Summary
Purpose
To study whether induction of prolonged (>30-min duration) in vitro electrographic seizure discharges resembling status epilepticus (SE) is graded or all-or-none, and to determine the critical factors mediating SE induction.
Methods
Prolonged electrographic seizure discharges were induced in combined hippocampal–entorhinal cortical (HEC) brain slices by electrical stimulation of the Schaeffer collaterals. Discharges were recorded by using field-potential electrodes in the dentate gyrus, CA3, CA1, and entorhinal cortex. Slices were prepared from rats that were (a) 21- to 30-day-old naive, (b) 60- to 120-day old naive, (c) epileptic, and (d) status post a prior traumatic brain injury.
Results
Induction of SE discharges was dependent on the duration, but not amplitude of the preceding stimulus train–induced afterdischarge in HEC slices from 21- to 30-day-old control, brain-injured, and epileptic animals, but not from 60- to 120-day-old animals. In slices from 21- to 30-day-old control animals, once afterdischarges exceeded 4 min in duration, SE was induced in 50% of slices, and after ≥6 min 37 s seizure activity; SE was induced in 95% of slices. A defined SE threshold also was evident in brain-damaged rats, including rats in which an epileptic condition was induced by pilocarpine injection 4–16 weeks before recording, and rats subjected to a fluid percussive head trauma 1–8 weeks before recording. However, in these brain-damaged animals, mean SE threshold was considerably lower (24 and 44 s, respectively). HEC slices from 60- to 120-day-old controls for the brain-injured and epileptic animals did not develop SE even after 20 stimulations, demonstrating the pronounced effect of brain injury and epilepsy on the development of SE in the HEC slice preparation compared with that in age-matched controls.
Conclusions
In vitro, SE discharges have a defined temporal threshold for initiation. Once a seizure exceeds 6–7 min in duration in control animals, and 30–55 s in brain-damaged animals, the probability of SE induction is greatly increased. This demonstrates that brain injury lowers the afterdischarge duration required to produce SE and suggests that brains injured from trauma or SE are more susceptible to develop status epilepticus.
Keywords: Epilepsy, Hippocampus, Status epilepticus, Threshold, Traumatic brain injury
Status epilepticus (SE) is recognized as a neurologic emergency associated with significant morbidity and mortality (1–4) SE is defined as continuous seizure activity lasting > 30 min, or intermittent seizure activity without regaining consciousness lasting > 30 min (5,6). This may be an arbitrary threshold value for diagnosis of SE. Most neurologists agree that seizures lasting > 30–60 min have transitioned into SE. However, in the clinic, most patients who are admitted evidencing continuous seizure activity are aggressively treated as if they are in SE. Studies in humans (7,8) and animals (9–11) have addressed the need to evaluate what constitutes a rational time threshold for the development and diagnosis of SE and indicated that the rigid 30-min time factor in the definition of SE may require more careful evaluation and support from actual data from human and animal studies. Implicit in the determination of this threshold is the concept that, after a seizure duration of a defined length, the probability of spontaneous seizure remission before a 30- to 60-min time point becomes vanishingly small.
The seizure-duration threshold sufficient to transition from “normal” seizures to SE is obviously difficult to determine in a clinical setting, because it necessarily involves untreated prolonged seizures experienced by patients participating in the study.Given the morbidity associated with SE, this obviously raises ethical issues. In initial experiments, this type of study might best be done in experimental animal models of epilepsy. We have developed an in vitro model of SE, in which rat hippocampal–entorhinal cortical (HEC) slices are induced to generate long duration (> 30 min) electrographic seizure activity by electrical stimulation (12,13). This model satisfies the temporal requirement for SE, and evidences a similar five-stage progression of activity seen in the clinic (14) and in SE models in vivo (15). SE discharges in HEC slices activate entorhinal cortex, dentate gyrus, CA3, and CA1 (13), and appear electrographically similar to complex partial SE recorded in humans (16–18).
In the present study, we used this in vitro model of SE to address three research questions: (a) Is SE an all-or-none event with a defined threshold? (b) If so, what is this threshold? and (c) Does this SE threshold change in animals experiencing conditions predisposing them to SE?
METHODS
Slice preparation and recording
Slice preparation and recording were conducted as described (12,13). In brief, rats (Sprague–Dawley) were anesthetized, decapitated, and the brain removed and hemisected. Naive rats were 21–30 days old, and experimental control rats were 60- to 120-day-old animals. Epileptic and traumatic brain-injured rats (see later) were 60–120 days old. Slices (450 µm) were prepared on a vibratome (Lancer 1000, St. Louis, MO, U.S.A.), at an angle 12 degrees from horizontal (rostral up) in oxygenated sucrose artificial cerebrospinal fluid (aCSF), comprising (in mM): 200 sucrose, 3 KCl, 1.25 Na2PO4, 26 NaHCO3, 10 glucose, 0.5 MgCl2, and 2 CaCl2. The magnesium concentration was adjusted in the aCSF to reduce Mg2+- mediated block of presynaptic Ca2+ channels, and thus enhance polysynaptic activity in HEC slices (19). At concentrations of magnesium of 1–4 mM, the HEC slices still developed SE, but the percentage of slices developing SE was slightly reduced from almost 100% in 21- to 30-day-old control, brain injury, and epileptic HEC slices to ~85% of the slices. The concentration of magnesium did not affect the all-or-none response, but it did have a slight effect on the number of slices that transitioned from a single seizure to SE. The concentration of magnesium used in these studies was optimized in previous studies to develop models of epileptogenesis and SE (12,13). Slices were then transferred to an incubator in normal aCSF, with 130 NaCl replacing the sucrose, and used as needed. For recording, slices were transferred to a 35°C interface slice recording chamber (Haas type; Medical Systems, Greenvale, NY, U.S.A.), and a bipolar tungsten stimulating electrode was placed in the Schaeffer collaterals of CA1, and tungsten electrodes were placed in CA1, CA3, dentate gyrus, and entorhinal cortex for simultaneous extracellular field potential recordings (Fig. 1). Electrical stimulation consisted of 2-s, 60-Hz, 8- to 10-V stimulus trains (100-µs pulse width) delivered to the Schaeffer collaterals at 10- to 30-min intervals. Electrographic SE in these in vitro slices was defined as > 30 min of unremitting generalized epileptiform discharges.
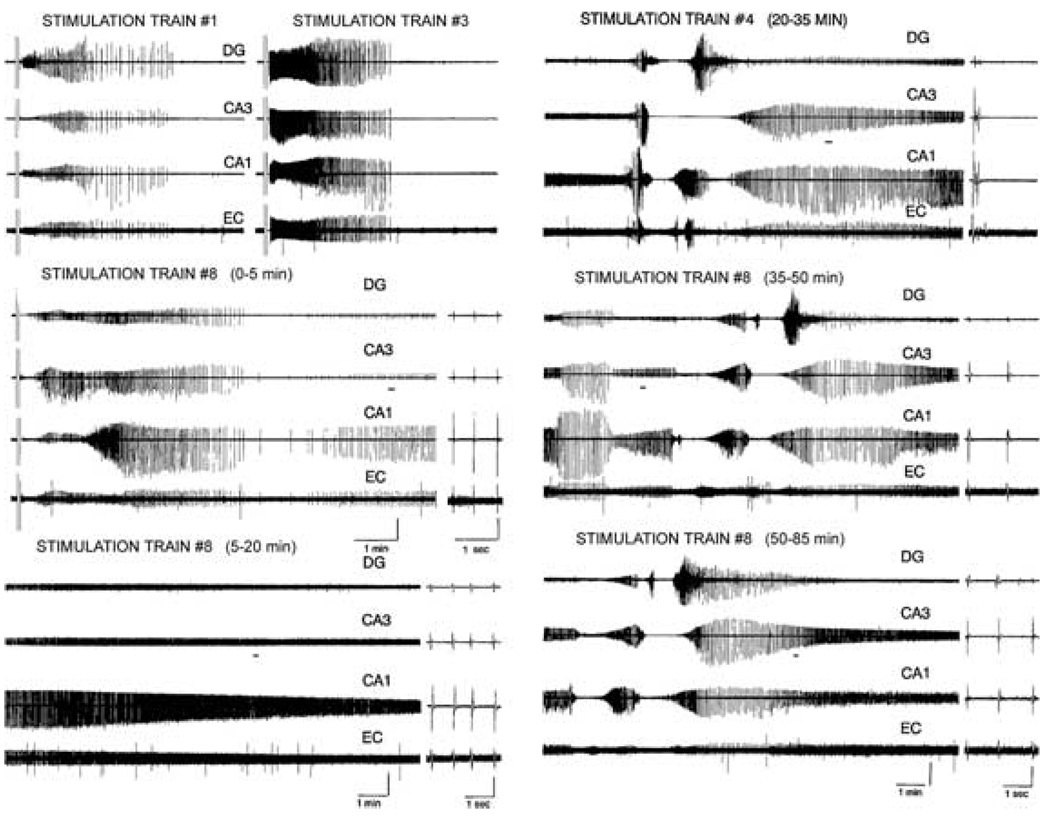
FIG. 1.
Electrographic seizure discharges >30 min in duration fit the definition of status epilepticus (SE). Self-sustaining epileptic activity (SE) triggered by repeated stimulus trains in a hippocampal–entorhinal cortical slice prepared from a 30-day-old animal. Continuous SE was triggered after the eighth stimulus train, which persisted for >1 h. The increasing duration of the afterdischarges can be seen in the earlier stimulus trains. DG, dentate gyrus; EC, entorhinal cortex.
Fluid percussion injury
The fluid percussive injury protocol was identical to that described previously (19). Rats were anesthetized with isoflurane, incubated, and mechanically ventilated. A craniotomy was performed over the right parietal cortex leaving the dura intact, and a rigid plastic tube (modified Luer-lock connector) was secured over the skull opening with dental acrylic. Injury was produced with a calibrated fluid percussion device by the descent of a metal pendulum striking a fluid-filled cylinder attached to the injury tube bonded to the rat’s skull. This injects a small volume of saline epidurally, producing a brief displacement and deformation of the brain (20,21). The resulting pressure pulse was measured extracranially by using a transducer. Within 5 min after completion of the surgical preparation, isoflurane was discontinued, and animals were subjected to the fluid pulse, sufficient to produce a moderate magnitude of injury (2.0 ± 0.05 atm), associated with transient traumatic unconsciousness and motor and memory deficits lasting for ≥ 5 and 15 days, respectively (3,21–25). Rats were allowed to recover for 7 days after injury before being used experimentally.
Preparation of chronically epileptic rats
The pilocarpine rat model of chronic temporal lobe epilepsy used in the present study was adopted (26) and has been described in detail in (19,27). In brief, 60- to 90-day-old female adult rats were injected with scopolamine methyl nitrate (1 mg/kg, i.p.) and then with pilocarpine (350 mg/kg, i.p.) 30 min later. This induced generalized convulsive SE within 10–30 min after injection. Animals were allowed to seize for 1 h, and then diazepam (DZP) was administered at 1, 3, and 5 h to block seizures. SE usually stopped within 3–5 h. Animals were then allowed to recover, and weak animals were given a sucrose/powdered milk mixture for several days to facilitate survival. Beginning 2 weeks after pilocarpine injection, animals were video-monitored for 8 h/day until at least one spontaneous behavioral seizure was observed. Animals were then deemed to be epileptic and were used for slice studies. Seizure frequency in this epileptic population of rats was determined to be 0.8 seizures/day, from 24-h video monitoring (27).
RESULTS
Data in the present study are reported from experiments conducted on 74 HEC slices prepared from 74 different animals: 25 21- to 30-day-old controls (naive), 25 60- to 120-day-old control (naive), 12 brain-injured, and 12 epileptic animals. Our previous studies demonstrated that HEC slices from 21- to 30-day-old animals developed SE-like electrographic epileptiform discharges (Fig. 1) after repeated stimulation trains (13). This study was initiated to compare the effect of repeated stimulation trains to develop SE on HEC slices prepared from 21- to 30- day-old controls, from brain injured, from epileptic, and from age-matched 60- to 120-day-old controls for brain-injured and epileptic animals. All slices were recorded for ≥ 2–4 h and were healthy as assessed by input/output curves with large-amplitude fields and by the fact that all slices were recorded for several hours with no spreading depressions evident. Repeated stimulation trains in 21- to 30-day-old controls caused 23 (92%) of 25 to develop > 30-min duration SE-like electrographic epileptiform discharges (Fig. 2A). None of the 25 60- to 120- day-old slices developed SE-like electrographic epileptiform discharges with > 20 repeated stimulation trains (Fig. 2B). Repeated stimulation trains in the brain-injured and epileptic animals caused 100% (12 of 12) and 100% (12 of 12) of the slices from these animals to develop SE-like electrographic epileptiform discharges.
FIG. 2.
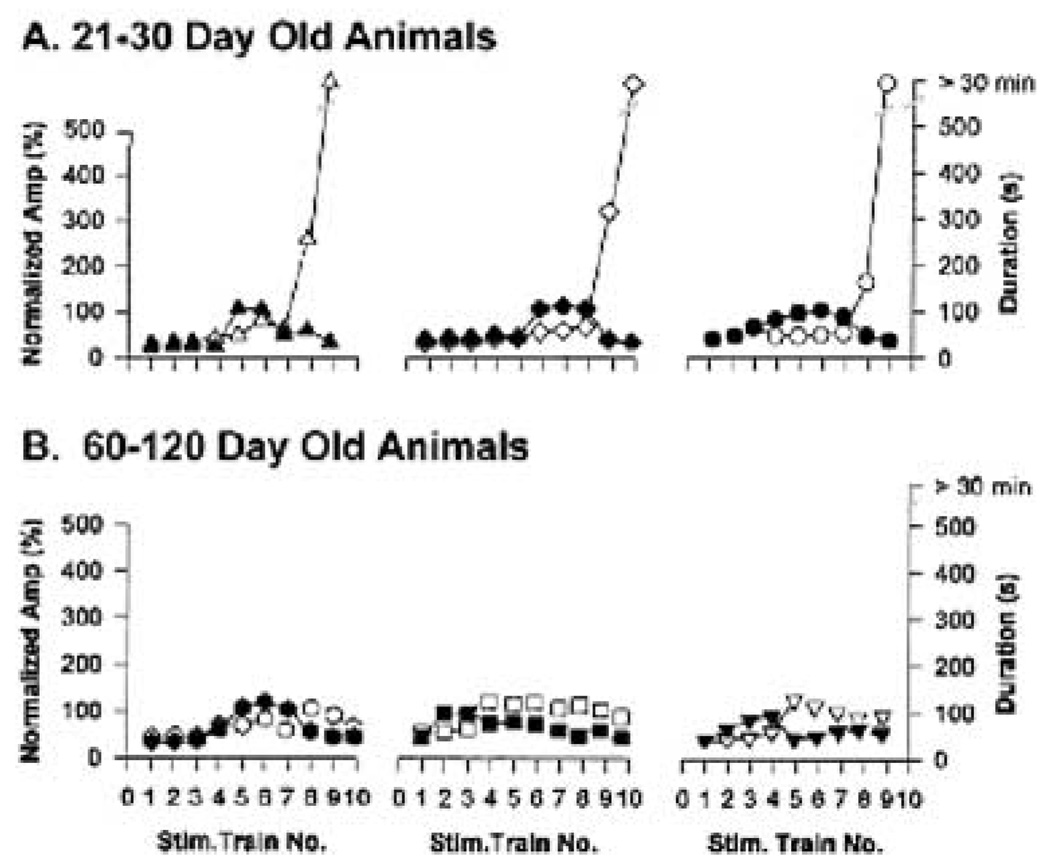
Afterdischarge duration and not amplitude is critical for induction of status epilepticus (SE) in hippocampal–entorhinal cortical slices from 21- to 30-day but not from 60- to 120-day-old animals. Plots of CA1 afterdischarge amplitude (normalized to maximal amplitude) and afterdischarge duration against the stimulus train number eliciting the afterdischarge for (A) three slices from 21- to 30-day-old animals, and (B) three slices from 60- to 120-day-old animals. Note that the afterdischarge amplitude plateaus several trains before the induction of SE in all slices, whereas afterdischarge duration continuously increases until SE is induced. Open symbols, the afterdischarge duration; solid (black) symbols, the normalized amplitude. The different symbols used in each graph highlight the fact that each plot presents data from an individual animal.
Long-duration SE-like activity in vitro
Repeated stimulus trains administered to the Schaeffer collaterals in HEC slices elicited electrographic epileptiform afterdischarges recorded throughout the HEC slice from the dentate gyrus, CA3, CA1, and entorhinal cortex, which gradually lengthened in duration and increased in amplitude (Fig. 1, stimulation trains 1 and 3). After several afterdischarges, a new pattern of delayed electrographic epileptiform activity was elicited, involving generation of very long-duration, continuous electrographic seizure activity at > 2 Hz (Fig. 1, stimulation train 8). This activity persisted continuously for > 30 min (65 min of activity is shown in Fig. 1). Although the activity intermittently waxed and waned in amplitude, accelerated and decelerated in frequency, and exhibited brief periods of depolarization block associated with ictal activity (Fig. 1 stimulation train 8, 20–35 min, 35–50 min, and 50–65 min), no periods of cessation of epileptic activity were noted. Thus these prolonged electrographic seizure discharges fulfill the temporal requirements for SE.
Increases in electrographic seizure duration but not amplitude are critical for SE induction
In examining the buildup of stimulation-induced afterdischarges preceding the induction of the SE discharges described earlier, increases in the duration but not in the amplitude of afterdischarges appeared to be the critical determinant for induction of SE. Figure 2A plots the afterdischarge duration and normalized CA1 afterdischarge amplitude in response to repeated Schaeffer collateral stimulus trains for three HEC slices prepared from control 21- to 30-day-old animals. Afterdischarge amplitude tended to increase with repeated stimulus trains, but then plateaued for several trains before the induction of SE. Similar plateaus in afterdischarge amplitude are evident for the three slices quantified in Fig. 2A. This contrasted with the plots of afterdischarge duration in the same slices. Afterdischarge duration continuously increased before the induction of SE, and therefore increases in afterdischarge duration appeared to be the primary factor determining when SE would be induced. In all three slices in Fig. 2A, afterdischarge duration increased from ~40 to 120 s continuously between stimulus trains 4 and 9, until SE was induced.
Induction of SE is an all-or-none event, with a defined afterdischarge-duration threshold
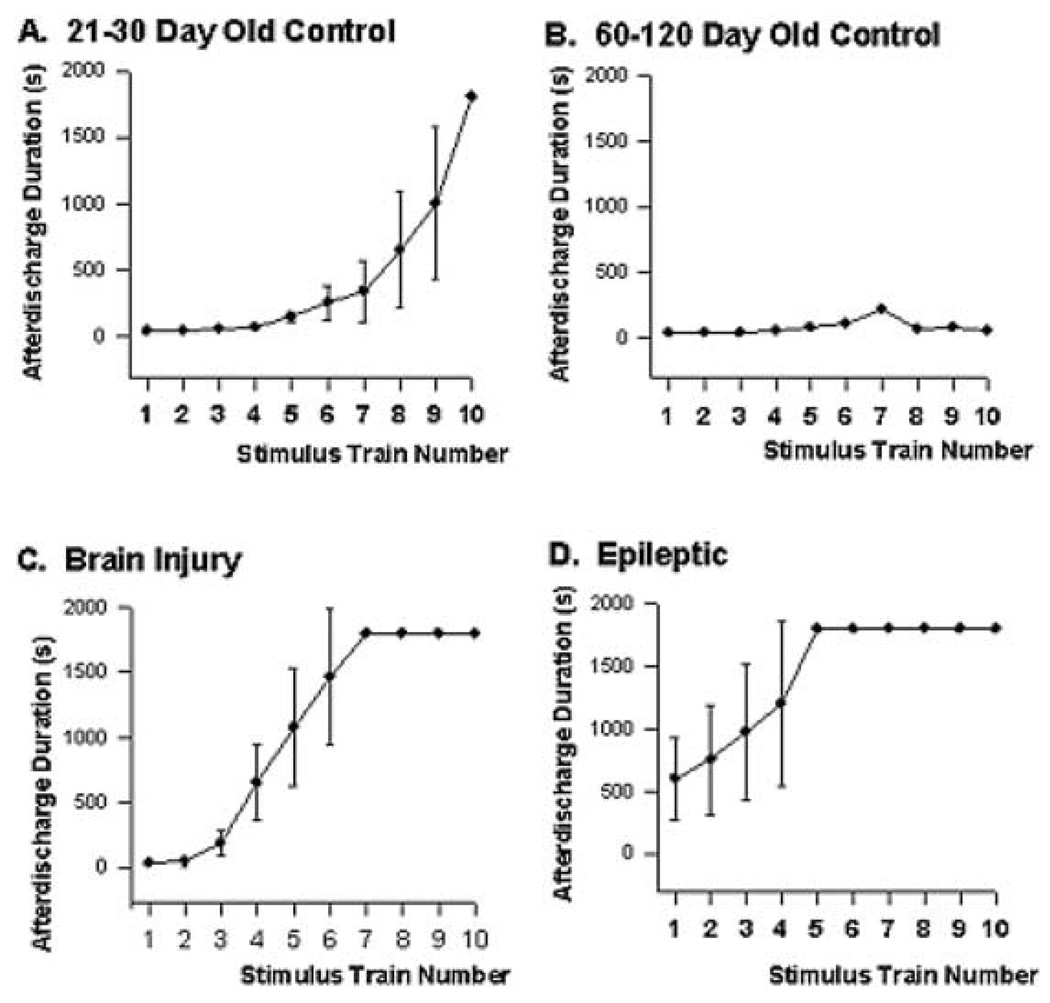
Although the afterdischarge duration increased steadily until SE was induced (Fig. 2A), the actual onset of SE was an all-or-none event. The mean and standard error of the CA1 afterdischarge amplitude for all 25 animals per stimulus train is provided in Fig. 3A. The CA1 afterdischarge amplitude increased from 10 to 80%of maximal amplitude between trains 7 and 10. In 21- to 30-day-old HEC slices from normal animals, afterdischarges increased rapidly immediately preceding the onset of SE. However, SE was defined as > 30 min of unremitting electrographic seizure activity (or > 1,800 s of activity) and, as can be seen from Fig. 3A, SE was triggered in an all-or-none manner. Ninety percent of slices prepared from control animals (mean ± 1 SD) triggered SE after an afterdischarge of 6 min 37 s (i.e., if an electrographic seizure lasted this long in a slice prepared from a 21- to 30-day-old control animal, the probability was 93% that SE would be induced with the following stimulation train). The mean duration of electrographic seizures preceding SE for the 21- to 30-day-old animals was 6 min 33 s (n = 25). Conversely, HEC slices prepared from 60- to 120-day-old normal animals did not develop sustained afterdischarges (Fig. 3B). Even after 20 stimulus trains, these slices did not develop SE. This result demonstrates that the adult brain is very resistant to developing SE. The 60- to 120-day-old HEC slices are the age-matched control slices for the brain-injured and epileptic animals. These models require several months for the animals to recover, so the older controls were used for comparison. No difference in response was observed between the 60- and 120-day-old animals or within this age group.
FIG. 3.
Quantification of stimulus train–induced electrographic seizure activity in hippocampal–entorhinal cortical slices prepared from 21- to 30-day-old control (A), 60- to 120-day-old control (B), brain-injured (C), and epileptic (D) animals. The graphs present a plot of Schaeffer collateral stimulus train number versus the duration of the afterdischarge elicited by the train. Recordings were stopped after 30 min of continuous electrographic seizure activity was triggered, representing the ceiling of activity duration (1,800 s). The status epilepticus (SE)-like discharges were triggered the earliest in the epileptic slices. The brain-injured slices also elicited prolonged discharges much earlier than the 21- to 30-day-old slices. The age-matched control slices (60–120 days old) for the brain-injured and epileptic animals did not develop SE. This demonstrates the marked effect of pilocarpine-induced SE and brain injury on the susceptibility of developing SE. The data give the means and standard errors of the afterdischarge duration in seconds.
Threshold afterdischarge durations for induction of SE differ between control and brain-damaged rats
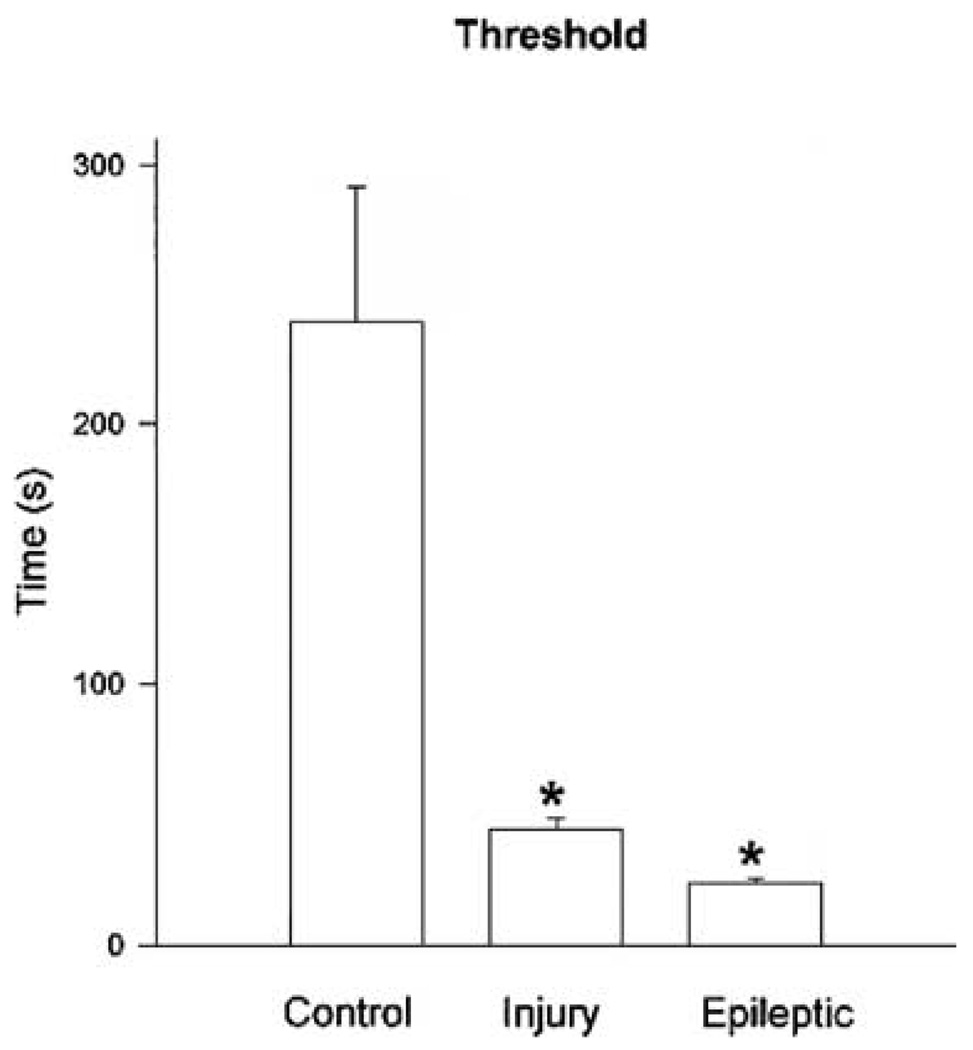
This all-or-none nature of SE induction was even more evident in brain-damaged animals, in which the afterdischarge duration threshold was 44 s in traumatic brain-injured rats and 24 s in epileptic rats. The mean duration of electrographic seizures preceding SE for the brain-injured animals was 53 s (n = 12); 11 (92%) of 12 slices from traumatic brain-injured rats that triggered SE discharges did so after an afterdischarge with a duration of < 60 s. The brain-injured slices reached the SE threshold much earlier than the 21- to 30-day-old slice (Fig. 4). Not surprisingly, slices prepared from epileptic animals triggered SE discharges after very few stimulus trains (one to five trains), because an afterdischarge duration normally achieved very rapidly (Fig. 4) could reach this SE threshold. This compared with control (six to 10 trains) and traumatic brain-injured animals (four to nine trains), in which a greater buildup in preceding afterdischarge duration was necessary before SE was induced. The mean duration of electrographic seizures preceding SE for the epileptic animal HEC slices was 38 s (n = 12); 11 (92%) of these 12 slices developed SE discharges.
FIG. 4.
Histogram quantitating differences in status epilepticus (SE) thresholds. Note the statistically significant differences in SE afterdischarge duration threshold between control, brain-injured, and epileptic hippocampal–entorhinal cortical (HEC) slices. Control slices had significantly higher thresholds than either brain-injured or epileptic HEC slices *(p < 0.0001, t test), and brain-injured slices had SE thresholds significantly longer than epileptic slices *(p < 0.005, t test).
DISCUSSION
In ratHEC slices, induction of prolonged electrographic seizure discharges fitting the definition of SE was all-or-none and had a defined threshold determined by the duration but not the amplitude of the previous afterdischarge. This afterdischarge duration threshold for SE induction was 4 min on average in HEC slices prepared from control rats, and 95% of the control slices triggered SE after afterdischarges of ≤ 7 min in duration. This contrasted with slices prepared from brain-damaged rats, in which SE thresholds were much lower. HEC slices prepared from rats that experienced a traumatic brain injury had a mean SE-induction threshold of 44 s, and 95% of these slices triggered SE after afterdischarges of < 1 min in duration, whereas HEC slices prepared from chronically epileptic rats had a mean SE threshold of 24 s, and 95% of slices triggered SE after afterdischarges of ≤ 30 sec in duration. Therefore this suggests that the clinical minimal seizure duration definition for diagnosis of SE (≥ 30 min) should be reexamined in clinical studies, because it appears in experimental studies that the probability that a seizure will terminate spontaneously after a seizure as ≥ 7 min in duration becomes vanishingly small. Furthermore, the threshold for induction of SE appears to be critically dependent on the history of the animal (or possibly the patient), and in SE-susceptible patients, this 7-min 95% confidence limit might be reduced to ≤ 1 min, again suggesting that further clinical studies may be necessary to address scientifically (rather than empirically) the issue of the definition of SE.
The long-duration electrographic seizure discharges recorded in HEC slices in the present study resemble SE for a number of reasons. First, these discharges fit the temporal definition of SE, in that they are ≥ 30 min of unremitting electrographic seizure activity. Second, the development and decay of these discharges transitions through a series of electrographic stages similar to SE described in humans (14) and in animals (15). Third, they resemble EEG activity described in patients with partial SE (16–18,28). SE in the HEC slices, like EEG recordings from patients with partial SE, demonstrated robust electrographic seizure activity that intermittently waxed and waned in amplitude, accelerated and decelerated in frequency, and exhibited brief periods of depolarization block associated with ictal activity. Finally, these in vitro SE discharges can be blocked by anticonvulsant benzodiazepines (BZDs) (12,13), drugs frequently used to treat SE. Therefore these in vitro SE discharges resemble the clinical condition in their frequency, duration, in their progression through a similar electrographic series of stages, and in their pharmacologic sensitivity.
The preceding afterdischarge duration but not amplitude determined whether SE would be induced by a given Schaeffer collateral stimulus train in HEC slices. Afterdischarge duration increased steadily before induction of SE, until a threshold value was exceeded, at which point SE followed. This contrasted with the afterdischarge amplitude, which grew with repeated Schaeffer stimulus trains, but virtually always plateaued at a maximal value several stimulus trains before SE was induced. This suggests that afterdischarge amplitude and duration are two independent phenomena, with properties dependent on different mechanisms. The amplitude of field potential recordings depends on the density of the electrical field sensed by the electrode, which, in laminar structures like the hippocampus, in turn depends on the number of neurons recruited into the discharge. During intense epileptiform activity in hippocampal slices, virtually the entire population of viable neurons may be participating in the generation of epileptic discharge, as assessed by sampling of neuronal firing behavior with an intracellular electrode (13), or computer modeling studies examining generation of epileptic discharges (4,29). Field potential recordings of the amplitude of epileptic discharges would therefore be expected to grow as more and more neurons are recruited into the discharge, but also to exhibit a definite maximal value, as most or all of the laminar neurons in the region sensed by the recording electrode become involved in the discharge. This contrasts with the afterdischarge duration, which depends on processes dampening or controlling synchronization of neuronal firing and can increase indefinitely. Several mechanisms have been hypothesized to be critical in epileptiform discharge generation and termination, including (but not limited to) γ-aminobutyric acid (GABA)ergic inhibition (30,31), activation of calcium-dependent potassium conductances (32,33), “h” current activation (34), relative levels of glutamatergic metabotropic receptor activation (35), activation of GABAB receptors (36), and activity-dependent alterations in extracellular and intracellular ion concentrations (37,38). In addition, the increased susceptibility of the injured brain may be influenced by the cell damage and reorganization of neuronal circuits that may occur after injury. We have shown that both the brain-trauma model and the pilocarpine SE model develop manifest significant cell loss in the hilar region of the ventral dentate gyrus and the CA3 region (19). The degree of injury and the resultant pathophysiologic changes resulting from this process may affect the susceptibility to develop SE.
Our working hypothesis is that a gradual and augmenting decrement in GABAergic inhibition during prolonged afterdischarges is the primary candidate mechanism for the threshold phenomenon in the induction of SE. The first postulate of this hypothesis is that GABAergic inhibition fades immediately during sustained activation, as is experienced during an epileptiform afterdischarge. The fact that BZD efficacy decreases dramatically during prolonged seizures supports the concept that GABAergic inhibition fades during seizures (39,40). This compromise in inhibitory efficacy results from a number of underlying mechanisms, including (but not limited to) accumulation of intracellular chloride concentrations (41–43), desensitization of GABA receptors (41,44), GABAB-mediated autoinhibition of GABA release (45), GABAA-receptor uncoupling and internalization (46), calcium-dependent regulation of GABA-receptor function (47,48), and altered phosphorylation state of GABA receptors (49,50). A second postulate of this hypothesis is that GABA-receptor activity is of primary functional importance in seizure termination. Once the strength of GABAergic inhibition has faded below a critical minimal value, seizure-termination mechanisms fail, and SE results. This phenomenon (reduced GABA efficacy triggering SE) would explain both the all-or-none nature of SE induction and the differences in SE thresholds in control, traumatic brain-injured, and epileptic animals. The traumatic brain-injured and epileptic animals used in the present study have already been demonstrated to have compromised inhibitory properties in various areas of the limbic system (19,27,51). Therefore, given that inhibitory efficacy is already compromised, it is expected that the degree of further decrement in inhibition necessary to reach the critical minimal value for SE to be induced would be considerably less, resulting in SE induction after shorter seizures in these animals. In addition, the decreased levels of GABAergic inhibition in the injured slices may reduce the susceptibility of the injured brains to BZDs. This is an area for future investigation. This “GABA hypothesis” of SE induction also could explain some of the susceptibility of certain populations of patients to SE. In epidemiologic studies, patients with epilepsy, traumatic brain injury, or cerebrovascular accidents are particularly likely to experience SE (1,2,52), and these conditions are associated with a loss in inhibitory efficacy in various areas of the brain (19,27,50,53,54,55).
In HEC slices prepared from normal rats, SE was induced in > 95% of slices by electrically induced afterdischarges of ≤ 7 min in duration. Because SE was an all-or-none phenomenon, this suggests that a diagnostic definition of SE as ≥ 30 min of continuous epileptic activity should be revised if similar mechanisms are evident in humans with epilepsy in clinical studies. The HEC slice model for the induction of SE in vitro (19) provides a useful preparation to investigate the mechanisms mediating the development of SE. However, it is not possible to translate findings from this model directly to the clinical area. In vitro models are useful to initiate studies on underlying mechanisms, but they do not represent the clinical situation. Careful prospective and retrospective clinical studies will be required to determine if similar seizure-duration thresholds are evident in human SE. In humans, if it is determined that the probability of spontaneous seizure termination becomes vanishingly small once a seizure reaches or exceeds a threshold duration, then the definition of SE should be operationally redefined based on the 50% threshold ± 1 SD, the seizure duration beyond which 95% of patients will be in SE. Clinical studies of this type must also consider the patient history in the analysis and determination of SE threshold, because the present study clearly demonstrated differing SE thresholds in brain-damaged rats compared with controls.
Acknowledgment
This study was supported by grants P50 NS-25630 (R.J.D. and D.A.C.), R01 NS-32403 (D.A.C.), and R01 NS-23350 (R.J.D.), all from NIH-NINDS, and the Sophie and Nathan Gumenick Neuroscience and Alzheimer’s Research Fund.
REFERENCES
- 1.DeLorenzo RJ, Towne AR, Pellock JM, et al. Status epilepticus in children, adults, and the elderly. Epilepsia. 1992;33:14–24. doi: 10.1111/j.1528-1157.1992.tb06223.x. [DOI] [PubMed] [Google Scholar]
- 2.Hauser WA. Status epilepticus: epidemiological considerations. Neurology. 1990;40 suppl 2:9–13. [PubMed] [Google Scholar]
- 3.Smith DH, Lowenstein DH, Gennerelli TA, et al. Persistent memory dysfunction is associated with bilateral hippocampal damage following experimental brain injury. Neurosci Lett. 1994;168:151–154. doi: 10.1016/0304-3940(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 4.Traub RD, Miles R, Wong RKS. Models of synchronized hippocampal bursts in the presence of inhibition, I: single population events. J Neurophysiol. 1987;58:739–751. doi: 10.1152/jn.1987.58.4.739. [DOI] [PubMed] [Google Scholar]
- 5.Epilepsy Foundation of America Working Group on Status Epilepticus. Treatment of convulsive status epilepticus. JAMA. 1993;270:854–859. [PubMed] [Google Scholar]
- 6.Gastaut H. Dictionary of epilepsy, part 1: definitions. Geneva: World Health Organization; 1973. [Google Scholar]
- 7.Shinnar S, Berg AT, Moshe SL, et al. How long do new-onset seizures in children last? Ann Neurol. 2001;49:659–664. [PubMed] [Google Scholar]
- 8.DeLorenzo RJ, Garnett LK, Towne AR, et al. Comparison of status epilepticus with prolonged seizure episodes lasting from 10 to 29 minutes. Epilepsia. 1999;40:164–169. doi: 10.1111/j.1528-1157.1999.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 9.Gruenthal M. Electrographic and histological characteristics of a model of limbic status epilepticus permitting direct control over seizure duration. Epilepsy Res. 1998;29:221–232. doi: 10.1016/s0920-1211(97)00085-5. [DOI] [PubMed] [Google Scholar]
- 10.Lothman EW, Berttram EH, Bekenstein JW, et al. Self-sustaining limbic status epilepticus induced by “continuous” hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–119. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- 11.Mazarati AM, Wasterlain CG, Sankar R, et al. Self-sustaining status epilepticus after brief electrical stimulation of the perforant path. Brain Res. 1998;801:251–253. doi: 10.1016/s0006-8993(98)00606-4. [DOI] [PubMed] [Google Scholar]
- 12.Rafiq A, Zhang Y-F, DeLorenzo RJ, et al. Long duration self-sustained epileptiform activity in the hippocampal-parahippocampal slice: a model of status epilepticus. J Neurophysiol. 1995;74:2028–2042. doi: 10.1152/jn.1995.74.5.2028. [DOI] [PubMed] [Google Scholar]
- 13.Mott DD, Xie C-W, Wilson WA, et al. GABAB autoreceptors mediate activity-dependent disinhibition and enhance signal transmission in the dentate gyrus. J Neurophysiol. 1993;69:674–691. doi: 10.1152/jn.1993.69.3.674. [DOI] [PubMed] [Google Scholar]
- 14.Treiman DM, Walton NY, Kendrick CW. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990;5:49–60. doi: 10.1016/0920-1211(90)90065-4. [DOI] [PubMed] [Google Scholar]
- 15.Lothman EW. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40 suppl 2:13–23. [PubMed] [Google Scholar]
- 16.Treiman DM, Delgado-Escueta AV. Complex partial status epilepticus. Jpn J Psychiatry. 1988;42:437–440. doi: 10.1111/j.1440-1819.1988.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 17.Wieser HG, Hailemariam S, Regard M, et al. Unilateral limbic epileptic status activity: stereo EEG, behavioral, and cognitive data. Epilepsia. 1985;26:19–29. doi: 10.1111/j.1528-1157.1985.tb05184.x. [DOI] [PubMed] [Google Scholar]
- 18.Williamson PD, Spencer DD, Spencer SS, et al. Complex partial status epilepticus: a depth electrode study. Ann Neurol. 1985;8:647–654. doi: 10.1002/ana.410180604. [DOI] [PubMed] [Google Scholar]
- 19.Coulter DA, Rafiq A, Shumate M, et al. Brain injury-induced enhanced limbic epileptogenesis: anatomical and physiological parallels to an animal model of temporal lobe epilepsy. Epilepsy Res. 1996;26:81–91. doi: 10.1016/s0920-1211(96)00044-7. [DOI] [PubMed] [Google Scholar]
- 20.Dixon CE, Lyeth BG, Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1989;67:1190–1197. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 21.McIntosh TK, Vink R, Noble L, et al. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 22.Hamon B, Stanton PK, Heinemann U. An N-methyl-d-aspartate receptor-independent excitatory action of partial reduction of extracellular Mg 2) in CA1-region of rat hippocampal slices. Neurosci Lett. 1987;75:240–245. doi: 10.1016/0304-3940(87)90304-1. [DOI] [PubMed] [Google Scholar]
- 23.Lyeth BG, Dixon CE, Hamm RJ, et al. Effects of anticholinergic treatment on transient behavioral suppression and physiological responses following concussive brain injury to the rat. Brain Res. 1988;448:249–258. doi: 10.1016/0006-8993(88)91104-3. [DOI] [PubMed] [Google Scholar]
- 24.Lyeth BG, Jenkins LW, Hamm RJ, et al. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- 25.Smith DH, Okiyama K, Thomas MJ, et al. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259–269. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- 26.Mello LEAM, Cavalheiro EA, Tan AM, et al. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs ZW, III, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- 28.Grand’Maison F, Reiher J, LeDuc CP. Retrospective inventory of clinical abnormalities in partial status epilepticus. Electroencephalogr Clin Neurophysiol. 1991;79:264–270. doi: 10.1016/0013-4694(91)90121-j. [DOI] [PubMed] [Google Scholar]
- 29.Whittington MA, Traub RD, Jefferys JG. Erosion of inhibition contributes to the progression of lowmagnesium bursts in rat hippocampal slices. J Physiol (Lond) 1995;486:723–734. doi: 10.1113/jphysiol.1995.sp020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapur J, Lothman EW. Loss of recurrent inhibition precedes delayed spontaneous seizures in the hippocampus after tetanic electrical stimulation. J Neurophysiol. 1983;61:427–434. doi: 10.1152/jn.1989.61.2.427. [DOI] [PubMed] [Google Scholar]
- 31.Korn SJ, Giacchino JL, Chamberlain NL, et al. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987;57:325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- 32.Alger BE, Williamson A. A transient calcium-dependent potassium component of the epileptiform burst after-hyperpolarization in rat hippocampus. J Physiol (Lond) 1988;399:191–205. doi: 10.1113/jphysiol.1988.sp017075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamberlain NL, Dingledine R. Control of epileptiform burst rate by CA3 hippocampal cell after-hyperpolarizations in vitro. Brain Res. 1989;492:337–346. doi: 10.1016/0006-8993(89)90917-7. [DOI] [PubMed] [Google Scholar]
- 34.Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron. 1996;17:297–308. doi: 10.1016/s0896-6273(00)80161-0. [DOI] [PubMed] [Google Scholar]
- 35.Merlin LR, Wong RKS. Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol. 1997;78:539–544. doi: 10.1152/jn.1997.78.1.539. [DOI] [PubMed] [Google Scholar]
- 36.Morrisett RA, Lewis DV, Swartzwelder HS, et al. Antiepileptic effects of GABAB receptor activation in area CA3 of rat hippocampus. Brain Res. 1993;600:235–242. doi: 10.1016/0006-8993(93)91378-6. [DOI] [PubMed] [Google Scholar]
- 37.Fisher RS, Pedley TA, Moody WJ, Jr, et al. The role of extracellular potassium in hippocampal epilepsy. Arch Neurol. 1976;33:76–83. doi: 10.1001/archneur.1976.00500020004002. [DOI] [PubMed] [Google Scholar]
- 38.Yaari Y, Konnerth A, Heinemann U. Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro, II: role of extracellular potassium. J Neurophysiol. 1986;56:424–438. doi: 10.1152/jn.1986.56.2.424. [DOI] [PubMed] [Google Scholar]
- 39.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walton NY, Treiman DM. Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Exp Neurol. 1988;101:267–275. doi: 10.1016/0014-4886(88)90010-6. [DOI] [PubMed] [Google Scholar]
- 41.Huguenard JR, Alger BE. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol. 1986;56:1–18. doi: 10.1152/jn.1986.56.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Kapur J, Coulter DA. Experimentally-induced status epilepticus acutely alters GABA receptor function in CA1 pyramidal neurons. Ann Neurol. 1995;38:893–900. doi: 10.1002/ana.410380609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarren M, Alger BE. Use-dependent depression of IPSPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1985;53:557–561. doi: 10.1152/jn.1985.53.2.557. [DOI] [PubMed] [Google Scholar]
- 44.Frosch MP, Lipton SA, Dichter MA. Desensitization of GABA-activated currents and channels in cultured cortical neurons. J Neurosci. 1992;12:3042–3053. doi: 10.1523/JNEUROSCI.12-08-03042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes EM., Jr Use-dependent regulation of GABAA receptors. Int Rev Neurobiol. 1996;39:53–76. doi: 10.1016/s0074-7742(08)60663-7. [DOI] [PubMed] [Google Scholar]
- 46.Chen CX, Wong RKS. Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J Physiol (Lond) 1995;482:353–362. doi: 10.1113/jphysiol.1995.sp020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Koninck Y, Mody I. The effects of raising intracellular calcium on synaptic GABAA receptor-channels. Neuropharmacology. 1996;35:1365–1374. doi: 10.1016/s0028-3908(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 48.Chen CX, Stelzer A, Kay AR, et al. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol (Lond) 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapur J, Macdonald RL. Cyclic AMP-dependent protein kinase enhances hippocampal dentate granule cell GABAA receptor currents. J Neurophysiol. 1996;76:2626–2634. doi: 10.1152/jn.1996.76.4.2626. [DOI] [PubMed] [Google Scholar]
- 50.Lowenstein DH, Thomas MJ, Smith DH, et al. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Towne AR, Pellock JM, Ko D, et al. Determinants of mortality in status epilepticus. Epilepsia. 1994;35:27–34. doi: 10.1111/j.1528-1157.1994.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 52.Gibbs JW, III, Sombati S, DeLorenzo RJ, et al. Physiological and pharmacological alterations in postsynaptic GABAA receptor function in a hippocampal culture model of chronic spontaneous seizures. J Neurophysiol. 1997;77:2139–2152. doi: 10.1152/jn.1997.77.4.2139. [DOI] [PubMed] [Google Scholar]
- 53.Mileson BE, Ehrmann ML, Schwartz RD. Alterations in the gammaaminobutyric acid-gated chloride channel following transient forebrain ischemia in the gerbil. J Neurochem. 1992;58:600–607. doi: 10.1111/j.1471-4159.1992.tb09761.x. [DOI] [PubMed] [Google Scholar]
- 54.Alger BE, Pitler TA. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends Neurosci. 1995;18:333–340. doi: 10.1016/0166-2236(95)93923-l. [DOI] [PubMed] [Google Scholar]
- 55.Hicks RR, Smith DH, Lowenstein DH, et al. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J Neurotrauma. 1993;10:405–414. doi: 10.1089/neu.1993.10.405. [DOI] [PubMed] [Google Scholar]