Abstract
Background:
We used data from the Minnesota Stroke Registry to examine rates of intravenous thrombolytic therapy in acute ischemic stroke and identifed opportunities to improve the utilization of this treatment.
Methods:
We analyzed a total of 1010 acute ischemic stroke patients who had been entered into the registry by 13 participating hospitals during the first three quarters of the 2008 calendar year.
Results:
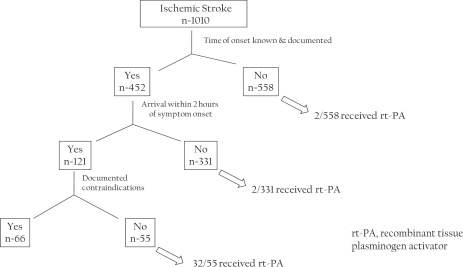
Of the 1010 patients only 121 (12%) came within 2 hours of symptom onset. Intravenous recombinant tissue plasminogen activator (rt-PA) was administered to 32/55 (58%) of the patients who arrived within 2 hours of symptom onset and met eligibility criteria for this treatment. The remaining 66 patients had a documented reason for non-treatment. The most common reason (22/66) for non-treatment was rapid resolution of symptoms or mild stroke. Out of those 22 patients, 20 were ambulating independently prior to admission and only 9/20 (45%) ambulated independently at discharge.
Conclusion:
Further community education on the need for immediate medical attention after stroke is needed. Patients appropriately excluded from rt-PA treatment due to mild deficits or rapidly improving symptoms seem to have poor discharge outcomes. This subgroup of patients will have to be studied further, preferably in the context of a clinical trial.
Keywords: Thrombolysis, acute stroke, ischemic stroke, registry, recombinant tissue plasminogen activator
The National Institutes of Neurological Disorders and Stroke (NINDS) recombinant tissue plasminogen activator (rt-PA) trial showed that acute ischemic stroke patients treated with intravenous (IV) rt-PA within 3 hours of symptom onset had significantly lower rates of disability compared to those treated with placebo.1 Multiple studies have subsequently confirmed the applicability of the NINDS trial results in the community setting.2, 3
Even though IV thrombolysis is now standard of care for eligible patients with acute ischemic stroke4, it is not being sufficiently utilized in the community. Partly in response to this underutilization, nationwide programs such as Get With The Guidelines - Stroke, Primary Stroke Center certification, and the Paul Coverdell National Acute Stroke Registry (PCNASR) advocate increased use of IV thrombolysis as a performance measure for quality improvement. We analyzed data from the Minnesota Stroke Registry (MSR), part of the PCNASR, to examine rates of IV thrombolytic use in Minnesota hospitals and identify opportunities to improve the utilization of this treatment.
Methods
The MSR collects quality of care information on patients hospitalized with acute strokes and transient ischemic attacks. Hospital participation is voluntary and trained abstractors from participating hospitals enter data via a web-based interface concurrent with or soon after patient care using standard data definitions provided by the Centers for Disease Control. The performance measure pertaining to thrombolytic treatment is the percentage of eligible acute ischemic stroke patients who arrive within 2 hours of time last known intact (denominator) and receive IV rt-PA within 3 hours of time last known intact (numerator).
Results
A total of 1010 acute ischemic stroke cases (as identified by admission diagnosis) were entered into the MSR by 13 participating hospitals through the third quarter of the 2008 calendar year. Of these, only 121 patients (12%) came within 2 hours of time last known intact. Figure 1 and Table 1 show the subgroups of patients who received rt-PA and the opportunities to improve the use of this treatment. Intravenous rt-PA was given to 32 of 55 patients who arrived within 2 hours of symptom onset and were considered eligible for this treatment. Hence, the performance measure was met in 58% of eligible patients. The remainder of 66 (of a total of 121) patients who came within 2 hours of symptom onset had a documented reason for non-treatment. The most common reason for non-treatment was rapid resolution of symptoms or mild stroke, (22 of 66; 33%). Of these 22 patients, 20 were ambulating independently prior to admission and only 9 of these 20 (45%) ambulated independently at discharge.
Figure 1.
Time of arrival and use of intravenous rt-PA in patients admitted with acute ischemic stroke in the Minnesota stroke registry
Table 1.
Minnesota Stroke Registry – reasons for thrombolytic non-treatment in patients arriving within 2 hours of symptom onset.
| Documented reason for exclusion† | Cases N (% of Total cases) |
|---|---|
| Contraindications | 22 (17) |
| CT findings (e.g. hemorrhage) | 4 (3) |
| Warnings | 8 (7) |
| Advanced age | 6 (5) |
| Complicating medical conditions, other illness | 2 (2) |
| Patient refusal | 10 (8) |
| Rapid improvement or mild stroke | 22 (18) |
| Other (including rt-PA administered at another facility) | 7 (6) |
| No documented reason | 23 (19) |
| Total non-treated cases | 89 (74) |
Some cases had multiple reasons for non-treatment
Abbreviations used: CT, computed tomography; rt-PA, recombinant tissue plasminogen activator
Discussion
Our data shows numerous opportunities to improve the use of IV rt-PA in the community. We focussed on two issues: First, early arrival of stroke patients to the emergency department clearly improves the likelihood of receiving intravenous thrombolysis. That the vast majority of patients with a documented time of symptom onset came after 2 hours of symptom onset indicates that community education on the need for immediate medical attention after stroke is sorely needed. Documentation by care providers regarding time of onset and reasons for non-treatment needs improvement to enable correct assessment of the number of eligible patients for rt-PA and true compliance to the thrombolytic performance measure. Second, patients appropriately (according to current treatment guidelines) excluded for mild deficits or rapidly improving symptoms, seem to have poor discharge outcomes. This has been noted in other stroke registries as well.5 This subgroup of patients will have to be studied, perhaps in the context of a clinical trial.
Disclosure/Acknowledgements
The first author was supported by an NINDS/NIH career development award K23NS051377 during this work. All authors were supported by grant U58DP000857 from the Centers for Disease Control and data was collected under this grant. The authors thank participating Minnesota hospitals for their commitment to the care of stroke patients in the community.
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-PA stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Charipar R, Charipar E. Administration of tissue plasminogen activator for acute ischemic stroke in a rural wisconsin hospital. WMJ. 2008;107:176–180. [PubMed] [Google Scholar]
- 3.Schmulling S, Grond M, Rudolf J, Heiss WD. One-year follow-up in acute stroke patients treated with rtPA in clinical routine. Stroke. 2000;31:1552–1554. doi: 10.1161/01.str.31.7.1552. [DOI] [PubMed] [Google Scholar]
- 4.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the american heart Association/American stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: The american academy of neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:478–534. doi: 10.1161/CIRCULATIONAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 5.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015–1020. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]