Abstract
Flotillins and caveolins represent two types of resident proteins associated with lipid rafts in mammalian cells, however, their possible cross-talk in regulating lipid raft functions remains poorly understood. In this report, we observed that siRNA-mediated down-regulation of flotillin-1 expression which disrupted lipid raft-mediated endocytosis of BODIPY FL C5-lactosylceramide also substantially decreased caveolin-1 level in SK-CO15 human intestinal epithelial cells. The decrease in caveolin-1 expression appeared to be specific for flotillin-1 knock-down and was not observed after down-regulation of flotillin-2. The decrease in caveolin-1 level in flotillin-1-depleted cells was not due to suppression of its mRNA synthesis and was not mimicked by cholesterol depletion of SK-CO15 cells. Furthermore, flotillin-1 dependent down-regulation of caveolin-1 was reversed after cell exposure to lysosomal inhibitor, chloroquine but not proteosomal inhibitor, MG262. Our data suggest that flotillin-1 regulates caveolin-1 level by preventing its lysosomal degradation in intestinal epithelial cells.
Keywords: Epithelial cells, Lipid rafts, Lactosyl ceramide internalization, Lysosomal degradation
The lipid rafts represent specialized dynamic areas (domains) of eukaryotic membranes which are characterized by unique chemical composition and physical properties [1,2]. The most vivid feature of lipid raft is their enrichment in particular lipids such as cholesterol and sphingolipids, which create a microenvironment for recruitment of various membrane and cytosolic proteins with high binding affinity to these lipids. By clustering/segregating different lipid and protein constituents of the membrane, rafts mediate many crucial cellular functions, including regulation of signaling by plasma membrane receptors, activity of ion pumps and channels and mediation of vesicle trafficking/fusion [3,4]. Although membrane rafts are readily formed in a pure lipid environments of liposomes, in living cells these lipid domains are frequently supported by specialized proteins which are important modulators of raft structure, stability and functions [5]. Resident lipid raft proteins flotillins and caveolins are co-expressed in many types of mammalian cells, and as such they are frequently enriched in the same cell membranes. However, several recent studies have demonstrated that flotillins and caveolins do not intermix and populate distinct subsets of lipid rafts [6–10].
The flotillin (also known as the reggie) protein family consists of two ubiquitously expressed isoforms, flotillin-1 and flotillin-2. Flotillins are evolutionary highly conserved proteins [11] that belong to the SPFH (for stomatins, prohibitins, flotillins, H.K/C) protein superfamily and have propensity for oligomerization [12,13]. Flotillins are enriched at the plasma membrane where they associate with the inner leaflet via hydrophobic amino acid stretches and posttranslationally attached acyl groups of palmitic and myristic acids [13–15]. At the plasma membrane, flotillin-1 and flotillin-2 are organized as homo- and hetero-tetramers and flotillin-2 is required for stabilization of flotillin-1 [12,16]. Flotillins readily induce membrane curvature and reportedly mediate a unique clathrin-independent endocytic pathway [6,9]. Beside their proposed role in endocytosis, flotillins are shown to participate in signaling by GPI-anchored plasma membrane proteins and in regulation of the actin cytoskeleton reorganizations, and actin-dependent cell adhesion and motility [15,11].
Caveolins are well-characterized resident lipid raft proteins, which participate in the formation of a distinct subset of the membrane rafts called caveolae [17–19]. Three isoforms of caveolin are known: ubiquitously expressed caveolin-1 and caveolin-2, and muscle-specific caveolin-3. Knock-down experiments in cultured cells and in vivo in genetically modified mice yielded important information about role of caveolins in caveolae formation at the plasma membrane and caveolar endocytosis [20]. For example, downregulation of caveolin-1 impaired albumin uptake in bovine aortic endothelial cells [21] and inhibited internalization of low-density lipoprotein receptor-related protein 6 in HeLa cells [22]. Similarly to flotillins, caveolin isoforms undergo homo- and hetero-association at the cell membranes. Such inter-isoform associations may stabilize caveolins in the cells since expression of caveolin-2 was shown to be strongly downregulated in caveolin-1 knock-down mice, possibly by accelerating its degradation, [20].
Although flotillins and caveolins occupy topographically distinct domains of the plasma membrane and do not physically associate [23,24,10,9], they may cooperate functionally by controlling different steps of protein endocytosis [25,26]. Therefore, it is important to know if these two types of lipid raft modulators cross-talk and influence each other expression and functions. Recent studies have demonstrated that cellular levels and localization of flotillin-1 and flotillin-2 do not depend on caveolin-1 expression in fibroblasts and epithelial cells [8,10]. However, whether flotillins regulate expression of caveolins has not been investigated. Here, we report a serendipitous observation that selective down-regulation of flotillin-1 expression in SK-CO15 human intestinal epithelial cells caused dramatic decrease in protein level of caveolin-1. Such decrease in caveolin-1 expression was not due to diminished mRNA level, but was a consequence of accelerated lysosomal degradation of this protein. These data provide the first evidence of an interplay between flotillins and caveolins and suggest that flotillin stabilizes caveolin-1 in living cells and therefore my regulate caveolin- 1 functions.
Materials and methods
Antibodies and other reagents
The following primary polyclonal (pAb) and monoclonal (mAb) antibodies were used for immunoblotting: flotillin-1 mAb clone 18, flotillin-2 mAb clone 29, caveolin- 1 pAb (BD Biosciences, San Jose, CA); α-actin pAb (Sigma–Aldrich, St. Louis, MO). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). BODIPY FL C5-lactosylceramide (BODIPY-LacCer) was obtained from Invitrogen (Carlsbad, CA), MG262 was purchased from Biomol Research Laboratories (Plymouth Meetings, PA), chloroquine, methyl-β-cyclodextrin and other reagents were obtained from Sigma.
Cell culture and RNA interference
SK-CO15 cells were provided by Dr. E. Rodriguez-Boulan, Weill Medical College of Cornell University, NY and were cultured in DMEM as described previously [27–29]. Cells were grown on 6-well plates or on collagen-coated cover slips for immunoblotting and immunofluorescence labeling respectively. RNA interference experiments were performed using siRNA SmartPools for human flotillin-1, flotillin-2, caveolin-1, and lamin A/C as control (Dharmacon, Lafayette, CO). SK-CO15 cells were plated at 60–70% confluency and were transfected next day with 50–100 nm of siRNA using DharmaFect 1 transfection reagent (Dharmacon) according to a manufacturer protocol. Cells were analyzed 72–98 h post-transfection. For the inhibitory analysis, transfected SK-CO15 cells were incubated with either 0.1 mM chloroquine or 10 nm MG262 for 22 h in DMEM. Cholesterol depletion was performed by treating cells with 5 mM methyl-β-cyclodextrin for 23 h in a serum-free DMEM.
Real-time RT-PCR
Total RNA was isolated from SK-CO15 cells using TRIsol LS reagent (Invitrogen) according to the manufacturer instruction. RNA samples treated with DNAseI (Invitrogen) to remove genomic DNA. One-step quantitative real-time RT-PCR was performed using BioRad iCycler and iScript One-step PT-PCR kit containing a SYBR Green dye (BioRad, Hercules, CA). Sequences for caveolin-1 and a housekeeping control 18S rRNA primers were published elsewhere [30,31], primer oligonucleotides were synthesized by IDT (Coralville, IA).
Immunoblotting
Cells were homogenized in Laemmli sample buffer containing a proteinase inhibitor cocktail (1:100, Sigma) and phosphatase inhibitor cocktails 1 and 2 (both at 1:200, Sigma). Lysates were supplemented with β-mercaptoethanol and boiled. SDS polyacrylamide gel electrophoresis and immunoblotting were conducted by standard protocols with 10–20 μg protein per lane. Proteins of interest were visualized after their transfer on nitrocellulose membrane using appropriate primary and HRP-conjugated secondary antibodies. Results shown are representative immunoblots of at least three independent experiments. Protein expression was quantified by densitometric analysis of immunoblot images using UN-SCAN-IT digitizing software (Silk Scientific, Orem, UT).
BODIPY-LacCer internalization assay
SK-CO15 cells were transfected with flotillin or control siRNAs, replated on coverslips 54 h post-transfection, and 18 h later were subjected to internalization assay. The assay was modified from protocol described elsewhere [32]. Briefly, SK-CO15 cells were washed in serum-free DMEM and incubated with 1 μM BODIPY-LacCer in the same media at for 30 min at 4 °C. Unbound BODIPY-LacCer was removed by washing cells with cold DMEM and internalization was started by placing cells at 37 °C for 5 min. Internalization was stopped by transferring cells back on ice and non-internalized BODIPY-LacCer was removed from the cell surface by incubating cells in the same medium supplemented with 5% fatty-acid free BSA for 1 h on ice, exchanging the medium for fresh every 10 min. Cells were fixed with 3.7% paraformaldehyde and were mounted on slides using ProLong Antifade medium (Invitrogen). The slides were examined using a Zeiss LSM510 laser scanning confocal microscope (Zeiss Microimaging Inc., Thornwood, NY) coupled to a Zeiss 100M axio-vert and 63× or 100× Pan-Apochromat oil lenses. Image analysis including pixel intensity measurement was performed using Axio-vision software (Release 4.3, Zeiss).
Results and discussion
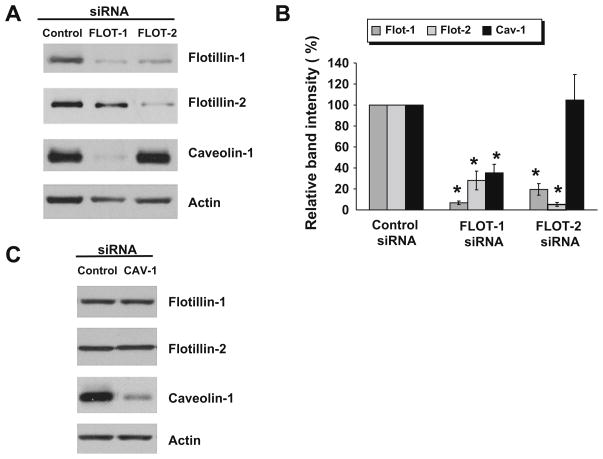
To examine possible relationships between two types of resident lipid raft proteins in intestinal epithelial cells, expression of flotillin isoforms and caveolin-1 was down-regulated using RNA interference in a SK-CO15 colonic epithelial cell line. Fig. 1A and B shows that transfection of SK-CO15 cells with flotillin-1 or flotillin- 2-specific siRNA SmartPools resulted in approximately 94% and 95% decrease in expression of targeted proteins, respectively. Furthermore, knock-down of either flotillin isoform mutually downregulated each other levels (Fig. 1A and B). This finding is consistent with previous report [33] and is likely to reflects known hetero- oligomerization of flotillin-1 and flotillin-2 which stabilizes them at cell membranes [16,12]. More interestingly, we observed that downregulation of flotillin-1 induced a substantial (~65%) decrease in the level of caveolin-1, whereas flotillin-2 knock-down did not affect caveolin-1 expression (Fig. 1A and B). Such a selective effect of flotillin-1 knock-down on caveolin-1 expression is surprising since we observed significant down-regulation of flotillin- 1 in flotillin-2-depleted cells (Fig. 1A and B). However, this observation can be explained by the different extent of flotillin-1 down-regulation. Indeed only ~6% of initial flotillin-1 remained in SK-CO15 cells treated with flotillin-1-specific siRNA, whereas approximately 20% of this isoform was detected in flotillin-2-depleted cells (Fig. 1B). Since flotillin-1 is an abundant intracellular protein, even 20% of its normal level may be sufficient in mediating some of its cellular activities and a significant depletion is required to demonstrate the entire functional effects of flotillin-1 knock-down. On the other hand, siRNA-mediated downregulation of caveolin-1 did not affect flotillin-1 or flotillin-2 protein level (Fig. 1C), which indicates a lack of mutual regulation of these two types of lipid raft proteins. To our best knowledge, this is the first report that flotillin-1 cross-talks with caveolin-1 by regulating its expression. Our findings are also in line with two recent studies of caveolin-1 null and caveolin-1 expressing fibroblasts and thyroid epithelial cells, which showed that cellular level and localization of flotillin-1 is regulated in caveolin-1-independent fashion [8,10].
Fig. 1.
Down-regulation of flotillin-1 expression decreases caveolin-1 level in intestinal epithelial cells. SK-CO15 cells were transfected with flotillin-1, flotillin-2, caveolin-1 or control siRNAs and expression of lipid raft-resident proteins in these cells was analyzed by immunoblotting 72 h post-transfection. Representative immunoblots (A and C) and densitometric quantification of immunoreactive bands (B) are shown. Note dramatic down-regulation of caveolin-1 expression in flotillin-1 but not flotillin-2-depleted cells and lack of effect of caveolin-1 knock-down on flotillin isoforms level. Data presented as means ± SE (n = 5), *P < 0.05 compared to the control siRNA-treated group.
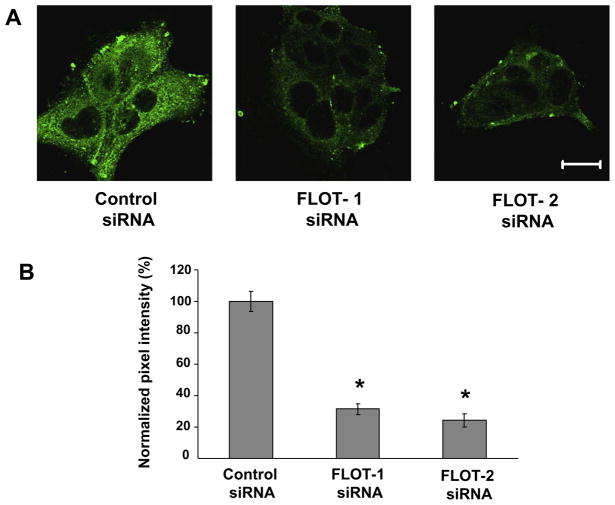
Given a putative role of flotillins in internalization of membrane raft associated lipids and proteins [6,34], we next investigated effects of flotillin isoforms knock-down on lipid raft-mediated endocytosis. This endocytic pathway was probed by tracking internalization of sphingolipid analog lactosylceramide conjugated with a BODIPY fluorescent dye [32]. Fig. 2 shows that down-regulation of flotillin-1 and flotillin-2 resulted in approximately 68% and 76% decrease in the intensity of intracellular BODIPY-LacCer signal, respectively, therefore indicating dramatic inhibition of this lipid raft marker endocytosis in flotillin-1 and flotillin-2-depleted SK-CO15 cells. Furthermore, our data suggest that decreased internalization of BODIPY-LacCer is a direct consequence of down-regulation of flotillins expression and it does not depend on caveolin-1 level.
Fig. 2.
Down-regulation of flotillin-1 and flotillin-2 expression inhibits lipid raft-mediated endocytosis. SK-CO15 cells were transfected with flotillin-1, flotillin-2 or control siRNAs and were subjected to the BODIPY-LacCer internalization assay 72 h post-transfection. Representative images of internalized LacCer (green, A) and pixel intensity quantification of internalized dye fluorescence (B) are shown. Note significant decrease in intracellular level of BODIPY-LacCer in flotillin-1 and flotillin-2 depleted cells. Data presented as means ± SE (n = 14), *P < 0.05 compared to the control siRNA-treated group. Bar, 20 μm.(For interpretation of color mentioned in this figure the reader is referred to the web version of the article.)
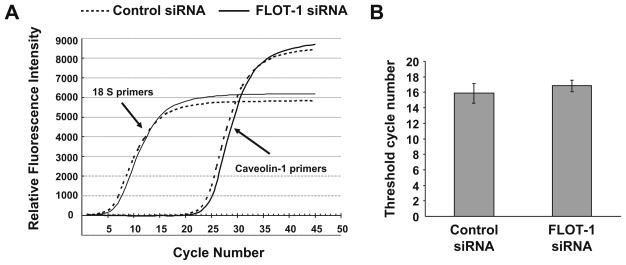
Given substantial and selective decrease of caveolin-1 expression in flotillin-1-depleted SK-CO15 cells, we next investigated possible mechanisms of such down-regulation. To distinguish between transcriptional and posttranslational mechanisms we analyzed expression of caveolin-1 mRNA by quantitative real time RT-PCR (Fig. 3A and B). RT-PCR reactions using caveolin-1-specific primers and total RNA isolated from SK-CO15 cells produced an amplicon that migrated as a single band of predicted size in 1.5% agarose gel and demonstrated a single maximum on its melting curve obtained using the BioRad iCycler (data not shown). Both parameters indicate high specificity of caveolin-1 mRNA amplification. A real time kinetic of caveolin-1 amplification was identical in RNA samples isolated from control and flotillin-1-depleted SKCO15 cells (Fig. 3A) and a calculated threshold cycle number did not differ significantly in these two experimental groups (Fig. 3B). These results indicate similar expression of caveolin-1 mRNA in control and flotillin-1-deficient SK-CO15 cells and suggest that the decrease in caveolin-1 protein level caused by flotillin- 1 knock-down is regulated on the posttranslational stage.
Fig. 3.
Flotillin-1 knock-down does not affect mRNA expression of caveolin-1. SK-CO15 cells were transfected with flotillin-1 or control siRNAs and 72 h post-transfection were processed for RNA isolation and real-time RT PCR quantification of caveolin-1 mRNA expression. Individual amplification curves for caveolin-1 and a housekeeping control, 18S rRNA (A) and calculated threshold cycle number for caveolin-1 amplification (B) are shown. Note identical threshold cycle number for caveolin-1 amplification in flotillin-1-depleted and control SK-CO15 cells. Data presented as means ± SE (n = 3).
The most likely mechanism of the posttranslational control of caveolin-1 level involves accelerated degradation of this protein. Indeed, increased degradation was previously shown to be responsible for decreased expression of caveolin-1 in different experimental conditions [35,36]. Therefore, we next examined the role of protein degradation in decreased caveolin-1 expression using selective inhibitors of the lysosomal and proteosomal degradation pathways [37,38]. Fig. 4A and B shows that lysosomal inhibitor chloroquine (0.1 mM) restored caveolin-1 expression in flotillin-1-depleted cells. By contrast, no significant change in caveolin-1 level was observed in these cells after exposure to a proteosomal inhibitor MG262 (10 nm, Fig. 4A and B). These results strongly suggest that depletion of flotillin-1 in SK-CO15 cells results in accelerated lysosomal degradation of caveolin-1. Our findings are consistent with previous studies which demonstrated that lysosomal, rather then proteosomal degradation of caveolin-1 occurred in vascular smooth muscle cells exposed to platelet-derived growth factor [35], or in epithelial cells depleted of certain caveolin- 1-regulating proteins [36,39].
Fig. 4.
Flotillin-1 knock-down decreases protein level of caveolin-1 by accelerating its lysosomal degradation in cholesterol-independent fashion. SK-CO15 cells were transfected with flotillin-1 or control siRNAs and 50 h post-transfection exposed to either lysosomal inhibitor chloroquine (CQ, 0.1 mM), proteosomal inhibitor MG262 (10 nm) or vehicle for additional 22 h. Expression of resident lipid raft proteins was analyzed by immunoblotting. Representative immunoblots (A) and densitometric quantification of immunoreactive bands (B) are shown. Note restoration of caveolin-1 expression in flotillin-1-depleted cells treated by chloroquine but not MG262. Data presented as means ± SE (n = 3), *P < 0.05 compared to the control siRNA-treated group. (C) SK-CO15 cells were incubated with either m-β-CD (5 mM) or vehicle for 22 h and expression of their resident lipid raft proteins was analyzed by immunoblotting. A representative immunoblot shows that m-β-CD treatment did not affect expression of caveolin-1 or flotillins in intestinal epithelial cells.
How does flotillin-1 knock-down destabilize caveolin-1 and trigger its lysosomal degradation in intestinal epithelial cells? One can suggest that flotillin-1 physically interacts with caveolin- 1 therefore stabilizing it at the plasma membrane and/or in other cellular compartments. However, this seems to be unlikely given several reports demonstrating lack of colocalization, cofractionation and physical association of flotillins and caveolin-1 in different types of epithelia [23,24,10,9]. Therefore, some indirect mechanisms should be involved in flotillin-1-dependent regulation of caveolin-1. Since both flotillins and caveolins are cholesterol-binding proteins, it is reasonable to suggest that they may influence each other by controlling the abundance and/or physical state of cholesterol at cell membranes. To test this possibility, we examined effects of cholesterol depletion on caveolin-1 expression using a cholesterol-extracting agent methyl-β-cyclodextrin (m-β-CD). A prolonged (~22 h) incubation of SK-CO15 cells with m-β-CD (5 mM) triggered some apoptotic events detected by PARP cleavage (data not shown), which indicated m-β-CD activity in our experimental conditions. Nevertheless, such cholesterol extraction did not affect levels of either caveolin-1 or flotillins (Fig. 4C). These data argue against the role of cholesterol in caveolin-1 destabilization caused by flotillin-1 depletion in SK-CO15 cells. Alternative mechanisms may be suggested based on a recently discovered wide repertoire of flotillin-1 functions, and particularly on its reported role in protein exocytosis and regulation of the actin cytoskeleton [15,11]. For example, since caveolin-1 was shown to constantly shuttle between Golgi and plasma membrane [5], defective vesicle exocytosis in flotillin-1-depleted cells may disrupt caveolin-1 trafficking and divert it into the lysosomal compartment. On the other hand, destabilization of the cortical actin cytoskeleton caused by flotillin-1 knock-down may increase internalization of caveolin-1 at the plasma membrane and direct it for degradation. Future studies are required to clarify exact mechanism of accelerated lysosomal degradation of caveolin-1 in flotillin-1-depleted cells. In conclusion, we report for the first time that flotillin-1 regulates caveolin-1 expression in intestinal epithelial cells. This regulation occurs on posttranslational level and involves protection of caveolin-1 from degradation in lysosomes. Through this mechanism flotillin-1 is likely to control organization and functions of caveolin-1-rich structures in epithelial cells.
Acknowledgments
The authors thank Dr. Enrique Rodriguez-Boulan for generous gift of SK-CO15 cells and Dr. Susan Voss for the excellent technical assistance. This work was supported by grants from the NIH (DK 59888) (A.N.) and CCFA (A.I.).
References
- 1.Pike LJ. Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci. 2005;118:1099–1102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- 4.Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–2104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Bauer M, Pelkmans L. A new paradigm for membrane-organizing and - shaping scaffolds. FEBS Lett. 2006;580:5559–5564. doi: 10.1016/j.febslet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 6.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald JL, Pike LJ. A simplified method for the preparation of detergent-free lipid rafts. J Lipid Res. 2005;46:1061–1067. doi: 10.1194/jlr.D400041-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Rajendran L, Le Lay S, Illges H. Raft association and lipid droplet targeting of flotillins are independent of caveolin. Biol Chem. 2007;388:307–314. doi: 10.1515/BC.2007.034. [DOI] [PubMed] [Google Scholar]
- 9.Frick M, Bright NA, Riento K, Bray A, Merrified C, Nichols BJ. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol. 2007;17:1151–1156. doi: 10.1016/j.cub.2007.05.078. [DOI] [PubMed] [Google Scholar]
- 10.Fernow I, Icking A, Tikkanen R. Reggie-1 and reggie-2 localize in non-caveolar rafts in epithelial cells: cellular localization is not dependent on the expression of caveolin proteins. Eur J Cell Biol. 2007;86:345–352. doi: 10.1016/j.ejcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Babuke T, Tikkanen R. Dissecting the molecular function of reggie/flotillin proteins. Eur J Cell Biol. 2007 doi: 10.1016/j.ejcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Solis GP, Hoegg M, Munderloh C, Schrock Y, Malaga-Trillo E, Rivera-Milla E, Stuermer CA. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403:313–322. doi: 10.1042/BJ20061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browman DT, Hoegg MB, Robbins SM. The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol. 2007;17:394–402. doi: 10.1016/j.tcb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Morrow IC, Parton RG. Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic. 2005;6:725–740. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 15.Langhorst MF, Reuter A, Stuermer CA. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell Mol Life Sci. 2005;62:2228–2240. doi: 10.1007/s00018-005-5166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoehne M, de Couet HG, Stuermer CA, Fischbach KF. Loss- and gain-of-function analysis of the lipid raft proteins Reggie/Flotillin in Drosophila: they are posttranslationally regulated, and misexpression interferes with wing and eye development. Mol Cell Neurosci. 2005;30:326–338. doi: 10.1016/j.mcn.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Parton RG, Hanzal-Bayer M, Hancock JF. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J Cell Sci. 2006;119:787–796. doi: 10.1242/jcs.02853. [DOI] [PubMed] [Google Scholar]
- 18.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 19.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez E, Nagiel A, Lin AJ, Golan DE, Michel T. Small interfering RNA-mediated down-regulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J Biol Chem. 2004;279:40659–40669. doi: 10.1074/jbc.M407051200. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of β-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Evans WEt, Coyer RL, Sandusky MF, Van Fleet MJ, Moore JG, Nyquist SE. Characterization of membrane rafts isolated from rat Sertoli cell cultures: caveolin and flotillin-1 content. J Androl. 2003;24:812–821. doi: 10.1002/j.1939-4640.2003.tb03132.x. [DOI] [PubMed] [Google Scholar]
- 24.Roitbak T, Surviladze Z, Tikkanen R, Wandinger-Ness A. A polycystin multiprotein complex constitutes a cholesterol-containing signaling microdomain in human kidney epithelia. Biochem J. 2005;392:29–38. doi: 10.1042/BJ20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fecchi K, Volonte D, Hezel MP, Schmeck K, Galbiati F. Spatial and temporal regulation of GLUT4 translocation by flotillin-1 and caveolin-3 in skeletal muscle cells. FASEB J. 2006;20:705–707. doi: 10.1096/fj.05-4661fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanchet MH, Le Good JA, Mesnard D, Oorschot V, Baflast S, Minchiotti G, Klumperman J, Constam DB. Cripto recruits Furin and PACE4 and controls Nodal trafficking during proteolytic maturation. EMBO J. 2008 doi: 10.1038/emboj.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bivic A, Real FX, Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci USA. 1989;86:9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov AI, McCall IC, Babbin B, Samarin SN, Nusrat A, Parkos CA. Microtubules regulate disassembly of epithelial apical junctions. BMC Cell Biol. 2006;7:12. doi: 10.1186/1471-2121-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS ONE. 2007;2:e658. doi: 10.1371/journal.pone.0000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayoral R, Fernandez-Martinez A, Roy R, Bosca L, Martin-Sanz P. Dispensability and dynamics of caveolin-1 during liver regeneration and in isolated hepatic cells. Hepatology. 2007;46:813–822. doi: 10.1002/hep.21746. [DOI] [PubMed] [Google Scholar]
- 31.Akpek EK, Jabs DA, Gerard HC, Prendergast RA, Hudson AP, Lee B, Whittum-Hudson JA. Chemokines in autoimmune lacrimal gland disease in MRL/MpJ mice. Invest Ophthalmol Vis Sci. 2004;45:185–190. doi: 10.1167/iovs.03-0812. [DOI] [PubMed] [Google Scholar]
- 32.Marks DL, Singh RD, Choudhury A, Wheatley CL, Pagano RE. Use of fluorescent sphingolipid analogs to study lipid transport along the endocytic pathway. Methods. 2005;36:186–195. doi: 10.1016/j.ymeth.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Chintagari NR, Gou D, Liu L. Knockdown of flotillin-2 inhibits lung surfactant secretion by alveolar type II cells. Cell Res. 2008;18:701–703. doi: 10.1038/cr.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payne CK, Jones SA, Chen C, Zhuang X. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic. 2007;8:389–401. doi: 10.1111/j.1600-0854.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson TE, Guicciardi ME, Gulati R, Kleppe LS, Mueske CS, Mookadam M, Sowa G, Gores GJ, Sessa WC, Simari RD. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signaling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol. 2003;23:1521–1527. doi: 10.1161/01.ATV.0000081743.35125.05. [DOI] [PubMed] [Google Scholar]
- 36.Forbes A, Wadehra M, Mareninov S, Morales S, Shimazaki K, Gordon LK, Braun J. The tetraspan protein EMP2 regulates expression of caveolin-1. J Biol Chem. 2007;282:26542–26551. doi: 10.1074/jbc.M702117200. [DOI] [PubMed] [Google Scholar]
- 37.Adams J, Behnke M, Chen S, Cruickshank AA, Dick LR, Grenier L, Klunder JM, Ma YT, Plamondon L, Stein RL. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett. 1998;8:333–338. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 38.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 39.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveolae formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]