses (Courtine et al. 2007; Dyhre-Poulsen et al. 2005). We observed that the MMRs elicited in the present study displayed these monosynaptic properties (Fig. 1) and phasic modulation during stepping in both NI and SCI subjects (Figs. 6 and 7). This suggests that the modulation of monosynaptic reflexes can be mediated in spinal circuits. The patterns of modulation, however, were less consistent across SCI subjects compared with the NI group (Figs. 6 and 7).
Source of phase-dependent MMR modulation
Individuals in the SCI group could display MMRs that were modulated with a magnitude as great as that observed in NI subjects (Fig. 8). However, the soleus MMR modulation index was significantly less in SCI subjects as a group than that in NI subjects during stepping. Reduced modulation of soleus H-reflexes has also been previously described in individuals with incomplete SCI (Boorman et al. 1992; Yang et al. 1991). Possible reasons for the reduced modulation in SCI populations include loss of regulating mechanisms of supraspinal origin, disrupted function of postlesional interneuronal circuits, and its dominant role in opposing gravity and postural control.
In the present study, the modulation of MMRs in SCI subjects during stepping cannot be attributed only to the excitation level of the motor pools (Duenas et al. 1990), as suggested in patients with spastic multiple sclerosis (Sinkjaer et al. 1995). In some instances the EMG activity was correlated to the MMR response in individuals with SCI, as observed in the NI group supporting an influence by the motor pool excitability. However, abnormal step cycle EMG was observed in SCI subjects (Fig. 5B) and was previously described by others (Barbeau and Norman 2003; Beres-Jones et al. 2003, 2004b; Dietz et al. 1994, 1995; Wernig et al. 1995), resulting in the observation that changes in MMR amplitude were not tightly linked to changes in EMG amplitude during stepping (Fig. 5B, Table 3B). This was also evident when comparing EMG and MMR amplitudes during standing to the stance and swing phases of stepping in both NI and SCI subjects (Fig. 5). These observations are consistent with the interpretation that presynaptic mechanisms may also play a role in the modulation of the MMR.
Different timing of MMR modulation during the step cycle between NI and SCI subjects may result from impaired presynaptic regulation. Premotoneuronal mechanisms that regulate transmission in the Ia afferent pathways, including presynaptic inhibition, recurrent inhibition, and disynaptic reciprocal inhibition (Capaday et al. 1990; Petersen et al. 1999), can be influenced supraspinally (Hultborn et al. 1976; Lundberg 1969; Nielsen and Kagamihara 1993b) and are impaired after some neurological injury and diseases (Ashby and Weins 1989; Boorman et al. 1996; Crone et al. 1994; Morita et al. 2001; Nielsen et al. 1995; Perez and Field-Fote 2003; Shefner et al. 1992; Sinkjaer et al. 1995). A common observation following spinal cord injury in animals and humans has been a less consistent coordination of the motor pools during stepping, as exemplified by our observation of covariation in the modulation patterns of dorsiflexor and plantarflexor muscles during stepping (Fig. 7B). Further studies are warranted to better understand the contribution of pre- and postsynaptic mechanisms in the generation of phase-dependent modulation of reflexes during stepping after SCI in humans.
Other factors could also have contributed to the differences observed in the SCI as compared with NI groups. For example, significant differences in the relative amplitude of the MMRs during stepping (Figs. 4 and 5) may reflect less limb load in individuals with SCI compared with that in NI individuals. The relative amplitudes of MMRs in multiple muscles were not different in the standing (Fig. 3) and prone position, thus indicating that reduced load was not a major contributor to the relative differences in the MMR amplitudes across motor pools. In addition, manual assistance also imposed sensory cues not experienced during normal walking, including cutaneous stimulation around the hips, knees, and ankles, including Achilles and hamstring tendon stimulation.
The stimulation used to evoke the apparent monosynaptic responses observed in the present study cannot be assumed to represent only Ia afferent activation, but may also excite group Ib and group II afferents in dorsal roots, intraspinal connections, and spinal interneurons, all of which may in turn have polysynaptic effects on the MMR. Hunter and Ashby (1994) examined responses to epidural spinal cord stimulation applied through electrodes chronically implanted in individuals for the clinical treatment of intractable pain. They suggested that single pulses of stimulation, administered at an intensity that produced paresthesia in the lower limbs, generated monosynaptic responses through the dorsal roots as well as antidromic activation of primary afferents within the dorsal columns (Hunter and Ashby 1994). Epidural spinal cord stimulation evoked monosynaptic reflexes bilaterally in hindlimb muscles of complete spinal rats with unilateral chronic deafferentation, demonstrating that intraspinal connections can also be activated by spinal stimulation (Lavrov et al. 2008). Given all of these observations it seems likely that percutaneous stimulation activates multiple spinal neuronal systems with dorsal root afferents potentially being a major source. Further, although the evoked responses appear to be predominantly monosynaptic, this cannot be assumed to be a response of only the group Ia afferents.
Habituation of evoked responses is possible when there is an insufficient interval between consecutive stimuli. In the present study, the schedule of stimulus delivery included an interstimulus interval of ≥3,000 ms or approximately every third step. Although a longer period between stimuli may be preferable, it is unclear exactly how long an interval is needed during the stepping. Hultborn and colleagues (1996) reported that at rest the soleus H-reflex was depressed for >10 s after dorsiflexion. During stepping, rhythmic flexion and extension around all joints, cutaneous sensation, and fluctuations in limb load may presumably deter the reflex responses from ever returning to baseline. Alternatively, the baseline may be resetting at each step. The interstimulus interval (ISI) used in the present study is comparable to ISIs used in previous studies assessing step-phase-dependent modulation of H-reflexes. Reported ISIs between pulses of peripheral nerve stimulation ranged from 400 to 2,000 ms during stepping in noninjured humans (Capaday and Stein 1986) and from 1,000 to 8,000 ms in patients with spasticity (Yang et al. 1991). In a more recent study Capaday and colleagues reported the stimuli was administered every one to five steps at random (Schneider et al. 2000). Those authors acknowledged using shorter intervals to minimize fatigue due to prolonged walking in their experiments with nondisabled subjects (Capaday and Stein 1986). This concern was especially relevant in our study with SCI subjects in whom decline of EMG amplitude during stepping (EMG exhaustion) has been reported (Dietz et al. 2009). Although we did not estimate the effects of any possible high-frequency inhibition of the MMR in the present study, the interval between pulses was sufficient to allow for deeply modulated responses in the step cycles of both subject groups.
Effect of training on reflex modulation
Differences in the amount of exposure to training or other rehabilitative interventions may have also contributed to the variation in response patterns among SCI subjects. Four subjects in the SCI group had participated in step or stand-training regimens in our lab, whereas other individuals had not (Fig. 9). Locomotor training has been effective in mediating functional reorganization of spinal circuits after spinal cord injury (Barbeau et al. 2002; Behrman and Harkema 2000, 2007; Dietz and Harkema 2004; Edgerton et al. 2008; Fung et al. 1994; Shields and Dudley-Javoroski 2006; Stein et al. 2002; Thompson et al. 2009; Trimble et al. 1998; Wernig et al. 1995). In this study we did not observe a correlation between modulation and number of training sessions. A limitation of this study in regard to assessing a training effect was the varied training experience and the low number of SCI subjects. Conceivably, the neurophysiological state of these individuals with chronic SCI is highly variable and to more clearly define the effect of training MMRs should be evaluated before and after the training intervention in the same individual. Additional investigation of the effect of locomotor training on monosynaptic reflex modulation is needed.
Concluding remarks
In NI individuals, the amplitude modulation of monosynaptic responses was proportional to and time-linked with the level of activation of each muscle studied. Significant phase-dependent modulation was also observed in individuals with clinically complete SCI, although with much less consistency. In addition, the modulation pattern in SCI was less synchronized with muscle activity, compared with NI patterns. These data provide evidence that in the absence of input from the brain the human lumbosacral spinal circuitry can gate afferent input as a function of the phase of the step cycle. This gating potential is likely to play an important role in processing complex sensory input among interneurons, which in turn coordinate those motor pools that contribute to locomotion.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-36854 and NS-1633, the Christopher and Dana Reeve Foundation, Roman Reed Spinal Cord Injury Research Fund of California Grant RR00865, the Russian Foundation for Basic Research and U.S. Civilian Research and Development Grant RUB1-2872-ST-07, Russian Foundation for Basic Research Grant 10-04-01172a, a predoctoral traineeship (T3 NS-7449) to C. J. Dy from the University of California, Los Angeles Training Program in Neural Repair, and a Dissertation Year Fellowship to C. J. Dy from the University of California, Graduate Division. S. J. Harkema is an endowed Owsley B. Frazier Chair in Neurological Rehabilitation.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank the patients, physical therapists, volunteers, and staff of the former UCLA Human Locomotion Research Center for the time and dedication to this study; C. Ferreira, R. van den Brand, E. Lan, and D. Lorenz for help in preparation of the data used in this study; and Dr. Igor Lavrov, Dr. Alan Garfinkel, and Dr. Claudia Angeli for suggestions on the manuscript.
REFERENCES
- Akazawa K, Aldridge JW, Steeves JD, Stein RB. Modulation of stretch reflexes during locomotion in the mesencephalic cat. J Physiol 329: 553–567, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby P, Wiens M. Reciprocal inhibition following lesions of the spinal cord in man. J Physiol 414: 145–157, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, Ladouceur M, Mirbagheri MM, Kearney RE. The effect of locomotor training combined with functional electrical stimulation in chronic spinal cord injured subjects: walking and reflex studies. Brain Res Brain Res Rev 40: 274–291, 2002. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Norman KE. The effect of noradrendergic drugs on the recovery of walking after spinal cord injury. Spinal Cord 21: 137–143, 2003. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412: 84–95, 1987. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther 80: 688–700, 2000. [PubMed] [Google Scholar]
- Behrman AL, Harkema SJ. Physical rehabilitation as an agent for recovery after spinal cord injury. Phys Med Rehabil Clin N Am 18: 183–202, 2007. [DOI] [PubMed] [Google Scholar]
- Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain 127: 2232–2246, 2004b. [DOI] [PubMed] [Google Scholar]
- Beres-Jones JA, Harkema SJ, Simonsen E, Dyhre-Poulsen P. Phase dependent modulation of H-reflex during stepping by the functionally isolated human spinal cord. Soc Neurosci Abstr 30: 601.6, 2004a. [Google Scholar]
- Beres-Jones JA, Johnson TD, Harkema SJ. Clonus after human spinal cord injury cannot be attributed solely to recurrent muscle-tendon stretch. Exp Brain Res 149: 222–236, 2003. [DOI] [PubMed] [Google Scholar]
- Boorman G, Becker WJ, Morrice BL, Lee RG. Modulation of the soleus H-reflex during pedalling in normal humans and in patients with spinal spasticity. J Neurol Neurosurg Psychiatry 55: 1150–1156, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman GI, Lee RG, Becker WJ, Windhorst UR. Impaired “natural reciprocal inhibition” in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr Clin Neurophysiol 101: 84–92, 1996. [DOI] [PubMed] [Google Scholar]
- Capaday C, Cody FW, Stein RB. Reciprocal inhibition of soleus motor output in humans during walking and voluntary tonic activity. J Neurophysiol 64: 607–616, 1990. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci 6: 1308–1313, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Schalk G, Stokes BT, Wolpaw JR. The interaction of a new motor skill and an old one: H-reflex conditioning and locomotion in rats. J Neurosci 25: 6898–6906, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Harkema SJ, Dy CJ, Gerasimenko YP, Dyhre-Poulsen P. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J Physiol 582: 1125–1139, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenna P, Frigo C. Excitability of the soleus H-reflex arc during walking and stepping in man. Exp Brain Res 66: 49–60, 1987. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J, Petersen N, Ballegaard M, Hultborn H. Disynaptic reciprocal inhibition of ankle extensors in spastic patients. Brain 117: 1161–1168, 1994. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. 79: 1329–1340, 1998. [DOI] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L. Locomotor activity in spinal man. Lancet 344: 1260–1263, 1994. [DOI] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol 37: 574–582, 1995. [DOI] [PubMed] [Google Scholar]
- Dietz V, Grillner S, Trepp A, Hubli M, Bolliger M. Changes in spinal reflex and locomotor activity after a complete spinal cord injury: a common mechanism? Brain 132: 2196–2205, 2009. [DOI] [PubMed] [Google Scholar]
- Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl Physiol 96: 1954–1960, 2004. [DOI] [PubMed] [Google Scholar]
- Duenas SH, Loeb GE, Marks WB. Monosynaptic and dorsal root reflexes during locomotion in normal and thalamic cats. J Neurophysiol 63: 1467–1476, 1990. [DOI] [PubMed] [Google Scholar]
- Dyhre-Poulsen P, Dy CJ, Courtine G, Harkema S, Gerasimenko YP. Modulation of multisegmental monosynaptic reflexes recorded from leg muscles during walking and running in human subjects (Abstract). Gait Posture 21: S66, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyhre-Poulsen P, Simonsen EB. H reflexes recorded during locomotion. Adv Exp Med Biol 508: 377–383, 2002. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Courtine G, Gerasimenko YP, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJK, Burdick JW, Roy RR. Training locomotor networks. Brain Res Rev 57: 241–354, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J, Barbeau H. Effects of conditioning cutaneomuscular stimulation on the soleus H-reflex in normal and spastic paretic subjects during walking and standing. J Neurophsyiol 72: 2090–2104, 1994. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods 157: 253–263, 2006. [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 77: 797–811, 1997. [DOI] [PubMed] [Google Scholar]
- Hofstoetter US, Minassian K, Hofer C, Mayr W, Rattay F, Dimitrijevic MMR. Modification of reflex responses to lumbar posterior root stimulation by motor tasks in healthy subjects. Artif Organs 32: 644–648, 2008. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res 108: 450–462, 1996. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. III. Effects from supraspinal pathways. Acta Physiol Scand 96: 368–391, 1976. [DOI] [PubMed] [Google Scholar]
- Jilge B, Minassian K, Rattay F, Dimitrijevic MMR. Frequency-dependent selection of alternative spinal pathways with common periodic sensory input. Biol Cybern 91: 359–376, 2004. [DOI] [PubMed] [Google Scholar]
- Kirshblum SC, Groah SL, McKinley WO, Gittler MS, Stiens SA. Spinal cord injury medicine. 1. Etiology, classification, and acute medical management. Arch Phys Med Rehabil 83, Suppl. 1: S50–S57, S90–S98, 2002. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Courtine G, Dy CJ, van den Brand R, Fong AJ, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR. Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. J Neurosci 28: 7774–7780, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol 92: 421–435, 1986. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the spinal cord. UCLA Forum Med Sci 11: 231–265, 1969. [PubMed] [Google Scholar]
- Maertens de Noordhout A, Rothwell JC, Thompson PD, Day BL, Marsden CD. Percutaneous electrical stimulation of lumbosacral roots in man. J Neurol Neurosurg Psychiatry 51: 174–181, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, Dimitrijevic MMR. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42: 401–416, 2004. [DOI] [PubMed] [Google Scholar]
- Minassian K, Persy I, Rattay F, Dimitrijevic MMR, Hofer C, Kern H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 35: 327–336, 2007. [DOI] [PubMed] [Google Scholar]
- Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain 124: 826–837, 2001. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol 464: 575–593, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Crone C. Changes in transmission across synapses of Ia afferents in spastic patients. Brain 118: 995–1004, 1995. [DOI] [PubMed] [Google Scholar]
- Perez MA, Field-Fote EC. Impaired posture-dependent modulation of disynaptic reciprocal Ia inhibition in individuals with incomplete spinal cord injury. Neurosci Lett 341: 225–228, 2003. [DOI] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. J Physiol 520: 605–619, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol 28: 345–376, 1987. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Berman SA, Sarkarati M, Young RR. Recurrent inhibition is increased in patients with spinal cord injury. Neurology 2: 2162–2168, 1992. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Logigian EL. Conduction velocity in motor, cutaneous afferent, and muscle afferent fibers within the same mixed nerve. Muscle Nerve 17: 773–778, 1994. [DOI] [PubMed] [Google Scholar]
- Shields RK, Dudley-Javoroski S. Musculoskeletal adaptations in chronic spinal cord injury: effects of long-term soleus electrical stimulation training. Neurorehabil Neural Repair 21: 169–179, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen EB, Dyhre-Poulsen P. Amplitude of the human soleus H reflex during walking and running. J Physiol 515: 929–939, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Toft E, Hansen HJ. H-reflex modulation during gait in multiple sclerosis patients with spasticity. Acta Neurol Scand 91: 239–246, 1995. [DOI] [PubMed] [Google Scholar]
- Stein RB, Capaday C. The modulation of human reflexes during functional motor tasks. Trends Neurosci 11: 328–332, 1988. [DOI] [PubMed] [Google Scholar]
- Stein RB, Chong SL, James KB, Kido A, Bell GJ, Tubman LA, Belanger M. Electrical stimulation for therapy and mobility after spinal cord injury. Prog Brain Res 137: 27–34, 2002. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Estabrooks KL, Chong S, Stein RB. Spinal reflexes in ankle flexor and extensor muscles after chronic central nervous system lesions and functional electrical stimulation. Neurorehabil Neural Repair 23: 133–142, 2009. [DOI] [PubMed] [Google Scholar]
- Trimble MH, Behrman AL, Flynn SM, Thigpen MT, Thompson FJ. Acute effects of locomotor training on overground walking speed and H-reflex modulation in individuals with incomplete spinal cord injury. J Spinal Cord Med 24: 74–80, 2001. [DOI] [PubMed] [Google Scholar]
- Troni W, Bianco C, Coletti Moja M, Dotta M. Improved methodology for lumbosacral nerve root stimulation. Muscle Nerve 19: 595–604, 1996. [DOI] [PubMed] [Google Scholar]
- Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on “rules of spinal locomotion” is effective in spinal cord injured persons. Eur J Neurosci 7: 823–829, 1995. [DOI] [PubMed] [Google Scholar]
- Yang JF, Fung J, Edamura M, Blunt R, Stein RB, Barbeau H. H-reflex modulation during walking in spastic paretic subjects. Can J Neurol Sci 18: 443–452, 1991. [DOI] [PubMed] [Google Scholar]