Abstract
Study Design
A case-control study of older adults with and without chronic low back pain (CLBP).
Objective
Compare and describe the radiographic severity of degenerative disc and facet disease in the lumbosacral spine of community-dwelling older adults with and without CLBP and to examine the relationship between spinal pathology and pain.
Summary of Background Data
Degenerative spinal pathology is often implicated as the primary reason for CLBP in older adults. Despite evidence that spinal pathology may be ubiquitous in older adults regardless of pain status, radiography continues to be heavily used in the diagnostic process.
Methods
Participants in this case-control study included 162 older adults (≥65) with CLBP and an age and gender matched pain-free group of 158 people. CLBP was characterized as pain of at least moderate intensity occurring daily or almost everyday for at least 3 months. Radiographic severity of disc and facet disease was graded using a reliable and valid system.
Results
Results demonstrated that the presence of degenerative disc and facet pathology in older adults is ubiquitous, regardless of clinical status, with greater than 90% demonstrating some level of degeneration. Higher radiographic severity scores were associated with the presence of CLBP. In fact, presence of severe disc pathology was associated with 2-fold greater odds of having CLBP. But, radiographic severity of disc and facet disease was not associated with pain severity among those with CLBP.
Conclusion
From a research perspective, radiographic evaluation of spinal pathology provides additional information about older adults with CLBP compared to pain-free individuals, but its clinical utility for diagnostic purposes is still in question.
Keywords: chronic low back pain, aging, degenerative disc disease, degenerative facet disease
In the industrialized parts of the world, low back pain (LBP) is extremely common with a prevalence ranging from 60% to 90%.1,2 Among older adults, LBP is the most frequently reported musculoskeletal problem and the third most frequently reported symptom of any kind.2,3 An estimated 42% of community dwelling older adults report having experienced at least 1 episode of LBP during the prior year.4 Nearly 20% of all visits to physicians for LBP involve individuals over age 65.2,5,6 The societal impact of LBP in older adults is demonstrated by Medicare data from 1991 to 2002 that shows a 132% increase in LBP patients and a 387% increase in related charges for LBP.7 Despite the fact that LBP is common and associated with poor outcomes in vulnerable older adults,8–11 little research has focused on LBP in people over the age of 65.2,12
It is difficult to identify a specific pathoanatomic cause of LBP in most cases, particularly in patients with chronic low back pain (CLBP), in whom psychological factors are thought to play a critical role in pain pathogenesis.13,14 Even so, degenerative spinal disease is often cited as the reason for CLBP, especially in older adults. Data indicate that degenerative pathology identified with advanced imaging (e.g., magnetic resonance imaging) has poor predictive validity for pain in older adults between the ages of 53 and 70.15 This, combined with the fact that important causes of pain, such as osteoporotic compression fractures, can be identified with plain radiograph examination, provides at least a partial explanation for why plain films of the lumbosacral spine are so commonly ordered by practitioners.
Degenerative spinal disease includes both degenerative disc disease (DDD) and degenerative facet disease (DFD) or facet osteoarthritis.16,17 DDD is common in the general population, particularly among older adults.18,19 The intervertebral disc has often been implicated as a source of LBP, but there is conflicting evidence regarding the relationship between DDD and pain.15,20 Facet pathology has also been suggested as a source of LBP21; and, like DDD, there is debate in the literature regarding its role as a primary cause of LBP.22,23 Although their diagnostic value in older adults with CLBP is unknown, radiographic imaging studies of the spine are heavily used in the diagnostic process. Preliminary evidence suggests that degenerative disease in older adults may be ubiquitous regardless of pain status15 and that patients with CLBP may be especially vulnerable to undergoing recurrent “diagnostic” procedures. Thus it is incumbent on our health system to ascertain the value of imaging studies of the spine in these patients.
We are unaware of any population-based studies examining radiographic DDD and DFD among older adults with and without CLBP. Our purpose was 2-fold: to compare and describe the radiographic severity of DDD and DFD in the lumbosacral spine of pain-free community dwelling older adults and those with CLBP, and to examine the relationship between degenerative disease of the lumbosacral spine and pain.
Materials and Methods
Subjects
The data available for analysis was taken from a large National Institutes of Health (NIH)-funded study performed at the University of Pittsburgh Pain Evaluation and Treatment Institute. The sample consisted of 320 English-speaking community dwelling older adults who had participated in a case-controlled observational study that compared subjects with CLBP, n = 162, to an age and gender matched pain-free group, n = 158, in physical and psychosocial functioning.24 The inclusion criterion for CLBP was LBP of at least moderate intensity that occurred daily or almost every day for at least the previous 3 months. Pain-free was defined as no pain or pain occurring less than once per week and of little intensity. Pain was screened with the pain thermometer.25 To ensure enrollment of older adults with mechanical CLBP, exclusion criteria included a history of back surgery or trauma, vertebral compression fractures, or known spinal pathology other than osteoarthritis. Additionally, subjects were excluded for cognitive impairment if the Folstein Mini-Mental State Examination was <21 adjusted for age and education. All participants signed informed consent. The study was approved by the University of Pittsburgh Institutional Review Board.
Procedures
All subjects were assessed for the following:
Radiographic Osteoarthritis Severity
Lumbosacral plain radiographs were scored using an established system that uses a 4 point scale of disease severity to evaluate the intervertebral disc and the facet joint (0 = no disease, 1 = mild disease, 2 = moderate disease, and 3 = severe disease). Films were scored by a radiologist who was masked to the clinical data and group assignment. Inter-rater reliability for radiograph scoring was found to be excellent and intraclass correlations were 0.85 for facet disease and 0.93 for disc disease.26 See Table 1 for operational definitions for disease scoring. Six interspaces (right and left facet joints and intervertebral disc at each level from T12–L1 to L5–S1) were scored. A summary score for the discs and the facets was calculated as the simple arithmetic sum of the points for the 6 discs and the 12 facet joints respectively.
Table 1.
Radiographic Scoring System for Degenerative Disc and Facet Disease
| Score | Operational Definition | |
|---|---|---|
| Facet joint | 0 | Normal |
| 1 | Narrowing of joint and/or mild eburnation | |
| 2 | Moderate narrowing + moderate eburnation or hypertrophy | |
| 3 | Severe osteoarthritis with narrowing, eburnation and osteophytes | |
| Intervertebral disc | 0 | No disease, defined by normal disc height, no spur formation, no eburnation, and no gas |
| 1 | Mild disease, defined by <25% disc space narrowing, small spur formation, minimal eburnation, and no gas | |
| 2 | Moderate disease, defined by 25%–75% disc space narrowing, moderate spur formation, moderate eburnation, and no gas | |
| 3 | Advanced disease, defined by >75% disc space narrowing, large spur formation, marked eburnation, gas present |
Disease severity is defined by the most severe radiographic component at a particular level.
Pain Severity
Was measured with the McGill Pain Questionnaire-Short Form (MPQ-SF) total pain intensity score. It is a widely used, validated, and reliable generic pain measure that has been used in an older population. It describes sensory and affective responses to pain. The range is 0 to 45 with higher being a worse score.27
Statistical Analysis
All analyses were performed using SAS software, version 9.1 (SAS Institute, Inc, Cary, NC). We compared radiographic scores for degenerative disc and facet disease (DDD and DFD, respectively) between pain-free participants and those with chronic LBP (CLBP) using analysis of covariance. All assumptions were met for analysis of covariance, including homogeneity of slopes. Comparisons were made for each individual level and for summary scores for the entire lumbosacral spine. χ2 analysis was used to evaluate the proportion of pain-free and CLBP participants who would be classified as having degenerative disc or facet disease. We examined proportions for those with any disc or facet disease versus no disease and for those with severe disease versus other severity categories. Odds ratios (OR) and 95% confidence intervals (CIs) were calculated using logistic regression to determine associations between back pain status and disc or facet disease. As in the proportion analyses, OR were calculated for the presence of any disease and for severe disease. Among those with LBP, we compared pain severity (MPQ-SF) based on presence of any degenerative disease or severe disease. We also used Spearman Rank Correlation Coefficients to examine the relationship between pain severity and radiographic summary scores for the discs and facets. To examine associations independent of other factors, all analyses were adjusted for age, gender, race, and body mass index.
Results
Characteristics of the study sample are shown in Table 2. CLBP and pain-free subjects were not significantly different from each other in age, gender, or ethnicity. However, the CLBP group was more overweight as compared to the pain-free group and had modestly lower Mini-Mental State Examination scores.
Table 2.
Demographic Characteristics
| Group |
|||
|---|---|---|---|
| Variable | CLBP | Pain-free | P |
| Sample size | 162 | 158 | |
| Age | |||
| Mean | 73.6 | 73.5 | 0.87 |
| SD | 5.2 | 4.8 | |
| Gender | |||
| Male | 83 | 94 | 0.16 |
| Female | 80 | 66 | |
| Ethnicity | |||
| White | 141 | 142 | 0.82 |
| African American | 18 | 15 | |
| Hispanic | 3 | 4 | |
| BMI | |||
| Mean | 29.0 | 26.1 | <0.001 |
| SD | 4.5 | 3.8 | |
| Folstein MMSE | |||
| Mean | 28.3 | 28.7 | 0.01 |
| SD | 1.3 | 1.2 | |
| MPQ-SF total score | |||
| Mean | 11.6 | — | |
| SD | 8.1 | — | |
| Pain duration in yr | |||
| Mean | 14.2 | — | |
| SD | 14.6 | — | |
BMI indicates body mass index; MMSE, Mini-Mental State Examination.
Table 3 delineates differences in radiographic scores for DDD between participants with CLBP and those who were pain-free at each spinal level. On examination of specific spinal levels, there was no significant difference at the T12–L1, L1–L2, or L5–S1 levels, but the CLBP group had higher DDD severity scores at the L2–L3 to L4–L5 levels (P < 0.05). The overall disc summary score was also higher for those with CLBP (P < 0.001). Table 4 shows differences in radiographic scores for DFD in bilateral facets of each spinal level. The only significant differences in facet disease severity were seen at the L3–L4 and L4–L5 levels on the left and at the L3–L4 level on the right (P < 0.05). The overall facet summary score for the entire lumbar spine was higher in those with CLBP compared to the pain-free (11.28 vs. 10.15, P = 0.015).
Table 3.
Radiographic Scores for Degenerative Disc Disease
| CLBP | Controls | P | |
|---|---|---|---|
| T12–L1 | 0.453 | 0.339 | >0.05 |
| L1–L2 | 0.801 | 0.711 | >0.05 |
| L2–L3 | 1.086 | 0.823 | 0.014 |
| L3–L4 | 1.253 | 0.867 | 0.0003 |
| L4–L5 | 1.434 | 1.068 | 0.0026 |
| L5–S1 | 1.074 | 0.949 | >0.05 |
| Disc summary score | 6.110 | 4.790 | 0.0005 |
Adjusted for age, sex, race, and body mass index.
Table 4.
Radiographic Scores for Degenerative Facet Disease
| Left Facet |
Right Facet |
|||||
|---|---|---|---|---|---|---|
| CLBP | Controls | P | CLBP | Controls | P | |
| T12–L1 | 0.011 | 0.027 | >0.05 | 0.019 | 0.019 | >0.05 |
| L1–L2 | 0.067 | 0.064 | >0.05 | 0.076 | 0.074 | >0.05 |
| L2–L3 | 0.196 | 0.128 | >0.05 | 0.209 | 0.127 | >0.05 |
| L3–L4 | 1.093 | 0.841 | 0.017 | 1.106 | 0.828 | 0.009 |
| L4–L5 | 2.048 | 1.862 | 0.027 | 1.996 | 1.878 | >0.05 |
| L5–S1 | 2.245 | 2.211 | >0.05 | 2.218 | 2.143 | >0.05 |
Adjusted for age, sex, race, and body mass index.
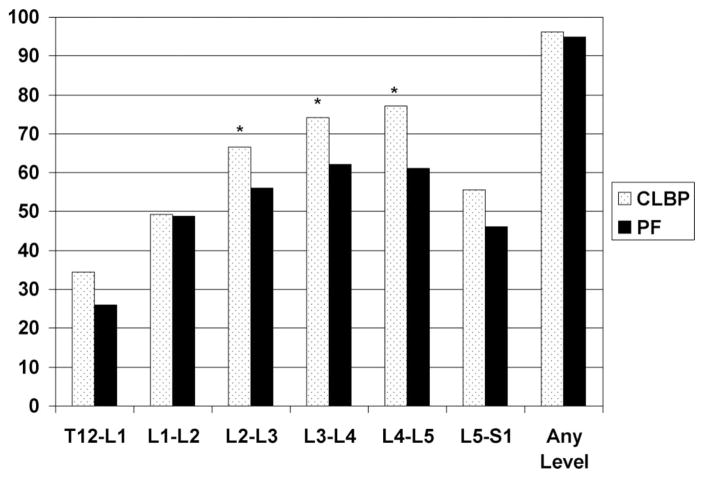
The proportion of people in the CLBP and pain-free groups classified as having “any DDD” (disc score >0 on a 0–3 scale at one or more individual spinal levels) are displayed in Figure 1. At the L2–L3 to L4–L5 levels, there is a higher proportion of disc disease in the CLBP group (P < 0.05). At least 95% of participants in both the CLBP group and the pain-free group would have at least 1 level classified as having any DDD.
Figure 1.
Percent of participants classified as having any DDD at a particular spinal level. PF = pain-free. Any disc disease: having a score of >0 on 0 to 3 scale at any spinal level. *P < 0.05.
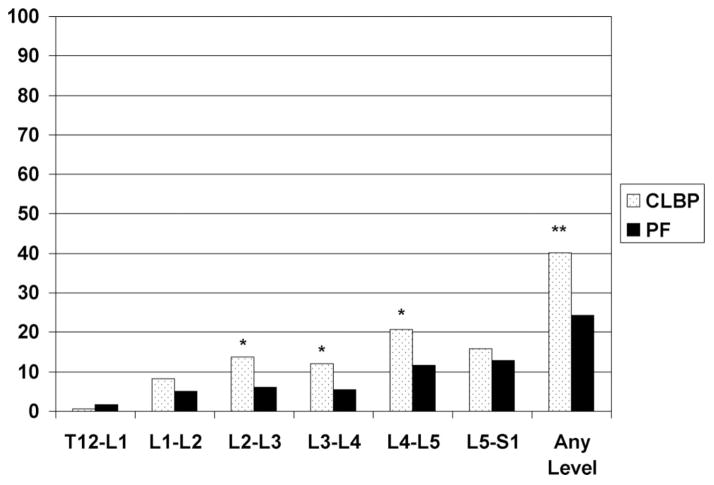
The proportion of people in the CLBP and pain-free groups classified as having “severe DDD” (disc score >2 at one or more given spinal levels) are displayed in Figure 2. Similar to the any DDD classification, there is a higher proportion of severe disease at the L2–L3 to L4–L5 levels (P < 0.05) in the CLBP group. Also, the proportion of individuals in the CLBP group with severe DDD at any level in the lumbar spine is significantly higher than in the pain-free group (P < 0.001).
Figure 2.
Percent of participants classified as having severe DDD at a particular spinal level. PF = pain-free. Severe disc disease: having a score of >2 on 0 to 3 scale at any spinal level. *P < 0.05, **P < 0.001.
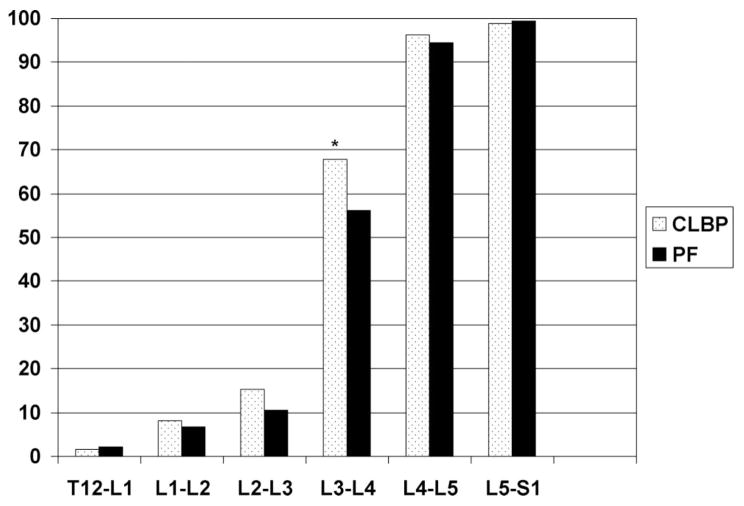
As seen in Figure 3, the proportion of individuals classified as having “any DFD” (facet score >0 on a 0–3 scale at an individual spinal level) increases at each successively caudal spinal level with the greatest increase occurring at the transition from the L2–L3 level to the L3–L4 level. Only at the L3–L4 level, there is a significant difference in the proportion of persons classified as having any DFD between the CLBP and pain-free groups. At the most caudal 2 levels of the lumbar spine, almost all participants (>93%), regardless of LBP status, would be classified as having some degree of DFD. Since there is no side-to-side difference, only right-sided facet data are presented in the figures.
Figure 3.
Percent of participants classified as having any right-sided DFD at a particular spinal level. PF = pain-free. Any facet disease: having a score of >0 on 0 to 3 scale at any spinal level. *P < 0.05.
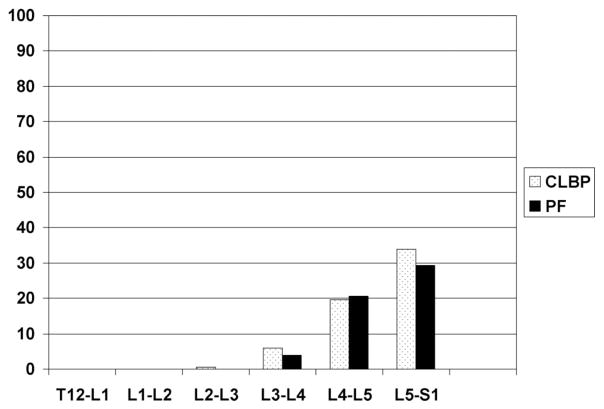
When considering “severe DFD” as seen in Figure 4, virtually none of the participants would be classified as having severe DFD based on examination of levels T12–L1 to L2–L3. There is an increasing prevalence of severe DFD in lower levels of the lumbar spine, but there are no differences in prevalence between the CLBP and pain-free groups.
Figure 4.
Percent of participants classified as having severe right-sided DFD at a particular spinal level. PF = pain-free. Severe facet disease: having a score of >2 on 0 to 3 scale at any spinal level.
The presence of any disc disease anywhere in the lumbar spine was not associated with significantly increased odds of having CLBP (OR: 1.35, 95% CI: 0.42, 4.57). However, presence of severe disc disease anywhere within the lumbar spine was associated with significantly increased odds of having CLBP by more than 2-fold (OR: 2.13, 95% CI: 1.27, 3.59). Occurrence of facet disease, in any form, was not associated with significantly increased odds of having CLBP (OR: 1.44, 95% CI: 0.89, 2.33) among community-dwelling older adults.
Among those with CLBP, there was no difference in pain severity as measured by the MPQ-SF based on the presence or absence of severe DDD or severe DFD at any spinal level (P > 0.05). Also, neither disc nor facet summary scores were significantly correlated with the MPQ-SF scores among those with CLBP (P > 0.05).
Discussion
To our knowledge, this is the first study to use a validated scoring system to examine the prevalence and severity of degenerative changes in the discs and facets of the lumbosacral spine in community-dwelling older adults with CLBP as compared to age- and sex-matched individuals who are pain-free. Our findings indicate that the burden of degenerative pathology in the lumbosacral spine does, at least in part, explain the presence or absence of pain. The radiographic scoring system developed by Weiner et al26 using both the disc and facet summary scores offers additional morphologic information about the spine that distinguishes older adults with CLBP from those who are pain-free; but, the disc summary score appears to offer better discriminant ability than the facet summary scores. It is important to note that radiographic severity of disc and facet disease was associated with CLBP status, but not with self-report measures of pain severity among those with CLBP.
When the radiographic scoring system is used to examine the proportion of people classified as having DDD, interesting patterns emerge. Within the entire sample, there is an increasing prevalence of DDD noted with caudal progression from the T12–L1 level to the L4–L5 level followed by a mild decrease at the L5–S1 level. This pattern is quite similar to the patterns seen in other studies by Peterson et al and Waris et al,20,28 which makes sense, given the sagittal plane orientation of the lumbosacral spine. Biomechanical studies have demonstrated that each successively caudal intervertebral disc in the lumbar spine bears progressively higher compressive loads down to the L5–S1 level. At L5–S1, there is a conversion from primarily compressive forces to shear forces as the distal lordosis dissipates.29 In fact, the highest shear forces in the spine occur at the L5–S1 level.29 This shift in type of forces may change the degenerative impact on the L5–S1 segment, thus explaining the pattern seen in our work and the work of others.
The presence of any degree of disc degeneration, from mild to severe, within the lumbosacral spine was quite common, in both those with and without CLBP; in fact, greater than 95% of the entire sample had some degree of disc degeneration. The presence of severe DDD was far less common. But when present, it was associated with more than 2-fold greater odds of having CLBP. The relationship between spinal pathology and CLBP status demonstrated in our study has been assumed, but the use of a reliable and validated radiographic scoring method to define severity of pathology has allowed us to establish the strength of this relationship. The lack of a relationship between radiographic severity and pain severity among those with CLBP is not surprising given the multidimensional nature of pain and pain perception.
In looking at degenerative facet pathology, there was a clear pattern of minimal degeneration in the first few segments (T12–L1 to L2–L3) followed by a rather steep rise in the prevalence of facet degeneration. At the most distal segment, L5–S1, nearly all participants in both pain groupings displayed some degree of degenerative change in bilateral facets. Other studies have demonstrated a similar degenerative pattern in the lumbar spine with the greatest degeneration in the lower segments.30–32 It has also been demonstrated that facet degeneration typically appears in the lumbosacral spine before the age of 30 and continues to increase into the 60+ age bracket when it becomes commonplace.30 Findings from a skeletal study by Eubanks et al also confirm our work. They indicated that facet osteophytosis begins at an early age stage in life, but, by the seventh decade of life, vertebral rim osteophytosis (suggestive of DDD) becomes more prevalent at all levels with the exception of L4–L5 and L5–S1, where facet osteophytosis is ubiquitous.31 This is exactly what we have found in our study.
It is interesting to note that there is a weak association between DFD and pain in this cohort. Total radiographic summary score for DFD was statistically higher for the CLBP group, but largely driven by differences at 1 level (L3–L4) and the clinical meaningfulness of this difference is questionable. The presence of severe DFD was not associated with significantly increased odds of having CLBP in this sample. This is likely due to the fact that DFD of any severity was equally prevalent in both those with CLBP and the pain-free. Facet pathology has been proposed as a pain generator for years, but reports are conflicting. There have been facet block studies which have demonstrated improved symptoms with injection of anesthetic and steroids, as well as a high prevalence of facet-related pain,21 but there have also been studies that have shown lower prevalence levels.22,33 Other work has shown that the best way to identify those who will respond favorably to facet block are those with specific clinical features, including relief with certain positions and lack of worsening with specific movements23; this indicates that other factors beyond facet pathology are involved in the development and perpetuation of CLBP.
A major advantage of this study is the use of a large community-dwelling sample of older men and women with specific data on radiographic severity of degenerative disc and facet disease as well as validated measures of pain. Nonetheless, limitations also must be considered in interpreting the findings. First, the cross-sectional nature of the data makes it impossible to identify disc or facet pathology as the cause of pain. Some might also question whether being more sedentary as a result of pain may lead to greater degenerative changes or if increased occupational workload might increase the risk of spinal degeneration. There is some evidence that occupational workload may influence spinal degeneration,34 but spinal degeneration is much more influenced by genetic factors.34,35 Another limitation of the study is the relatively high functional capacity of the cohort which limits the generalizability of our results. The reason for the high functional capacity is that the main study included a laboratory based lifting task, and in order to ensure the safety of participants, a number of exclusion criteria were used, e.g., postural instability, severe cardiac or respiratory disease, and vertebral compression fractures. The validity of the observed associations, therefore, should be replicated and extended in a cohort of older adults with greater medical comorbidity, disability, and coexisting nondegenerative spinal pathology such as osteoporosis.
In conclusion, disc and facet pathology were quite prevalent in the lumbosacral spines of community-dwelling older adults, regardless of their pain status. But, significant degenerative disc pathology in the lumbosacral spine, as measured by a validated radiographic scoring system, was clearly associated with the CLBP status. However, the degree of radiographic pathology was not associated with severity of pain among those with CLBP. From a research perspective, these findings are interesting and beg further investigation; but, these findings do not likely have clinical utility to the extent that we cannot use any 1 particular radiographic finding to explain pain in these older adults. Future work examining relationships between radiographic pathology and function among those with CLBP and the pain-free is warranted.
Key Points
More than 90% of older adults (≥65 years old) have some level of degenerative disc and facet pathology regardless of pain status.
Higher radiographic severity scores were associated with the presence of CLBP.
Presence of severe disc pathology was associated with 2-fold greater odds of having CLBP.
Radiographic severity of disc and facet disease was not associated with pain severity among those with CLBP.
Acknowledgments
Federal funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Supported by the National Institute on Aging, National Institutes of Health with a research grant (R01AG018299). Supported by grant number 1K12HD055931-01 (NICHD) (to G.H.) and grant number KL2 RR024154-03 (to N.M.) from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
The contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- 1.Brodke DS, Ritter SM. Nonsurgical management of low back pain and lumbar disk degeneration. Instr Course Lect. 2005;54:279–86. [PubMed] [Google Scholar]
- 2.Bressler HB, Keyes WJ, Rochon PA, et al. The prevalence of low back pain in the elderly: a systematic review of the literature. Spine. 1999;24:1813–9. doi: 10.1097/00007632-199909010-00011. [DOI] [PubMed] [Google Scholar]
- 3.Koch H, Smith MC. DHHS publication (PHS) 110. Hyattsville, MD: Advance Data from Vital and Health Statistics, National Center for Health Statistics (NCHS); 1985. Office-Based Ambulatory Care for Patients 75 Years Old and Over, National Ambulatory Medical Care Survey, 1980 and 1981; pp. 85–1250. [Google Scholar]
- 4.Weiner DK, Haggerty CL, Kritchevsky SB, et al. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC Cohort and implications for the future. Pain Med. 2003;4:311–20. doi: 10.1111/j.1526-4637.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 5.Cypress BK. Characteristics of physician visits for back symptoms: a national perspective. Am J Public Health. 1983;73:389–95. doi: 10.2105/ajph.73.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a US national survey. Spine. 1995;20:11–9. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Weiner DK, Kim Y, Bonino P, et al. Low back pain in older adults: are we utilizing healthcare resources wisely? Pain Med. 2006;7:143. doi: 10.1111/j.1526-4637.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 8.Leveille SG, Guralnik JM, Hochberg M, et al. Low back pain and disability in older women: independent association with difficulty but not inability to perform daily activities. J Gerontol A Biol Sci Med Sci. 1999;54:M487–93. doi: 10.1093/gerona/54.10.m487. [DOI] [PubMed] [Google Scholar]
- 9.Reid MC, Williams CS, Gill TM. Back pain and decline in lower extremity physical function among community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 2005;60:793–7. doi: 10.1093/gerona/60.6.793. [DOI] [PubMed] [Google Scholar]
- 10.Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2005;60:882–7. doi: 10.1093/gerona/60.7.882. [DOI] [PubMed] [Google Scholar]
- 11.Hicks GE, Simonsick EM, Harris TB, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60:1420–4. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- 12.Bigos SBO, Braen G, et al. Acute Low Back Problems in Adults. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services; 1994. [Google Scholar]
- 13.Reid MC, Williams CS, Concato J, et al. Depressive symptoms as a risk factor for disabling back pain in community-dwelling older persons. J Am Geriatr Soc. 2003;51:1710–7. doi: 10.1046/j.1532-5415.2003.51554.x. [DOI] [PubMed] [Google Scholar]
- 14.Waddell G, Newton M, Henderson I, et al. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157–68. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- 15.Jarvik JJ, Hollingworth W, Heagerty P, et al. The Longitudinal Assessment of Imaging and Disability of the Back (LAIDBack) Study: baseline data. Spine. 2001;26:1158–66. doi: 10.1097/00007632-200105150-00014. [DOI] [PubMed] [Google Scholar]
- 16.Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;165:110–23. [PubMed] [Google Scholar]
- 17.Valdes AM, Hassett G, Hart DJ, et al. Radiographic progression of lumbar spine disc degeneration is influenced by variation at inflammatory genes: a candidate SNP association study in the Chingford cohort. Spine. 2005;30:2445–51. doi: 10.1097/01.brs.0000184369.79744.a5. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura N, Dennison E, Wilman C, et al. Epidemiology of chronic disc degeneration and osteoarthritis of the lumbar spine in Britain and Japan: a comparative study. J Rheumatol. 2000;27:429–33. [PubMed] [Google Scholar]
- 19.Kellgren JH, Lawrence JS. Osteo-arthrosis and disk degeneration in an urban population. Ann Rheum Dis. 1958;17:388–97. doi: 10.1136/ard.17.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson CK, Bolton JE, Wood AR. A cross-sectional study correlating lumbar spine degeneration with disability and pain. Spine. 2000;25:218–23. doi: 10.1097/00007632-200001150-00013. [DOI] [PubMed] [Google Scholar]
- 21.Mooney V, Robertson J. The facet syndrome. Clin Orthop Relat Res. 1976;115:149–56. [PubMed] [Google Scholar]
- 22.Manchikanti L, Pampati V, Fellows B, et al. The diagnostic validity and therapeutic value of lumbar facet joint nerve blocks with or without adjuvant agents. Curr Rev Pain. 2000;4:337–44. doi: 10.1007/s11916-000-0016-4. [DOI] [PubMed] [Google Scholar]
- 23.Revel ME, Listrat VM, Chevalier XJ, et al. Facet joint block for low back pain: identifying predictors of a good response. Arch Phys Med Rehabil. 1992;73:824–8. [PubMed] [Google Scholar]
- 24.Rudy TE, Weiner DK, Lieber SJ, et al. The impact of chronic low back pain on older adults: a comparative study of patients and controls. Pain. 2007;131:293–301. doi: 10.1016/j.pain.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–4. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Weiner DK, Distell B, Studenski S, et al. Does radiographic osteoarthritis correlate with flexibility of the lumbar spine? J Am Geriatr Soc. 1994;42:257–63. doi: 10.1111/j.1532-5415.1994.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 27.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 28.Waris E, Eskelin M, Hermunen H, et al. Disc degeneration in low back pain: a 17-year follow-up study using magnetic resonance imaging. Spine. 2007;32:681–4. doi: 10.1097/01.brs.0000257523.38337.96. [DOI] [PubMed] [Google Scholar]
- 29.Keller TS, Colloca CJ, Harrison DE, et al. Influence of spine morphology on intervertebral disc loads and stresses in asymptomatic adults: implications for the ideal spine. Spine J. 2005;5:297–309. doi: 10.1016/j.spinee.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Eubanks JD, Lee MJ, Cassinelli E, et al. Prevalence of lumbar facet arthrosis and its relationship to age, sex, and race: an anatomic study of cadaveric specimens. Spine. 2007;32:2058–62. doi: 10.1097/BRS.0b013e318145a3a9. [DOI] [PubMed] [Google Scholar]
- 31.Eubanks JD, Lee MJ, Cassinelli E, et al. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop Relat Res. 2007;464:184–9. doi: 10.1097/BLO.0b013e3181583d4e. [DOI] [PubMed] [Google Scholar]
- 32.Tischer T, Aktas T, Milz S, et al. Detailed pathological changes of human lumbar facet joints L1–L5 in elderly individuals. Eur Spine J. 2006;15:308–15. doi: 10.1007/s00586-005-0958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manchukonda R, Manchikanti KN, Cash KA, et al. Facet joint pain in chronic spinal pain: an evaluation of prevalence and false-positive rate of diagnostic blocks. J Spinal Disord Tech. 2007;20:539–45. doi: 10.1097/BSD.0b013e3180577812. [DOI] [PubMed] [Google Scholar]
- 34.Videman T, Battie MC, Ripatti S, et al. Determinants of the progression in lumbar degeneration: a 5-year follow-up study of adult male monozygotic twins. Spine. 2006;31:671–8. doi: 10.1097/01.brs.0000202558.86309.ea. [DOI] [PubMed] [Google Scholar]
- 35.Battie MC, Videman T, Gibbons LE, et al. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration: a study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine. 1995;20:2601–12. [PubMed] [Google Scholar]