Abstract
The primary center of serotonin (5-HT) projections to the forebrain is the dorsal raphe nucleus (DR), a region known for its role in the limbic stress response. The ventromedial subregion of the DR (vmDR) has the highest density of 5-HT neurons and is the major target in experiments that involve the DR. However, studies have demonstrated that a variety of stressors induce activation of neurons that is highest in the lateral wing subregion (lwDR) and includes activation of lwDR 5-HT neurons. Despite the functional role that the lwDR is known to play in stress circuits, little is known about lwDR 5-HT neuron physiology. Whole cell patch clamp electrophysiology in mice revealed that lwDR 5-HT cells have active and passive intrinsic membrane properties that make them more excitable than vmDR 5-HT neurons. In addition, lwDR 5-HT neurons demonstrated faster in vitro firing rates. Finally, within the vmDR there was a positive correlation between rostral position and increased excitability, among several other membrane parameters. These results are consistent with stressor induced patterns of activation of 5-HT neurons that includes, in addition to lwDR neurons, a small subset of rostral vmDR neurons. Thus increased intrinsic excitability likely forms a major part of the mechanism underlying the propensity to be activated by a stressor. The membrane properties identified in lwDR recordings may thereby contribute to a unique role of lwDR 5-HT neurons in adaptive responses to stress and in the pathobiology of stress-related mood disorders.
INTRODUCTION
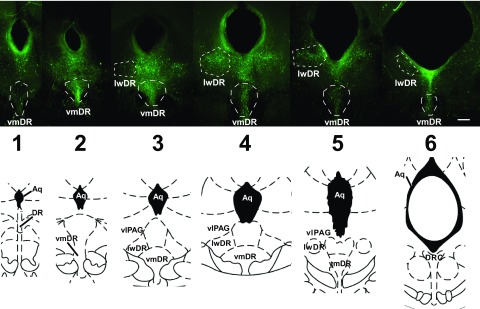
As the primary center of serotonin (5-HT) projections to the forebrain, the dorsal raphe nucleus (DR) is known for modulating the limbic system in response to stressors and for its putative role in stress-related mood disorders such as anxiety and depression. There is a topographical organization within the dorsomedial, ventromedial (vmDR), and lateral “wing” (lwDR) subregions of the DR such that subpopulations of neurons innervate distinct targets, receive disparate afferent input, and are likely to be differentially regulated in stress-related pathology (Abrams et al. 2004; Johnson et al. 2004; Lowry et al. 2008; Michelsen et al. 2007). A subregion that is poised to have a crucial role in the neural circuits that mediate the response to stressors is the lwDR, a subregion whose 5-HT neurons overlap with the ventrolateral periaqueductal gray (Fig. 1) (Paxinos and Franklin 2001; Paxinos and Watson 1997).
Fig. 1.
5-Hydroxytryptamine (5-HT, serotonin) neurons extend across the rostrocaudal extent of the dorsal raphe (DR). Top panel: images of 200 μm thick slices across the rostrocaudal extent of the DR following immunohistochemical detection of tryptophan hydroxylase. White dotted lines indicate the regions targeted for electrophysiology (scale bar: 208 μm). Bottom panel: corresponding brain atlas images (Paxinos and Watson 1996). Numbers indicate rostrocaudal level used for correlative analysis. Aq, aqueduct; DRC, dorsal raphe, caudal part; lwDR, lateral wing of the DR; vlPAG, ventrolateral periaqueductal gray; vmDR, ventromedial DR.
Several types of stressors, including exposure to an open field arena, social stress, swim stress, and interoceptive stressors along with several anxiogenic drugs activate topographically organized subpopulations of DR neurons, particularly within the lwDR subregion (Abrams et al. 2005; Berton et al. 2007; Chung et al. 2000; Gardner et al. 2005; Hale et al. 2008; Johnson et al. 2004, 2008; Martinez et al. 1998; Roche et al. 2003). To our knowledge, there are few stressors that do not activate the lwDR. Gene markers of neuronal activation implicate the non-5-HT neurons of the lwDR in swim stress (Roche et al. 2003); however, recent studies revealed that 5-HT neurons are an important part of the swim stress-activated circuitry whose activation may be masked by 5-HT1A receptor-mediated inhibitory feedback mechanisms (Commons 2008). Interestingly, the majority of 5-HT neurons activated by swim stress are not located in the 5-HT-dense vmDR, but rather are found in the lwDR. Intravenous lactate induces panic-like responses in a rat model of panic disorder, but has little effect on control animals (Johnson and Shekhar 2006; Shekhar et al. 2006). This mild stressor activates lwDR 5-HT cells in control animals but fails to activate lwDR 5-HT cells in the model of panic, implicating lwDR activation in the normative response to innocuous stressors (Johnson et al. 2008).
Thus lwDR 5-HT neurons likely play a distinctive, crucial role in the response to stressors. However, because the vast majority of electrophysiological studies of 5-HT neurons target the midline DR, and especially the vmDR, surprisingly little is known about the physiology of lwDR 5-HT neurons. It is unclear whether the distinct role of lwDR 5-HT cells in stress-related circuits might be due to fundamental differences in neuron physiology. We hypothesized that there are intrinsic membrane properties of lwDR 5-HT neurons that distinguish them from vmDR neurons and that contribute to their activation by stressors. To the best of our knowledge, these experiments also represent the first characterization of membrane properties and 5-HT1A autoreceptor-mediated responses of immunohistochemically identified 5-HT cells in the mouse DR. Part of this work was previously presented as an abstract (Crawford and Beck 2008).
METHODS
Animals
Adult male 5-HT-YFP mice or their wild-type littermates were used at 2–4 mo of age. These mice contain 5-HT neurons that express yellow fluorescent protein (YFP) under the control of the 5-HT specific Pet-1 promoter (Scott et al. 2005). The mice are on a background containing predominantly C-57/Black6. Mice were housed in a standard animal facility with lights on 06:00 to 18:00. Animal protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Whole cell electrophysiology
Electrophysiology recordings were conducted as previously described (Beck et al. 2004). In brief, mice were killed using decapitation. While submerged in a cold artificial cerebrospinal fluid (aCSF) solution, in which NaCl is replaced with sucrose (248 mM), the midbrain was rapidly dissected, blocked, and cut with a Leica VT1000s vibratome (Leica Microsystems, Bannockburn, IL), to generate 200 μm thick brain slices. Slices were maintained in aCSF bubbled with 95% O2-5% CO2 at 36°C for 1 h and then at room temperature until used. Individual slices were then placed in a recording chamber and continuously perfused with 32–34°C aCSF solution bubbled with 95% O2-5% CO2, with a solution flow rate of 1.5–2 ml/min. Neurons were visualized using Nikon E600 (Optical Apparatus, Ardmore, PA) upright microscope fitted with a ×40 water-immersion objective, differential interference contrast, and infrared filter. Ventromedial and lateral wing DR 5-HT neurons were targeted based on expression of the YFP transgene and later confirmed based on the expression of the synthetic enzyme marker, tryptophan hydroxylase. To visualize YFP-positive 5-HT cells for recording, a fluorescent lamp and yellow-fluorescence filter were used. The image was generated on a computer monitor using a charge-coupled device camera and Nikon Elements software (Optical Apparatus). Whole cell recording pipettes fabricated on a P-97 pipette puller (Sutter Instrument, Novato, CA) had a resistance of 6–10 MΩ when filled with an intracellular solution of (in mM) 130 K-gluconate, 5 NaCl, 10 Na-phosphocreatinine, 1 MgCl2, 0.02 EGTA, 10 HEPES, 2 MgATP, 0.5 Na2GTP, and 0.1% biocytin (pH 7.3). Recordings were collected on-line with a Multiclamp 700B amplifier, Digidata 1320 A/D converter, and Clampex 9.0 software (Molecular Devices, Union City, CA). Membrane properties of the cell were monitored, as was the access resistance of the patch pipette during recordings that lasted a minimum of 20 min. Any cell that showed physiological signs of instability or depolarizing resting membrane potential above −50 mV was excluded from data analysis. Reported values do not incorporate a junction potential of approximately +15 mV, as calculated using Clampex software. Composition of the aCSF was (in mM): 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2.0 MgSO4, 2.5 CaCl2, 10 dextrose, and 26 NaHCO3. Following the experiment, DR slices were fixed for 2–3 h with 4% paraformaldehyde and processed for immunohistochemical detection of tryptophan hydroxylase (TPH) and the biocytin-filled, recorded cell.
In vitro extracellular electrophysiology
Raphe slices from 5-HT-YFP mice were prepared and visualized as described earlier. YFP-labeled neurons were targeted with extracellular pipettes fabricated on a Sutter Instrument pipette puller and filled with 150 mM NaCl. The electrode was visually placed on top of the YFP neuron. Firing activity was restored to 5-HT neurons using bath application of 1 μM phenylephrine HCl. Any neurons with unstable firing rates over the course of the experiment were deemed unhealthy and excluded.
Data analysis
Current clamp recordings using visualized whole cell patch clamp techniques were analyzed using Clampfit 9.0 (Molecular Devices). Resting membrane potential (RMP), action potential (AP) threshold, AP duration, afterhyperpolarization (AHP) amplitude, and the time it takes for the AHP to depolarize to one-half its peak amplitude (AHP t1/2) were measured directly from traces as previously described (Beck et al. 2004). A voltage–current (V–I) graph was generated using current pulses ranging from −100 to 0 pA. Membrane input resistance (IR) was determined from the slope of the linear portion of the plot of the peak voltage induced by each current step. Frequency–intensity plots were obtained by measuring the number of action potentials generated by depolarizing current steps ranging from 0 to +80 pA in 20 pA increments. Average firing rate (Hz) was determined by the number of APs generated over the 630 ms current pulse. Gain was determined from the slope of the frequency–intensity plot. The time constant tau was obtained from an exponential fit of the membrane potential during the first 300 ms of a −20 pA hyperpolarizing current pulse. AP and AHP characteristics were determined from action potentials generated by injecting just enough current through the recording electrode to elicit a single action potential. AP amplitude, AP duration, and AHP amplitude were measured in relation to the AP threshold. AHP t1/2 is the duration of the AHP measured from its peak to half-amplitude. In experiments using CdCl2, AP duration was determined at half-amplitude. 5-HT1AR-mediated responses were measured with current clamp recordings using 400 ms, −30 pA current pulses at 10-s intervals to monitor resistance along with changes in membrane potential. Data generated by extracellular recordings were analyzed using the Mini Analysis Program (Synaptosoft, Decatur, GA). Reported values are means ± SE, unless otherwise noted; P values were generated using Student's t-test unless otherwise noted and P < 0.05 was deemed significant. Outlying values were included for the purpose of understanding the heterogeneity of 5-HT neurons. Any data that showed non-Gaussian distribution were compared using nonparametric statistical tests. Additional statistical analysis was performed using Prism (GraphPad Software, La Jolla, CA).
Immunohistochemistry
Immunohistochemical identification of each neuron recorded in patch clamp configuration was completed as previously described (Beck et al. 2004; Kirby et al. 2008; Lemos et al. 2006). In brief, a standard immunohistochemistry protocol was used on 200 μm thick slices using mouse anti-TPH (1:200, Sigma) along with secondary donkey anti-mouse Alexa Fluor 488 (1:200, Invitrogen) and streptavidin-conjugated Pacific Blue (1:100, Invitrogen). Images were captured using a Leica DMR fluorescent microscope (Leica Microsystems, Bannockburn, IL) and OpenLab 3.0.9 software (Improvision, Lexington, MA) and then confirmed on a Leica DMIRE2 confocal microscope (Leica Microsystems) using Leica confocal software (version 2.5, Leica Microsystems).
Drugs
All chemicals for making the sucrose aCSF, aCSF, and extracellular electrolyte solution were purchased from Fisher Scientific (Pittsburgh, PA). All chemicals for the intracellular electrolyte solution, 5-carboxamidotryptamine (5-CT), cadmium chloride, phenylephrine HCl, and N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl(cyclohexanecarboxamide (WAY-100635) were purchased from Sigma-Aldrich (St. Louis, MO). Apamin bicuculline, (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid HCl (CGP 55845), d-2-amino-5-phosphonopentanoic acid (d-AP5), 6,7-dinitroquinoxaline-2,3-dione (DNQX), and 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD 7288) were purchased from Tocris Bioscience (Ellisville, MO).
RESULTS
YFP-labeled 5-HT neurons of the vmDR and lwDR subregions were targeted throughout the rostrocaudal extent of the DR. A total of 85 neurons were recorded from 41 mice; the 5-HT identity of all neurons was confirmed by immunohistochemical detection of tryptophan hydroxylase. Data recorded from YFP-labeled 5-HT neurons and from unlabeled 5-HT neurons from wild-type offspring of the same mouse line did not differ (data not shown). Subregions were defined according to the distribution of 5-HT neurons as shown in Fig. 1.
Under current clamp, 5-HT cells in the mouse showed passive and active electrophysiological properties resembling those reported in rat, including large input resistance, wide AP, large slow AHPs, and lack of spontaneous firing in brain slice preparations (Beck et al. 2004; Kirby et al. 2003; Marinelli et al. 2004; Vandermaelen and Aghajanian 1983). In vmDR, as expected, there were a few distinctions between rat and mouse 5-HT neurons. Compared with the values reported in immunohistochemically identified vmDR 5-HT neurons in our previously published rat studies (n = 33, Beck et al. 2004), vmDR 5-HT neurons of the mouse (n = 19) differed in input resistance (637 ± 32 MΩ in rat vs. 419.5 ± 34.3 MΩ in mouse, P < 0.0001), tau (51 ± 3.0 ms in rat vs. 23.4 ± 1.9 ms in mouse, P < 0.0001), AP amplitude (69 ± 1.4 mV in rat vs. 56.6 ± 1.6 mV in mouse, P < 0.0001), and AHP amplitude (16 ± 0.8 mV in rat vs. 29.3 ± 1.6 mV in mouse, P < 0.0001). Current clamp recordings also revealed a small subset of confirmed 5-HT cells (5 of 19 vmDR and 3 of 17 lwDR cells) that departed from the classical description for 5-HT neurons and demonstrated an inward rectification current, visible in the nonlinear V–I plot in response to hyperpolarizing current steps (Supplemental Fig. S1, A and B).1 Another small subset of 5-HT neurons (2 of 19 vmDR cells) featured a delayed-onset inward rectification Ih current (Supplemental Fig. S1C). The cell used to generate traces for Supplemental Fig. S1C was not included in other experiments. Previously, inward rectification currents and Ih currents were reported in the rat vmDR, but only in non-5-HT neurons (Beck et al. 2004; Kirby et al. 2003). The other passive and active membrane properties of these few 5-HT cells did not differ from other 5-HT neurons (data not shown); these neurons were therefore included in further analysis.
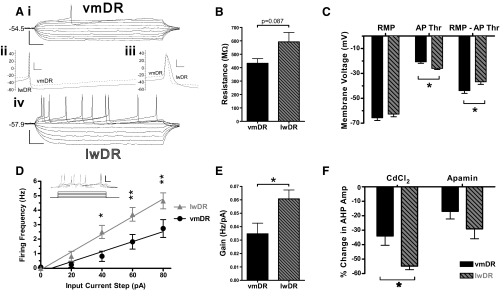
The electrophysiological characteristics of lwDR 5-HT neurons were compared with those of vmDR 5-HT neurons (Table 1). All measured parameters demonstrated a single Gaussian distribution with the exception of lwDR resistance, vmDR tau, vmDR AP duration, and vmDR gain, each of which contained one to two outliers. Thus comparisons of resistance, tau, AP duration, and gain were conducted using nonparametric tests. Lateral wing 5-HT neurons had a trend toward a larger membrane resistance compared with that of vmDR neurons (P = 0.087, Mann–Whitney test) (Table 1, Fig. 2B). This was consistent with the larger time constant (tau) observed in lwDR neurons (P = 0.013, Mann–Whitney test) (Table 1).
Table 1.
Membrane characteristics of 5-HT neurons of the vmDR and lwDR
| RMP, mV | Res, MOhm | Tau, ms | AP Thr, mV | AP Amp, mV | AP Dur, ms | AHP Amp, mV | AHP t1/2, ms | |
|---|---|---|---|---|---|---|---|---|
| vmDR | −66.2 ± 2.1 | 432.4 ± 34.9 | 23.4 ± 1.9 | −20.5 ± 1.3 | 56.6 ± 1.6 | 1.8 ± 0.1 | 29.4 ± 1.1 | 149.1 ± 13.0 |
| lwDR | −63.3 ± 1.9 | 592.0 ± 70.2 | 30.6 ± 2.4* | −25.1 ± 1.2* | 62.2 ± 1.7* | 2.4 ± 0.1** | 33.7 ± 1.6* | 224.9 ± 14.4*** |
Values are means ± SE; vmDR, n = 19; lwDR, n = 17.
P < 0.05;
P < 0.01;
P < 0.001. RMP, resting membrane potential; Res, resistance; AP Thr, action potential threshold; AP Amp, AP amplitude; AP Dur, AP duration; AHP Amp, afterhyperpolarization amplitude; AHP t1/2, AHP duration measured at half-height.
Fig. 2.
Intrinsic membrane properties differentiate lwDR 5-HT neurons from vmDR 5-HT neurons. lwDR, lateral wings of the DR; vmDR, ventromedial DR. A: representative data traces from vmDR and lwDR neurons demonstrate changes in membrane potential in response to various input current steps (i, iv, scale bar 50 mV, 50 ms), whereas enlarged traces demonstrate the afterhyperpolarization (AHP, ii, scale bar: 20 mV, 20 ms) and action potential (AP, iii, scale bar: 20 mV, 2 ms). Graphs in B–E depict average characteristics (means ± SE) for all recorded vmDR neurons (black, n = 19) and lwDR neurons (gray with diagonal lines, n = 17). B: there was a trend toward a larger membrane resistance in lwDR neurons. C: summary bar graph depicting resting membrane potential (RMP), AP threshold (AP Thr), and activation gap (RMP–AP Thr). AP threshold was lower in lwDR than that in vmDR, resulting in a smaller activation gap. D: frequency–intensity plots of vmDR and lwDR neurons demonstrate the increased excitability of lwDR neurons given 40, 60, or 80 pA of input current. *P < 0.05, **P < 0.01, 2-way ANOVA, Bonferroni posttest. Inset: the input current steps and current clamp traces used to generate frequency–intensity plots (scale bar: 50 mV, 50 ms). E: bar graph showing that the gain, i.e., slope of the frequency–intensity plot, was significantly greater in lwDR neurons. F: bar graph summarizing the effects of CdCl2 and apamin on AHP amplitude (Amp) in vmDR (n = 5) and lwDR (n = 5) neurons. Two-way ANOVA revealed that the effect of the Ca-channel blockers on AHP amplitude was significantly larger in lwDR neurons than that in vmDR neurons. Also, the effect of CdCl2 was significantly larger than the effect of apamin. *P < 0.05, Bonferroni posttest.
There were also differences in active properties of the neurons, as measured from APs generated by current injection through the recording electrode. 5-HT cells of the lwDR showed the broad AP and large amplitude AHP, characteristic of 5-HT neurons (Beck et al. 2004; Kirby et al. 2003) (Table 1). Notably, the APs of lwDR 5-HT neurons had a more hyperpolarized threshold of activation (P = 0.011), larger amplitude (P = 0.022), and longer duration (P = 0.002, Mann–Whitney test) (Table 1, Fig. 2A). Although the average RMP was comparable between the two subregions (P = 0.301), the lower AP threshold in lwDR neurons resulted in a significantly lower difference between the resting membrane potential and the AP threshold (RMP–AP Thr), a measure we term “activation gap” (P = 0.033) (Fig. 2C). The AHP of lwDR 5-HT cells had a larger amplitude (P = 0.034) and a longer duration, measured at half-amplitude (AHP t1/2, P = 0.001, Table 1).
In 5-HT neurons, influx of Ca2+ during the AP repolarizing phase results in a characteristic shoulder that increases AP duration (Aghajanian and Vandermaelen 1982; Vandermaelen and Aghajanian 1983). In addition, increased intracellular Ca2+ activates Ca-dependent K channels that shape the AHP of 5-HT neurons (Aghajanian 1985; Freedman and Aghajanian 1987); the amplitude and duration of the late component of the AHP are enhanced by calcium-induced calcium release (CICR) from intracellular stores (Pan et al. 1994). However, because electrophysiological characterization of 5-HT neurons has historically focused on midline raphe neurons in the rat, it is unknown whether Ca2+ contributes to the AP and AHP in lwDR 5-HT neurons and whether the differences in the shape of lwDR AP and AHP from the vmDR neurons could be explained by a difference in calcium influx.
To test this, we assessed active membrane properties in vmDR and lwDR 5-HT neurons before and after bath application of the Ca-channel blocker CdCl2 or apamin, a selective blocker of Ca-dependent small conductance K channels (SK channels). Occasional neurons demonstrated an obvious shoulder during the repolarization phase of the AP and did show a reduction in AP duration in the presence of 100 μM CdCl2 (data not shown). However, there was no significant effect of CdCl2 on average AP duration in either the vmDR (n = 5, P = 0.799) or the lwDR (n = 5, P = 0.184, Fig. 2F or Supplemental Fig. S2). Furthermore, the average AP duration of lwDR 5-HT neurons was still longer than that of vmDR neurons in the presence of CdCl2 (1.8 ± 0.1 ms, n = 5 vmDR vs. 2.5 ± 0.2 ms, n = 5, lwDR, P = 0.004). There was no effect of CdCl2 on AP threshold or AP amplitude (data not shown). Likewise, 100 nM apamin had no significant effect on AP duration, AP threshold, or AP amplitude (data not shown).
The shape of AHPs was indeed modulated by Ca2+ because the presence of either CdCl2 or apamin decreased the AHP amplitude in both subregions, with significantly larger effects in the lwDR (Fig. 2F and Supplemental Fig. S2, two-way ANOVA, P = 0.0082, main effect of subregion). Irrespective of the subregion, the effect of apamin on AHP amplitude was significantly smaller than the effect of CdCl2 (Fig. 2F, two-way ANOVA, P = 0.0013, main effect of drug type). The effect of CdCl2 was significantly greater in lwDR neurons than that in vmDR 5-HT cells (Fig. 2F, Bonferroni posttest, P < 0.05) such that the average AHP amplitudes of the two subregions were nearly equalized in the presence of CdCl2 (18.3 ± 1.6 mV in vmDR vs. 15.1 ± 1.0 mV in lwDR, t-test, P = 0.120). More specifically, the late component of the AHP mediated by CICR (Pan et al. 1994) was absent in both vmDR and lwDR neurons in the presence of the Ca-channel blockers (Supplemental Fig. S2). In the vmDR a small amplitude early component of the AHP persisted, which was shorter in duration with a faster rise toward RMP than that observed in the control condition (Supplemental Fig. S2). This early component of the AHP was completely attenuated by CdCl2 in the lwDR, but not by apamin (Supplemental Fig. S2). Even a supramaximal concentration of apamin (300 nM) failed to recapitulate the effect of CdCl2 on AHP amplitude (data not shown). Collectively, these data suggest that there are apamin-insensitive Ca channels that contribute to the AHP of 5-HT neurons and these channels may be more abundant in the lwDR.
These data confirm that, in the mouse, Ca2+ influx contributes to the shape of the AHP in lwDR 5-HT neurons as it does in vmDR 5-HT neurons. The larger reduction seen in lwDR AHP amplitude produced by Ca-channel blockers parallels the larger AHP amplitude seen under baseline conditions and suggests an enhanced contribution of Ca2+ to the shape of AHPs of lwDR neurons compared with vmDR neurons.
Frequency–intensity plots, constructed from the mean firing frequency in response to square current pulses of increasing amplitude, showed that lwDR 5-HT cells had steeper slopes (i.e., increased gain) than those of vmDR 5-HT cells (P = 0.042, Mann–Whitney test, Fig. 2, D and E). In addition, lwDR neurons demonstrated increased excitability compared with that of vmDR neurons, in that they reached higher firing rates given the same magnitude of input (Fig. 2D). This difference was significant at current steps ranging from 40 to 80 pA (Fig. 2D).
Pharmacologic blockade of synaptic input supports the intrinsic nature of distinctive membrane characteristics
The increase in excitability of lwDR 5-HT neurons is likely due to an array of active and passive membrane properties that are presumed to be intrinsic to the recorded neurons. However, in the slice preparation, 5-HT neurons receive both glutamatergic and GABAergic input (Lemos et al. 2006; Liu et al. 2000; Pan and Williams 1989). It is unknown whether spontaneous synaptic input to 5-HT neurons may affect membrane characteristics. Thus we conducted an additional experiment to verify that differences between vmDR and lwDR membrane properties were indeed independent of synaptic input. We measured passive and active membrane properties after bath application of 10 μM d-AP-5, 20 μM bicuculline, 10 μM CGP 55845, and 10 μM DNQX to block synaptic input mediated by N-methyl-d-aspartate (NMDA), γ-aminobutyric acid types A and B (GABAA and GABAB, respectively), and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, respectively. After blockade of synaptic input, lwDR 5-HT cells maintained their differences in active and passive cell characteristics compared with vmDR neurons (Table 2) as well as their higher excitability in F–I plots (20 pA: 0.0 ± 0.0 vs. 1.6 ± 0.5 Hz, P = 0.007; 40 pA: 0.0 ± 0.0 vs. 3.5 ± 1.1 Hz, P = 0.005; 60 pA: 0.8 ± 0.5 vs. 5.4 ± 1.2 Hz, P = 0.007; 80 pA: 2.12 ± 0.8 vs. 6.7 ± 1.7 Hz, P = 0.007, vmDR vs. lwDR, respectively). Furthermore, there were no significant differences in passive membrane properties between synaptic blockade and the control condition in vmDR neurons (n = 4) or lwDR neurons (n = 5; data not shown). This suggests that the synaptic input that is present in the in vitro slice preparation cannot account for the increased excitability of lwDR 5-HT neurons and that differences in excitability are due primarily to the differences in their intrinsic membrane properties.
Table 2.
Differences in lwDR membrane characteristics remain despite blockade of major synaptic input
| RMP, mV | Res, MOhm | Tau, ms | AP Thr, mV | AP Amp, mV | AP Dur, ms | AHP Amp, mV | AHP t1/2, ms | Firing Rate @80 pA, Hz | Gain, Hz/pA | |
|---|---|---|---|---|---|---|---|---|---|---|
| vmDR | −66.1 ± 4.8 | 441.5 ± 49.2 | 22.8 ± 4.5 | −21.4 ± 1.3 | 59.5 ± 2.2 | 1.9 ± 0.1 | 27.2 ± 1.8 | 134.1 ± 41.7 | 2.1 ± 0.8 | 0.03 ± 0.01 |
| lwDR | −60.6 ± 4.6 | 667.2 ± 34.2** | 37.2 ± 3.5* | −24.8 ± 1.3 | 67.4 ± 2.7* | 3.0 ± 0.2*** | 29.8 ± 1.3 | 259.4 ± 15.5* | 6.7 ± 1.7* | 0.09 ± 0.02* |
Values are means ± SE; vmDR, n = 5; lwDR, n = 6.
P < 0.05;
P < 0.01;
P < 0.001. Student's t-test was used to compare attributes of vmDR neurons versus lwDR neurons following pharmacological blockade of major synaptic input to 5-HT neurons. See Table 1 for abbreviations.
5-HT neurons of the lwDR demonstrate faster in vitro firing rates
The increased excitability of lwDR 5-HT neurons suggests that lwDR cells would have faster baseline firing rates in the intact brain. Although the noradrenergic input that drives DR 5-HT firing activity in vivo is severed in the slice preparation, we can examine 5-HT neuron firing rates in vitro using α1-adrenergic agonists (Vandermaelen and Aghajanian 1983). Extracellular recordings of YFP-labeled 5-HT neuron AP firing rates were obtained in raphe slices following bath application of 1.0 μM phenylephrine HCl (Fig. 3). In the vmDR, 5-HT neurons fired at a rate of 1.1 ± 0.3 Hz (n = 7, Fig. 3), which is consistent with several reports of in vivo firing rates of vmDR 5-HT neurons (Aghajanian and Haigler 1974; Allers and Sharp 2003). However, lwDR 5-HT neurons demonstrated significantly faster firing rates (2.2 ± 0.4 Hz, n = 6, P = 0.042, Fig. 3). This suggests that the differences in intrinsic excitability of DR 5-HT neurons directly affect the in vitro firing rate, also likely contributing to differences in 5-HT cell firing rate and 5-HT output in vivo.
Fig. 3.
The firing rate of lwDR 5-HT neurons in vitro is faster than that of vmDR 5-HT neurons. A: extracellular single unit recordings after bath application of 1.0 μM phenylephrine HCl show the firing rate of a vmDR 5-HT neuron (scale bar: 0.2 mV, 2.56 s; inset scale bar: 0.2 mV, 2.6 ms) and lwDR neuron (scale bar: 0.4 mV, 2.56 s; inset scale bar: 0.4 mV, 2.6 ms). Insets show the characteristic features of the AP waveform. Differences in amplitude were attributed to electrode position. B: summary graph depicting the average firing rate (means ± SE) of recorded vmDR and lwDR neurons. The firing rate of lwDR neurons (n = 6) was significantly higher than that of vmDR neurons (n = 7).
Autoreceptor-mediated responses of vmDR and lwDR 5-HT neurons
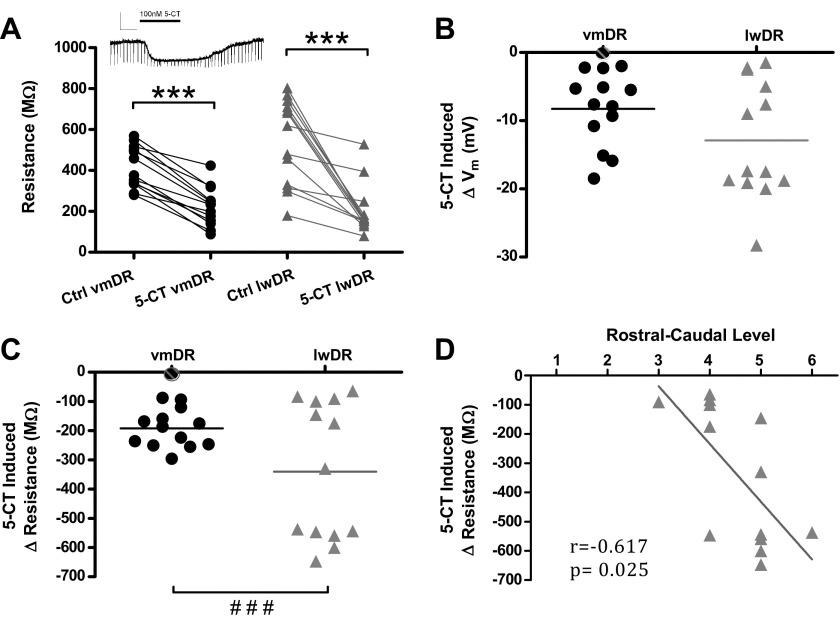
The 5-HT1A autoreceptor-mediated response is a defining characteristic of vmDR 5-HT neurons, but has yet to be characterized in lwDR 5-HT neurons. It is unknown whether there are differences in autoreceptor responses that may contribute to the increased excitability of lwDR 5-HT neurons. During current clamp recordings at the neuron's RMP, bath application of the 5-HT1,7R agonist 5-CT (100 nM) was applied following assessment of membrane characteristics. 5-CT elicited a measurable membrane hyperpolarization and decrease in membrane resistance in all recorded 5-HT neurons, except 1 vmDR 5-HT neuron (Fig. 4). Each hyperpolarization was within the linear range of the V–I plot generated for each neuron (data not shown). The magnitude of the hyperpolarization in vmDR and lwDR neurons is shown in Fig. 4B. Because the lwDR responses were not normally distributed, the nonparametric Mann–Whitney U test was used to compare the two groups, revealing no significant difference in 5-HT1AR-mediated hyperpolarization (P = 0.182). Membrane resistance, measured by the change in membrane potential elicited by a periodic 30 pA current pulse, was used to quantify change in the resistance imparted by the G protein coupled K channels that mediate the 5-HT1AR response (Fig. 4, A and C). The average change in resistance in vmDR 5-HT neurons was −192.1 ± 18.2 MΩ (baseline resistance 415.2 ± 28.4 MΩ, n = 13), whereas the average change in lwDR 5-HT cells was −340.3 ± 65.1 MΩ (baseline resistance, 544.1 ± 58.5 MΩ, n = 12); there was no significant difference (Mann–Whitney test, P = 0.383). However, the variance of lwDR 5-HT1AR-mediated responses was significantly larger than that of vmDR responses (F-test, P < 0.0001, Fig. 4C). Responses within the lwDR were distributed into two major groups according to the magnitude of the 5-CT-induced change in resistance, where large responses were above the mean and small responses below the mean. The response of a single cell that was near the mean was not included in either group in further analysis. Within the lwDR, small 5-HT1AR-mediated responses (109.8 ± 17.0 MΩ, n = 6) were significantly smaller than those measured in the vmDR (P = 0.013), whereas large responses were significantly larger (572.6 ± 17.6 MΩ, n = 6, P < 0.0001). Although those cells with large and small responses did not differ according to any other parameters, there was a significant correlation between larger 5-HT1AR-mediated responses and more caudal localization within the lwDR (Fig. 4D, Spearman's r = −0.617, P = 0.025). In addition, larger 5-HT1A autoreceptor responses were also correlated with higher initial membrane resistance (Spearman's r = 0.571, P = 0.041) and larger AHP amplitude (Spearman's r = 0.610, P = 0.027). Other than the 5-CT-induced change in resistance, no parameter was correlated to rostrocaudal position in the lwDR. No correlation between the magnitude of the 5-HT1AR-mediated response and rostrocaudal position or initial membrane resistance was seen in the vmDR (rostral position: r = 0.09, P = 0.762; initial membrane resistance: r = 0.196, P = 0.563).
Fig. 4.
5-HT1A receptor-mediated responses in 5-HT neurons. A: graph depicting the membrane resistance before (Ctrl) and after 5-CT bath administration of 100 nM 5-CT in vmDR and lwDR recordings. 5-CT decreased membrane resistance in both vmDR and lwDR 5-HT neurons. Inset: raw data trace of membrane hyperpolarization and decreased resistance generated by administration of 5-CT (scale bar: 20 mV, 50 ms). B: graph depicting the distribution of the change in membrane potential, i.e., membrane hyperpolarization, for all recorded neurons following administration of 5-CT. C: graph depicting the distribution of the change in membrane resistance induced by 5-CT in the vmDR and the lwDR. In the lwDR the change in resistance was distributed into 2 major groups. ###P < 0.001, F-test for differences in variance. D: correlation between the change in resistance and position along the rostrocaudal axis for lwDR neurons. The magnitude of the 5-CT response in lwDR cells was correlated to the rostrocaudal position, where cells with the largest 5-CT responses were located in the caudal regions of the DR. Rostrocaudal levels indicated in Fig. 1. Correlation determined by Spearman's test.
To ensure that 5-HT1AR-mediated responses in the lwDR were mediated by the same underlying inward rectifying potassium current seen in previous studies (Innis et al. 1988; Okuhara and Beck 1994), voltage ramps were conducted in several cells to measure current elicited before and after 5-CT administration. The 5-CT activated current was determined by subtracting the 5-CT response from the baseline current and the reversal potential for the 5-CT-induced current was determined by the x-intercept of the plot of activated current versus input voltage (data not shown). The reversal potential was comparable in the vmDR (−78.9 ± 1.3 mV, n = 4) and the lwDR (−82.8 ± 5.0 mV, n = 4 P = 0.481). Given the junction potential of approximately +15 mV (see methods), the reversal potentials lie close to the theoretical equilibrium potential for K+ (−99.1 mV, determined by the Nernst equation). This suggests that the G protein coupled inward rectifying K channels responsible for 5-HT1AR responses in other brain regions (Innis et al. 1988; Okuhara and Beck 1994) also underlie the responses measured in both vmDR and lwDR neurons.
Because 100 nM 5-CT elicited changes in resistance that ranged from −64.6 to −647.5 MΩ, we wanted to know whether this broad range of responses represented differences in the concentration–response curve of individual neurons. First, to determine whether 5-HT1AR-mediated responses desensitize in the mouse DR, repeated doses of 5-CT were administered and responses measured by both current clamp and voltage clamp recordings in 5-HT neurons. Although the 5-HT1AR-mediated response did not desensitize in some cells, the responses of several 5-HT neurons in both the vmDR and lwDR did desensitize. In the vmDR, two of three 5-HT neurons demonstrated a degree of desensitization, in which the second response was on average 81.4 ± 4.8% of the first response. In the lwDR, two of three 5-HT neurons demonstrated a degree of desensitization, in which the second response was on average 82.3 ± 8.3% of the first response (data not shown). These results precluded repeated application of 5-CT to obtain data for the construction of concentration–response curves in individual cells.
Distinguishing characteristics are found among another stress-activated subpopulation of DR neurons
We hypothesize that the distinctive intrinsic membrane properties described earlier are a crucial part of the mechanism that makes lwDR neurons more excitable in slice preparations and preferentially activated by stressors in the intact brain. We therefore expect that other subpopulations of 5-HT neurons activated by stressors would have intrinsic membrane properties that are similar to those of lwDR 5-HT cells. Rostral vmDR 5-HT neurons are activated by several stressors, albeit to a lesser extent than lwDR 5-HT neurons (Bouwknecht et al. 2007; Commons 2008; Hale et al. 2008). To understand whether intrinsic membrane properties may also contribute to rostral vmDR activation, we analyzed the correlation between vmDR cellular characteristics and the position of the recorded neuron along the rostrocaudal axis. Indeed, a more rostral position within the vmDR was positively correlated with a smaller activation gap, larger tau, longer AHP duration, and, most notably, increased firing rate elicited by 80 pA of current (Fig. 5) and larger gain (Spearman's r = −0.562, P = 0.012, data not shown). There was also a trend toward a correlation between rostral position and larger resistance (Pearson's r = −0.373, P = 0.115) as well as lower AP threshold (r = 0.396, P = 0.116).
Fig. 5.
Rostral position was correlated to several intrinsic membrane properties of vmDR 5-HT neurons. Graphs demonstrate the correlation between vmDR membrane properties and rostrocaudal position as defined in Fig. 1. Rostral position was correlated to (A) faster firing rates in response to an 80 pA input current step, (B) smaller magnitude activation gap, (C) larger tau, and (D) longer AHP t1/2. See results for abbreviations. Correlation determined by Pearson's test in A, B, and D and by Spearman's test in C due to the non-Gaussian distribution of tau values.
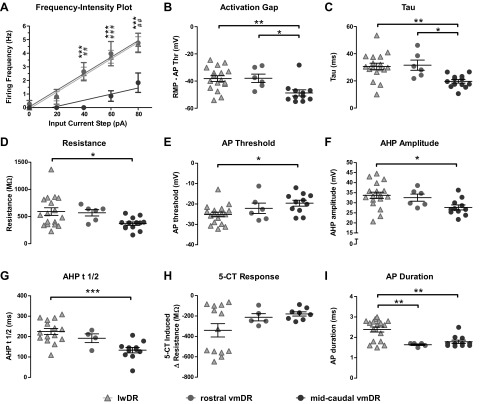
We then conducted a direct comparison between lwDR cells, rostral vmDR neurons (levels 1 and 2 in Fig. 1), and the remaining midcaudal vmDR neurons (levels 3–6 in Fig. 1). Two-way ANOVA with Bonferroni posttests were used to evaluate the frequency intensity plots, whereas one-way ANOVA analyses with Tukey–Kramer posttests were used for all other comparisons. The frequency–intensity plot of rostral vmDR cells closely resembled lwDR cells, indicating both an increased excitability and increased gain when compared with those of midcaudal vmDR neurons (Fig. 6A). In both rostral vmDR and lwDR neurons, the firing rate during an 80 pA input current step was larger than that of midcaudal vmDR cells (P = 0.005, rostral vs. midcaudal, P < 0.05; lwDR vs. midcaudal, P < 0.01, Bonferroni posttests), as was the gain (P = 0.001, rostral vs. midcaudal, P < 0.05, lwDR vs. midcaudal, P < 0.01, Tukey–Kramer posttests). Both lwDR and rostral vmDR neurons demonstrated a smaller activation gap and larger tau than those of midcaudal vmDR cells (Fig. 6, B and C). For several parameters, rostral vmDR cells did not differ from either group, whereas lwDR cells remained significantly different from midcaudal vmDR cells; these parameters included resistance, AP threshold, AHP amplitude, and AHP t1/2 (Fig. 6, D–G). The AP duration and 5-HT1AR-mediated responses did not differ between rostral and midcaudal vmDR cells and remained distinguishing factors for lwDR 5-HT neurons (Fig. 6, H and I). Thus the rostral vmDR cells share many of the same membrane properties that typified lwDR 5-HT neurons, including increased excitability, larger gain, smaller activation gap, and larger tau than those of midcaudal vmDR neurons. The common parameters of lwDR and rostral vmDR neurons suggest a common physiological mechanism for the stress-induced neuronal activation that has been observed in both subpopulations.
Fig. 6.
Unlike midcaudal vmDR 5-HT neurons, rostral vmDR 5-HT neurons were comparable to lwDR 5-HT neurons according to several parameters. Graphs demonstrate the comparison between all lwDR neurons, rostral vmDR neurons (levels 1 and 2), and midcaudal vmDR neurons (levels 3–6; see Fig. 1). A: the frequency–intensity plot demonstrates that rostral vmDR cells had increased excitability and gain that were comparable to those of lwDR cells and were distinct from midcaudal vmDR cells. ***P < 0.001, lwDR vs. midcaudal vmDR, 2-way ANOVA Bonferroni posttest; ##P < 0.01, ###P < 0.001, rostral vmDR vs. midcaudal vmDR, 2-way ANOVA, Bonferroni posttest. B: the activation gap and (C) tau values of lwDR and rostral vmDR cells differed significantly from those of midcaudal vmDR cells. D–G: for several parameters, lwDR cells differed significantly from midcaudal vmDR cells but rostral vmDR cells did not differ from either group. These included (D) resistance, (E) AP threshold, (F) AHP amplitude, and (G) AHP t1/2 (time it takes for the AHP to depolarize to one-half its peak amplitude). H: the bimodal distribution of 5-CT responses was unique to the lwDR, whereas rostral vmDR neurons remained comparable to midcaudal vmDR neurons. I: both rostral and midcaudal vmDR neurons remained distinct from lwDR neurons in AP duration. One-way ANOVA and Tukey–Kramer posttest used for B–I. *P < 0.05, **P < 0.01, ***P < 0.001 obtained from posttests.
DISCUSSION
Behavioral studies have previously demonstrated that stressors activate lwDR 5-HT cells more than vmDR 5-HT cells (Commons 2008; Johnson et al. 2008); however, a mechanism underlying this difference has not been identified. In this study, whole cell patch clamp electrophysiology experiments demonstrated that lwDR 5-HT neurons possess distinctive membrane properties that make lwDR neurons more excitable than vmDR neurons. The increased excitability is intrinsic to the lwDR 5-HT neuron (i.e., independent of synaptic input) and is accompanied by faster firing rates demonstrated in vitro.
Active and passive membrane properties likely contribute to excitability
Among passive membrane properties, the resistance was notably larger in lwDR neurons and likely contributed to the increased excitability seen in frequency–intensity plots. In addition, the larger tau seen in lwDR cells would result in greater summation of postsynaptic potentials, such as would occur with the full complement of synaptic connections of the intact brain.
Analysis of active membrane properties revealed that the AP threshold was more hyperpolarized in lwDR neurons; this could also contribute to increased excitability. The lower AP threshold in lwDR 5-HT neurons may be due to differences in the kinetics of the voltage-gated Na channels or in the subtypes of Na channels expressed in the lwDR. Because AP amplitude was measured relative to threshold, the lower AP threshold of lwDR neurons contributed to the larger AP amplitude; however, it would not account for the larger AHP amplitude. In the presence of Ca-channel blockers, lwDR 5-HT neurons demonstrated a larger decrease in AHP amplitude than that in vmDR neurons. In addition, the effect of apamin was only a fraction (∼53%) of that of CdCl2, which may indicate that apamin-insensitive Ca channels also contribute to the AHP in 5-HT neurons. Based on these data we propose that the influence of Ca2+ influx is greater in the lwDR than that in the vmDR, possibly due to an increased Ca2+ conductance, increased membrane expression of Ca channels, or increased release of calcium-induced calcium release. The AHP of lwDR neurons may therefore be more sensitive to signaling that alters intracellular Ca2+ levels, including but not limited to neurotransmitter receptor pathways (Pan et al. 1994).
Blockade of SK channels in midline DR 5-HT neurons results in burst firing patterns in vivo and irregular firing patterns in vitro (Crespi 2009; Rouchet et al. 2008). The data presented earlier constitute the first evidence that apamin-sensitive SK channels are present in lwDR neurons in the mouse, where they likely contribute to regular firing patterns as they do in midline 5-HT neurons of the rat. In the presence of apamin a small early component of the AHP persisted in all recorded neurons. The early component also persisted in vmDR recordings following CdCl2 application, but was attenuated by CdCl2 in the lwDR. This finding is consistent with midline DR studies in which apamin blocks only the late component of the AHP (Pan et al. 1994) and blocks only 80–90% of the postactivation outward current that underlies the AHP (Freedman and Aghajanian 1987). One explanation for the persistent early AHP component is that some of the channels involved in the falling phase of the action potential 1) drive the early component of the AHP, 2) are not Ca dependent, and 3) are more abundant in vmDR neurons than in lwDR neurons. Another explanation is that an inward tail current, as has been demonstrated in midline DR neurons (Penington and Kelly 1993), is larger in lwDR neurons and attenuates the early AHP in this subpopulation. Further experiments will help distinguish between these possibilities.
The physiological significance of differences in membrane properties between the DR subregions was demonstrated by in vitro recordings of firing rate. Extracellular single unit recordings demonstrated that lwDR 5-HT neurons have a faster firing rate than that of vmDR 5-HT neurons. This suggests that the increased intrinsic excitability of lwDR 5-HT neurons has significant functional consequences, resulting in increased sensitivity to the noradrenergic signals required for the characteristic firing pattern of 5-HT neurons (Baraban and Aghajanian 1980, 1981; Vandermaelen and Aghajanian 1983). The increased excitability of lwDR neurons was independent of synaptic input, although additional neurotransmitters may modulate 5-HT activity in vivo. With the full complement of synaptic connectivity in the intact brain, the increased gain of lwDR 5-HT neurons would translate into increased 5-HT output in response to excitatory modulating input from stress-activated afferents.
Correlative analysis of vmDR 5-HT neurons throughout the rostrocaudal axis revealed that vmDR cells with more rostral position tended to be more excitable. In addition, the rostral subset of vmDR neurons was largely comparable to that of lwDR neurons and significantly more excitable than the midcaudal subset of vmDR 5-HT neurons. Rostral vmDR 5-HT neurons are a stressor-activated subpopulation (Bouwknecht et al. 2007; Commons 2008; Hale et al. 2008), thought to be part of the ascending mesostriatal serotonergic system related to behavioral arousal and motor function (Imai et al. 1986a,b; Loughlin and Fallon 1982; Lowry 2002; Steinbusch et al. 1981; Waselus et al. 2006). Interestingly, the distinctive membrane properties of rostral vmDR—i.e., firing rate, activation gap, AHP duration, and tau—were among the distinguishing properties of lwDR 5-HT neurons. This finding further supports the hypothesis that this constellation of membrane characteristics, which typifies lwDR 5-HT neurons, forms the physiological mechanism underlying increased responsiveness to stress. Furthermore, increased AP duration and large 5-CT responses were restricted to lwDR cells and absent in rostral vmDR cells, suggesting that prolonged AP duration and enlarged 5-HT1AR-mediated responses do not play a crucial role in the increased excitability of 5-HT neurons.
Heterogeneous subpopulations of DR 5-HT neurons are topographically organized
The heterogeneity of 5-HT neurons has become more evident over recent years. Extracellular in vivo recordings in the DR paired with juxtacellular labeling has revealed small subsets of 5-HT neurons with unique properties, including fast firing rates, bursting activity, and synchronization with theta rhythm (Allers and Sharp 2003; Hajos et al. 2007; Kocsis et al. 2006). Previously we found differences between median raphe and vmDR 5-HT membrane properties in the rat (Beck et al. 2004). The results reported here are the first to be obtained from immunohistochemically identified 5-HT cells in the mouse and provide additional evidence for the heterogeneity of 5-HT neurons. Subsets of neurons found mostly in the vmDR demonstrated rectification or Ih currents (Supplemental Fig. S1) that are typically seen in non-5-HT neurons (Beck et al. 2004).Thus there is likely a topographic distribution of the channels that mediate these currents or differential posttranslational modification of these channels within 5-HT cells.
Our experiments also highlighted a subset of lwDR neurons with larger 5-HT1AR responses than those of vmDR 5-HT neurons. Correlation analysis showed that lwDR neurons demonstrating large membrane resistance and more caudal position were most likely to have large 5-HT1AR-mediated responses. Neurons with large 5-HT1AR responses may have increased receptor expression, efficacy of coupling to Gi/o effector molecules, and/or affinity to the agonist 5-CT. We could not obtain reliable concentration–response data from individual cells because of desensitization of the 5-HT1AR-mediated response. The desensitization is consistent with a previous study that demonstrated that 5-HT1AR in the DR internalize when activated (Riad et al. 2001). Techniques other than those used in the preceding experiments may yield more useful insight with respect to the relative abundance of 5-HT1AR in the lwDR as well as the efficacy of other components of the signaling pathway.
Implications for the neural circuits that mediate stress
Although a wide range of stressors preferentially activate lwDR neurons (Berton et al. 2007; Chung et al. 2000; Commons 2008; Gardner et al. 2005; Johnson et al. 2004, 2008; Martinez et al. 1998, 2002; Roche et al. 2003), the data presented here fill the void in our understanding of why lwDR 5-HT neurons are more responsive. The intrinsic membrane properties of lwDR neurons contribute to a heightened gain, enabling lwDR 5-HT cells to convert converging input from a stress-activated circuit into greater output in projection regions that are responsible for physiological and behavioral responses to stressors.
A few tracer studies in the rat describe the afferent input to the DR with respect to the lwDR subregion or the rostrocaudal axis. The major limbic forebrain input to the DR extends from the lateral habenula and medial prefrontal cortex, regions that have a higher density of input to the lwDR than to the vmDR (Araki et al. 1988; Peyron et al. 1998; Varga et al. 2003). The locus coeruleus, long thought to be the primary driver of spontaneous firing in the raphe, exhibits input of equal density to the vmDR and lwDR (Kim et al. 2004). Inputs from the medial amygdala primarily innervate the vmDR, whereas the lateral portion of the medial amygdala and the central amygdala innervate the lwDR (Lee et al. 2007). In addition, fibers containing the stress hormone corticotropin releasing factor (CRF) course from the interfascicular region of the rostral vmDR, through the middle DR, toward the dorsolateral edge of the caudal lwDR (Kirby et al. 2000; Valentino et al. 2001; Waselus and Van Bockstaele 2007). Thus to complement their unique intrinsic physiology, there are also anatomic factors that make lwDR neurons well suited for integrating input known to be part of the neural circuitry that mediates stress—i.e., input from CRF fibers, the limbic forebrain, the central amygdala, and afferents with diffuse projections throughout the DR.
Although the DR is known for its forebrain serotonergic projections, only vmDR and dorsomedial neurons project to the cortex, whereas lwDR projections are primarily to subcortical targets (Lowry et al. 2008). The lwDR descending output to the dorsolateral periaqueductal gray (dlPAG) and the rostral ventrolateral medulla (RVLM) is a crucial part of the circuitry that mediates the response to stressors. Serotonergic projections from the lwDR inhibit the dlPAG and RVLM, thereby attenuating sympathoexcitatory behavioral responses and decreasing vasopressor responses to stress (Bago and Dean 2001; Bago et al. 2002; Beckett and Marsden 1997; Beckett et al. 1992; Johnson et al. 2004; Underwood et al. 1999). The emerging hypothesis is that the lwDR 5-HT neurons are activated and help inhibit sympathetic excitation as part of the normative response to innocuous stressors. This circuit is dysregulated in a rat model of panic disorder, in which decreased activation of lwDR neurons accompanies excessive panic-like responses to a mild stressor (Johnson et al. 2008).
In summary, the increased intrinsic responsiveness of lwDR neurons distinguishes them from vmDR neurons and likely underlies both their increased in vitro firing rate and increased propensity to be activated by an array of stressors. Their anatomic location near putative stress-related inputs in concert with increased intrinsic gain make lwDR 5-HT neurons particularly suited for generating output to sympathomotor targets and forebrain regions involved in adaptive responses to stress. Because the excitability of lwDR 5-HT neurons is an underpinning of the normative response to stressors, dysregulation of lwDR neurons likely contributes to the pathobiology of anxiety and other stress-related mood disorders.
GRANTS
This work was supported by National Institute of Mental Health Grants MH-075047 to S. G. Beck and MH-082611 to L. K. Crawford.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Tecott and E. Deneris for providing the founders for the colony of mice used in these experiments.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience 133: 983–997, 2005. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann NY Acad Sci 1018: 46–57, 2004. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK. Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature 315: 501–503, 1985. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Haigler HJ. l-Tryptophan as a selective histochemical marker for serotonergic neurons in single-cell recording studies. Brain Res 81: 364–372, 1974. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Vandermaelen CP. Intracellular identification of central noradrenergic and serotonergic neurons by a new double labeling procedure. J Neurosci 2: 1786–1792, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience 122: 193–204, 2003. [DOI] [PubMed] [Google Scholar]
- Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res 441: 319–330, 1988. [DOI] [PubMed] [Google Scholar]
- Bago M, Dean C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by 5-HT(1A) receptors in the RVLM. Am J Physiol Regul Integr Comp Physiol 280: R976–R984, 2001. [DOI] [PubMed] [Google Scholar]
- Bago M, Marson L, Dean C. Serotonergic projections to the rostroventrolateral medulla from midbrain and raphe nuclei. Brain Res 945: 249–258, 2002. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Suppression of serotonergic neuronal firing by alpha-adrenoceptor antagonists: evidence against GABA mediation. Eur J Pharmacol 66: 287–294, 1980. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Noradrenergic innervation of serotonergic neurons in the dorsal raphe: demonstration by electron microscopic autoradiography. Brain Res 204: 1–11, 1981. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. J Neurophysiol 91: 994–1005, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett S, Marsden CA. The effect of central and systemic injection of the 5-HT1A receptor agonist 8-OHDPAT and the 5-HT1A receptor antagonist WAY100635 on periaqueductal grey-induced defence behaviour. J Psychopharmacol 11: 35–40, 1997. [DOI] [PubMed] [Google Scholar]
- Beckett SR, Lawrence AJ, Marsden CA, Marshall PW. Attenuation of chemically induced defence response by 5-HT1 receptor agonists administered into the periaqueductal gray. Psychopharmacology (Berl) 108: 110–114, 1992. [DOI] [PubMed] [Google Scholar]
- Berton O, Covington HE, 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron 55: 289–300, 2007. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull 72: 32–43, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Martinez M, Herbert J. c-fos expression, behavioural, endocrine and autonomic responses to acute social stress in male rats after chronic restraint: modulation by serotonin. Neuroscience 95: 453–463, 2000. [DOI] [PubMed] [Google Scholar]
- Commons KG. Evidence for topographically organized endogenous 5-HT-1A receptor-dependent feedback inhibition of the ascending serotonin system. Eur J Neurosci 27: 2611–2618, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LK, Beck SG. Murine serotonin neurons have distinct membrane properties: implications for the neural circuitry mediating stress. Program No. 160.11. 2008 Abstract Viewer and Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2008. Online. [Google Scholar]
- Crespi F. Apamin increases 5-HT cell firing in raphe dorsalis and extracellular 5-HT levels in amygdala: a concomitant in vivo study in anesthetized rats. Brain Res 1281: 35–46, 2009. [DOI] [PubMed] [Google Scholar]
- Freedman JE, Aghajanian GK. Role of phosphoinositide metabolites in the prolongation of afterhyperpolarizations by alpha 1-adrenoceptors in rat dorsal raphe neurons. J Neurosci 7: 3897–3906, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience 136: 181–191, 2005. [DOI] [PubMed] [Google Scholar]
- Hajos M, Allers KA, Jennings K, Sharp T, Charette G, Sik A, Kocsis B. Neurochemical identification of stereotypic burst-firing neurons in the rat dorsal raphe nucleus using juxtacellular labelling methods. Eur J Neurosci 25: 119–126, 2007. [DOI] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA, Evans AK, Stamper CE, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience 157: 733–748, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Park MR, Steindler DA, Kitai ST. The morphology and divergent axonal organization of midbrain raphe projection neurons in the rat. Brain Dev 8: 343–354, 1986a. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol 243: 363–380, 1986b. [DOI] [PubMed] [Google Scholar]
- Innis RB, Nestler EJ, Aghajanian GK. Evidence for G protein mediation of serotonin- and GABAB-induced hyperpolarization of rat dorsal raphe neurons. Brain Res 459: 27–36, 1988. [DOI] [PubMed] [Google Scholar]
- Johnson P, Lowry C, Truitt W, Shekhar A. Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J Psychopharmacol 22: 642–652, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann NY Acad Sci 1018: 58–64, 2004. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Shekhar A. Panic-prone state induced in rats with GABA dysfunction in the dorsomedial hypothalamus is mediated by NMDA receptors. J Neurosci 26: 7093–7104, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res 1026: 56–67, 2004. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci 28: 12927–12937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience 116: 669–683, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology 22: 148–162, 2000. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Varga V, Dahan L, Sik A. Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci USA 103: 1059–1064, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Eum YJ, Jo SM, Waterhouse BD. Projection patterns from the amygdaloid nuclear complex to subdivisions of the dorsal raphe nucleus in the rat. Brain Res 1143: 116–125, 2007. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Pan YZ, Ma X, Lamy C, Akanwa AC, Beck SG. Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur J Neurosci 24: 3415–3430, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT(2) receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res 873: 34–45, 2000. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Fallon JH. Mesostriatal projections from ventral tegmentum and dorsal raphe: cells project ipsilaterally or contralaterally but not bilaterally. Neurosci Lett 32: 11–16, 1982. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol 14: 911–923, 2002. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A. Topographic organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Serotonin and Sleep: Molecular, Functional and Clinical Aspects, edited by Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt DJ. Basel, Switzerland: Birkhäuser-Verlag, 2008, p. 25–67. [Google Scholar]
- Marinelli S, Schnell SA, Hack SP, Christie MJ, Wessendorf MW, Vaughan CW. Serotonergic and nonserotonergic dorsal raphe neurons are pharmacologically and electrophysiologically heterogeneous. J Neurophysiol 92: 3532–3537, 2004. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress 5: 3–13, 2002. [DOI] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci 10: 20–33, 1998. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus–from silver stainings to a role in depression. Brain Res Rev 55: 329–342, 2007. [DOI] [PubMed] [Google Scholar]
- Okuhara DY, Beck SG. 5-HT1A receptor linked to inward-rectifying potassium current in hippocampal CA3 pyramidal cells. J Neurophysiol 71: 2161–2167, 1994. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Grudt TJ, Williams JT. Alpha 1-adrenoceptors in rat dorsal raphe neurons: regulation of two potassium conductances. J Physiol 478: 437–447, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT. GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J Neurophysiol 61: 719–726, 1989. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 2001. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 1997. [Google Scholar]
- Penington NJ, Kelly JS. Ionic dependence of a slow inward tail current in rat dorsal raphe neurones. J Physiol 464: 33–48, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82: 443–468, 1998. [DOI] [PubMed] [Google Scholar]
- Riad M, Watkins KC, Doucet E, Hamon M, Descarries L. Agonist-induced internalization of serotonin-1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors). J Neurosci 21: 8378–8386, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci 23: 970–977, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouchet N, Waroux O, Lamy C, Massotte L, Scuvee-Moreau J, Liegeois JF, Seutin V. SK channel blockade promotes burst firing in dorsal raphe serotonergic neurons. Eur J Neurosci 28: 1108–1115, 2008. [DOI] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA 102: 16472–16477, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, Gehlert DR, DiMicco JA. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci 26: 9205–9215, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch HW, Nieuwenhuys R, Verhofstad AA, Van der Kooy D. The nucleus raphe dorsalis of the rat and its projection upon the caudatoputamen. A combined cytoarchitectonic, immunohistochemical and retrograde transport study. J Physiol (Paris) 77: 157–174, 1981. [PubMed] [Google Scholar]
- Underwood MD, Arango V, Bakalian MJ, Ruggiero DA, Mann JJ. Dorsal raphe nucleus serotonergic neurons innervate the rostral ventrolateral medulla in rat. Brain Res 824: 45–55, 1999. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol 435: 450–463, 2001. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res 289: 109–119, 1983. [DOI] [PubMed] [Google Scholar]
- Varga V, Kocsis B, Sharp T. Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex and lateral habenula on single neurons in the dorsal raphe nucleus. Eur J Neurosci 17: 280–286, 2003. [DOI] [PubMed] [Google Scholar]
- Waselus M, Galvez JP, Valentino RJ, Van Bockstaele EJ. Differential projections of dorsal raphe nucleus neurons to the lateral septum and striatum. J Chem Neuroanat 31: 233–242, 2006. [DOI] [PubMed] [Google Scholar]
- Waselus M, Van Bockstaele EJ. Co-localization of corticotropin-releasing factor and vesicular glutamate transporters within axon terminals of the rat dorsal raphe nucleus. Brain Res 1174: 53–65, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.