Abstract
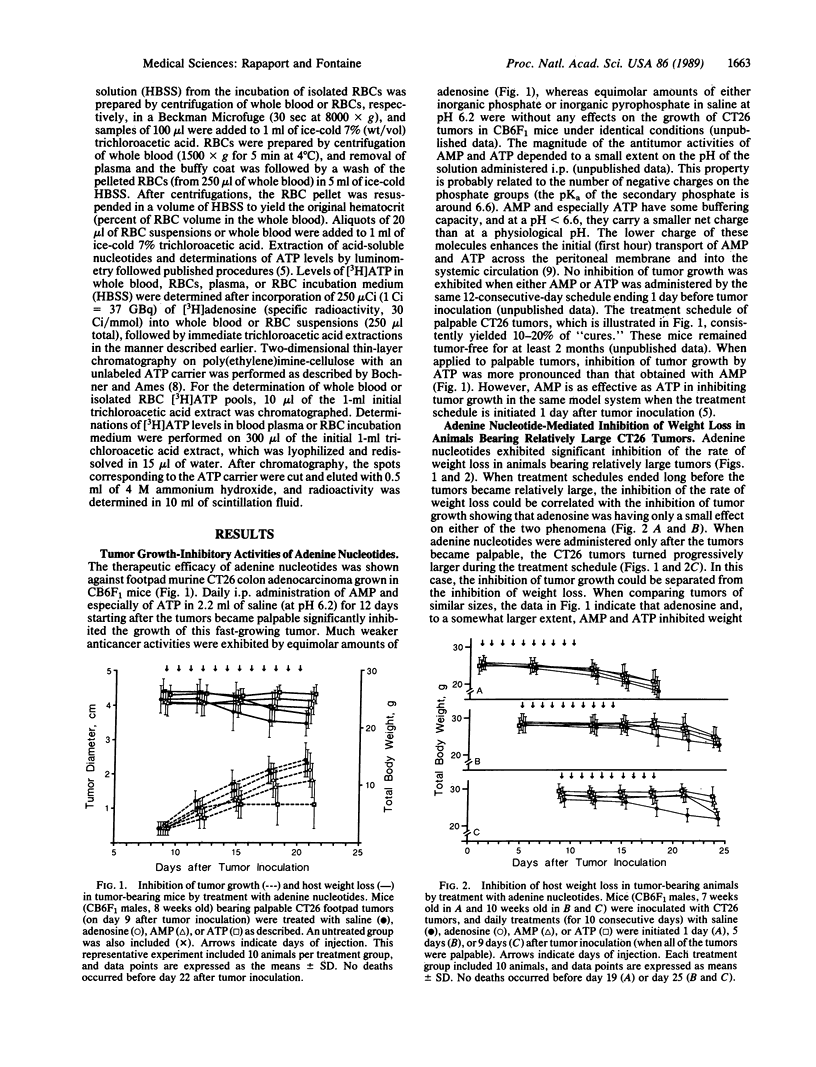
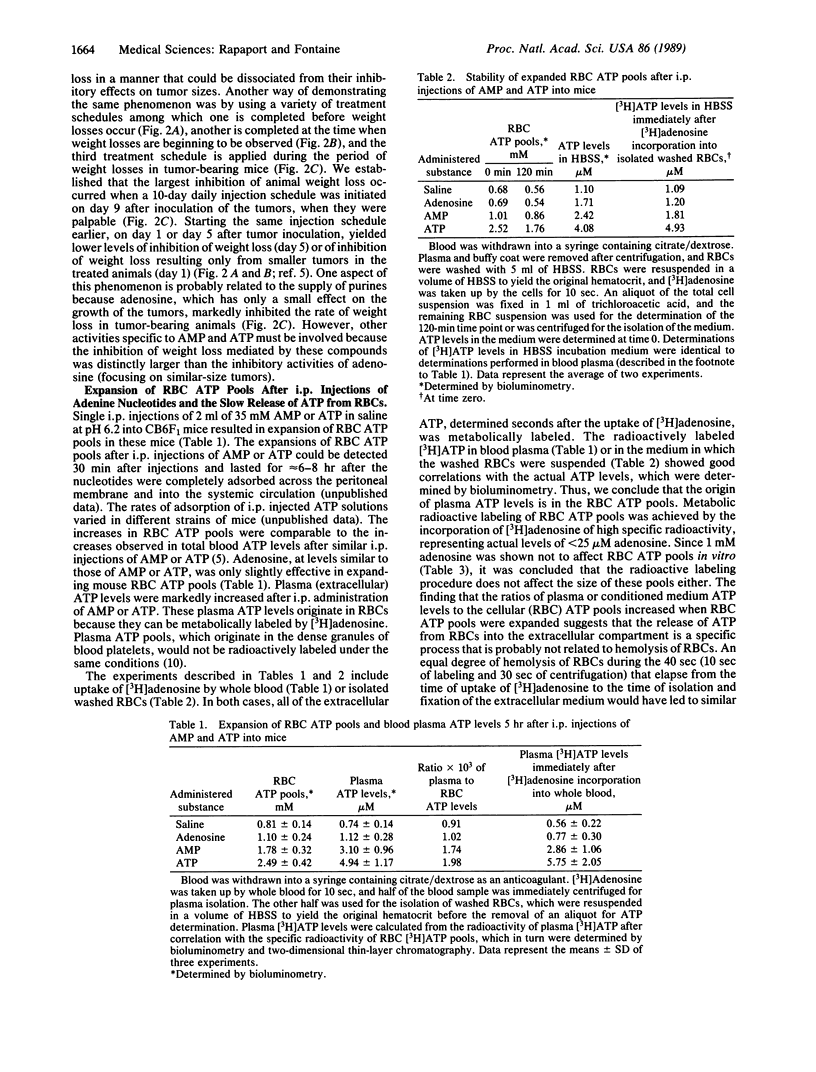
ATP and AMP exhibit significant anticancer activities against established footpad CT26 colon adenocarcinoma in CB6F1 mice. Adenosine, inorganic phosphate, and inorganic pyrophosphate were without such effects under identical conditions. Daily intraperitoneal injections of adenine nucleotides in large volumes of saline, starting after the tumors became palpable, resulted in inhibition of tumor growth and a few "cures." The treatment was not toxic to the host as determined by changes in body weights. Weight loss observed in animals upon progression of the fast-growing CT26 tumors was slowed markedly in adenine nucleotide-treated mice. The inhibition of weight loss in tumor-bearing mice was shown to be neither the cause nor the effect of the inhibition of tumor growth. Intraperitoneal injections of AMP or ATP but not of adenosine yielded expansions of erythrocyte ATP pools in host animals. The expanded erythrocyte ATP pools are stable over a period of hours, while slowly releasing micromolar amounts of ATP into the blood plasma compartment, leading to several-fold increases in plasma (extracellular) ATP levels. Based on previous studies in which 1-5 microM extracellular ATP effectively inhibited the growth of a variety of tumor cells in several in vitro systems, it is suggested that similar levels of ATP in blood plasma account for the anticancer activities observed in a murine host.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Born G. V., Bergquist D., Arfors K. E. Evidence for inhibition of platelet activation in blood by a drug effect on erythrocytes. Nature. 1976 Jan 22;259(5540):233–235. doi: 10.1038/259233a0. [DOI] [PubMed] [Google Scholar]
- Born G. V., Wehmeier A. Inhibition of platelet thrombus formation by chlorpromazine acting to diminish haemolysis. Nature. 1979 Nov 8;282(5735):212–213. doi: 10.1038/282212a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Costa G. Cachexia, the metabolic component of neoplastic diseases. Cancer Res. 1977 Jul;37(7 Pt 2):2327–2335. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Chan T. Pyrimidine starvation induced by adenosine in fibroblasts and lymphoid cells: role of adenosine deaminase. Science. 1973 Nov 23;182(4114):836–837. doi: 10.1126/science.182.4114.836. [DOI] [PubMed] [Google Scholar]
- Heppel L. A., Weisman G. A., Friedberg I. Permeabilization of transformed cells in culture by external ATP. J Membr Biol. 1985;86(3):189–196. doi: 10.1007/BF01870597. [DOI] [PubMed] [Google Scholar]
- Holmsen H. Platelet metabolism and activation. Semin Hematol. 1985 Jul;22(3):219–240. [PubMed] [Google Scholar]
- Lüthje J., Ogilvie A. Catabolism of Ap3A and Ap4A in human plasma. Purification and characterization of a glycoprotein complex with 5'-nucleotide phosphodiesterase activity. Eur J Biochem. 1985 May 15;149(1):119–127. doi: 10.1111/j.1432-1033.1985.tb08901.x. [DOI] [PubMed] [Google Scholar]
- Meyskens F. L., Williams H. E. Adenosine metabolism in human erythrocytes. Biochim Biophys Acta. 1971 Jun 30;240(2):170–179. doi: 10.1016/0005-2787(71)90654-x. [DOI] [PubMed] [Google Scholar]
- Mills G. C., Schmalstieg F. C., Trimmer K. B., Goldman A. S., Goldblum R. M. Purine metabolism in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2867–2871. doi: 10.1073/pnas.73.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett D., Dean B. The function of adenosine deaminase in the human erythrocyte. Biochem Biophys Res Commun. 1977 Jul 11;77(1):374–378. doi: 10.1016/s0006-291x(77)80207-6. [DOI] [PubMed] [Google Scholar]
- Rapaport E. Compartmentalized ATP pools produced from adenosine are nuclear pools. J Cell Physiol. 1980 Nov;105(2):267–274. doi: 10.1002/jcp.1041050210. [DOI] [PubMed] [Google Scholar]
- Rapaport E. Experimental cancer therapy in mice by adenine nucleotides. Eur J Cancer Clin Oncol. 1988 Sep;24(9):1491–1497. doi: 10.1016/0277-5379(88)90340-9. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Fishman R. F., Gercel C. Growth inhibition of human tumor cells in soft-agar cultures by treatment with low levels of adenosine 5'-triphosphate. Cancer Res. 1983 Sep;43(9):4402–4406. [PubMed] [Google Scholar]
- Rapaport E., Garcia-Blanco M. A., Zamecnik P. C. Regulation of DNA replication in S phase nuclei by ATP and ADP pools. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1643–1647. doi: 10.1073/pnas.76.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport E. Treatment of human tumor cells with ADP or ATP yields arrest of growth in the S phase of the cell cycle. J Cell Physiol. 1983 Mar;114(3):279–283. doi: 10.1002/jcp.1041140305. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C. Increased incorporation of adenosine into adenine nucleotide pools in serum-deprived mammalian cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1145–1147. doi: 10.1073/pnas.75.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres I. J., Litterst C. L., Guarino A. M. Transport of model compounds across the peritoneal membrane in the rat. Pharmacology. 1978;17(6):330–340. doi: 10.1159/000136874. [DOI] [PubMed] [Google Scholar]
- Weisman G. A., De B. K., Friedberg I., Pritchard R. S., Heppel L. A. Cellular responses to external ATP which precede an increase in nucleotide permeability in transformed cells. J Cell Physiol. 1984 May;119(2):211–219. doi: 10.1002/jcp.1041190211. [DOI] [PubMed] [Google Scholar]


