Abstract
This review summarizes the current thinking about the causes of anemia universally experienced by preterm infants in the early postnatal weeks. In addition to describing developmentally determined physiologic processes contributing to anemia of prematurity, this review discusses clinically important nonphysiologic contributors to anemia experienced by preterm infants during the neonatal period. Chief among these and an important contributor to the need for red blood cell transfusions is the heavy laboratory phlebotomy loss sustained shortly after birth, when neonatal cardiorespiratory illness is most severe. Understanding and recognizing the physiologic and nonphysiologic processes contributing to anemia encountered in early postnatal life is important in knowing which treatment and prevention modalities are likely to be most effective in different clinical situations. The evaluation of rare and uncommon acquired and genetic causes of anemia in newborns are not covered in this review.
Introduction
Immediately following birth, all infants universally experience a decrease in hemoglobin (Hb) that results in varying degrees of anemia. The rapidity with which this anemia develops and its ultimate severity are determined by a combination of multiple physiologic and nonphysiologic processes. Preterm infants are especially vulnerable to these processes for two reasons. First, the severity of the developmental postnatal decrease in Hb is most pronounced in the least mature infants, placing them at high risk of developing clinically significant anemia. Second, as a group, preterm infants are particularly prone to developing severe cardiorespiratory and infectious illnesses, the diagnosis and management of which requires frequent laboratory assessment, resulting in heavy phlebotomy loss. It is the combination of developmentally regulated physiologic processes (commonly referred to as anemia of prematurity [AOP]) along with concomitant pathologic and iatrogenic processes that contribute to the progressive anemia experienced by virtually all preterm infants. The prevention and treatment of neonatal anemia is the subject of a companion article in this issue.
Definition and Natural History of AOP
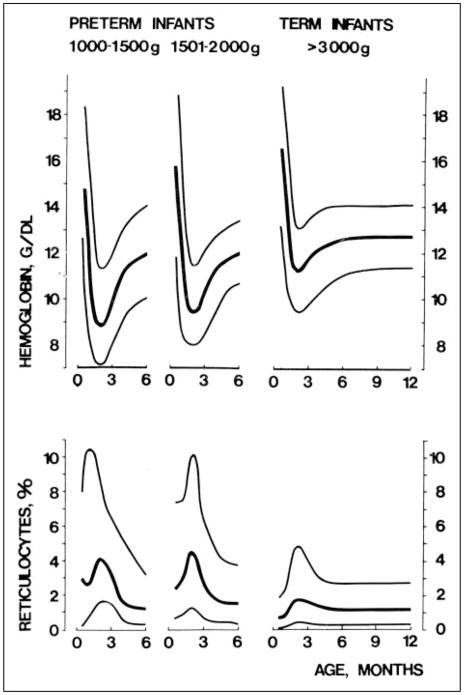
The current use of the term AOP to describe the postnatal decrease in Hb experienced by virtually all preterm infants should be understood from a historical perspective. For more than a century, clinicians have recognized that both term and preterm infants experience a progressive decline in Hb concentration during the 8 to 10 weeks immediately following birth (Fig. 1). (1)(2) This is the greatest normal change in Hb value for any period of life. In healthy term infants, clinical signs or symptoms of anemia are absent; this normal decline in Hb is referred to as “physiologic” or “early anemia of infancy.” Among term infants, Hb values fall from 14.6 to 22.5 g/dL (146 to 225 g/L) at birth to a low of 10.0 to 12.0 g/dL (100 to 120 g/L) by 8 to 10 weeks of age. After reaching this nadir, values gradually increase, eventually reaching adult Hb values within the first 2 years after birth.
Figure 1.
Hemoglobin concentrations and reticulocyte counts in preterm and term infants during the first 6 postnatal months. Median values and 95% confidence limits are indicated for each of three birthweight groups represented. Reprinted with permission from Dallman PR. Anemia of prematurity. Ann Rev Med. 1981;32:143–160.
Among preterm infants, the expected postnatal decline in Hb is more severe than for term infants (Fig. 1), and unlike term infants, the more rapid and profound fall in Hb may be accompanied by clinical signs of anemia. Even in the absence of iatrogenic laboratory phlebotomy loss, preterm infants experience a more rapid and severe decrease in Hb than term infants, often to a nadir of 7.0 to 8.0 g/dL (70 to 80 g/L). The lowest values are encountered in the smallest, least mature preterm infants. In evaluating the Hb and reticulocyte data shown in Figure 1 for very low-birthweight (VLBW) preterm infants, it is important to note that these data were derived from relatively healthy surviving preterm infants cared for in the early 1970s. Hence, they do not accurately reflect the more rapid Hb decline among many of today’s critically ill preterm infants. (1)
With increasing survival of smaller, less mature infants, a new group of preterm infants has emerged: extremely low-birthweight (ELBW) infants weighing less than 1,000 g. Before the era of infant ventilators, surfactant, and other new therapies and technologies that are taken for granted in developed countries today, most ELBW infants did not survive. Hence, there were no “normative” data to include from this group in Figure 1.
To refer to the postnatal decrease in Hb universally experienced by ELBW and VLBW infants—and by heavier, critically ill, less preterm infants—as AOP is misleading. This is because the developmentally based, physiologic factors responsible for the postnatal decrease in Hb in all preterm infants commonly are over-shadowed by the contribution of concomitant nonphysiologic factors and conditions (Fig. 2). These include phlebotomy loss attributable to laboratory testing—euphemistically referred to as “bleeding/hemorrhaging into the laboratory”—and clinical conditions such as sepsis and inadequate nutrition. Failure to recognize nonphysiologic contributors to neonatal anemia results in missed opportunities to apply effective therapies for individual or for groups of preterm infants.
Figure 2.
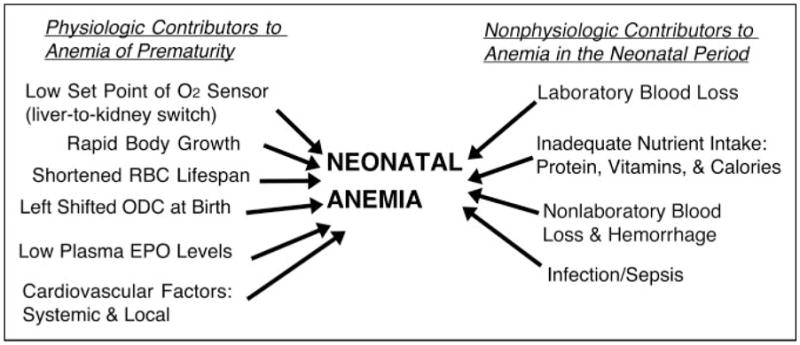
Pathophysiology of neonatal anemia. Contributors to anemia that develops during the neonatal period include physiologic and nonphysiologic factors. Physiologic factors that are developmentally regulated are viewed as likely contributors to anemia of prematurity. Those that are nonphysiologic are acquired and are more amenable to intervention; some are iatrogenic. The contributors included in the figure are operative at varying times, depending on the developmental and clinical circumstances, with many occurring simultaneously. The effect of each on the severity of anemia varies. During the first postnatal weeks, when severe neonatal cardiorespiratory illness is at its peak and frequent laboratory testing is most intense, phlebotomy loss among preterm infants is typically the most important contributor to neonatal anemia and the need for red blood cell transfusion. EPO=erythropoietin, ODC=oxygen dissociation curve, RBC=red blood cell
Developmental Processes Contributing to AOP
The normal postnatal decline in Hb values in preterm infants, referred to as AOP, is understood best as the result of simultaneous physiologic processes related to birth and the transition to extrauterine life. Normal fetal hematologic data have aided in our understanding of physiologic processes contributing to AOP in that advancing gestation is associated with decreasing growth velocity and reticulocyte and nucleated red blood cell (RBC) counts. This fetal process is paralleled by postnatal reticulocyte counts in the least mature preterm infants being two- to threefold greater than their term counter-parts (Fig. 1).
Following birth, as oxygen dependency switches from placenta-based to lung-based, an immediate and abrupt increase in oxygen tension (PaO2) occurs as arterial Hb becomes fully saturated with oxygen. With normal cardiac function, oxygen delivery to the tissues increases, bringing about an abrupt decline in plasma erythropoietin (EPO) concentration to subnormal adult concentrations, which is followed shortly thereafter by a marked decline in reticulocyte and nucleated RBC counts after 5 to 7 days of age. During this period, the percentage of erythroid precursor cells in the bone marrow declines from 35% at birth to 15% or less after the first postnatal week.
These peripartum events occur in the context of several additional developmentally regulated processes that contribute further to the physiologic postnatal decline in Hb. The processes include: 1) a shorter fetal and neonatal RBC survival compared with adults (~60 days versus ~120 days); 2) a progressive shift of the oxygen dissociation curve resulting from the switch from high oxygen-affinity HbF to low oxygen-affinity HbA from ~34 to 36 weeks’ gestation to ~3 to 4 month post-term; 3) a postnatal increase in intraerythrocyte 2,3-diphosphoglycerate, allowing greater ease in releasing oxygen from Hb; 4) adaptive cardiovascular mechanisms compensating for the reduced oxygen-carrying capacity of anemia; and 5) low circulating plasma concentrations of EPO relative to the degree of anemia compared with adults. (3)
Low Neonatal Plasma EPO Values: Physiologic or Pathologic?
The case for whether low endogenous plasma EPO values observed in preterm infants constitute a true pathologic state has yet to be made convincingly. This is a potentially important question because of its implications for the recommended use of recombinant human EPO to treat neonatal anemia. Although low plasma EPO values are viewed as pathologic by many, multiple lines of evidence indicate that low EPO values in anemic infants represent a normal physiologic response when placed in the context of normal development. First, a lower tissue oxygenation set point (ie, a lower responsiveness to tissue hypoxia) is likely in the fetus and preterm neonate compared with older, more mature individuals. (3) This may be the result of the developmentally regulated switch from the low oxygen set point of the liver to the high set point of the kidney. During this time (ie, from late fetal life to early infancy), the primary site of EPO production shifts from the liver to the kidney. A lower oxygen set point may be an important fetal mechanism for avoiding fetal polycythemia-hyperviscosity syndrome. Second, because the preterm infant’s liver functions both as a hematopoietic organ and as an organ of EPO production, low plasma values may not reflect local EPO concentration in proximity to the region where EPO is produced and acts as a paracrine hormone, exerting its effect by stimulating erythroid progenitors to expand in numbers and mature. Third, because some data indicate that fetal erythroid progenitors are more sensitive to EPO than adult progenitors, even low plasma concentrations of EPO may be capable of stimulating adequate erythropoiesis. Fourth, the higher reticulocyte counts observed in preterm relative to term infants at the nadir of their respective postnatal Hb values suggest that preterm infants have a proportionately greater erythropoietic capacity than their term counterparts. Finally, because EPO clearance is increased two- to fourfold in preterm infants relative to adults, low endogenous plasma EPO values may reflect a greater rate of EPO elimination and consequently a greater endogenous EPO production rate. In this regard, it is possible—if not likely—that increased EPO clearance observed in infants is a consequence of the relatively greater erythroid progenitor-rich red bone marrow volume needed in early life to keep up with rapid growth velocity during infancy.
Nonphysiologic Contributors to Anemia in Early Postnatal Life
In addition to the physiologic contributors to AOP, nonphysiologic factors contribute to the development of anemia during the neonatal period (Fig. 2). These factors exacerbate AOP by greatly accelerating the rate and magnitude of the postnatal decrease in Hb. As a result, they frequently are greater contributors to the development of clinically significant anemia managed with RBC transfusion.
First and foremost among these nonphysiologic factors is blood loss accompanying frequent laboratory testing. Strong evidence supports nonphysiologic factors as primary contributors to the development of anemia and to the large numbers of RBC transfusions received by critically ill VLBW infants. Foremost among these is “bleeding into the laboratory” during the early weeks after birth, when blood sampling for patient monitoring is at its zenith. Several compelling lines of evidence support this speculation:
Reports of the relatively large volumes of blood removed. Estimates of laboratory phlebotomy loss among infants in the neonatal intensive care unit during the first 6 weeks after birth range from 11 to 22 mL/kg per week (~15% to 30% of an infant’s total blood volume) (Table). For a 1-kg infant, 6 to 7 mL of blood drawn for laboratory testing is equivalent to one 450-mL adult whole blood donor unit.
The similar temporal patterns in the intensity of laboratory phlebotomy loss and the administration of RBC transfusions. Approximately 50% of all RBC transfusions administered to VLBW infants are given in the first 2 weeks after birth, and 70% are administered within the first month.
The close temporal relationship between the volume of blood removed for laboratory testing and the volume of blood transfused back to the infant. In several studies, highly significant direct correlations have been observed between blood volumes removed and transfused (ie, correlation coefficients of 0.8 to 0.9).
Table.
Studies of Neonates Weighing <1,500 g Reporting Laboratory Phlebotomy and Red Blood Cell Transfusion Data
| Authors | N | Infant Study Population | Postnatal Age | Mean Birth Weight (g) | Weekly Phlebotomy Loss (mL/kg) | Weekly RBC Transfusion Volume (mL/kg) | Correlation Phlebotomy versus Transfusion r† |
|---|---|---|---|---|---|---|---|
| Blanchette and Zipursky, 1984 (4) | 57 | <1,500 g | Birth to 6 wk | Not reported | 11.1 | 6.7 | 0.82 |
| Obladen et al, 1988 (5)* | 60 | <1,500 g | Birth to 4 wk | 1,161 | 12.7 | 10.6 | 0.91 |
| Ringer et al, 1998 (6) | 270 | Hospital A: <1,500 g | Birth to 2 wk | 1,073 | 8.2 | 15.7 | Not reported |
| Hospital B: <1,500 g | Birth to 2 wk | 978 | 21.4 | 16.8 | Not reported | ||
| Alagappan et al, 1998 (7) | 80 | <1,250 g | Birth to 2 wk | 948 | 20.7 | 21.7 | Not reported |
| Maier et al, 1998 (8)* | 79 | 500 to 1,000 g | Birth to 9 wk | 800 | 4.7 | 4.9 | Not reported |
| Widness et al, 2005 (9) | 47 | 500 to 1,000 g + umbilical artery catheter | Birth to 2 wk | 734 | 36.7 | 22.2 | 0.73 |
| Mean | 949 | 16.5 | 14.1 | 0.82 | |||
| SD | 161 | 10.8 | 6.9 | 0.09 |
European study
Correlation coefficient for phlebotomy volume versus transfusion volume
RBC=red blood cell, SD=standard deviation
Other less important, but still clinically significant pathologic conditions include hemorrhage, infection/sepsis, inadequate nutrient intake, and cardiorespiratory disease. The developmental immaturity of preterm infants places them at greater risk for all of these conditions relative to their term counterparts. Because these and other disease processes are often active at the same time, it is difficult to identify the predominant contributor(s) to anemia.
Summary
Anemia develops in the context of ongoing developmentally regulated processes that contribute to the postnatal decrease in Hb values observed during the first 10 to 12 weeks after birth. Applying a designation of AOP to all preterm infants who have low Hb values often is misleading. This is because nonphysiologic factors such as blood loss attributable to laboratory testing, sepsis, and undernutrition exert a greater effect than physiologic factors such as decreased sensitivity to tissue hypoxia, shortened RBC survival, left-shifted oxygen dissociation curve, low plasma, rapid growth, and cardiovascular factors. The relative contribution of low circulating EPO concentrations to the development of pathologic anemia in preterm infants remains controversial. AOP and the “early anemia of infancy,” its less severe counterpart in healthy term infants, are both self-limited conditions that resolve by 3 months of age.
Acknowledgments
Author Disclosure: Dr Widness has disclosed that this work was supported, in part, by USPHS NIH P01 HL46925. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
Abbreviations
- AOP
anemia of prematurity
- ELBW
extremely low-birthweight
- EPO
erythropoietin
- Hb
hemoglobin
- RBC
red blood cells
- VLBW
very low-birthweight
Footnotes
Objectives After completing this article, readers should be able to:
1 Define the clinical course and contributors to anemia of prematurity (AOP).
2 Define the clinical course and contributors to nonphysiologic anemia occurring during the neonatal period.
3 Distinguish between physiologic and nonphysiologic mechanisms contributing to AOP.
References
- 1.Dallman PR. Anemia of prematurity. Annu Rev Med. 1981;32:143–160. doi: 10.1146/annurev.me.32.020181.001043. [DOI] [PubMed] [Google Scholar]
- 2.Ohls RK. Evaluation and treatment of anemia in the neonate. In: Christensen RD, editor. Hematologic Problems of the Neonate. 1. Philadelphia, Pa: WB Saunders; 2000. pp. 137–170. [Google Scholar]
- 3.Kling PJ, Schmidt RL, Roberts RA, Widness JA. Serum erythropoietin levels during infancy: associations with erythropoiesis. J Pediatr. 1996;128:791–796. doi: 10.1016/s0022-3476(96)70331-1. [DOI] [PubMed] [Google Scholar]
- 4.Blanchette V, Zipursky A. Assessment of anemia in newborn infants. Clin Perinatol. 1984;11:489–516. [PubMed] [Google Scholar]
- 5.Obladen M, Sachsenweger M, Stahnke M. Blood sampling in very low birth weight infants receiving different levels of intensive care. Eur J Pediatr. 1988;147:399–404. doi: 10.1007/BF00496419. [DOI] [PubMed] [Google Scholar]
- 6.Ringer SA, Richardson DK, Sacher RA, Keszler M, Churchill WH. Variations in transfusion practice in neonatal intensive care. Pediatrics. 1998;101:194–200. doi: 10.1542/peds.101.2.194. [DOI] [PubMed] [Google Scholar]
- 7.Alagappan A, Shattuck KE, Malloy MH. Impact of transfusion guidelines on neonatal transfusions. J Perinatol. 1998;18:92–97. [PubMed] [Google Scholar]
- 8.Maier RF, Obladen M, Kattner E, et al. High- versus low-dose erythropoietin in extremely low birth weight infants. The European Multicenter rhEPO Study Group. J Pediatr. 1998;132:866–870. doi: 10.1016/s0022-3476(98)70320-8. [DOI] [PubMed] [Google Scholar]
- 9.Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005;115:1299–1306. doi: 10.1542/peds.2004-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]