Abstract
This longitudinal study examined the impact of drug use and abuse on medication adherence among 150 HIV-infected individuals, 102 who tested urinalysis positive for recent illicit drug use. Medication adherence was tracked over a 6-month period using an electronic monitoring device (MEMS caps). Over the 6-month study drug-positive participants demonstrated significantly worse medication adherence than did drug-negative participants (63 vs. 79%, respectively). Logistic regression revealed that drug use was associated with over a fourfold greater risk of adherence failure. Stimulant users were at greatest risk for poor adherence. Based upon within-participants analyses comparing 3-day adherence rates when actively using versus not using drugs, this appears to be more a function of state rather than trait. These data suggest that it is the acute effects of intoxication, rather than stable features that may be characteristic of the drug-using populace, which leads to difficulties with medication adherence.
Keywords: HIV infection, AIDS, Medication adherence, Drug use, Methamphetamine, Cocaine
Introduction
The introduction of highly active antiretroviral therapy (HAART) has substantially reduced HIV/AIDS associated morbidity and mortality (Garcia et al., 2002; Hogg et al., 1998; Ory & Mack, 1998; Palella et al., 1998). Early optimism concerning the benefits of these medications has been tempered, however, by evidence suggesting that even modest or occasional nonadherence can greatly diminish the benefits of treatment and lead to serious personal and public health consequences. Research has demonstrated that suboptimal adherence (i.e., taking less than 90–95% of prescribed doses) is associated with increased risk of adverse virologic and clinical outcomes, including increased viral replication and the development of drug-resistant HIV strains (Gifford et al., 2000; Liu et al., 2001; Wainberg & Friedland, 1998), as well as a host of clinically significant health-related setbacks (Paterson et al., 2000). Unfortunately, poor adherence to antiretroviral medication is a common problem, with as many as half of individuals on combination therapies failing to take their medication in accordance with dosage, time, and dietary instructions (Murphy et al., 2001; Nieuwkerk et al., 2001).
Given the relationship between suboptimal medication adherence and adverse clinical outcomes in HIV, efforts to identify factors predictive of adherence are clearly needed. Previous studies have examined the role of demographic characteristics (e.g., age, gender, ethnicity, socioeconomic status), psychiatric disorders, health beliefs, social support, regimen complexity, and cognitive functioning in predicting HAART compliance (Arnsten et al., 2002; Berg et al., 2004; Chesney, Morin, & Sherr, 2000; Gifford et al., 2000; Golin et al., 2002; Hinkin et al., 2002, 2004; Howard et al., 2002; Kalichman, Ramachandran, & Catz, 1999; Rabkin & Chesney, 1999; Simoni, Frick, & Huang, 2006; Smith, Rapkin, Morrison, & Kammerman, 1997; Stein et al., 2000).
Central to the present study is the finding that drug use and abuse may also be related to poorer medication adherence among HIV infected adults (Arnsten et al., 2001, 2002; Hinkin et al., 2004; Howard et al., 2002, Tucker et al., 2004). There are several potential mechanisms by which substance use may impact adherence behavior, including neurocognitive deficits, psychosocial impairment, and exacerbation of psychiatric dysfunction (Chang et al., 2002; Volkow et al., 2001). However, the literature describing the impact of drug use and abuse on adherence in HIV has been variable. While some studies have found an association between the two, other investigations have demonstrated no relationship between drug use and adherence (Crisp, Williams, Timpson, & Ross, 2004; Mohammed et al., 2004). Such discordant findings may be explained, at least in part, by differences in the measurement of antiretroviral adherence behavior across studies. More specifically, while self report provided the sole index of medication use in those studies failing to find a relationship between drug use and adherence behavior, computerized assessment of medication adherence using Medication Event Monitoring System (MEMS) caps was employed in those investigations observing a significant relationship between the two (Hinkin et al., 2004; Howard et al., 2002).
Multiple studies have demonstrated that the method employed to measure medication adherence may yield significantly different results (Arnsten et al., 2001; Bangsberg, Bronstone, & Hofmann, 2002; Bangsberg et al., 2000; Garber et al., 2004; Kimmerling, Wagner, & Ghosh-Dastidar, 2003; Levine et al., in press; Paterson, Potoski, & Capitano, 2002; Wohl et al., 2003). Electronic monitoring devices such as MEMS caps, the technique used in the present study to measure HAART adherence, employ a computer chip embedded in the top of a pill bottle to automatically record the date, time, and duration of pill bottle opening. Although such electronic monitoring devices have their limitations (Burke, 2001) and may underestimate actual adherence rates, several studies have shown that they may be more accurate than pill counts or self-report, both of which appear to overestimate adherence rates (Burney, Krishnan, Ruffin, Zhang, & Brenner, 1996; Liu et al., 2001; O'Brien, Petrie, & Raeburn, 1992; Stephenson, Rowe, Haynes, Macharia, & Leon, 1993).
Along similar lines, studies investigating the prevalence of drug abuse in various populations have also demonstrated a lack of concordance between patient's self-report and actual drug use status based on objective measurements, such as urine toxicology screens (Chermack et al., 2000; Ehrman, Robbins, & Cornish, 1997; Fendrich, Johnson, Wislar, Hubbell, & Spiehler, 2004; Kilpatrick, Howlett, Sedgwick, & Ghodse, 2000; Lu, Taylor, & Riley, 2001; Tassiopoulos et al., 2004). Given that drug-using individuals appear to under-report the frequency and quantity of their drug use in some circumstances, urinalysis was used to measure recent drug use in the current investigation.
The present study attempts to describe the relationship between both recent drug use and abuse/dependence and medication adherence in a cohort of HIV infected men and women. Medication adherence in this study was assessed using MEMS caps over a 6-month time period. Specifically, this paper will describe (a) the relationship between drug use and overall adherence; (b) the association between drug use and rate of decline in adherence rates; (c) the differential impact of stimulant use versus other drugs on adherence; (d) whether adherence rates vary as a function of recency of use; and (e) the impact of substance abuse and dependence on adherence. It is expected that current drug users and abusers, particularly stimulant users, will demonstrate poorer adherence behavior than non-drug users and non-stimulant users.
Method
Participants and Procedures
A total of 150 HIV-seropositive adults were enrolled in the current study from 2001 to 2005. Participants were recruited using fliers soliciting HIV-infected drug users that were posted in infectious disease clinics at university-affiliated medical centers and from community agencies in the Los Angeles area that specialize in providing services to HIV-infected individuals. Inclusion criteria included: both male and female gender, those who were HIV positive, 18 years of age or older, and receiving highly active antiretroviral therapy. Participants had to be responsible for administering their own medications, agree to use the MEMS cap device for the duration of the investigation, and demonstrate the manual dexterity necessary for opening their pill bottle. Individuals were excluded from participation in the study if they met DSM-IV-TR criteria for current psychotic spectrum disorders, seizure disorder, stroke, closed-head injury with loss of consciousness in excess of 30 min, or any other neurological disease, CNS opportunistic infection, or neoplasm.
At the time of participation, 68% of participants met Centers for Disease Control and Prevention diagnostic criteria for AIDS (1993) and all participants had been prescribed a regimen of HAART, defined here as a combination of three or more antiretroviral drugs, including protease inhibitors (PI), non-nucleoside reverse transcriptase inhibitors (NNRTI), nucleoside analogue reverse transcriptase inhibitors (NRTI), and nucleotide analogue reverse transcriptase inhibitors (NtRTI). The average number of HIV-related medications prescribed was 5.35 (SD = 3.4) and the majority of participants (79.3%) had been on antiretroviral regimens for more than 3 years prior to their participation in the study. The number of pills taken each day for all medications ranged from 1 to 40 with an average of 11.2 (SD = 7.3). The virologic status of participants varied widely, with CD4 counts ranging between 20 and 2000 (Median = 375.0, IQR = 238.0–608.0). The sample included 64% African Americans (N = 94), 14.7% Caucasians (N = 22), 14% Hispanics (N = 21), 4% Asians/Pacific Islanders (N = 6), and 3.3% multiracial participants (N = 5). Women comprised 17.3% of the sample (N = 26). Mean age of participants was 41.3 years (6.9) with a range of 18–61. The majority of participants had completed high school with a mean of 12.9 (2.0) years of education. Premorbid intellectual functioning, as estimated with the North American Adult Reading Test (NAART), was in the average range with a estimated mean Verbal IQ score of 104.9 (Range = 86.7–127.1). Overall prevalence of substance abuse disorders was relatively high, with 29.3% (N = 44) of participants meeting DSM-IV diagnostic criteria for current drug abuse or dependence. Additional demographic data are presented in Table 1.
Table 1.
Sample demographics and clinical characteristics
| Variable | Drug-positive (N = 102) Mean (SD) | Drug-negative (N = 48) Mean (SD) |
|---|---|---|
| Age | 41.4 (6.8) | 41.2 (7.3) |
| Years of education* | 12.7 (1.9) | 13.5 (2.2) |
| NAART VIQ score | 104.2 (8.6) | 106.7 (9.6) |
| BDI-II total score | 14.3 (9.7) | 11.5 (8.0) |
| # HIV Medications | 5.4 (3.4) | 5.2 (3.3) |
| # Pills taken per day | 10.7 (6.9) | 12.0 (8.1) |
| Years on HAART | 6.2 (4.3) | 7.2 (4.0) |
| CD4 count | 442.3 (271.3) | 471.6 (379.8) |
| % of Sample | % of Sample | |
| Female | 16.7 | 18.8 |
| MSM | 65.3 | 66.0 |
| AIDS diagnosis | 67.7 | 72.9 |
| Ethnicity** | ||
| African-American | 73.5 | 43.8 |
| Hispanic | 9.8 | 22.9 |
| Caucasian | 10.8 | 22.9 |
| Asian/Pacific Islander | 2.0 | 8.3 |
| Multiracial | 3.9 | 2.1 |
| Unemployment | 72.5 | 66.7 |
| SCID alcohol abuse/dependence | 8.5 | 4.2 |
| SCID drug abuse/dependence** | 43.6 | 6.3 |
| Good medication adherence( ≥ 90%)** | 18.6 | 50.0 |
Statistical significance at the < .05 level
Statistical significance at the < .01 level
Study participants were seen a total of seven times, including a baseline visit and six monthly follow-up visits. After providing written informed consent, participants completed self-report questionnaires and two structured diagnostic interviews as described below. Urine samples were also collected under the supervision of a staff member. Participants then received instructions regarding the use of MEMS caps and were scheduled to return for a follow-up visit 1 month later. At each follow-up visit, MEMS cap data were collected and urine samples were obtained. Each participant received a total of $475 for their participation in the study, payment of which was dispensed across the seven study visits.
Measures
Drug Use/Abuse
Urine toxicology screening was used as the objective measurement of recent drug use in this study. Urine samples were collected from all participants under direct staff supervision at baseline and during each follow-up visit. These samples provided data regarding the presence of opioids, methamphetamine, benzoylecgonine (a cocaine metabolite), tetrahydrocannabinol (THC), phencyclidine (PCP), and barbiturates. Opioids, methamphetamine and cocaine are typically detectable 2–3 days after last use; PCP up to a week after last use; and THC and barbiturates are detectable up to a month after last use.
Psychiatric Symptomatology
In order to determine whether participants met diagnostic criteria for substance abuse or dependence, a modification of the Structured Clinical Interview for Diagnosis (SCID; Spitzer et al., 1992) was conducted with each participant under the supervision of a licensed clinical psychologist (S.A.C.) with expertize in diagnostic interviewing.
Medication Adherence
MEMS caps were used to assess participants’ HAART adherence over the 6-month duration of the study. MEMS caps employ a pressure-activated microprocessor in the medication bottle cap that automatically records the date, time, and duration of bottle opening. Data are retrieved from the cap using a specially designed communication module connected to a personal computer. For the majority of participants (65.3%), MEMS caps were employed to track adherence to protease inhibitors. For those participants on a protease-sparing regimen, MEMS caps were used to track adherence to another antiretroviral medication (e.g., nucleoside reverse transcriptase inhibitor or nonnucleoside reverse transcriptase inhibitor). Participants were instructed to take their MEMS-monitored medication as prescribed by their physician, not to open the bottles for any reason other than removing a dose, and to refill the bottle at a time when they ordinarily took a dose. They were also cautioned against pocket dosing (i.e., removing more than one dose at a time for later use). Data was downloaded from the MEMS cap monthly, at each of the six return visits. Adherence rates were calculated by dividing actual dose events by prescribed doses during the 1-month period between visits. Participants who took at least 90% of their prescribed doses across the 6-month study were classified as good adherers. Those who took less than 90% of doses were classified as poor adherers. Only data from those whose MEMS adherence rates were greater than 10% overall for the course of the study was used (N = 150) as those with lower rates (N = 4) were suspected of failing to follow the MEMS cap instructions.
Data Analysis
Descriptive statistics were conducted on all variables to summarize the data. The sample was then divided into two groups as a function of urinalysis wherein “drug use” was defined as any positive toxicology screen between baseline and visit 7. Comparisons of demographic variables between drug-positive and drug-negative participants were performed using one-way analysis of variance (ANOVA) for continuous variables (e.g., age, education) and chi-square frequency analysis for categorical variables (e.g., ethnicity, gender). Between group differences in the rate of medication adherence was then assessed using repeated measures and mixed model ANOVA. Paired t-tests were conducted among stimulant users to compare participants’ 3-day adherence rates immediately prior to a positive toxicology screen versus their 3-day adherence rates immediately prior to a negative toxicology screen. The relative risk of adherence failure associated with drug use status was determined using binary logistic regression analyses using standard entry criteria with race and education entered first as control variables followed by drug use status. Adherence failure in these analyses was defined as an overall adherence rate of less than 90% of doses taken across the 6-month study. Adherence behavior was then further analyzed by forming two new groups on the basis of SCID data (i.e., substance abusing/dependent versus non substance abusing/dependent) and conducting ANOVA and logistic regression analyses in the same manner described above.
Results
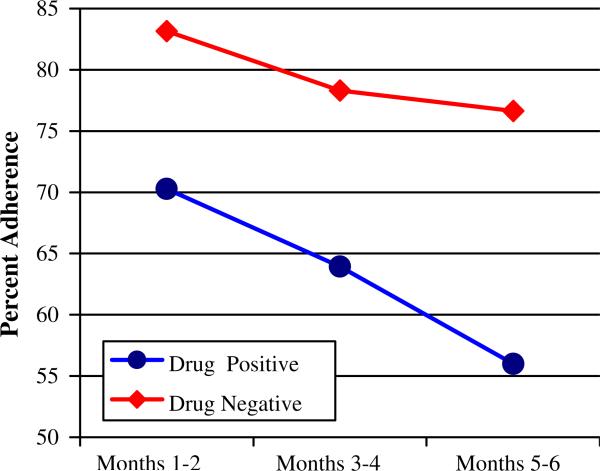
Table 2 contains adherence data across the 6-month study period for both the drug-positive and drug-negative groups. Results of repeated measures ANOVA revealed a main effect for drug use with the drug-positive group demonstrating significantly poorer medication adherence than did the drug-negative group, F(1,148) = 13.9, P < .01, η2 = .09. Averaged across the 6-month study, the drug-negative group's adherence rate was 79% as compared to 63% for the drug-positive group. A main effect for time was also present, F(2,147) = 18.4, P < .01, η2 = .11. Over time, adherence rates for the entire sample dropped from 74.4% for the first 2 months, to 68.5% for months 3–4, down to 62.6% for months 5 and 6 of the study. As can be seen in Fig. 1, comparison of the rate of decline in adherence between the drug-positive versus drug-negative groups revealed a trend towards a steeper rate of decline in adherence amongst the drug-positive group, F(2,147) = 2.89, P = .06. While adherence rates among the drug-negative group only dropped 6% points (from 83% to 77% across the 6 month sampling period), adherence rates for the drug-positive group dropped more than twice as much (from 70% to 56%).
Table 2.
Medication adherence rates for drug-positive and drug-negative participants across the 6-month study period
| Month of study | Drug-positive (N = 102) | Drug-negative (N = 48) |
|---|---|---|
| Month 1 | 72.6 | 86.3 |
| Month 2 | 68.0 | 80.2 |
| Month 3 | 66.1 | 78.7 |
| Month 4 | 63.6 | 77.8 |
| Month 5 | 59.2 | 78.8 |
| Month 6 | 54.3 | 75.3 |
| Mean adherence (Months 1–6) | 63.6 | 79.8 |
Fig. 1.
Medication adherence rates among drug-positive and drug-negative participants
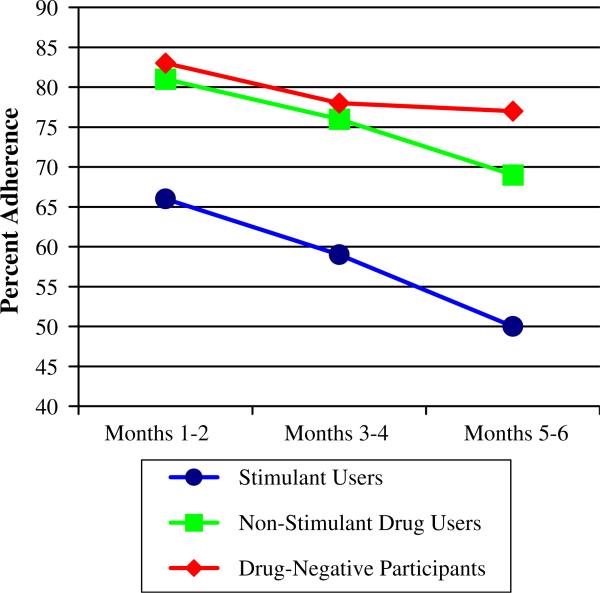
Given that the majority of participants within the drug-positive group were stimulant users (i.e., cocaine and/or methamphetamine), the next series of analyses compared stimulant users versus other-drug users who were toxicology negative for recent stimulant use versus drug free participants. The adherence rates for these three groups are depicted in Fig. 2. Results of mixed model ANOVA again revealed a main effect for time, F(2,146) = 21.8, P < .01, η2 = .13 with adherence rates for all three groups declining over time. A main effect for group was also found, F(2,147) = 12.9, P < .01, η2 = .15. Follow-up pairwise comparisons revealed that the stimulant positive group's adherence rate was significantly lower than both the other-drug-positive group (P = .001) as well as the non-drug group (P < .001). Of note, adherence rates for the drug-negative and the stimulant negative/other drug-positive group did not statistically differ.
Fig. 2.
Medication adherence rates among stimulant users, non-stimulant drug users, and drug-negative participants
Having determined that stimulant use in particular, rather than drug use in general, was associated with poorer medication adherence, the next series of analyses explored whether adherence rates differed as a function of whether participants only used cocaine versus using cocaine and methamphetamine. Of the 71 stimulant users, 19(27%) tested positive for cocaine, 50(70%) tested positive for both cocaine and meth-amphetamine, and 2(3%) tested positive for amphetamines. For the purpose of this analysis we compared the 19 cocaine only participants with the 50 participants who tested positive for both cocaine and methamphetamine, omitting the 2 methamphetamine only participants. Between group comparisons revealed a trend toward the cocaine + methamphetamine group evidencing poorer adherence than did the cocaine only group, F(1,67) = 3.6, P = .06, η2 = .05. The mean adherence rate for the cocaine only group was 68.1% vs. 54.5% for the cocaine + methamphetamine group.
To determine the relative risk of adherence failure associated with drug use, a logistic regression analysis was performed. For the purpose of this analysis, adherence failure was defined as an overall adherence rate of less than 90% of doses taken. Results of logistic regression revealed that drug use was associated with a 4.1 times greater risk of being a poor adherer, Wald = 11.3, P < .01, OR = 4.1; 95% CI = 1.8–9.3. Only 18.6% of the drug-positive group were classified as good adherers versus 50% of the drug-negative group. Follow-up logistic regression analyses comparing stimulant users to drug free participants found stimulant use to be associated with a 7.0 greater risk of poor adherence relative to a non-drug using contrast group, Wald = 13.8, P < .01; OR = 7.0, 95% CI = 2.5–19.3.
The next analysis was conducted to explore whether lower levels of adherence among the stimulant using group was attributable to the adverse effects of drug use itself or instead was a marker of a trait that is characteristic of individuals who use stimulants, even when they are clean. This analysis was therefore confined to participants who tested positive for stimulant use on at least one study visit and who also tested clean on at least one other occasion (N = 41). Three-day adherence rates for visits at which participants tested stimulant positive were grouped and averaged, as were adherence rates for visits at which the same participants were stimulant negative on urine toxicology assays. Three-day adherence rates were employed because that corresponds to the duration that stimulant use can be detected after ingestion. Hence, for each participant this yielded two adherence rates corresponding to when they were and were not using stimulant drugs. The three-day mean adherence rate for participants who tested positive for recent stimulant use was 51.3% compared to a three-day mean adherence rate of 71.7% for the same participants when they had not recently used stimulants. Paired t-tests revealed this to be a statistically significant difference, t(40) = –3.8, P < .01.
The above analyses examined the relationship between medication adherence and recent drug use without regard to whether participants’ drug use rose to the level of abuse or dependence. Based upon a structured psychiatric diagnostic interview (SCID-IV), 31% of participants were found to meet DSM-IV-TR diagnostic criteria for drug abuse/dependence (hereafter termed the drug abuse group). Results of mixed model ANOVA comparing current substance abusers to non-abusers revealed a main effect for group, F(1,140) = 7.1, P < .01, η2 = .05. As anticipated, the abuse/dependence group evidenced poorer medication adherence than did the non-abuse group (61.0% vs. 72.7%, respectively). While adherence rates declined for both groups, a significant Group by Time interaction effect was found, F(2,139) = 4.2, P < .05, η2 = .03. Although the non-abuse group's mean adherence rates dropped 10% points, from 77.4% to 68.4%, the drug abuse group experienced a more precipitous decline with their mean adherence rate dropping over 18% points (from 70.1% to 51.3%). Logistic regression analyses revealed that participants who met DSM-IV-TR diagnostic criteria for drug abuse/dependence had a 3.3 times greater risk of poor adherence relative to participants without a current drug abuse diagnosis, (Wald = 4.7, P = .03; OR = 3.3, 95% CI = 1.1–9.8).
Discussion
This study examined the relationship between drug use and medication adherence among a cohort of HIV-infected adults. Not surprisingly, and consistent with findings from other longitudinal studies of adherence (Howard et al., 2002), adherence rates declined over time across the entire sample, regardless of drug use status. Consistent with our predictions, any current drug use, as determined by urine toxicology, as well as the presence of a current substance use disorder (abuse/dependence), as determined by diagnostic interview, was associated with poorer adherence to HAART. Current drug users were over four times more likely to show suboptimal levels of adherence (defined here as fewer than 90% doses taken) compared to those whose urine toxicology showed no recent drug use. The poor levels of medication adherence across the entire cohort was discouraging to observe. Within the Drug-positive group, only 18.6% were classified as good adherers whereas only 50% of the Drug-negative group were able to maintain 90+% overall adherence rates. The use of stimulants (i.e., cocaine or methamphetamine) proved to be particularly disruptive of adherence in this sample of HIV infected adults, as persons whose urine screens were positive for stimulants were seven times more likely to have poor adherence than those without positive urines. Among the subset of participants with stimulant-positive toxicology reports at some point during the study, adherence was most dramatically affected around periods of active use. Finally, from a longitudinal perspective, stimulant users had a more precipitous decline in adherence over the 6-month course of the study than did non-users and results were generally similar when using either toxicology-defined or diagnosis-defined groupings.
That stimulant use, including cocaine, may adversely impact medication adherence is generally consistent with the extant literature (e.g., Arnsten et al., 2002; Halkitis, Kutnick, & Slater, 2005; Ingersoll, 2004; Sharpe, Lee, Nakashima, Elam-Evans, & Fleming, 2004). Some have suggested that disruptions to sleep and eating patterns and the increased level of environmental instability attendant to stimulant abuse may drive lower adherence rates (Reback, Larkins, & Shoptaw, 2003). In the current study, those individuals who used cocaine + methamphetamine were at particularly high risk for poor adherence, raising the possibility that methamphetamine may exert greater “lifestyle disruption” than cocaine. However, because the vast majority of methamphetamine users in our study were also using cocaine we cannot rule out that their lower adherence rates (compared to non-drug users and also to cocaine only users) might merely reflect the additive or synergistic impact of polystimulant use. Regardless, our findings converge with results from several studies that have found both cocaine and methamphetamine abuse associated with decreased overall adherence (Arnsten et al., 2002; Colfax & Shoptaw, 2005; Reback et al., 2003; Sharpe et al., 2004). Our findings differ from those of Matthews et al. (2002) who did not find methamphetamine use to be associated with poorer HAART adherence. However, this difference can be attributed to differences in methods of assessing substance use, with their group relying on self-reported methamphetamine use data within the past 30 days, while we used both self-report and urine toxicology to assess substance use/abuse. This further underscores how different findings can emerge as a function of the method by which substance use/abuse is measured.
The methodology employed in the current study has a number of advantages over previous studies that warrant discussion. First, drug use was assessed via multiple methods, including both urine toxicology and structured diagnostic interview, allowing for greater accuracy in the grouping of our participants. The use of urine toxicology reports allowed for a finer-grained analysis of actual documented substance use in close temporal proximity to when MEMS adherence data were collected. As noted above, studies that have failed to find a relationship between substance use and adherence in HIV-infected adults have relied on self-reported drug use behaviors (Crisp et al., 2004; Matthews et al., 2002; Mohammed et al., 2004) which is known to be particularly unreliable among active substance abusers (Chermack et al., 2000; Ehrman et al., 1997; Tassiopoulos et al., 2004). In addition, our study showed the differential impact of a specific class of drug (stimulants) upon adherence, and suggested that perhaps not all drugs have equally disruptive effects. Finally, medication adherence was monitored via an objective, electronic monitoring system (MEMS caps) throughout the entire course of the 6-month study. This design allows for the ability to look at the trajectory of adherence failure over time and go beyond the many studies that have examined adherence behavior cross-sectionally.
We are not the first to posit that poor adherence among substance users in general may be due to the inconsistent, unpredictable, and chaotic lifestyles led by many substance abusers (e.g., see Tucker et al., 2004). Adherence to HAART generally requires meticulous attention to timing of doses, to dietary considerations, and to the coordination of multiple medications. Also, depression and cognitive impairment may contribute to the lowered rates of adherence among active drug users (DiMatteo, Lepper, & Croghan, 2000), and cocaine users in particular (Arnsten et al., 2002, Hinkin et al., 2004). Our group and others have documented that methamphetamine use among HIV infected adults significantly increases the risk for cognitive impairment (Gonzalez et al., 2004; Letendre et al., 2005; Levine et al., 2006; Rippith et al., 2004) and rates of psychiatric disturbance are elevated among HIV seropositive participants (Hinkin, Castellon, Atkinson, & Goodkin, 2003). Our group continues to examine the degree to which all of these factors may interact to impact adherence.
To our knowledge, this study is the first to go beyond group comparisons and begin to explore the same individuals’ adherence patterns during both drug-using and non-drug-using time points. Over the course of 6-months, a number of participants produced both “clean” and “dirty” urines – and adherence rates were markedly lower in these participants when examining the days surrounding the “dirty” toxicology screens. This would suggest that it is the behaviors associated with active drug use that adversely impact HAART adherence rather than personality or trait-like characteristics of the participants themselves. This provocative, albeit preliminary, finding would lend support to the notion that substance abuse treatment may be a vital prerequisite for successful HAART adherence.
This study raises several important lines of future inquiry. Consistent with prior work by our group with a different cohort (Hinkin et al., 2002) as well as findings from other research groups (e.g., Howard et al., 2002) we again found adherence rates to significantly decline over time. This finding suggests that benefits to adherence that may be derived from close monitoring are quite transient, and that this trend is exacerbated by even periodic drug use. It may also be that over time participants become less willing to utilize MEMS caps as instructed. This would result in the appearance of a decline in adherence rates over time that might not accurately reflect actual adherence rates. Further research is needed to untangle this conundrum. Also unclear is whether certain classes of drugs are more detrimental than others to HAART adherence and, if so, what are the mechanisms driving that relationship? We did not have adequate power to compare cocaine only versus methamphetamine only groups of substance users/abusers to more conclusively determine specific drug effects on adherence. Similarly, we did not have sufficient numbers of opiate abusers to more conclusively explore the impact of opiate use on adherence.
Another critical question is whether risk factors can be identified that could predispose some active drug users to adherence failure. Cognitive dysfunction, psychiatric disturbance, severity of drug use, and presence of social support might all mediate the impact of substance use on adherence. We believe it is unlikely that a single mechanism explains the adverse impact of active substance use on adherence. Accordingly, multifactorial models should be employed in future studies. Other limitations include the manner in which participants were recruited. By specifically recruiting HIV positive drug-users, the cohort who volunteered may have been biased towards heavier users. While none of the participants were homeless, we did not obtain information regarding stability of housing. Chaotic home environments, which may be more common among drug users, could be expected to adversely impact on a number of health behaviors, including medication adherence. Finally, these data raise the intriguing possibility that adherence to HAART may rebound with abstinence from active substance use. As such, harm reduction interventions aimed at either fostering sobriety or reducing the deleterious impact of continuing drug use on health behavior appear necessary to maximize adherence to antiviral regimens.
Acknowledgments
This study was supported by a grant from the National Institute on Drug Abuse (RO1 DA13799) to CHH. The authors would like to thank Marta Robinet, Adam Perkins, and Oscar Ureño for their research assistance.
Contributor Information
Charles H. Hinkin, David Geffen School of Medicine, University of California at Los Angeles, 760 Westwood Plaza, Room C8-747, Los Angeles, CA 90024, USA VA Greater Los Angeles Health Care System, Los Angeles, USA.
Terry R. Barclay, David Geffen School of Medicine, University of California at Los Angeles, 760 Westwood Plaza, Room C8-747, Los Angeles, CA 90024, USA
Steven A. Castellon, David Geffen School of Medicine, University of California at Los Angeles, 760 Westwood Plaza, Room C8-747, Los Angeles, CA 90024, USA VA Greater Los Angeles Health Care System, Los Angeles, USA.
Andrew J. Levine, David Geffen School of Medicine, University of California at Los Angeles, 760 Westwood Plaza, Room C8-747, Los Angeles, CA 90024, USA
Ramani S. Durvasula, California State University at Los Angeles, Los Angeles, USA
Sarah D. Marion, Point Loma University, San Diego, USA
Hector F. Myers, University of California at Los Angeles, Los Angeles, USA Research Center on Ethnicity, Health & Behavior, Charles R. Drew University of Medicine, Los Angeles, USA.
Douglas Longshore, David Geffen School of Medicine, University of California at Los Angeles, 760 Westwood Plaza, Room C8-747, Los Angeles, CA 90024, USA.
References
- Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Change CJ, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clinical Infectious Diseases. 2001;33(18):1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten J, Demas P, Grant R, Gourevitch M, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of General Internal Medicine. 2002;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Demas PA, Howard AA, Schoenbaum EE, Gourevitch MN, Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. Journal of General Internal Medicine. 2004;19(11):1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR, Bronstone A, Hofmann R. A computer based assessment detects regimen misunderstandings and nonadherence for patients on HIV antiretroviral therapy. AIDS Care. 2002;14(1):3–15. doi: 10.1080/09540120220097892. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- Burke LE. Electronic measurement. In: Burke LE, Ockene IS, editors. Compliance in healthcare and research. Futura Publishing Company; Armonk, NY: 2001. [Google Scholar]
- Burney KD, Krishnan K, Ruffin MT, Zhang D, Brenner DE. Adherence to single daily dose of aspirin in a chemoprevention trial. An evaluation of self-report and microelectronic monitoring. Archives of Family Medicine. 1996;5:297–300. doi: 10.1001/archfami.5.5.297. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, et al. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Research: Neuroimaging. 2002;114(2):65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Chesney M, Morin M, Sherr L. Adherence to HIV combination therapy. Social Science & Medicine. 2000;50:1599–1605. doi: 10.1016/s0277-9536(99)00468-2. [DOI] [PubMed] [Google Scholar]
- Chermack ST, Roll J, Reilly M, Davis L, Kilaru U, Grabowski J. Comparison of patient self-reports and urinalysis results obtained under naturalistic methadone treatment conditions. Drug & Alcohol Dependence. 2000;59(1):43–49. doi: 10.1016/s0376-8716(99)00106-4. [DOI] [PubMed] [Google Scholar]
- Colfax G, Shoptaw S. The methamphetamine epidemic: Implications for HIV prevention and treatment. Current HIV/AIDS Reports. 2005;2(4):194–199. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- Crisp BR, Williams M, Timpson S, Ross MW. Medication compliance and satisfaction with treatment for HIV disease in a sample of African-American crack cocaine smokers. AIDS and Behavior. 2004;8(2):199–206. doi: 10.1023/B:AIBE.0000030250.33931.af. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Cornish JW. Comparing self-reported cocaine use with repeated urine tests in outpatient cocaine abusers. Experimental and Clinical Psychopharmacology. 1997;5(2):150–156. doi: 10.1037//1064-1297.5.2.150. [DOI] [PubMed] [Google Scholar]
- Fendrich M, Johnson TP, Wislar JS, Hubbell A, Spiehler V. The utility of drug testing in epidemiological research: Results from a general population survey. Addiction. 2004;99:197–208. doi: 10.1111/j.1360-0443.2003.00632.x. [DOI] [PubMed] [Google Scholar]
- Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: A summary of the literature. Medical Care. 2004;42(7):649–652. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- Garcia de Olalla P, Knobel H, Carmona A, Gnelar A, Lopez-Colmes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. Journal of Acquired Immune Deficiency Syndrome. 2002;30:105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patient on multidrug antiretroviral regimens. Journal of Acquired Immune Deficiency Syndrome. 2000;23:386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- Golin CE, Liu H, Hays HD, Miller LG, Beck CK, Ickovics J, et al. A prospective study of predictors of adherence to combination antiretroviral medication. Journal of General Internal Medicine. 2002;17:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, et al. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug and Alcohol Dependence. 2004;76(2):181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Kutnick AH, Slater S. The social realities of adherence to protease inhibitor regimens: Substance use, health care and psychological states. Journal of Health Psychology. 2005;10(4):545–558. doi: 10.1177/1359105305053422. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Atkinson JH, Goodkin K. Neuropsychiatric aspects of HIV infection among older adults. Journal of Clinical Epidemiology. 2001;54(Suppl 1):S44–52. doi: 10.1016/s0895-4356(01)00446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, et al. Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(1):S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RS, Heath KV, Yip B, Craib KJ, O'Shaughnessy MV, Schechter MT, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. Journal of the American Medical Association. 1998;279(6):450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- Howard AA, Arnsten JH, Lo Y, Vlahov D, Rich JD, Schuman P, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- Ingersoll K. The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS Care. 2004;16(2):199–211. doi: 10.1080/09540120410001641048. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. Journal of General Internal Medicine. 1999;14:267–273. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick B, Howlett M, Sedgwick P, Ghodse AH. Drug use, self report and urinalysis. Drug and Alcohol Dependence. 2000;58:111–116. doi: 10.1016/s0376-8716(99)00066-6. [DOI] [PubMed] [Google Scholar]
- Kimmerling M, Wagner G, Ghosh-Dastidar B. Factors associated with accurate self-reported adherence to HIV antiretrovirals. International Journal of STD & AIDS. 2003;14:281–284. doi: 10.1258/095646203321264917. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, et al. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: Biological correlates of disease. AIDS. 2005;19(Suppl 3):S72–S78. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- Levine A, Hardy D, Miller E, Castellon S, Longshore D, Hinkin C. The effect of recent stimulant use on sustained attention in HIV-infected adults. Journal of Clinical and Experimental Neuropsychology. 2006;28:29–42. doi: 10.1080/13803390490918066. [DOI] [PubMed] [Google Scholar]
- Levine A, Hinkin C, Marion S, Keuning A, Castellon S, Lam M, et al. Adherence to antiretroviral medications in HIV: Differences in data collected via self-report and electronic monitoring. Health Psychology. 2006 doi: 10.1037/0278-6133.25.3.329. In press. [DOI] [PubMed] [Google Scholar]
- Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- Lu NT, Taylor BG, Riley KJ. The validity of adult arrestee self-reports of crack cocaine use. American Journal of Drug & Alcohol Abuse. 2001;27(3):399–419. doi: 10.1081/ada-100104509. [DOI] [PubMed] [Google Scholar]
- Matthews WC, Mar-Tang M, Ballard C, Colwell B, Abulhosn K, Noonan C, et al. Prevalence, predictors, and outcomes of early adherence after starting or changing antiretroviral therapy. AIDS Patient Care and STDs. 2002;16(4):157–172. doi: 10.1089/10872910252930867. [DOI] [PubMed] [Google Scholar]
- Mohammed H, Kieltyka L, Richardson-Alston G, Magnus M, Fawal H, Vermund SH, et al. Adherence to HAART among HIV-infected persons in Rural Louisiana. AIDS Patient Care and STDs. 2004;18(5):289–296. doi: 10.1089/108729104323076025. [DOI] [PubMed] [Google Scholar]
- Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MR, Flanigan TP, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patient with advanced HIV disease. Annals of Internal Medicine. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- Nieuwkerk PY, Sprangers MA, Burger DM, Hoetelmans RM, Hugen PW, Danner SA, et al. Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort. Archives of Internal Medicine. 2001;161:1962–1968. doi: 10.1001/archinte.161.16.1962. [DOI] [PubMed] [Google Scholar]
- O'Brien MK, Petrie K, Raeburn J. Adherence to medication regimens: Updating a complex medical issue. Medical Care Review. 1992;49:435–454. doi: 10.1177/002570879204900403. [DOI] [PubMed] [Google Scholar]
- Ory MG, Mack KA. Middle-aged and older people with AIDS. Research on Aging. 1998;20:653–664. [Google Scholar]
- Palella FJ, Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. New England Journal of Medicine. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Paterson D, Potoski B, Capitano B. Measurement of adherence to antiretroviral medications. JAIDS. 2002;31:S103–S106. doi: 10.1097/00126334-200212153-00003. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Chesney M. Treatment adherence to HIV medications: The Achilles heel of the new therapeutics. In: Ostrow D, Kalichman S, editors. Behavioral and mental health impacts of new HIV therapies. Plenum Press; New York: 1999. [Google Scholar]
- Reback CJ, Larkins S, Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS Care. 2003;15(6):775–785. doi: 10.1080/09540120310001618621. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. Journal of Community Health. 2004;29(2):117–27. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychology. 2006;25(1):74–81. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MY, Rapkin BD, Morrison A, Kammerman S. Zidovudine adherence in persons with AIDS: Relation of patient beliefs about medications to self-termination of therapy. Journal of General Internal Medicine. 1997;12:216–223. doi: 10.1046/j.1525-1497.1997.012004216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First M,B. The Structured Clinical Interview for DSM-III–R (SCID): I. History, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stein MD, Rich JD, Maksad J, Chen MH, Hu P, Sobota M, et al. Adherence to antiretroviral therapy among HIV-infected methadone patients: Effect of ongoing illicit drug use. American Journal of Drug and Alcohol Abuse. 2000;26(2):195–205. doi: 10.1081/ada-100100600. [DOI] [PubMed] [Google Scholar]
- Stephenson BJ, Rowe BH, Haynes RB, Macharia WM, Leon G. Is this patient taking the treatment as prescribed? Journal of the American Medical Association. 1993;269:2779–2781. [PubMed] [Google Scholar]
- Tassiopoulos KB, Bernstein J, Heeren T, Levenson S, Hingson R, Bernstein E. Hair testing and self report of cocaine use by heroin users. Addiction. 2004;99(5):590–597. doi: 10.1111/j.1360-0443.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Orlando M, Burnam MA, Sherbourne CD, Kung F, Gifford AL. Psychosocial mediators of antiretroviral nonadherence in HIV-positive adults with substance use and mental health problems. Health Psychology. 2004;23(4):363–370. doi: 10.1037/0278-6133.23.4.363. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wainberg MA, Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance. Journal of the American Medical Association. 1998;279:1977–1983. doi: 10.1001/jama.279.24.1977. [DOI] [PubMed] [Google Scholar]
- Wohl D, Stephenson B, Golin C, Kiziah N, Rosen D, Ngo B, et al. Adherence to directly observed antiretroviral therapy among human immunodeficiency virus-infected prison inmates. Clinical Infectious Diseases. 2003;36:1572–1576. doi: 10.1086/375076. [DOI] [PubMed] [Google Scholar]