Abstract
Several groups have recently shown that 17β-estradiol is protective in spinal cord injury (SCI). Testosterone can be aromatized to 17β-estradiol and may increase estrogen-mediated protection. Alternatively, testosterone has been shown to increase excitotoxicity in models of central nervous system (CNS) injury. These experiments test the hypothesis that endogenous testosterone in male rats alters 17β-estradiol-mediated protection by evaluating a delayed administration over a clinically relevant dose range and manipulating testicular-derived testosterone. Adult male Sprague Dawley rats were either gonadectomized or left gonad-intact prior to SCI. SCI was produced by a midthoracic crush injury. At 30 min post SCI, animals received a subcutaneous pellet of 0.0, 0.05, 0.5, or 5.0 mg of 17β-estradiol, released over 21 days. Hindlimb locomotion was analyzed weekly in the open field. Spinal cords were collected and analyzed for cell death, expression of Bcl-family proteins, and white-matter sparing. Post-SCI administration of the 0.5- or 5.0-mg pellet improved hindlimb locomotion, reduced urinary bladder size, increased neuronal survival, reduced apoptosis, improved the Bax/Bcl-xL protein ratio, and increased white-matter sparing. In the absence of endogenous testicular-derived androgens, SCI induced greater apoptosis, yet 17β-estradiol administration reduced apoptosis to the same extent in gonadectomized and gonad-intact male rats. These data suggest that delayed post-SCI administration of a clinically relevant dose of 17β-estradiol is protective in male rats, and endogenous androgens do not alter estrogen-mediated protection. These data suggest that 17β-estradiol is an effective therapeutic intervention for reducing secondary damage after SCI in males, which could be readily translated to clinical trials.
Key words: apoptosis, Bcl-xL, Bax, estrogen, serum levels
Introduction
Spinal cord injury (SCI) is a devastating and debilitating condition that affects an estimated 12,000 new patients each year in the United States (National Spinal Cord Injury Statistical Center, 2008). Recently, the effectiveness of high-dose methylprednisolone therapy, the standard of care for reducing secondary damage after SCI, has come under reassessment (George et al., 1995; Hall and Springer, 2004; Hugenholtz, 2003; Lee et al., 2007; Suberviola et al., 2008), which has rekindled interest in developing novel therapeutic interventions. There is an emerging and somewhat controversial literature suggesting that female hormones, in particular 17β-estradiol (E2), could be protective agents in SCI. In support of this hypothesis, a comparison of male and female rodents with SCI indicated that female rats and mice showed less tissue damage and improved hindlimb locomotor function compared to male cohorts (Farooque et al., 2006; Hauben et al., 2002). Similarly, in a model of cervical SCI, female rats showed improved respiratory recovery compared to male or ovariectomized counterparts (Doperalski et al., 2008). Several studies have reported that 17β-estradiol administration reduces secondary injury and promotes functional recovery after SCI, but the dosing and administration timing vary substantially between studies. For example, we have previously shown that pretreatment with a sustained physiological dose of 17β-estradiol to adult or aged female rats decreased apoptosis in the gray matter, increased white-matter sparing, and increased recovery of hindlimb locomotion (Chaovipoch et al., 2006). Similarly, Webb and colleagues (2006) found that pre-injury administration with a sustained physiological dose of 17β-estradiol reduced the severity of autonomic dysreflexia following SCI in male mice. Yune and colleagues (2004) demonstrated that pre-SCI treatment with a single intravenous low dose (100 μg/kg) of 17β-estradiol in male rats reduced lesion size, reduced apoptotic cell death, increased expression of the anti-apoptotic gene Bcl-2, and improved hindlimb locomotion. In a subsequent study using the same dosing paradigm, this group reported that up-regulation of Bcl-2 expression induced by 17β-estradiol is mediated through PIcK/AKt-dependent CREB activation (Yune et al., 2008). Other work has shown that a single injection of a high dose (4 mg/kg) of 17β-estradiol given 15 min and 24 h after SCI to male rats decreased infiltration of macrophages and microglia, decreased myelin loss, and reduced apoptosis by inhibition of calpain activation (Sribnick et al., 2006). Similarly, a single injection of 4 mg/kg of 17β-estradiol immediately after SCI reduced lesion sized and promoted functional recovery (Ritz and Hausmann, 2008). Administration of 300 μg/kg 17β-estradiol to male mice 1 h before and at 3 and 6 h after SCI was shown to reduce apoptosis in the gray and white matter, reduce expression of pro-apoptotic Bax, increase expression of anti-apoptotic Bcl-2, and reduce inflammation. Co-administration of the estrogen receptor antagonist ICI 182,780 eliminated these effects (Cuzzocrea et al., 2008). In contrast to all these studies, Swartz and colleagues (2007) reported that pretreatment with a sustained administration of either a physiological or superphysiological 17β-estradiol did not confer tissue sparing or functional recovery in male or female rats. Thus most previous work has demonstrated that pre- and/or immediate post-SCI administration of 17β-estradiol at either physiological or superphysiological doses reduces secondary injury after SCI.
A primary goal of this study was to evaluate the effectiveness of post-injury administration of 17β-estradiol at both a time and dose range that are more clinical relevant than previous work. In selecting the earliest, clinically feasible, post-SCI administration time, a recent meta-analysis of the average pre-hospital time of emergency medical services (EMS) was considered. These data show that the average EMS response time is 5 min in urban areas and 8 min in rural settings, and that the average on-scene time before transport is 13–15 min (Carr et al., 2006). This suggests that EMS could administer a non-invasive treatment within 30 min after SCI; therefore the 17β-estradiol administration time used in this study was 30 min post SCI. The second consideration was to conduct a dose–effect analysis using doses that are similar to the range in clinically approved 17β-estradiol transdermal compounds typically used for estrogen replacement therapy such as Vivelle-Dot® (Novogyne Pharmaceuticals, East Hanover, NJ), EstroGel® (Ascend Therapeutics Pharmaceuticals, Herndon, VA), or Elestrin® (Nycomed, Zurich, Switzerland), as indicated in the publically available, full prescribing information prescription insert. Assuming a 65-kg patient, this range is 0.002–0.16 mg/kg per day. In this study, we mimicked this clinical dose range and evaluated the efficacy of three doses of 17β-estradiol ranging from 0.008–0.8 mg/kg per day, based on an average body weight of 300 g in male rats. Thus these experiments were designed to evaluate the effect of post-SCI administration of 17β-estradiol in a rodent model using a clinically relevant time of administration and dose range in male rats, controlling for testicular-derived endogenous androgens.
Epidemiological data from the National Spinal Cord Statistics Data Center indicate that over 80% of SCI patients are male (National Spinal Cord Injury Statistical Center, 2008). Notably, the male hormone testosterone is readily converted to 17β-estradiol by aromatase in neurons and astrocytes, which suggests that endogenous androgen levels in males may affect 17β-estradiol-mediated protection after SCI (Roselli, 2007). Testosterone in males is physiologically secreted by the testes and adrenal glands, and acts primarily via androgen receptors. Data suggests that testosterone levels are reduced in male patients and animals after SCI, especially in the acute period (Clark et al., 2008; Rouleau et al., 2007; Schopp et al., 2006). There is some evidence that testosterone is protective against central nervous system (CNS) damage such as neurodegenerative diseases (Bialek et al., 2004; Gold and Voskuhl, 2006; Hammond et al., 2001) and oxidative stress (Chisu et al., 2006). With regard to acute CNS injury, Ogata and colleagues (1993) found that testosterone protected spinal cord motor neurons from glutamate toxicity in vitro. Kujawa and colleagues (1989, 1991, 1995) demonstrated that superphysiological doses of exogenous testosterone propionate reduce motor neuron loss and promote regeneration and functional recovery in a hamster axotomy model. In a subsequent study, this group demonstrated that exogenous administration of testosterone conferred protection after axotomy with similar efficacy to the protective properties of 17β-estradiol (Huppenbauer et al., 2005). Alternatively, other data show that testosterone can induce cell death. For example, superphysiological levels of testosterone have been shown to induce neuronal and glial cell death and to amplify excitotoxicity in vitro (Caruso et al., 2004; Gatson and Singh, 2007; Orlando et al., 2007). Furthermore, Yang and colleagues (2002, 2005) have demonstrated that chronic testosterone replacement increased lesion volume in a middle cerebral artery occlusion model in male rats, and that gonadectomy or administration of 17β-estradiol reduced lesion volume, which suggests that testosterone may be deleterious in CNS injury. Thus the effects of testosterone on estrogen-mediated protection in CNS injury remain controversial and unclear. Since previous work has shown that 17β-estradiol is protective in SCI in male animals, we hypothesized that endogenous testicular-derived androgens, especially testosterone, could alter these protective effects, and consequently evaluated the effect of endogenous androgens on 17β-estradiol-mediated protection in male rats after SCI in the current study.
Methods
Experimental animals
All experimental protocols were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals, and were carried out with approval from the Institutional Animal Care and Use Committee of the University of California, Davis or the University of Alabama, Birmingham. Two hundred and fifty adult male Sprague-Dawley rats (2 months old, 250–300 g) were maintained on a diet of purified chow and water ad libitum and a 12-h light/dark cycle. Rats were either gonadectomized or left gonad-intact and were then randomized into either an uninjured control group that received a laminectomy only (LAM) or an SCI group that also received a subcutaneous implantation of a commercially available matrix-driven delivery system pellet designed to release either 0.0, 0.05, 0.5, or 5.0 mg of 17β-estradiol over 21 days, which corresponds to a daily dose of 0.0 0.008, 0.08, or 0.8 mg/kg per day respectively, based on a body weight of 300 g (Innovative Research of America, Sarasota, FL).
Surgical procedures
Gonadectomy (orchidectomy)
To eliminate the endogenous, testicular-derived androgens (i.e., testosterone), adult male Sprague-Dawley rats were bilaterally gonadectomized 1 week prior to SCI using standard surgical procedures under inhaled isoflurane anesthesia (4% induction, 2% maintenance).
Spinal cord injury and estrogen treatment
Crush injury to the mid-thoracic spinal cord was performed, with slight modification, as previously described (Chaovipoch et al., 2006). Briefly, rats were anesthetized by an intraperitoneal (i.p.) injection of ketamine/xylazine mixture (70/30 mg/kg). Body temperature was maintained with an anal temperature probe and heating pad equipped with a temperature feedback control system (CMA Microdialysis, Stockholm, Sweden). The skin and muscle were incised to expose the vertebral column. A laminectomy was performed in the mid-thoracic region (T8–T9). Micro-dissector forceps (0.6-mm-wide smooth tip, RS-5096; Roboz Surgical Instrument Co., Gaithersburg, MD) with a detent were carefully placed by hand between the dura and laminae. Proper placement was ensured by visual inspection. The spinal cord was extradurally compressed for 3 sec. The forceps laterally compressed the spinal cord to a blade separation of 0.5 mm, as measured with micro-calipers. Following compression, hemostasis was secured. The incised erector spinae musculature and skin were then closed in layers with chromic absorbable surgical suture (3/0 absorbable chromic catgut suture; CP Medical, Portland, OR). At 30 min post SCI, rats received a subcutaneous pellet of 0.0, 0.05, 0.5, or 5 mg/kg E2, which are designed to be released over 21 days. The gonad-intact groups were treated identically. After surgery, rats received Ringer's solution for fluid replacement and hydration, 5 mg/kg carprofen (Pfizer Animal Health, New York) and 0.1 mg/kg enrofloxacin (Bayer Health Care, Inc., Shawnee, KS) by subcutaneous injection (s.c.), for post-operative fluid replacement and prevention of pain and infection respectively. The bladders of paraplegic animals were emptied by gentle abdominal compression three times per day (at 9:00, 14:00, and 19:00 h).
Behavior and physiology analysis
Basso, Beattie, Breshnahan locomotor test
Hindlimb locomotor function after SCI injury was evaluated using the Basso, Beattie, Breshnahan (BBB) open-field locomotor test (Basso et al., 1995). Animals (n = 12 per group) were placed in a 1.2-m diameter metal, smooth-surfaced activity chamber for 4 min, and hindlimb movement was scored by two trained investigators who were naïve to the treatment of the animal. All discrepancies in scores were resolved by discussion between the raters at the conclusion of the test and scored to the deficit. Scores were generated for each hindlimb and averaged. The scale is based on well-defined operational definitions that are used to rank hindlimb movements, ranging from no movement (0) to normal walking with coordinated fore–hindlimb stepping and normal paw placement (21) (Basso et al., 1995). Scores from 0 to 7 evaluate hindlimb joint movements (hip, knee, and ankle) at the early stage of recovery. Scores from 8 to 13 evaluate stepping and coordination by indicating the return of paw placement and coordinated movements with the forelimbs. Scores 14–21 evaluate the return of toe clearance during stepping predominant paw position, trunk stability, and tail position. BBB scores were obtained on post-SCI days 3, 7, 14, 21, and 28. Additionally, a transformation of the BBB scale developed specifically to improve the metric properties in evaluation of animals with moderate to severe SCI was used as previously described (Ferguson et al., 2004).
Wet-tissue weight of urinary bladder
At 28 days post SCI, animals (n = 12 per group) were deeply anesthetized with Fatal Plus (100 mg/kg i.p.; Vortech Pharmaceuticals, Dearborn, MI) and perfused intracardially with cold 0.1 M phosphate buffer saline, pH 7.4, followed by cold 4% paraformaldehyde for 20 min. The urinary bladder was removed, drained of residual liquid, blot-dried, and weighed by an investigator naïve to the treatment condition of the animal.
Analysis of plasma testosterone and 17β-estradiol levels
Additional animals (n = 3 per condition) were used to determine plasma levels of testosterone and 17β-estradiol. These male rats were either gonadectomized or left gonad-intact as described above. All animals received a mid-thoracic SCI and post-SCI estrogen treatment as described above. At 4 days post SCI, animals were anesthetized (4% isoflurane, 2% maintenance, via a nose cone), and 1 mL of blood was removed from the femoral artery. Serum was separated from blood cells by centrifugation, rapidly frozen over dry ice, and stored at −20°C until further analysis. Serum testosterone and 17β-estradiol concentrations were determined by using competitive enzyme immunoassay (EIA) kits specific for each hormone according to the manufactures instructions (#582401.1 testosterone; #582251.1 estradiol; Cayman Chemical Company, Ann Arbor, MI). Samples were analyzed in triplicate.
Histological analysis
Spinal cord tissue preparation
At 4 and 28 days post SCI, animals (n = 10 per group) were deeply anesthetized and perfused as described above. The spinal cords were collected and the location of the SCI was marked with tissue-marking dye (TBS®; Triangle Biomedical Sciences, Croasdaile, NC). The spinal-cord tissue was post-fixed for 24 h at 4°C in 4% paraformaldehyde and subsequently cryoprotected in an increasing gradient of 10%–30% sucrose for 24 h at 4°C. The tissue was then frozen over dry ice and blocked into 3-mm segments corresponding to the epicenter of lesion and sections immediately rostral and caudal to the epicenter. Tissue segments were then embedded in OCT-Compound (Tissue-Tex; Fisher Scientific, Pittsburg, PA) and stored at −80°C until serial random sectioning. Serial 30-μm sections were sectioned on a cryostat (Leica Instruments, Nusloch, Germany), then mounted on 1% gelatin-coated slides and stored at −20°C until further histological analysis.
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling histochemistry
Apoptosis was detected by a terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) assay, according to the manufacturer's directions (ApopTag Peroxidase In Situ Apoptosis Detection Kit; Chemicon/Millipore, Inc., Billerica, MA). The sections were encircled with a hydrophobic barrier (ImmEdge Pen; Vector Laboratories, Burlingame, CA) and then dried at 60°C for 15 min. Tissue sections were rehydrated through graded alcohols, and endogenous peroxidase activity was blocked with 2% and then 3% H2O2. The sections were washed and then permeabilized overnight at 4°C with NeuroPore (Trevigen, Inc., Gaithersberg, MD). Tissue sections were washed and then incubated in 1X terminal deoxynucleotidyl transferase (TdT) labeling buffer for 10 min at room temperature and then labeled with DNA label reaction mix for 1.5 h at 37°C. Incubation in stop buffer for 5 min at room temperature terminated the labeling reaction. Following washes, sections were incubated with Vector Strep-avidin HRP kit (Vector Laboratories) for 45 min at room temperature, and developed in Vector SG chromagen (Vector Laboratories) for 9 min at room temperature. Tissue sections were then rinsed and dried overnight at room temperature. The sections were counterstained in neutral red (Aniara, Inc., Mason, OH) for 20 sec at room temperature followed by dehydration through graded alcohol to xylene and then coverslipped with Permount mounting media (Fischer Scientific, Pittsburg, PA).
Cresyl violet histochemistry
The histological processing of tissue with cresyl violet demonstrates Nissl substance, which is primarily comprised of rough endoplasmic reticulum and is lost after neuronal injury or axonal degeneration (Carson, 1990). For cresyl violet histochemistry, tissue was washed and dried overnight before staining. Sections were dehydrated through graded alcohol to xylene for two changes of 5 min each, and then rehydrated through graded alcohol to water. Sections were stained in 0.1% aqueous cresyl fast violet (EM Science, Gibbstown, NJ) in sodium acetate buffer for 5 min, followed by differentiation in 95% ethanol with 0.2% HCl for 4–5 min. Differentiation was performed such that both the Nissl substance and the cell nuclei were demonstrated. Slides were washed in graded alcohol and xylene and coverslipped with Permount mounting media (Fischer Scientific).
Myelin basic protein immunohistochemistry
Sections were encircled with a hydrophobic barrier (ImmEdge Pen, Vector Laboratories) and then dried at 60°C for 15 min. Slides were rehydrated through graded alcohols and endogenous peroxidase activity was blocked with 0.5% and 1% H2O2. Sections were then washed, and non-specific reactivity was blocked with 3% horse serum + 0.3% Triton X + 3% bovine serum albumin at 37°C for 1 h. Tissue sections were rinsed and then incubated in mouse monoclonal anti-MBP antibody (1:1000, ab24567-250; Abcam, Cambridge, MA) for 48 h at 4°C. Thereafter, the sections were washed and incubated in anti-mouse secondary antibody at 37°C for 2 h, followed by incubation with Avidin-biotin complex (1:20, Vector Elite ABC Kit; Vector Laboratories) at room temperature for 2 h and developed in Vector SG chromagen (Vector Laboratories) for 7 min at room temperature. Sections were rinsed, dried overnight, and coverslipped with Permount mounting media (Fischer Scientific).
Analysis of protein expression by Western blotting
Tissue lysis
Animals (n = 5 per group) were euthanized and perfused with cold saline, and a 3-mm section of spinal cord at the epicenter of the lesion was collected. Each 3-mm piece of spinal-cord tissue was placed in 300 μl of lysis buffer, which contained 100 mM Tris pH 7.5 + 1% SDS supplemented with protease inhibitor cocktail (Complete Mini Protease Cocktail Tablets; Roche Diagnostics, Indianapolis, IN). Tissue was homogenized for 30 sec using a manual homogenizer, and then sonicated for 10 sec over ice. Homogenate was centrifuged at 14,000g for 5 min. The supernatant was collected and protein assay performed (Bio-Rad, Hercules, CA). Samples were diluted to 2 mg/mL protein with lysis buffer and stored at −80°C until analyzed.
Gel electrophoresis and protein transfer
Using a standard tank electrophoresis system (Bio-Rad), precast 4–20% Tris-HCL one-dimensional gels were loaded with 25 μg of protein in 40 μL per well. A molecular weight standard protein ladder was used in one lane on each gel. Electrophoresis was conducted at 120 V for 1 h at room temperature. After the completion of electrophoresis, the gels and polyvinylidene fluoride (PVDF) membrane were equilibrated in transfer buffer + 20% methanol. The proteins were electrophoretically transferred from gel to membrane for 2 h at 200 mA on ice.
Immunoblotting
The transfer membrane was blocked for 2 h in filtered 5% milk in TBST. After blocking, 3 mL of blocking buffer + 1° antibody was added to a 50 mL conical tube. The membrane was incubated with primary antibody in the rotating conical tube overnight at 4°C. Following washes in TBST (10 min per wash), the membrane was probed with the appropriate 2° antibody for 1 h at room temperature.
Visualization and relative optical density
All immunoblots were developed using Pierce Femto Max SuperSignal (Thermo-Fischer Scientific, Rockford, IL) and imaged using the Kodak Image Station (4000 MM Pro; Carestream Molecular Imaging, Rochester, NY). Densitometry of each blot was performed using UN-SCAN-IT gel: Gel Analysis Software (Silk Scientific, Inc., Orem, UT). Relative optical density values for each band were normalized to the corresponding control band (GAPDH). Positive control proteins were used for each antibody. The antibodies used were anti-Bax (1:1000, AB# 2727; Cell Signaling Technology, Danvers, MA) with the CTLL-2 as the positive control, anti Bcl-xL (1:1000 AB# AMO5; Calbiochem/EMD Chemicals, Gibbstown, NJ) with HeLa as the positive control, anti-ERα (1:100, AB# M7047; Dako, Inc., Carpinteria, CA) with ovary extracts as the positive control, and GAPDH (1:1000, AB# 9485; AbCam, Inc., Cambridge, MA).
Quantification of histological markers
Quantification of cell number with optical fractionator probe
Stereological counting was conducted on an Olympus BX-51 microscope (Center Valley, PA) linked to a MicroFire® true-color CCD digital camera (Optronics, Goleta, CA) using StereoInvestigator software (Microbrightfield, Inc., Williston, VT) at 200–400 × magnification to obtain unbiased optical fractionator estimates of the total number of neurons in the ventral horn at the lesion epicenter. For analysis of cresyl violet histochemistry, only neurons with an ovoid, spherical, triangular, or multipolar profile, and with a soma diameter larger than 10 μm, an intact cell membrane, and a clearly defined nucleus were counted. Beginning at a randomly chosen first section at the rostral end of the lesion epicenter, cells were counted in every 10th section throughout the rostral-caudal extent of the lesion (3 mm total tissue) in the ventral horn. All assessments were performed by an investigator naïve to the treatment of the animal. Similarly, TUNEL-positive nuclei were quantified in adjacent serial random sections and visualized at 400 × magnification. Only nuclei with a blebbed appearance and staining intensity 2 × greater than background were counted.
Quantitative analysis of spared myelin
Digital images of tissue processed with immunohistochemistry for myelin basic protein were captured with an Olympus BX-51 microscope (100–200 ×) linked to a MicroFire true-color CCD digital camera using StereoInvestigator software. The Cavalieri probe was used to determine the area of intact white matter at the epicenter of the lesion. Serial transverse sections throughout the rostral-caudal extent of the lesion were analyzed, and the intensity of immunohistochemistry of myelin basic protein reactivity and morphology were used to determine the border between intact and damaged myelin. The section with the smallest white matter area was designated as the epicenter, and the area of that section and the serial sections immediately rostral and caudal were averaged to obtain the percentage of the spared white matter area at the epicenter for each animal. All assessments were conducted by an investigator naïve to the treatment group.
Statistical analysis
All data were analyzed using SigmaStat Advisory Statistics for Science software (V3.5; Systat Software, Inc, Point Richmond, CA) on a personal computer. Significance was set at p < 0.05. Data are presented as mean ± standard error of the mean (SEM). Data for behavioral and physiological outcome measures were analyzed with repeated measures one-way analysis of variance (ANOVA), followed by a Holm-Sidak's post-hoc analysis for multiple comparisons versus a control group. Data for histological measures were analyzed with a one-way ANOVA, followed by the Holm-Sidak's method.
Results
Post spinal cord injury estrogen and testosterone levels
Serum levels of testosterone and 17β-estradiol were measured at 4 days post SCI using an EIA specific for each hormone (Table 1). In male rats that received a gonadectomy prior to SCI, testosterone levels ranged from 10.7 to 18.8 pg/mL as compared to levels in gonad intact males that ranged from 122.5 to 143.6 pg/mL, which indicates that bilateral surgical removal of the testes significantly reduced serum testosterone, as anticipated. Additionally, the post-SCI administration of 17β-estradiol did not significantly alter serum testosterone levels as no significant differences in serum testosterone levels between E2 groups were observed. In contrast, post-SCI administration of 17β-estradiol of either the 0.5 or 5.0 mg pellet significantly increased serum levels of 17β-estradiol levels in both the gonadectomized and gonad-intact male rats (as indicated by *, Table 1). However, when comparing gonadectomized to gonad-intact rats at each dose of 17β-estradiol, no significant differences in serum E2 were found. These data suggest that testosterone levels did not affect serum levels of 17β-estradiol.
Table 1.
Effect of Gonadectomy and Post-SCI Administration of 17β-Estradiol on Serum Levels of Testosterone and 17β-Estradiol
| Experimental condition | Serum testosterone (pg/mL) | Serum 17β-estradiol (pg/mL) |
|---|---|---|
| GDX + 0.0 mg | 13.3 ± 6.0 | 15.4 ± 5.4 |
| GDX + 0.05 mg | 15.0 ± 5.3 | 37.0 ± 5.3 |
| GDX + 0.5 mg | 18.8 ± 2.6 | 168.4 ± 18.7* |
| GDX + 5.0 mg | 10.7 ± 3.5 | 1945.7 ± 604.4* |
| Intact + 0.0 mg | 122.5 ± 6.7 | 23.6 ± 2.5 |
| Intact + 0.05 mg | 142.1 ± 17.9 | 26.1 ± 1.2 |
| Intact + 0.5 mg | 143.6 ± 8.1 | 147.7 ± 30.4* |
| Intact + 5.0 mg | 131.9 ± 2.4 | 1856.8 ± 308.1* |
On day 4 post SCI, blood was drawn from the femoral artery from male rats that received a gonadectomy (GDX) or that remained gonad-intact (intact). Serum levels of testosterone and 17β-estradiol were measured by an EIA. Mean levels ± SEM are presented for each experimental treatment group. *Significantly different than corresponding vehicle group (GDX + 0.0 mg or Intact + 0.0 mg). N = 3 for all groups.
Post spinal cord injury estrogen administration increased functional recovery of hindlimb locomotion
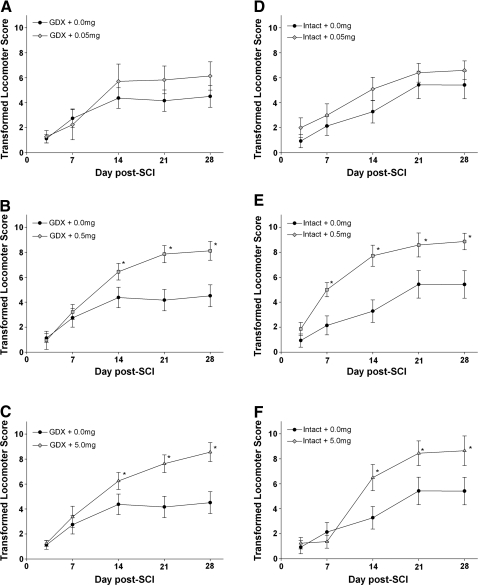
The BBB open-field locomotor scale was used to evaluate hindlimb locomotor performance following experimental SCI as previously described (Basso et al., 1995; Chaovipoch et al., 2006). In rats that received a gonadectomy prior to injury, post-SCI administration of either the 0.5- or 5.0-mg pellet of E2 significantly improved the BBB open-field scores on days 14, 21, and 28 compared to the group receiving the placebo (0.0 mg) pellet (Fig. 1B,C). Similarly, in gonad-intact male rats, administration of the 0.5- or 5.0-mg pellet of E2 significantly improved the BBB open-field locomotor score compared to the placebo group on post-SCI days 7, 14, 21, and 28, and days 14, 21, and 28 respectively (Fig. 1E,F). In both the gonadectomized and gonad-intact groups, animals receiving either the 0.5- or 5.0-mg pellet of E2 recovered to an average score of 8, which corresponds to plantar placement of the hind paws without weight support, compared to the placebo-treated animals' average recovery score of 4, which corresponds to slight movement of all three joints of the hindlimb (hip, knee, and ankle). The laminectomy groups scored 21 on all days tested, which was significantly higher than all injured groups. No differences were found between laminectomy animals treated with E2 versus the placebo animals, suggesting that E2 did not affect locomotion in uninjured animals (data not shown). No significant differences were observed when comparing gonadectomized and gonad-intact groups at any dose of 17β-estradiol or on any day of recovery. Taken together, these data indicate that the medium and high dose of E2 administered after SCI significantly improved recovery of hindlimb locomotion and that the presence of gonad-derived testosterone did not change the level of functional recovery induced by post-SCI administration of 17β-estradiol.
FIG. 1.
Effect of post-SCI administration of 17β-estradiol (E2) on the Basso, Beattie, Bresnahan (BBB) score. Hindlimb locomotor function was assessed by two raters naïve to the treatment of the animal, and scores were transformed to improve the metric properties in evaluation of animals with moderate to severe SCI as previously described (Ferguson et al., 2004). Mean scores ± SEM for post-SCI days 3, 7, 14, 21, and 28 are shown for gonadectomized (GDX) male rats in A–C and gonad-intact (intact) male rats in D–F. Data from the each of the vehicle groups (GDX + 0.0 mg or Intact + 0.0 mg) are repeated for comparison to groups treated with 17β-estradiol. *Significantly different than placebo (0.0 mg) pellet on that day. N = 12 for all groups.
Post spinal cord injury estrogen administration decreases bladder volume
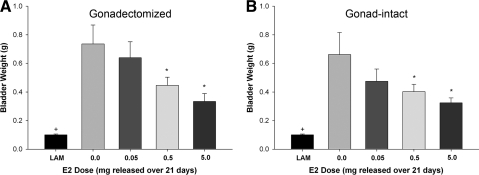
Wet-tissue weight of the urinary bladder has been previously used as in indicator of lower urinary-tract function after SCI, with larger weights corresponding to less function (Pikov and Wrathall, 2001). After euthanasia, the urinary bladder was extracted and the wet-tissue weight was obtained. We found that the average weight of the urinary bladder of rats receiving only a laminectomy was 0.10 ± 0.005 g in the gonadectomized group and 0.11 ± 0.01 g in the gonad-intact group, and that the laminectomy animals had significantly lower bladder weights than all SCI groups (Fig. 2A,B). In the gonadectomized rats, post-SCI administration of either the 0.5- or 5.0-mg pellet significantly reduced bladder weight compared to the 0.0-mg group at 28 days after injury (Fig. 2A). Similarly, in gonad-intact animals, post-SCI administration of the 0.5- or 5.0-mg E2 pellet reduced bladder weight compared to animals receiving the 0.0-mg pellet (Fig. 2B). No significant differences were observed when comparing gonadectomized and gonad-intact groups at any dose of 17β-estradiol. These data indicate that the medium and high dose of estrogen administered after SCI significantly decreased the weight of the urinary bladder, and that the presence of gonad-derived testosterone did not change the estrogen-mediated recovery of lower urinary-tract function.
FIG. 2.
Effect of post-SCI administration of 17β-estradiol (E2) on wet-tissue weight of the urinary bladder at 28 days post SCI. After euthanasia, the urinary bladder was extracted and the wet-tissue weight was obtained. Average tissue weight of the urinary bladder is shown for gonadectomized animals (A) and for gonad-intact animals (B). *Significantly lower than the placebo (0.0 mg) pellet group. +Laminectomy only (LAM) had significantly lower bladder weight than all SCI groups. N = 12 for all groups.
Post spinal cord injury estrogen administration increases white-matter sparing
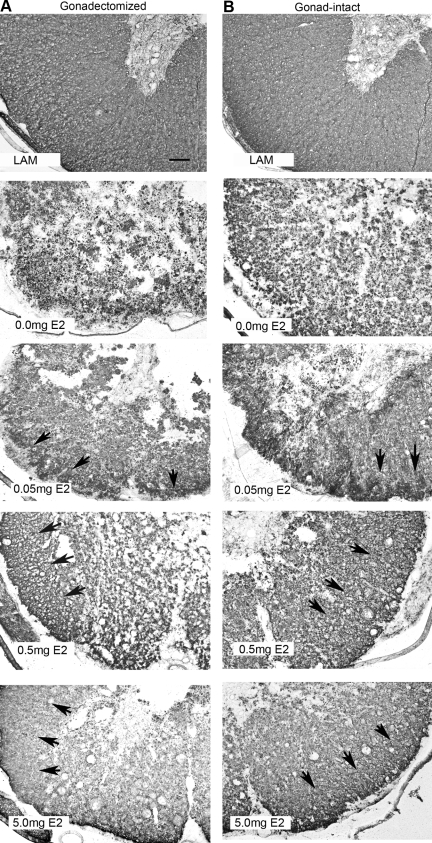
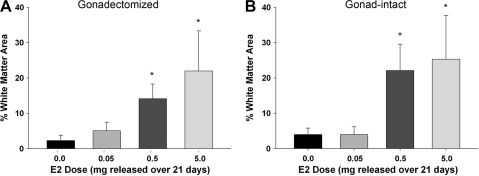
Previous work has shown that preservation of white matter is highly correlated with the recovery of hindlimb and lower urinary-tract function in the rodent mid-thoracic spinal cord injury model (Basso, 2000; Fehlings and Tator, 1995; Noble and Wrathall, 1987; Pikov and Wrathall, 2002). Consequently, we evaluated the effect of post-SCI E2 administration on the percentage of white-matter area present in the injury epicenter at 28 days after SCI. Serial transverse sections throughout the rostral-caudal extent of the lesion were processed with immunohistochemistry for myelin basic protein, and the three sections with the lowest area (epicenter) were used for calculations. Representative micrographs from the epicenter at 28 days post SCI are shown in Figure 3. The percentage of white matter for rats that received only a laminectomy was 76.3 ± 0.4% for gonadectomized and 78.5 ± 0.7% for gonad-intact rats (data not shown). In the gonadectomized males, the percentage of intact white matter in the placebo-treated group was 2.2 ± 0.7%. Post-SCI administration of either the 0.5- or 5.0-mg E2 pellet significantly increased the percentage of white matter to 14.1 ± 1.8% and 22.0 ± 5.1% respectively. Administration of the 0.05-mg pellet did not significantly increase white-matter sparing (Fig. 4A). Similarly, in the gonad-intact male rats, the percentage of white matter in the 0.5-mg group was 22.1 ± 3.3% and 25.3 ± 5.6% in the 5.0-mg group, significantly higher than the area in the placebo group (4.0 ± 0.8%). However, the 0.05-mg E2 pellet did not significantly increase white matter area (Fig. 4B). Additionally, no significant differences were observed when comparing gonadectomized versus gonad-intact groups at any dose of 17β-estradiol evaluated. In summary, these data indicate that post-SCI administration of either the 0.5- or 5.0-mg E2 pellet causes significant preservation of white matter, and that levels of gonad-derived testosterone did not alter estrogen-induced white-matter sparing.
FIG. 3.
Representative micrographs of immunohistochemistry for myelin basic protein at 28 days post SCI. Micrographs (100 ×) of coronal tissue sections from gonadectomized male rats (A) show intact myelin in tissue from animals that received a laminectomy only (LAM, top panel). Little or no intact myelin was identified in the SCI + 0.0 mg E2 or SCI + 0.05 mg E2 groups (middle two panels), and a larger area of intact myelin was observed in tissue from SCI + 0.5 mg E2 or SCI + 5.0 mg E2 animals (lower two panels, border indicated by arrows). Similarly, in gonad-intact male animals (B), less intact myelin was detected in the SCI + 0.0 mg E2 or SCI + 0.05 mg E2 groups (middle two panels), and more intact myelin was found in the SCI + 0.5 mg E2 or SCI + 5.0 mg E2 groups (lower two panels, border indicated by arrows). Bar = 100 μm.
FIG. 4.
Effect of post-SCI administration of 17β-estradiol (E2) on white-matter sparing at the lesion epicenter. Unbiased stereology using the Cavalieri probe was used to determine the area of intact white matter. The section with the smallest area was designated as the epicenter and averaged with the area of the serial sections immediately rostral and caudal to obtain the percentage of the spared white-matter area for each animal. Mean percentage of white-matter area ± SEM are shown for gonadectomized (A) and gonad-intact (B) groups. *Significantly different than placebo (0.0 mg) pellet group. N = 10 for all groups.
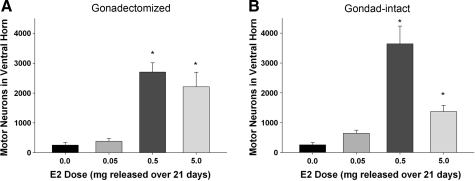
Estrogen increases neuronal survival in the ventral horn
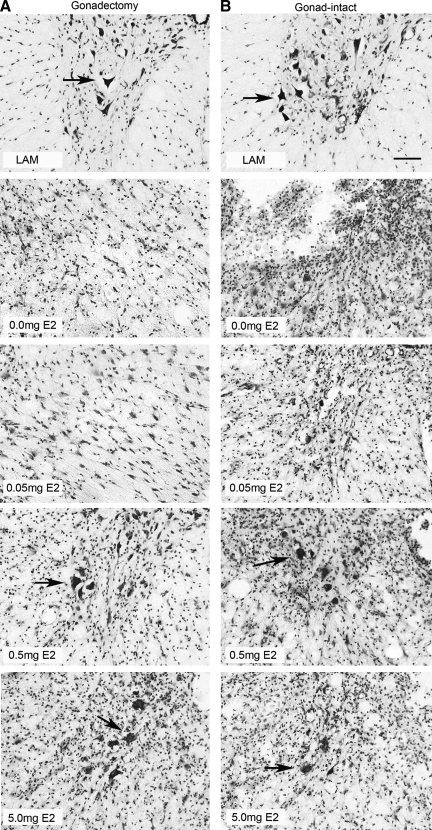
Serial transverse sections of spinal cord through the epicenter of the lesion were processed with cresyl violet histochemistry for identification of Nissl substance at 28 days post SCI, and neurons were identified by morphology and cell size. In gonadectomized rats, more neurons were present in the ventral horn of rats administered either the 0.5- or 5.0-mg estrogen pellet compared to rats administered the placebo (0.0 mg) or low dose (0.05 mg; Fig. 5A). Quantification of the number of motor neurons in the ventral horn with unbiased stereology indicated that significantly more neurons were present in the gonadectomized (8671.3 ± 662.6) or gonad-intact (8486.3 ± 644.9) laminectomy groups than in any injury group (data not shown). Additionally, in the gonadectomized rats, significantly more neurons were present in either the 0.5- or 5.0-mg E2 pellet groups than in the placebo (0.0 mg) group (Fig. 6A). A similar trend was seen in gonad-intact rats in that more neurons were visualized in the 0.5- and 5.0-mg treatment groups than in the placebo (0.0 mg) or 0.05-mg treatment groups (Fig. 5B). This observation was confirmed with unbiased stereological counts of neurons in the ventral horn, as significantly more neurons were counted in the 0.5- and 5.0-mg estrogen groups than in the placebo group (Fig. 6B). When gonadectomized and gonad-intact groups were compared at each dose of 17β-estradiol, no significant differences were found, suggesting that the absence of gonad-derived testosterone did not affect estrogen-mediated protection of motor neurons in the ventral horn.
FIG. 5.
Representative micrographs of cresyl violet histochemistry for Nissl substance at 28 days post SCI. Micrographs (200 ×) of transverse tissue sections from gonadectomized male rats (A) illustrate the presence of identifiable neurons (indicated by arrow) in the ventral horn of tissue from the laminectomy only (LAM, top panel), SCI + 0.5 mg E2, or SCI + 5.0 mg E2 (lower two panels) groups. Very few neurons were identified in tissue from the SCI + 0.0 mg E2 or SCI + 0.05 mg E2 groups (middle two panels). Similarly, micrographs (200 ×) from gonad-intact male rats (B) show the presence of neurons (indicated by arrow) in the laminectomy (LAM, upper panel), SCI + 0.5 mg E2, and SCI + 5.0 mg groups (lower two panels), and very few neurons present in the SCI + 0.0 mg E2 or SCI + 0.05 mg E2 groups (middle two panels). Bar = 50μm.
FIG. 6.
Effect of post-SCI administration of 17β-estradiol (E2) on neuronal survival in the ventral horn at 28 days post SCI. Unbiased stereology using the optical fractionator probe was used to determine the number of surviving neurons in the ventral horns. The mean number of motor neurons ± SEM in the ventral horn is shown for gonadectomized (A) and gonad-intact (B) male rats. *Significantly different than placebo (0.0 mg) pellet group. N = 10 for all groups.
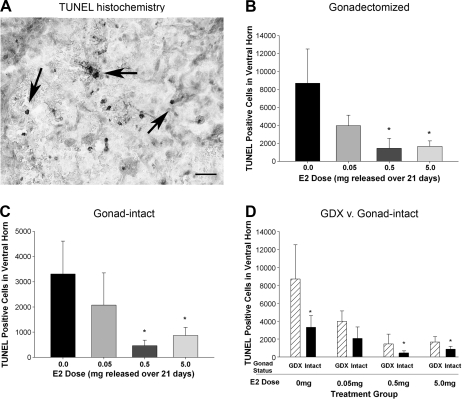
Estrogen treatment decreases apoptotic cell death in the ventral horn
Previous work has demonstrated that SCI induces apoptosis (Beattie et al., 2000; Keane et al., 2006). Since a primary hypothesized mechanism of estrogen protection in CNS injury is reduction of apoptosis (Wise et al., 2001), we evaluated the effect of post-SCI administration of E2 on apoptotic cell death in the ventral horn at 4 days post SCI. Serial sections throughout the epicenter of the lesion were analyzed using a TUNEL assay, and the number of TUNEL-positive cells in the ventral horns was quantified by an investigator naïve to the experimental group using unbiased stereology. A representative micrograph showing TUNEL histochemistry at 4 days post SCI in a spinal-cord section from an animal in the gonadectomy and 0.0 mg E2 groups is shown in Fig. 7A. In animals that received a laminectomy only, very few TUNEL+ cells were counted (159.6 ± 70.2 for the gonadectomy group and 190.3 ± 98.8 for the gonad-intact group), which was significantly lower than in all SCI groups (data not shown). In gonadectomized rats, post-SCI administration of either the 0.5- or 5.0-mg E2 pellet significantly reduced the number of TUNEL+ cells in the ventral horn compared to the placebo (0.0 mg) group (Fig. 7B). In gonad-intact male rats, post-SCI administration of either the 0.5- or 5.0-mg E2 pellet caused a significant reduction in the number of TUNEL+ cells in the ventral horn after SCI (Fig. 7C). Interestingly, when the effect of gonad status was compared across all 17β-estradiol treatment groups, significantly fewer TUNEL+ cells were counted in the gonad-intact than in the gonadectomized condition in the 0.0-, 0.5-, and 5.0-mg estrogen groups. A similar non-significant reduction in the number of TUNEL+ cells was found in gonad-intact male rats compared to the gonadectomized rats in the 0.05-mg estrogen group (Fig. 7D). Taken together, these data suggest the medium and high dose of estrogen reduce apoptotic cell death in the gray matter, and that significantly less apoptotic cell death occurs in the presence of gonad-derived testosterone.
FIG. 7.
Effect of post-SCI administration of 17β-estradiol (E2) on apoptosis in the ventral horn at 4 days post SCI. A representative micrograph (400 ×) from a gonadectomized male rat treated with the placebo (0.0 mg) E2 pellet showing darkly stained TUNEL-positive nuclei with a blebbed appearance (dark arrows) and lighter outline of cell soma visualized with neutral red counterstaining (A). Bar = 20 μm. TUNEL+ cells in the ventral horn were counted with unbiased stereology using the optical fractionator probe. The mean number of TUNEL+ cells ± SEM in the ventral horn is shown for gonadectomized (B) and gonad-intact (C) male rats. *Significantly different than placebo (0.0 mg) pellet group. N = 10 for all groups. The stereological counts of TUNEL+ cells was compared across estrogen treatment (D) using the same data from panels B and C. *Significant difference between gonadectomized (GDX) and gonad-intact group at that E2 dose.
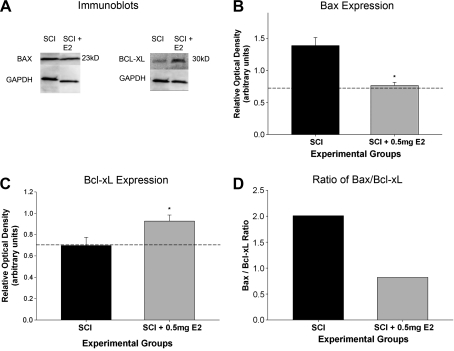
Since we found that E2 reduced apoptotic cell death in the ventral horn after SCI (Fig. 7), we hypothesized that that expression of Bcl-2 family proteins after SCI is also affected by administration of 17β-estradiol. To test this hypothesis, we conducted immunoblots for expression of the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-xL on tissue extracted 7 days post SCI at the lesion epicenter from gonad-intact animals that received either a 0.0- or 0.5-mg E2 pellet (Fig. 8A). We found that post-SCI administration of the 0.5-mg E2 pellet significantly reduced Bax levels compared to the 0.0-mg group (Fig. 8B). Additionally, we found that post-SCI administration of the 0.5-mg E2 pellet significantly increased the expression of Bcl-xL compared to injured animals receiving the 0.0-mg E2 pellet (Fig. 8C). A “rheostat” hypothesis has been proposed in which the ratio of pro- and antiapoptotic proteins determines the susceptibility of a cell to apoptosis (Yang and Korsmeyer, 1996). Therefore, we calculated the ratio of the protein expression of Bax/Bcl-xL for rats administered either the placebo (0.0 mg) or 0.5-mg E2 pellet after SCI. The Bax/Bcl-xL ratio for the animals that received the placebo E2 pellet was higher than that for animals receiving the 0.5-mg E2 pellet (Fig. 8D). This suggests that post-SCI administration of 17β-estradiol reduces the apoptotic susceptibility in the spinal cord after traumatic injury.
FIG. 8.
Effect of post-SCI administration of 17β-estradiol on Bcl-2 family proteins at the epicenter of the lesion. Immunoblots for expression of the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-xL were conducted on spinal-cord tissue from the epicenter of the lesion in animals that received either the placebo pellet (0.0 mg) or the 0.5-mg E2 pellet (SCI + E2, A). Mean ± SEM relative optical density measurements were normalized to GAPDH for Bax expression (B) or Bcl-xL expression (C), with laminectomy values indicated by the dotted line. *Significant difference between placebo (0.0 mg) and 0.5-mg pellet groups (panels B and C). The ratio of Bax/Bcl-xL was calculated from the normalized relative optical density values (D). N = 5 animals per group, immunoblots in duplicate.
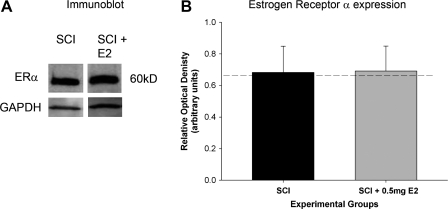
Estrogen receptors are present in the spinal cord of adult male rats
The estrogen receptor is the main target of 17β-estradiol binding in the adult CNS. Thus we evaluated the effect of SCI and E2 administration on the expression of the estrogen receptor alpha (ERα) in male rats. We conducted Western blotting experiments to evaluate expression of the ERα in tissue extracted from laminectomy or SCI at 7 days post SCI in animals receiving either the 0.0- or 0.5-mg pellet of 17β-estradiol after SCI. We found that the ERα is robustly expressed in the spinal cord of adult male animals regardless of the SCI (Fig. 9).
FIG. 9.
Effect of post-SCI administration of 17β-estradiol (E2) on estrogen receptor alpha (ERα) expression at the epicenter of the lesion. Immunoblots for expression of the ERα were conducted on spinal-cord tissue from the epicenter of the lesion in animals that received either the placebo pellet (0.0 mg) or the 0.5 mg E2 pellet (SCI + E2, A). Mean ± SEM relative optical density measurements were normalized to GAPDH for ERα expression (B) with laminectomy values indicated by the dotted line. N = 5 animals per group, immunoblots in duplicate.
Discussion
In this work, we demonstrate that a delayed, post-injury administration of a clinically relevant dose of 17β-estradiol reduces secondary damage and promotes functional recovery after SCI. Specifically, we show that administration of either the 0.5- or 5.0-mg slow-release pellet: improves hindlimb locomotion, as evaluated with the BBB open-field score; increases white-matter sparing at the lesion epicenter; and increases neuronal survival in the ventral horn. Additionally, we found that post-SCI administration of 17β-estradiol significantly decreases apoptosis in the ventral horn and favorably modulates the ratio of pro- and anti-apoptotic Bcl-2 family proteins. We also show that eliminating testicular-derived androgens significantly increases apoptosis but does not alter the protective effect of 17β-estradiol on apoptotic cell death, which suggests that testosterone and 17β-estradiol may act by separate mechanisms to reduce apoptosis after SCI.
Estrogen promotes tissue sparing and associated functional recovery
Although we found that post-SCI administration of 17β-estradiol increased both neuronal survival in the gray matter and white-matter sparing, previous research using the midthoracic rodent model of SCI has shown that return of function of hindlimb locomotion is largely dependent upon preservation of white matter (Fehlings and Tator, 1995; Gorska et al., 2009; Noble and Wrathall, 1987). For example, Kloos and colleagues (2005) demonstrated that in a T8 SCI increasing the contusional impact force/ displacement of the spinal cord resulted in decreased percentage of white matter area and a corresponding decrease in BBB score. They reported that female animals with 4% white-matter sparing after SCI had a corresponding BBB score of 8–9 (Kloos et al., 2005). These values are similar, albeit slightly lower, than white-matter sparing values we observed in male rats with crush injury in the present study. We observed that animals in the SCI + 0.0 mg E2 group had an average spared white-matter percentage of 2.2% with a corresponding BBB score of 4. We also found that rats receiving either a 0.5- or 5.0-mg pellet of E2 reached a BBB recovery score of 8, with a corresponding percentage of white-matter area of 14–22%, which is consistent with the idea that the BBB open-field score is related to white-matter sparing in a midthoracic SCI model. Thus we interpret the functional improvement in hindlimb locomotion reported here with post-SCI administration of 17β-estradiol in male rats as being related to the percentage of spared white matter.
Recovery of hindlimb locomotion is an important and widely used measurement of functional recovery after SCI in rodent models. Yet the relationship between spared tissue and recovery of hindlimb locomotion can be complicated by integration of upper and lower motor neuronal circuitry including segmental, intersegmental, and supraspinal elements (Dietz et al., 1999; Gorska et al., 2007, 2009) In contrast, visceral functions, such as lower urinary-tract function, are controlled by simpler reflexive circuits, and can be used as a less complicated indicator of functional recovery after SCI (Pikov et al., 1998). Additionally, evaluation of lower urinary-tract function after SCI is highly clinically relevant, as indicated from a survey of SCI patients who rate restoration of voluntary control of bladder function as a high recovery priority (Anderson, 2004). Urine storage and voiding are key functions of the lower urinary tract, and measuring bladder weight in rats after SCI has previously been used as an indicator of lower urinary-tract functional recovery, with lower bladder weights associated with functional recovery (Keirstead et al., 2005; Pikov and Wrathall, 2001, 2002; Pikov et al., 1998). The bladder weights that we report for the uninjured, laminectomy-only groups (0.1 g) were similar to those reported previously by others in their evaluation of micturition and lower urinary-tract function in rats after SCI (Keirstead et al., 2005; Pikov and Wrathall, 2001, 2002). Interestingly, we found that post-SCI administration of either the 0.5- or 5.0-mg pellet of 17β-estradiol significantly reduced bladder weight measured at 28 days post SCI. Although we are the first to report that estrogen promotes recovery of lower urinary-tract function after CNS injury, these findings are similar to a previous report that administration of a superphysiological dose of 17β-estradiol administered at the time of pudendal-nerve crush injury promotes recovery of urethral function in female rats (Ahmed et al., 2006), Even though our results are intriguing and demonstrate that post-SCI administration of estrogen promotes functional recovery after SCI, a more rigorous analysis of lower urinary-tract function, including urodynamic measures such as ultrasound of the bladder (Keirstead et al., 2005), EMG-analysis of detrusor-external urethral sphincter coordination (de Groat et al., 1990), or telemetric monitoring of corpus spongiosum penis pressure (Nout et al., 2006), would more thoroughly assess and quantify the protective effect of 17β-estradiol on lower urinary-tract function after SCI.
Estrogen, endogenous androgens, and apoptosis
It has been over a decade since the earliest reports of apoptotic cell death in SCI, and reduction and/or regulation of apoptosis remains a therapeutic target (Beattie, 2004; Springer, 2002). The Bcl-2 protein family includes both pro- and anti-apoptotic members, which regulate the permeability of the mitochondrial outer membrane through control of the formation of a permeability transition pore (Antignani and Youle, 2006). Complexes formed by proteins containing BH3, including Bax and Bad, facilitate the release of cytochrome C from mitochondria and are pro-apoptotic. Anti-apoptotic members of the Bcl-2 family, such as Bcl-2 and Bcl-xL, inhibit the pore-forming function of BH-3 and prevent the formation of the permeability transition pore and release of apoptotic inducing factors (Chang et al., 1999; Harris and Thompson, 2000; Rosse et al., 1998; Zhang et al., 2005). In the adult CNS, Bcl-xL is the predominant Bcl-2 family protein expressed, and is the main regulator of apoptosis (Alonso et al., 1997; Hamner et al., 1999; Motoyama et al., 1995; Parsadanian et al., 1998). SCI has been shown to decrease neuronal and glial expression of Bcl-xL at time points associated with increased apoptosis (Nesic-Taylor et al., 2005; Qiu et al., 2001). We report here that the medium and high doses of 17β-estradiol significantly increase expression of Bcl-xL and favorably modulate the ratio of Bax to Bcl-xL, suggesting that regulation of apoptosis may be a fundamental mechanism of estrogen-mediated protection after SCI. Similar to our findings, other work has demonstrated that administration of 17β-estradiol reduced expression of Bax and increased expression Bcl-2 after SCI (Cuzzocrea et al., 2008; Sribnick et al., 2006; Yune et al., 2004, 2008). Taken together, our current work and these previous studies indicate that one mechanism by which 17β-estradiol confers protection after SCI is by reduction of apoptosis via regulation the expression of pro- and antiapoptotic proteins, presumably by activation of estrogen receptors that are present in the spinal cord of adult male rats without significant alteration in expression levels by SCI.
The effects of testosterone in CNS injury remain controversial. Since testosterone can be aromatized to estrogen (Azcoitia et al., 2001), we hypothesized that endogenous testosterone would increase estrogen-mediated protection. However, previous work has shown that endogenous or exogenous testosterone increases excitotoxicity and lesion volume in a MCAO rodent model of ischemia (Yang et al., 2002, 2005). Thus we evaluated the effect of endogenous, testicular-derived testosterone on estrogen-mediated protection and outcome after SCI. We found that removal of endogenous androgens increases apoptosis after SCI, but that post-SCI administration of 17β-estradiol is similarly protective regardless of androgen status. Specifically, we show that in gonadectomized male rats, post-SCI administration of the 0.5- or 5.0-mg pellet of 17β-estradiol caused 83% or 80% reduction in apoptosis in the gray matter respectively, compared to an 86% (0.5-mg group) and 73% (5.0-mg group) reduction in gonad-intact male rats. We also report that the total number of apoptotic cells counted in gonad-intact male rats was significantly lower than in gonadectomized cohorts at each dose of 17β-estradiol. Taken together, these data suggest that endogenous testicular-derived testosterone is protective against apoptosis, and that 17β-estradiol-mediated protection is not affected by endogenous androgens. We speculate that testosterone and estrogen are likely acting via different pathways to induce protection. In support of this idea, recent observations suggest that nonaromatizable androgens mimic the protective properties of testosterone (Pike et al., 2008), and that activation of the androgen receptor stimulates mitogen-activated protein kinase signaling (MAPK) and subsequent inactivation of the proapoptotic protein Bad (Nguyen et al., 2005).
Implications of evaluated dose response and post spinal cord injury administration time
Previous work examining the protective potential of 17β-estradiol has typically examined only one or two doses, and mainly initiated the estrogen treatment before or immediately after the SCI (Chaovipoch et al., 2006; Cuzzocrea et al., 2008; Ritz and Hausmann, 2008; Sribnick et al., 2005; Webb et al., 2006; Yune et al., 2004). However, failures to examine a dose–response relationship and appropriate therapeutic window have been discussed as pre-clinical design inadequacies that can limit successful translation of potential therapeutics for SCI (Faden and Stoica, 2007). Consequently, a main objective of this study was to evaluate the effectiveness of 17β-estradiol using a clinically relevant, delayed post-SCI administration time, and evaluating a dose–response relationship using a range of doses that more closely mimics clinically available estrogen compounds. Since our chosen route of administration was subcutaneous, we selected a dose range for evaluation that closely coincided with clinically approved 17β-estradiol transdermal compounds typically used for estrogen replacement therapy, such as Vivelle-Dot (Novogyne Pharmaceuticals), EstroGel (Ascend Therapeutics Pharmaceuticals), or Elestrin (Nycomed), administered in the range of 0.002–0.16 mg/kg per day. We measured plasma levels in the male rats after administration of the various doses of 17β-estradiol and found that the plasma levels associated with the 0.5-mg pellet dose (147–168 pg/mL) are similar to those reported in healthy post-menopausal females using these medications, which have maximum concentration (Cmax) ranges from 67–145 pg/mL. These data confirm that the dosing paradigm we evaluated was similar to dosing that can be achieved using 17β-estradiol formulations currently clinically available. Our data imply that delayed post-SCI administration of 17β-estradiol is beneficial as an acute treatment to reduce secondary damage and promote functional recovery after SCI at doses currently used clinically, which may enhance and accelerate the translation of 17β-estradiol as a therapeutic intervention for use in SCI patients.
Acknowledgments
We wish to give special thanks to Tracy D'Alessandro for surgical assistance. This work was supported by R21NS052559, Roman Reed Spinal Cord Research Act of California, and the NIH Neuroscience Blueprint Core Grant NS57098 to the University of Alabama, Birmingham.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahmed Y. Lin D.L. Ferguson C. Esparza N. Damaser M.S. Effect of estrogen on urethral function and nerve regeneration following pudendal nerve crush in the female rat. J. Urol. 2006;175:1948–1952. doi: 10.1016/S0022-5347(05)00894-3. [DOI] [PubMed] [Google Scholar]
- Alonso G. Guillemain I. Dumoulin A. Privat A. Patey G. Immunolocalization of Bcl-xL/S in the central nervous system of neonatal and adult rats. Cell Tissue Res. 1997;288:59–68. doi: 10.1007/s004410050792. [DOI] [PubMed] [Google Scholar]
- Anderson K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Antignani A. Youle R.J. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr. Opin. Cell Biol. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Azcoitia I. Sierra A. Veiga S. Honda S. Harada N. Garcia-Segura L.M. Brain aromatase is neuroprotective. J. Neurobiol. 2001;47:318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Neuroanatomical substrates of functional recovery after experimental spinal cord injury: Implications of basic science research for human spinal cord injury. Phys. Ther. 2000;80:808–817. [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Beattie M.S. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol. Med. 2004;10:580–583. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Beattie M.S. Farooqui A.A. Bresnahan J.C. Review of current evidence for apoptosis after spinal cord injury. J. Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- Bialek M. Zaremba P. Borowicz K.K. Czuczwar S.J. Neuroprotective role of testosterone in the nervous system. Pol. J. Pharmacol. 2004;56:509–518. [PubMed] [Google Scholar]
- Carr B.G. Caplan J.M. Pryor J.P. Branas C.C. A meta-analysis of prehospital care times for trauma. Prehosp. Emerg. Care. 2006;10:198–206. doi: 10.1080/10903120500541324. [DOI] [PubMed] [Google Scholar]
- Carson F.L. Histotechnology: A Self-Instructional Text. Chicago: ASCP Press; 1990. pp. 170–171. [Google Scholar]
- Caruso A. Di Giorgi Gerevini V. Castiglione M. Marinelli F. Tomassini V. Pozzilli C. Caricasole A. Bruno V. Caciagli F. Moretti A. Nicoletti F. Melchiorri D. Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J. Neurochem. 2004;88:1179–1185. doi: 10.1046/j.1471-4159.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- Chang B.S. Kelekar A. Harris M.H. Harlan J.E. Fesik S.W. Thompson C.B. The BH3 domain of Bcl-x(S) is required for inhibition of the antiapoptotic function of Bcl-x(L) Mol. Cell Biol. 1999;19:6673–6681. doi: 10.1128/mcb.19.10.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaovipoch P. Jelks K.A. Gerhold L.M. West E.J. Chongthammakun S. Floyd C.L. 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J. Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- Chisu V. Manca P. Lepore G. Gadau S. Zedda M. Farina V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3-nitro-l-tyrosine incorporation into alpha-tubulin in a mouse neuroblastoma cell line. Arch. Ital. Biol. 2006;144:63–73. [PubMed] [Google Scholar]
- Clark M.J. Schopp L.H. Mazurek M.O. Zaniletti I. Lammy A.B. Martin T.A. Thomas F.P. Acuff M.E. Testosterone levels among men with spinal cord injury: Relationship between time since injury and laboratory values. Am. J. Phys. Med. Rehabil. 2008;87:758–767. doi: 10.1097/PHM.0b013e3181837f4f. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S. Genovese T. Mazzon E. Esposito E. Di P. R. Muia C. Crisafulli C. Peli A. Bramanti P. Chaudry I.H. Effect of 17beta-estradiol on signal transduction pathways and secondary damage in experimental spinal cord trauma. Shock. 2008;29:362–371. doi: 10.1097/shk.0b013e31814545dc. [DOI] [PubMed] [Google Scholar]
- de Groat W.C. Kawatani M. Hisamitsu T. Cheng C.L. Ma C.P. Thor K. Steers W. Roppolo J.R. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. Syst. 1990;30(Suppl):S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- Dietz V. Nakazawa K. Wirz M. Erni T. Level of spinal cord lesion determines locomotor activity in spinal man. Exp. Brain Res. 1999;128:405–409. doi: 10.1007/s002210050861. [DOI] [PubMed] [Google Scholar]
- Doperalski N.J. Sandhu M.S. Bavis R.W. Reier P.J. Fuller D.D. Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats. Respir. Physiol. Neurobiol. 2008;162:160–167. doi: 10.1016/j.resp.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden A.I. Stoica B. Neuroprotection: Challenges and opportunities. Arch. Neurol. 2007;64:794–800. doi: 10.1001/archneur.64.6.794. [DOI] [PubMed] [Google Scholar]
- Farooque M. Suo Z. Arnold P.M. Wulser M.J. Chou C.T. Vancura R.W. Fowler S. Festoff B.W. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord. 2006;44:182–187. doi: 10.1038/sj.sc.3101816. [DOI] [PubMed] [Google Scholar]
- Fehlings M.G. Tator C.H. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Ferguson A.R. Hook M.A. Garcia G. Bresnahan J.C. Beattie M.S. Grau J.W. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J. Neurotrauma. 2004;21:1601–1613. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- Gatson J.W. Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–2464. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- George E.R. Scholten D.J. Buechler C.M. Jordan-Tibbs J. Mattice C. Albrecht R.M. Failure of methylprednisolone to improve the outcome of spinal cord injuries. Am. Surg. 1995;61:659–663. [PubMed] [Google Scholar]
- Gold S.M. Voskuhl R.R. Testosterone replacement therapy for the treatment of neurological and neuropsychiatric disorders. Curr. Opin. Investig. Drugs. 2006;7:625–630. [PubMed] [Google Scholar]
- Gorska T. Chojnicka-Gittins B. Majczynski H. Zmyslowski W. Overground locomotion after incomplete spinal lesions in the rat: Quantitative gait analysis. J. Neurotrauma. 2007;24:1198–1218. doi: 10.1089/neu.2006.0219. [DOI] [PubMed] [Google Scholar]
- Gorska T. Chojnicka-Gittins B. Majczynski H. Zmyslowski W. Recovery of overground locomotion following partial spinal lesions of different extent in the rat. Behav. Brain Res. 2009;196:286–296. doi: 10.1016/j.bbr.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Springer J.E. Neuroprotection and acute spinal cord injury: A reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J. Le Q. Goodyer C. Gelfand M. Trifiro M. LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Hamner S. Skoglosa Y. Lindholm D. Differential expression of bcl-w and bcl-x messenger RNA in the developing and adult rat nervous system. Neuroscience. 1999;91:673–684. doi: 10.1016/s0306-4522(98)00642-3. [DOI] [PubMed] [Google Scholar]
- Harris M.H. Thompson C.B. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000;7:1182–1191. doi: 10.1038/sj.cdd.4400781. [DOI] [PubMed] [Google Scholar]
- Hauben E. Mizrahi T. Agranov E. Schwartz M. Sexual dimorphism in the spontaneous recovery from spinal cord injury: A gender gap in beneficial autoimmunity? Eur. J. Neurosci. 2002;16:1731–1740. doi: 10.1046/j.1460-9568.2002.02241.x. [DOI] [PubMed] [Google Scholar]
- Hugenholtz H. Methylprednisolone for acute spinal cord injury: Not a standard of care. CMAJ. 2003;168:1145–1146. [PMC free article] [PubMed] [Google Scholar]
- Huppenbauer C.B. Tanzer L. DonCarlos L.L. Jones K.J. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: Equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J. Neurosci. 2005;25:4004–4013. doi: 10.1523/JNEUROSCI.5279-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane R.W. Davis A.R. Dietrich W.D. Inflammatory and apoptotic signaling after spinal cord injury. J. Neurotrauma. 2006;23:335–344. doi: 10.1089/neu.2006.23.335. [DOI] [PubMed] [Google Scholar]
- Keirstead H.S. Fedulov V. Cloutier F. Steward O. Duel B.P. A noninvasive ultrasonographic method to evaluate bladder function recovery in spinal cord injured rats. Exp. Neurol. 2005;194:120–127. doi: 10.1016/j.expneurol.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kloos A.D. Fisher L.C. Detloff M.R. Hassenzahl D.L. Basso D.M. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp. Neurol. 2005;191:251–265. doi: 10.1016/j.expneurol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kujawa K.A. Emeric E. Jones K.J. Testosterone differentially regulates the regenerative properties of injured hamster facial motoneurons. J. Neurosci. 1991;11:3898–3906. doi: 10.1523/JNEUROSCI.11-12-03898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa K.A. Kinderman N.B. Jones K.J. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp. Neurol. 1989;105:80–85. doi: 10.1016/0014-4886(89)90174-x. [DOI] [PubMed] [Google Scholar]
- Kujawa K.A. Tanzer L. Jones K.J. Inhibition of the accelerative effects of testosterone on hamster facial nerve regeneration by the antiandrogen flutamide. Exp. Neurol. 1995;133:138–143. doi: 10.1006/exnr.1995.1016. [DOI] [PubMed] [Google Scholar]
- Lee H.C. Cho D.Y. Lee W.Y. Chuang H.C. Pitfalls in treatment of acute cervical spinal cord injury using high-dose methylprednisolone: A retrospect audit of 111 patients. Surg. Neurol. 2007;68(Suppl 1):S37–S41. doi: 10.1016/j.surneu.2007.06.085. [DOI] [PubMed] [Google Scholar]
- Motoyama N. Wang F. Roth K.A. Sawa H. Nakayama K. Nakayama K. Negishi I. Senju S. Zhang Q. Fujii S. Lon D.Y. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal cord injury information network. 2008.
- Nesic-Taylor O. Cittelly D. Ye Z. Xu G.Y. Unabia G. Lee J.C. Svrakic N.M. Liu X.H. Youle R.J. Wood T.G. McAdoo D. Westlund K.N. Hulsebosch C.E. Perez-Polo J.R. Exogenous Bcl-xL fusion protein spares neurons after spinal cord injury. J. Neurosci. Res. 2005;79:628–637. doi: 10.1002/jnr.20400. [DOI] [PubMed] [Google Scholar]
- Nguyen T.V. Yao M. Pike C.J. Androgens activate mitogen-activated protein kinase signaling: Role in neuroprotection. J. Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- Noble L.J. Wrathall J.R. An inexpensive apparatus for producing graded spinal cord contusive injury in the rat. Exp. Neurol. 1987;95:530–533. doi: 10.1016/0014-4886(87)90162-2. [DOI] [PubMed] [Google Scholar]
- Nout Y.S. Leedy G.M. Beattie M.S. Bresnahan J.C. Alterations in eliminative and sexual reflexes after spinal cord injury: Defecatory function and development of spasticity in pelvic floor musculature. Prog. Brain Res. 2006;152:359–372. doi: 10.1016/S0079-6123(05)52024-7. [DOI] [PubMed] [Google Scholar]
- Ogata T. Nakamura Y. Tsuji K. Shibata T. Kataoka K. Steroid hormones protect spinal cord neurons from glutamate toxicity. Neuroscience. 1993;55:445–449. doi: 10.1016/0306-4522(93)90513-f. [DOI] [PubMed] [Google Scholar]
- Orlando R. Caruso A. Molinaro G. Motolese M. Matrisciano F. Togna G. Melchiorri D. Nicoletti F. Bruno V. Nanomolar concentrations of anabolic-androgenic steroids amplify excitotoxic neuronal death in mixed mouse cortical cultures. Brain Res. 2007;1165:21–29. doi: 10.1016/j.brainres.2007.06.047. [DOI] [PubMed] [Google Scholar]
- Parsadanian A.S. Cheng Y. Keller-Peck C.R. Holtzman D.M. Snider W.D. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J. Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C.J. Nguyen T.V. Ramsden M. Yao M. Murphy M.P. Rosario E.R. Androgen cell signaling pathways involved in neuroprotective actions. Horm. Behav. 2008;53:693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikov V. Wrathall J.R. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: Effect of injury severity. J. Neurosci. 2001;21:559–569. doi: 10.1523/JNEUROSCI.21-02-00559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikov V. Wrathall J. R. Altered glutamate receptor function during recovery of bladder detrusor-external urethral sphincter coordination in a rat model of spinal cord injury. J. Pharmacol. Exp. Ther. 2002;300:421–427. doi: 10.1124/jpet.300.2.421. [DOI] [PubMed] [Google Scholar]
- Pikov V. Gillis R.A. Jasmin L. Wrathall J.R. Assessment of lower urinary tract functional deficit in rats with contusive spinal cord injury. J. Neurotrauma. 1998;15:375–386. doi: 10.1089/neu.1998.15.375. [DOI] [PubMed] [Google Scholar]
- Qiu J. Nesic O. Ye Z. Rea H. Westlund K.N. Xu G.Y. McAdoo D. Hulsebosch C.E. Perez-Polo J.R. Bcl-xL expression after contusion to the rat spinal cord. J. Neurotrauma. 2001;18:1267–1278. doi: 10.1089/089771501317095304. [DOI] [PubMed] [Google Scholar]
- Ritz M.F. Hausmann O.N. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Roselli C.F. Brain aromatase: Roles in reproduction and neuroprotection. J. Steroid Biochem. Mol. Biol. 2007;106:143–150. doi: 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse T. Olivier R. Monney L. Rager M. Conus S. Fellay I. Jansen B. Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- Rouleau P. Ung R.V. Lapointe N.P. Guertin P.A. Hormonal and immunological changes in mice after spinal cord injury. J. Neurotrauma. 2007;24:367–378. doi: 10.1089/neu.2006.0117. [DOI] [PubMed] [Google Scholar]
- Schopp L.H. Clark M. Mazurek M.O. Hagglund K.J. Acuff M.E. Sherman A.K. Childers M.K. Testosterone levels among men with spinal cord injury admitted to inpatient rehabilitation. Am. J. Phys. Med. Rehabil. 2006;85:678–684. doi: 10.1097/01.phm.0000228617.94079.4a. [DOI] [PubMed] [Google Scholar]
- Springer J.E. Apoptotic cell death following traumatic injury to the central nervous system. J. Biochem. Mol. Biol. 2002;35:94–105. doi: 10.5483/bmbrep.2002.35.1.094. [DOI] [PubMed] [Google Scholar]
- Sribnick E.A. Matzelle D.D. Ray S.K. Banik N.L. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J. Neurosci. Res. 2006;84:1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sribnick E.A. Wingrave J.M. Matzelle D.D. Wilford G.G. Ray S.K. Banik N.L. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J. Neurosci. Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Suberviola B. Gonzalez-Castro A. Llorca J. Ortiz-Melon F. Minambres E. Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury. 2008;39:748–752. doi: 10.1016/j.injury.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Swartz K.R. Fee D.B. Joy K.M. Roberts K.N. Sun S. Scheff N.N. Wilson M.E. Scheff S.W. Gender differences in spinal cord injury are not estrogen-dependent. J. Neurotrauma. 2007;24:473–480. doi: 10.1089/neu.2006.0167. [DOI] [PubMed] [Google Scholar]
- Webb A.A. Chan C.B. Brown A. Saleh T.M. Estrogen reduces the severity of autonomic dysfunction in spinal cord-injured male mice. Behav. Brain Res. 2006;171:338–349. doi: 10.1016/j.bbr.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Wise P.M. Dubal D.B. Wilson M.E. Rau S.W. Liu Y. Estrogens: Trophic and protective factors in the adult brain. Front. Neuroendocrinol. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- Yang E. Korsmeyer S.J. Molecular thanatopsis: A discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- Yang S.H. Liu R. Wen Y. Perez E. Cutright J. Brun-Zinkernagel A.M. Singh M. Day A.L. Simpkins J.W. Neuroendocrine mechanism for tolerance to cerebral ischemia-reperfusion injury in male rats. J. Neurobiol. 2005;62:341–351. doi: 10.1002/neu.20103. [DOI] [PubMed] [Google Scholar]
- Yang S.H. Perez E. Cutright J. Liu R. He Z. Day A.L. Simpkins J.W. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J. Appl. Physiol. 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- Yune T.Y. Kim S.J. Lee S.M. Lee Y.K. Oh Y.J. Kim Y.C. Markelonis G.J. Oh T.H. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J. Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
- Yune T.Y. Park H.G. Lee J.Y. Oh T.H. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through Phosphoinositide-3-Kinase/Akt-dependent CREB activation. J. Neurotrauma. 2008;25:1121–1131. doi: 10.1089/neu.2008.0544. [DOI] [PubMed] [Google Scholar]
- Zhang X. Chen Y. Jenkins L.W. Kochanek P.M. Clark R.S. Bench-to-bedside review: Apoptosis/programmed cell death triggered by traumatic brain injury. Crit. Care. 2005;9:66–75. doi: 10.1186/cc2950. [DOI] [PMC free article] [PubMed] [Google Scholar]