Abstract
Post-traumatic morbidity reduces the quality of life for traumatic brain injury (TBI) survivors by altering neuropsychological function. After midline fluid percussion injury (FPI), diffuse pathology in the ventral posterior thalamus suggests that somatosensory whisker function may be impaired post-injury. The goals of the present study were to design and validate a task to detect injury-induced somatosensory morbidity (Experiment 1), and to evaluate preliminary applications of the task (Experiment 2). In Experiment 1, male Sprague-Dawley rats were subjected to moderate FPI (∼1.9 atm) or sham injury. Over an 8-week time course, the whiskers on both mystacial pads were stimulated manually with an applicator stick in an open field for three 5-min periods. Behavioral responses in this whisker nuisance task were recorded using objective criteria (max score = 16). Sham animals were ambivalent or soothed by whisker stimulation (4.0 ± 0.8), whereas brain-injured rats showed aggravated responses at 1 week (6.7 ± 0.9), which became significant at 4 weeks (9.5 ± 0.5) and 8 weeks (8.4 ± 1.1) compared to sham injury, indicating chronic injury-induced sensory sensitivity. Total free serum corticosterone levels indicated a significant stress response in brain-injured (125.0 ± 17.7 ng/mL), but not uninjured animals (74.2 ± 12.2 ng/mL) in response to whisker stimulation. In Experiment 2, to evaluate applications of the whisker nuisance task, four additional uninjured and brain-injured groups were subjected to mild brain injury only, shaved whiskers after moderate brain injury, repeated whisker nuisance task stimulation after moderate brain injury, or regular opportunities for tactile exploration of an enriched environment after moderate brain injury over 4 weeks post-injury. The whisker nuisance task has the sensitivity to detect mild brain injury (7.7 ± 1.0), but morbidity was not mitigated by any of the neurorehabilitative interventions. Following diffuse brain injury, the whisker nuisance task is a promising tool to detect post-traumatic morbidity and the efficacy of therapeutic interventions that may restore discrete circuit function in brain-injured patients.
Key words: corticosterone, physical therapy, plasticity, rehabilitation
Introduction
Each year, the majority of the 1.4 million Americans who sustain a traumatic brain injury (TBI) survive (Langlois et al., 2004). Diffuse, and especially mild, brain injury constitutes a majority (over 80%) of all human TBIs that result from acceleration-deceleration forces, such as those typically associated with motor vehicle accidents, auto-pedestrian contact, and falls (Langlois et al., 2004). Individuals with diffuse TBI may not receive medical care at the time of the injury, but days, weeks, or even months later may begin to articulate transient and mild to ongoing and debilitating post-traumatic morbidity, as acute functional deficits subside (Langlois et al., 2004; McAllister, 1992; Shaw, 2002). Post-traumatic morbidity is the long-term behavioral and psychological consequence of injury-related pathological processes that impair brain circuit activation and function. Morbidities are grouped together in post-concussion syndrome symptoms, which include problems with cognition (concentration, memory, and reasoning), sensory processing (light and sound), communication (expression and understanding), and behavior or mental health (depression, anxiety, personality changes, aggression, impulsivity, irritability, and mood swings) (McAllister, 1992; Shaw, 2002). The prevalence of post-traumatic morbidity ranges from 20–50% (McAllister, 1992). These accumulating morbidities reduce quality of life for TBI survivors. To date, post-traumatic morbidity in animals has been far less obvious or enduring.
Historically, laboratory examinations have focused on post-traumatic deficits that demonstrate early onset and delayed resolution (Fujimoto et al., 2004). Examples include neuromotor (McIntosh et al., 1989), balance (Hamm, 2001), and even learning (Lifshitz et al., 2007b). These deficits have been associated with injury-induced neuropathology and thereby promoted neuroprotective strategies. This study describes a late-onset, persistent sensory morbidity involving the facial whiskers after diffuse brain injury.
Rodents use their mystacial (facial) whiskers to navigate their environment by locating and identifying objects (position, size, texture, and shape) through tactile whisking (Brecht et al., 1997; Carvell and Simons, 1990; Guic-Robles et al., 1989). In the whisker system (Bernardo and Woolsey, 1987; Woolsey and Van der, 1970), whisker deflection drives mechanoreceptors on sensory axons of trigeminal ganglion neurons, which project to the brainstem trigeminal complex (Henderson and Jacquin, 1995). The whisker-barrel circuit incorporates topographic projections to the ventral posterior medial thalamus and primary somatosensory barrel cortex (Chmielowska et al., 1989; Land et al., 1995; Woolsey and Van der, 1970). This circuit is equally susceptible to the vascular, neuronal, glial, and axonal injuries associated with diffuse brain injury. Axotomy, neuronal atrophy, and chronic neuroinflammation in the ventral posterior medial thalamus predicts functional deficits associated with sensory stimulation of the whiskers (Kelley et al., 2006, 2007; Lifshitz et al., 2007a). In the absence of acute neuronal death or eventual reduction in number (Lifshitz et al., 2007a; Singleton et al., 2002), circuit disruption, rather than circuit destruction, may ensue and mediate functional impairments. Circuit disruption and consequent neuroplastic responses may establish new maladaptive connections over time (Christman et al., 1997; Hulsebosch et al., 1998; Povlishock et al., 1992), from which injury-induced morbidity emerges.
Experimental manipulations of rodent whiskers influence animal exploratory and initiation behavior (Dyck, 2004), but in the context of diffuse brain injury, the neural circuitry itself would be disrupted, rather than the whiskers. Although not examined in diffuse TBI, focal brain injury results in chronic attenuated metabolic activation by whisker stimulation (Dietrich et al., 1994; Dunn-Meynell and Levin, 1995; Passineau et al., 2000), and marked regenerative responses (Emery et al., 2000). Brain injury also produces a chronic somatomotor deficit in tasks in which rats remove tape from the vibrissae or forepaws (Dunn-Meynell and Levin, 1995; Riess et al., 2001). Injury-induced pathology within this circuit is highlighted by the differences in motor cortex stimulation intensity required to produce a visually detected vibrissa response (Ip et al., 2003). The whisker-barrel circuit affords discrete cytological landmarks, large representation, and direct access to circuit activation, which leave it susceptible to injury and make it optimal for investigation.
In this study we explore the hypothesis that the somatosensory whisker circuit can be exploited to develop and validate a task to detect injury-induced behavioral morbidity in a rat model of moderate diffuse TBI (Experiment 1). Further, the task can be applied to evaluate injury severity and neurorehabilitative interventions targeted at sensory input through the facial whiskers to mitigate morbidity (Experiment 2). The whisker nuisance task is presented to detect injury-induced sensory sensitivity in diffuse brain-injured rats.
Methods
Midline fluid percussion brain injury
Adult male Sprague-Dawley rats (350–375 g) were subjected to midline fluid percussion injury (FPI) consistent with methods described previously (Hosseini and Lifshitz, 2009; Lifshitz et al., 2007a; Lifshitz, 2008). Final animal numbers are indicated in the results section for each study. Briefly, the rats were anesthetized with 5% isoflurane in 100% oxygen and maintained at 2% via nose cone. During surgery, body temperature was maintained with a Deltaphase® isothermal heating pad (Braintree Scientific Inc., Braintree, MA). With the head held in position with a head holder assembly (Kopf Instruments, Tujunga, CA), a midline scalp incision exposed the skull. A 4.8-mm circular craniotomy was performed (centered on the sagittal suture midway between the bregma and the lambda), without disrupting the underlying dura or superior sagittal sinus. An injury hub was fabricated from the female portion of a Luer-Lock needle hub, which was cut, beveled, and scored to fit within the craniotomy. A skull screw was secured in a 1-mm hand-drilled hole into the right frontal bone. The injury hub was affixed over the craniotomy using cyanoacrylate gel and methyl-methacrylate (Hygenic Corp., Akron, OH) was applied around the injury hub and screw. The incision was sutured at the anterior and posterior edges and topical lidocaine ointment was applied. Then the animals were returned to a warmed holding cage and monitored until ambulatory (approximately 60–90 min).
For injury induction, the animals were re-anesthetized with 5% isoflurane 60–90 min after surgery to standardize anesthesia levels at the time of injury. The dura was inspected through the injury-hub assembly, which was then filled with normal saline and attached to the male end of the fluid percussion device (Custom Design and Fabrication, Virginia Commonwealth University, Richmond, VA). As reflexive responses returned, an injury of moderate (1.9–2.0 atm) or mild severity (1.1–1.2 atm) was administered by releasing the pendulum onto the fluid-filled cylinder. The animals were monitored for the presence of a forearm fencing response and the return of the righting reflex as indicators of injury severity (Hosseini and Lifshitz, 2009). Sham animals were connected to the FPI device, but the pendulum was not released. The injury-hub assembly was removed en bloc, integrity of the dura was observed, bleeding was controlled with Gelfoam (Pharmacia, Kalamazoo, MI), and the incision was stapled closed. Moderate brain-injured animals had righting reflex recovery times >6 min, mild-injured animals had righting reflex times between 2 and 4 minutes, and sham-injured animals recovered within 15 sec. After recovery of the righting reflex, the animals were placed in a warmed holding cage before being returned to the vivarium. Surgical recovery was monitored postoperatively for 3 days, for which no overt differences (e.g., weight, coat, movement, and grooming) were observed between the animal groups. Sutures were removed 7–10 days post-injury as needed. The experiments were conducted in accordance with National Institutes of Health and institutional guidelines concerning the care and use of laboratory animals. Adequate measures were taken to minimize pain or discomfort.
Experiment 1: Design and validation of the whisker nuisance task
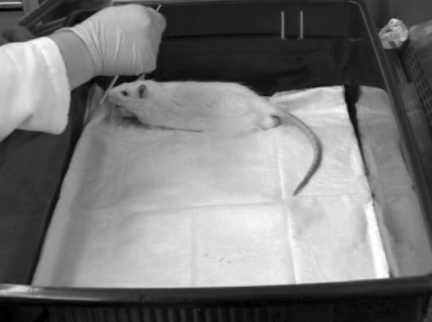
Behavioral responses to whisker stimulation are observed during the whisker nuisance task. At 1, 4, or 8 weeks after moderate midline fluid percussion brain or sham injury, the rats were acclimated for 5 min to a plastic test cage (57.1 × 39.4 × 15.2 cm) lined with an absorbent pad (Fig. 1). The whiskers of both mystacial pads were manually stimulated with a wooden applicator stick for three consecutive 5-min periods (15 min total). Between periods (approximately 30 sec), stimulation is absent while behavioral observations are recorded for the previous 5-minute period of whisker stimulation. Behavioral testing was conducted at the same time of day for all animals by one or more observers who were blinded to injury status. All behavioral sessions were videotaped for archival storage.
FIG. 1.
Photograph of a diffuse brain-injured rat in the whisker nuisance task. Whisker stimulation is conducted by manually deflecting the whiskers on both sides of the face with a wooden applicator stick for 15 min while the animal explores an open test cage. Behavioral performance is scored using the criteria described in the text and Table 1.
Based on preliminary observations, scoring criteria in eight categories were developed (Table 1). The predominant behavioral responses were recorded for freezing, stance and body position, breathing, whisker position, whisking response, evasiveness, response to stick presentation, and grooming, on 0–2 point non-parametric scales (0 = absent, 1 = present, and 2 = profound). Normal behavioral responses to stimulation were assigned a zero value, whereas meaningful abnormal behavioral responses were assigned a value of 2. The maximum whisker nuisance score is 16. High scores (8–16) indicate abnormal responses to the stimulation, in which the rat freezes, becomes agitated or is aggressive. Low scores (0–4) indicate normal responses, in which the rat is either soothed or indifferent to the stimulation. The average of the total whisker nuisance scores for each animal was incorporated into a Kruskal-Wallis non-parametric analysis of variance (ANOVA) to determine changes in comparison to sham animals, followed by a Dunn's multiple comparison post-test with Bonferroni correction (p < 0.05 was considered significant).
Table 1.
Observed Behavioral Criteria Used To Generate the Whisker Nuisance Score
| P1 | P2 | P3 | |
|---|---|---|---|
| Freezing | |||
| Walking around, exhibits curious behavior | 0 | 0 | 0 |
| Slow or stationary, limited curiosity, cautious | 1 | 1 | 1 |
| Freezing, defensive, and fearful | 2 | 2 | 2 |
| Stance and body position | |||
| Relaxed, looking skyward, forepaws under body | 0 | 0 | 0 |
| Cowering, guarded, grounded forepaws | 2 | 2 | 2 |
| Breathing | |||
| Normal range | 0 | 0 | 0 |
| Forced, gasping | 2 | 2 | 2 |
| Whisker position | |||
| Fully protracted (both sides) | 0 | 0 | 0 |
| Protraction and retraction | 1 | 1 | 1 |
| Fully retracted (both sides) | 2 | 2 | 2 |
| Whisking response | |||
| Standard whisking, normal movement | 0 | 0 | 0 |
| Tremors, twitching | 1 | 1 | 1 |
| None, stopped | 2 | 2 | 2 |
| Evading stimulation | |||
| No evasive behavior | 0 | 0 | 0 |
| Escape behavior or directed movement to avoid/protect whiskers | 2 | 2 | 2 |
| Response to stick presentation | |||
| Ambivalence or curiosity about stick | 0 | 0 | 0 |
| Avoiding and anxiety or biting and attacking or freezing | 2 | 2 | 2 |
| Grooming | |||
| No, minimal, or normal grooming | 0 | 0 | 0 |
| Irritated scratching/rubbing/pulling | 2 | 2 | 2 |
P1, P2, P3 refer to periods 1–3, each lasting 5 min. Normal behavioral responses to whisker stimulation are assigned a value of zero; abnormal responses were assigned a value of 2. A separate whisker nuisance score is obtained for each period and averaged for a single animal. The maximum score is 16.
Experiment 2: Applications of the whisker nuisance task
At 4 weeks post-injury, mild brain-injured animals were evaluated by the whisker nuisance task to determine the injury severity dependence of the task.
Separate groups of animals had their whiskers shaved immediately after sham or moderate brain injury to minimize somatosensory circuit activation. The whiskers were shaved every 3–4 days until 22 days post-injury to allow the whisker nuisance task to be conducted.
In separate uninjured and moderate brain-injured animals, the somatosensory circuit was repeatedly activated by conducting the whisker nuisance task 15 min a day, three times per week for 3 weeks post-injury (nine total sessions). This neurorehabilitation paradigm reproduces an outpatient physical therapy program, since the diffuse brain-injured rats are mobile, feeding, and grooming.
A group of sham and moderate brain-injured animals were exposed to an enriched environment to enable voluntary whisker stimulation during tactile exploration. To follow an outpatient physical therapy paradigm, the animals were placed in the enriched environment for 45 min, three times per week for 3 weeks, beginning 1 week post-injury (nine total sessions). Enriched environments include fresh bedding and a unique set and arrangement of enrichment objects (e.g., plastic igloos, tubes, and gnaw bones) in an oversized plastic cage.
All animals were evaluated using the whisker nuisance task at 4 weeks post-injury. Whisker nuisance scores were incorporated into a Kruskal-Wallis non-parametric ANOVA to determine changes between group (sham versus injury), and therapy (rehabilitation versus none), followed by a Dunn's multiple comparison post-test (p < 0.05 considered significant).
Corticosterone assay
Fifteen minutes after the whisker nuisance task, the animals were euthanized by an overdose of sodium pentobarbital (150 mg/kg IP), and then a cardiac blood sample was collected. Separate groups of animals were not exposed to the whisker nuisance task prior to blood collection. Blood collection occurred during the middle of the dark phase (10:00 am–2:00 pm). Histopathology is not presented in this report. The blood samples were centrifuged (3000g for 10 min) and the serum was stored at −20°C. A commercially available competitive immunoassay was conducted according to the manufacturer's protocol for the quantitative determination of corticosterone (no. 900-097; Assay Designs, Inc. Ann Arbor, MI). The kit uses an anti-corticosterone polyclonal antibody to bind standards and samples. The enzyme reaction generates a yellow color that is inversely proportional to the corticosterone concentration and is read on a microplate reader (405 nm). All samples were diluted 1:5 (80%) in order to stay within the sensitivity of the assay (32–20,000 pg/mL). Statistically significant differences were analyzed by two-way ANOVA (injury × whisker nuisance), followed by a least significant difference planned comparison post-test with p < 0.05.
Results
Experiment 1: Development of the whisker nuisance task
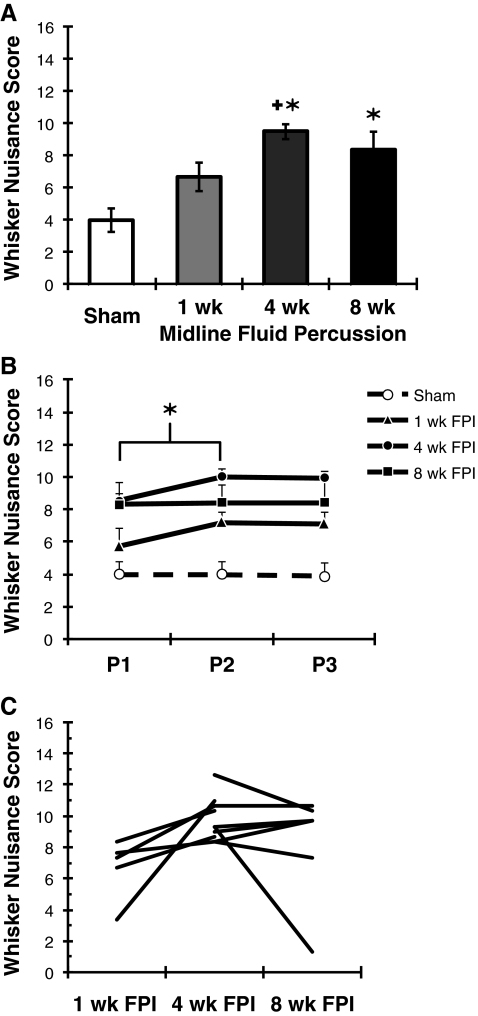
At 1-week (n = 10), 4-week (n = 17), and 8-week (n = 7) time points after moderate diffuse brain injury or sham injury (n = 9), the whiskers on both mystacial pads were stimulated with an applicator stick in an open field to evaluate sensory sensitivity. The behavioral responses during the whisker nuisance task, as described here, were quantified by a blinded observer using categorical, objective criteria (Table 1). Sham animals were either ambivalent to or soothed by whisker stimulation. In chronic diffuse brain-injured animals, whisker stimulation resulted in an agitated response, indicated by huddled posture, forced breathing, fear-like freezing, and exploratory escape behavior. The whisker nuisance score combines behavioral observations made across eight categories, for which lower numbers indicate observations typical of uninjured animals. The whisker nuisance task has the sensitivity to detect significant injury-induced behavioral morbidity in moderate brain-injured animals at 4–8 weeks post-injury, compared to sham animals (KW (3,43) = 22.42; p = 0.0001; Fig. 2A). At 1 week after moderate injury, whisker nuisance scores are above sham values (p = 0.055), and significantly lower than scores at 4 weeks post-injury (p = 0.027; Fig. 2A).
FIG. 2.
Time course of the whisker nuisance response in diffuse brain-injured rats. (A) The whisker nuisance task can detect injury-induced sensitivity to whisker stimulation up to 8 weeks after moderate, midline fluid percussion injury (FPI). Uninjured sham control animals were soothed by the stimulation (low scores). At 1 week after brain injury, the behavioral responses are not significantly different from sham animals. By 4 weeks and through 8 weeks post-injury, brain-injured rats responded to whisker stimulation by freezing, guarding mystacial pads, or acting aggressive (higher scores). At 4 weeks after injury, behavioral performance is significantly different from the sham and 1-week post-injury groups. The sensory sensitivity to whisker stimulation is maintained through 8 weeks post-injury (mean ± standard error of the mean [SEM]; *p < 0.05 compared to sham animals; +p < 0.05 compared to 1 week post-FPI by Kruskal-Wallis analysis of variance [ANOVA] and Mann-Whitney U test). (B) Behavioral responses to whisker stimulation do not habituate over the three consecutive testing periods (P1–P3; mean ± SEM). The observation of behaviors in additional scoring categories contribute to the significant increase in the whisker nuisance score between P1 and P2 (*p < 0.05 by Friedman ANOVA). (C) Whisker nuisance persists once morbidity manifests. A subset of brain-injured animals was evaluated twice by the whisker nuisance task (the lines represent individual animals). Whisker nuisance scores are generally maintained over time. All animals shown here were brain-injured.
In re-viewing videotape of the whisker nuisance task, the movement-related and abnormal breathing behaviors serve as the first signs of nuisance. Meaningful expression of these aberrant responses emerges within 2 min after whisker stimulation begins.
Analysis of behavioral performance over the three testing periods and over time post-injury indicates no habituation in task performance. Between the first and second period (Fig. 2B), whisker nuisance scores showed a small but significant increase across all groups (Friedman ANOVA, χ2 (2,42) = 6.92, p < 0.03). For individual brain-injured animals repeatedly evaluated at 1 and 4 weeks or 4 and 8 weeks post-injury (Fig. 2C), performance in the whisker nuisance task remained stable, suggesting that structural reorganization may mediate the responses to whisker stimulation.
The whisker nuisance score is a composite across eight behavioral categories. A component analysis was conducted to determine if the principal findings were driven by performance in a single category. Scores for individual categories were subtracted from the whisker nuisance score and statistical significance was re-evaluated. Removing “response to stick,” “grooming,” or “whisker position” from the whisker nuisance score decreases the task's ability to detect a difference between sham and brain-injured rats (p values decrease by 0.0003). Removing “freezing,” “stance and body position,” or “whisker response” from the whisker nuisance score improves the task's ability to detect an injury-related difference (p values increase by 0.00004). However, these small changes in the significance level indicate that no single component drives the whisker nuisance score.
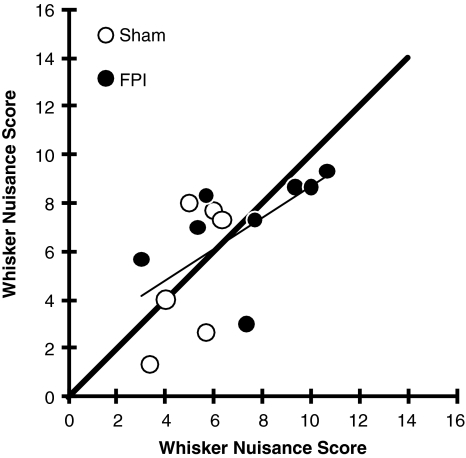
To demonstrate the objectivity of the scoring criteria, a subset of animals were scored in the whisker nuisance task simultaneously by two observers (A.M.L. and K.C.S.M.). The linear regression between the scores was significant (r2 = 0.349; p < 0.05), with an equal number of scores above and below a line of equality (Fig. 3).
FIG. 3.
Inter-rater reliability of whisker nuisance scoring. Independent scoring for 14 animals (6 sham, open circles; 8 FPI, closed circles) by two observers demonstrates an equal number of scores above and below a line of equality (thick black line). The linear regression is significant (r2 = 0.349; p < 0.05; FPI, fluid percussion injury).
Based on the variance in the data, the study achieved 100% power to detect a 3-point difference between sham and brain-injury at 4 weeks post-injury, with a significance level (alpha) of 0.05 (two-tailed). A power analysis indicated that sample sizes of 10 in each group had an 80% power to detect a 2-point difference from sham animals, with a significance level of 0.05 (two-tailed using G*Power v3.0.1 software; Heinrich Heine Universität, Düsseldorf, Germany; Faul et al., 2007). A 2-point difference amounts to the meaningful expression of a single behavioral observation (a score of 2 in one category), or the emergence of two separate behavioral responses.
Behavioral responses to whisker stimulation indicate a delayed onset (>1 week post-injury) and persistence through 8 weeks post-injury once behavioral morbidity emerges.
Exclusivity of sensory sensitivity to tactile whisker stimulation
Widespread pathology in the diffusely-injured brain, particularly in sensory and motor pathways, could explain the behavioral responses to whisker stimulation in brain-injured animals. Therefore we evaluated behavioral responses to whisker versus forepaw stimulation, and tactile stimulation versus cold sensitivity, in moderate brain-injured and uninjured rats at 4 weeks post-injury. Tactile stimulation discriminated brain-injured from uninjured rats (KW (1,8) = 20.66; p = 0.002), without a distinction between whisker and forepaw stimulation (KW (1,8) = 0.82; p < 0.4; data not shown). Diffuse pathology in adjacent thalamic domains (ventral posterior medial and lateral nuclei) likely explains this concordance (Lifshitz et al., 2007a). Practically, the face provides easier access for tactile stimulation. Initial attempts to automate stimulation by oscillating magnetic fields in a gaussian coil caused vibration, rather than deflection of the whiskers (Melzer et al., 1985), did not elicit aberrant behavioral responses. Similarly, whisker deflection is superior to electrical stimulation to promote facial nerve recovery (Skouras et al., 2009).
Behavioral responses in brain-injured animals could arise from pain associated with trigeminal nerve injury. Allodynia (hypersensitivity) in the whisker pad was evaluated by cold (acetone) and pressure (von Frey filaments) stimuli (Choi et al., 1994; Ren, 1999; Vos et al., 1994). Briefly, two drops of acetone were applied to either the whisker pad or forepaw using laboratory tubing (PE50) connected to a 1-cc syringe. The rapid evaporation of the acetone results in a localized cold stimulus that permits behavioral observations, including shaking, lifting, evasion, licking, and whisking. Sham and moderate brain-injured animals respond similarly to acetone application pre-injury and at 4 weeks post-injury (data not shown). Hypersensitivity to pressure was determined by applying von Frey filaments of increasing thickness to the whisker pad to elicit an aversive twitch, shake, or movement at 3 weeks after sham or brain injury (Ren, 1999; Vos et al., 1994). All animals progressed to the stiffest hair without aversive responses, indicating an absence of peripheral nerve pain and hypersensitivity to whisker pad pressure (data not shown).
Anxiety appears to be a component of the whisker nuisance behavior. A separate group of uninjured and moderate brain-injured animals was evaluated in an open-field locomotor test for 60 min, as previously described (Wooters and Bardo, 2009). During this undisturbed, extended observation period, the amount of time spent in the periphery versus the center of the arena was assessed. Brain-injured animals (n = 7–8) spent less time in the arena's center at 4 days post-injury, and more time at 1 and 4 weeks compared to sham animals (n = 6; F (3,25) = 1.55; p < 0.23; data not shown). The acute increase and chronic decreases in open field exploration after diffuse TBI are small and not statistically significant, and thus are likely not meaningful differences from sham animals. More sensitive and selective tasks would be warranted to explore post-traumatic anxiety in the diffuse brain-injured rodent.
Experiment 2: Applications of the whisker nuisance task
In the naïve adult rodent, the somatosensory map demonstrates use-dependent plasticity (Feldman and Brecht, 2005). The modulation of activity in the somatosensory whisker circuit through sensory deprivation or stimulation may influence the course of injury-induced structural changes, and thus the extent of post-traumatic behavioral deficits.
Chronic post-traumatic morbidity exists regardless of injury severity
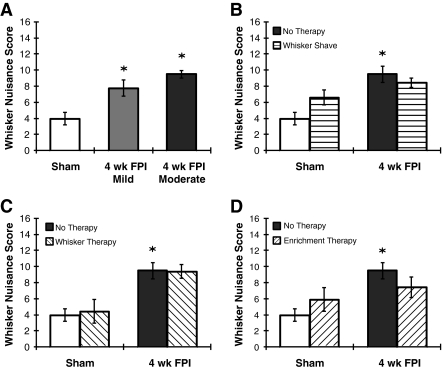
At 4 weeks after mild diffuse brain injury (7.7 ± 1.0; n = 13), behavioral responses to whisker stimulation are significantly different from sham animals (KW (2,39) = 16.29; p = 0.0003; Fig. 4A). Whisker nuisance scores between mild and moderate (n = 17) brain-injured animals at 4 weeks post-injury are not statistically significantly different.
FIG. 4.
Applications of the whisker nuisance task. (A) At 4 weeks after mild fluid percussion injury (FPI; ∼1.1 atm), whisker nuisance scores are significantly different from sham animals (*p < 0.05 by Kruskal-Wallis ANOVA and Mann-Whitney U test), but not moderately-injured animals. (B) To minimize sensory stimulation post-injury, sham and moderate brain-injured rats' whiskers were shaved every 3–4 days until 22 days post-injury to allow whisker regrowth. The whisker nuisance scores for sham rats whose whiskers were shaved were increased (p = 0.10). For FPI rats, performance was unaffected. (C) Sham and moderate brain-injured rats were subjected repeatedly to the whisker nuisance task (15 min per day, three times per week) as a therapeutic intervention to alleviate post-traumatic morbidity. Whisker therapy may not mitigate post-traumatic morbidity. (D) Sham and moderate brain-injured rats were placed individually in an enriched environment allowing them to voluntarily stimulate their whiskers through tactile exploration for 45 min 3 days a week for 4 weeks. At 4 weeks, no significant change in whisker nuisance scores was observed. The sham and 4-week post-moderate FPI without therapeutic intervention data are copied from Figure 2 for comparison (all values are mean ± standard error of the mean).
Preliminary whisker deprivation and stimulation paradigms do not affect behavioral performance
The prominence of the mystacial whiskers on the rodent's face affords sensory deprivation or direct stimulation to rehabilitate the circuit through activity-related plasticity. Using the whisker nuisance task at 4 weeks post-injury as an outcome, sham and moderate brain-injured rats were subjected to preliminary rehabilitative paradigms involving repeated whisker shaving (Fig. 4B), repeated involuntary manual whisker stimulation (Fig. 4C), or tactile exploration of an enriched environment (Fig. 4D). All preliminary rehabilitative paradigms were compared to no-therapy control animals (Fig. 2A). All therapies resulted in a significant injury effect, without specific effects of the therapy. Repeated shaving of all whiskers for 3 weeks post-injury (n = 6 FPI and n = 7 sham) non-significantly improves the outcome in brain-injured rats, but worsens performance in uninjured rats (KW (3,39) = 19.59, p = 0.0002). Repeated whisker stimulation (15 min three times per week; n = 7 FPI and n = 3 sham) does not influence performance in the whisker nuisance task in moderately brain-injured animals (KW (3,36) = 19.05, p = 0.0003). Regular exposure to an enriched environment containing foraging, housing, and tube objects to promote tactile exploration (45 min three times per week; n = 6 FPI and n = 4 sham) non-significantly improves the outcome in brain-injured rats, but worsens performance in uninjured rats (KW (3,36) = 15.66, p = 0.0013). Following diffuse brain injury, the whisker nuisance task remains a promising tool to evaluate refinements in rehabilitative therapies.
Corticosterone levels
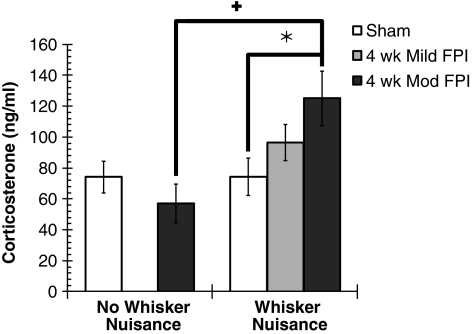
Corticosterone is the major glucocorticoid linking the hypothalamic-pituitary-adrenal axis, and it also serves in multiple brain-behavior interactions. In the present context, circulating corticosterone levels can indicate the physiological stress associated with the presence or absence of whisker stimulation prior to perfusion. Total corticosterone levels are significantly affected by whisker stimulation in moderate brain-injured rats (F (1,37) = 4.70, p = 0.037; Fig. 5). In the absence of whisker stimulation (n = 9 FPI and n = 8 sham), corticosterone levels are similar between uninjured (74.3 ±10.3 ng/mL) and brain-injured animals (56.9 ± 12.5 ng/mL). As predicted from the behavioral responses in uninjured sham animals, corticosterone levels are not affected by whisker stimulation (74.2 ± 12.2 ng/mL; n = 13). At 4 weeks after diffuse brain injury, corticosterone levels in response to whisker stimulation are elevated significantly, by 68% (125.0 ± 17.7 ng/mL; n = 13) above the levels seen in uninjured sham animals that received similar whisker stimulation. By comparison, corticosterone levels after whisker stimulation in mild-injured animals were elevated non-significantly, by 30% (96.6 ± 11.7 ng/mL) above the levels in uninjured sham animals. These data indicate that whisker stimulation evokes a physiological stress response in diffuse brain-injured rats, which parallels the behavioral observations seen during the whisker nuisance task.
FIG. 5.
Total free serum corticosterone as measured by enzyme immunoassay. Circulating stress hormone levels are unchanged in non-stimulated brain-injured animals compared to uninjured sham control at 4 weeks post-injury. Corticosterone levels in sham animals are unaffected by the whisker nuisance task. Exposure to the whisker nuisance task significantly elevates serum corticosterone levels compared to uninjured (*p < 0.05) and non-stimulated (+p < 0.05) animals (mean ± standard error of the mean; two-way analysis of variance and least significant difference planned comparison post-test; FPI, fluid percussion injury).
Discussion
In the evaluation of diffuse brain injury, the whisker nuisance task is a promising tool to detect post-traumatic morbidity and evaluate the efficacy of therapeutic interventions. The whisker nuisance task can identify the onset and persistence of post-traumatic sensory sensitivity in diffuse brain-injured rats at 4–8 weeks post-injury. Similarly, morbidity after experimental spinal cord injury, such as neuropathic pain, develops over a 4- to 6-week time course from plasticity-dependent processes (Nesic et al., 2005; Wang and Thompson, 2008). Analogous to the behavioral responses seen during the whisker nuisance task, brain-injured survivors typically show signs of agitation, particularly a heightened sensitivity to sensory stimulation (Bohnen et al., 1991; Waddell and Gronwall, 1984). Our laboratory is exploring unregulated neuroplastic responses to diffuse injury as a potential biological basis for the observed whisker nuisance behavior.
In midline fluid percussion, injury-induced histopathology is uncomplicated by contusion, cavitation, or overt hemorrhage (McGinn et al., 2009; Povlishock and Katz, 2005), and represents the majority of TBI cases, which are diffuse and of mild to moderate severity. At the microscopic level, the primary forces of the injury cause traumatic axonal and vascular injury (Farkas and Povlishock, 2007; Kelley et al., 2006; Povlishock and Stone, 2001; Singleton et al., 2002). The consequent axotomy, neuronal atrophy, and chronic neuroinflammation without neuronal loss in the ventral posterior nucleus of the thalamus indicate that neurological function involving the whiskers may be susceptible to injury (Kelley et al., 2007; Lifshitz et al., 2007a).
Nature of the chronic sensory sensitivity
Whisker stimulation in brain-injured animals shows late-onset, robust, and persistent aberrant behavioral responses. Neurobehavioral measures employed previously in experimental TBI studies are typically early-onset, subtle, and transient (Fujimoto et al., 2004; Hamm, 2001; Hogg et al., 1998; Sanders et al., 2001), resembling post-traumatic deficits rather than morbidity. Brain injury survivors exhibit similar sensory hypersensitivity and agitation with sensory overload (Bohnen et al., 1991; Waddell and Gronwall, 1984).
Eight behavioral components comprise the whisker nuisance score, and each component contributes to the overall score. The breadth and scope of behavioral responses to whisker stimulation vary between animals after brain injury, but have been reliably observed to some extent in all brain-injured rats. The high inter-rater reliability highlights the simplicity of the scoring criteria, for which variability is reduced by averaging scores over three periods. Ultimately, the whisker nuisance scores for sham and brain-injured rats do not exhibit floor or ceiling effects, thereby permitting the detection of both improvements and declines in post-traumatic morbidity after mild or moderate injury.
The behavioral responses remain specific to tactile whisker stimulation, since aberrant behaviors were not as evident or profound with cold sensitivity, pressure, or forepaw stimulation. Thus the behavioral responses likely arise from central circuit reorganization, rather than injury to peripheral components of the whisker circuitry (Ren, 1999).
The sensitivity to whisker stimulation remains below behavioral detection around 1 week post-injury, only emerging by 4 weeks post-injury. Circuit reorganization after mechanical injury that involves synaptic pruning, subsequent sprouting, and the emergence of chronic pain likely begins around 3 days post-injury, and evolves over weeks (Bothwell et al., 2001; Nesic et al., 2005; Wang and Thompson, 2008). Once circuits restructure, behavioral morbidity likely persists, as demonstrated over testing periods and time post-injury, without habituation or sensitization of behavioral performance. Yet the delayed emergence indicates that underlying cellular processes (likely structurally reorganizational in nature) may be amenable to therapeutic intervention.
Behavioral responses indicate that whisker stimulation may elicit anxiety in brain-injured animals. The open-field test cage used for whisker stimulation is anxiety-inducing (Isaac et al., 1989). However, the time spent in the center versus the periphery during an open-field examination (without whisker stimulation) does not differ between uninjured and brain-injured animals. Yet the physiological response to whisker stimulation is significantly enhanced in brain-injured animals, as evidenced by elevated corticosterone levels. Stimulation-induced rises in serum corticosterone could be interpreted as a response to uncontrollable stress (Ordyan and Zhukov, 1998). In this way, serum corticosterone levels may serve as an objective measure of whisker nuisance. Brain-injured patients exhibited 55–103% increases in serum corticosterone when challenged with adrenocorticotropic hormone years post-injury (Kleindienst et al., 2009), which matched the acute injury-induced rise in corticosterone.
Similarly to the neuromotor score for brain injury and the Basso-Beattie-Bresnahan scale for spinal cord injury (Basso et al., 1995; Fujimoto et al., 2004), the scoring criteria are subjective and could benefit from more objective, quantitative methodology, such as ultrasonic vocalization (Jourdan et al., 1995) or serum corticosterone. Task automation could improve reliability and reproducibility. However, automated mechanical whisker stimulation has only proven possible in anesthetized animals (Gonzalez and Sharp, 1985), and electromagnetic whisker stimulation results in whisker vibration rather than deflection (Melzer et al., 1985). In preliminary observations, mice have proven too small and agile to manually stimulate their whiskers. Parallel studies could examine the motor control of whisking, since tactile exploration involves sensorimotor feedback.
Injury-induced, aberrant responses to whisker stimulation indicate an altered perception of sensory stimuli, possibly derived from maladaptive circuits tapping into existing fear, anxiety, or pain circuitry (Craig, 2002). Sensory information processing in the adult nervous system depends on identifying the appropriate signals among the inherent noise (Faisal et al., 2008). As noise and error accumulate in a diffusely-injured neural circuit, information becomes more difficult to extract, such that brain-injured animals become agitated by the seemingly non-noxious whisker stimulation. In this way, the morbidity elicited by the whisker nuisance task resembles the agitation observed in overstimulated brain-injured patients (Waddell and Gronwall, 1984), akin to sensory gating failures in schizophrenia (Cromwell et al., 2008). The defensive freezing (learned helplessness) response could be interpreted as allodynia or neuropathic pain, in which normally innocuous stimuli produce subjective symptomatology (Scholz and Woolf, 2007; Vierck and Light, 2000). Behavioral morbidities may emerge as a consequence of global activation and an inability to discern useful information from elevated background noise, as has been demonstrated in sensory integration disorder, a component of autism spectrum disorder (Iarocci and McDonald, 2006). It follows that discrete activation of other sensory, endogenous, or motor circuits may result in other post-traumatic morbidities. Less likely, diffuse injury could fracture the sensory-motor integration necessary for tactile exploration, which was not observed in animals exposed to an enriched tactile environment. Restructured circuits may underlie the behavioral responses to whisker stimulation.
Initial neurorehabilitation paradigms may not mitigate morbidity and adversely affect sham performance
The present neurorehabilitation strategies after moderate diffuse TBI are a first step in a long process of incorporating aspects of clinical care in the laboratory to determine the necessary and sufficient components of therapeutic intervention. For diffuse brain injury, rehabilitative and/or pharmacological strategies, rather than surgical intervention, remain the primary treatment options. Neurorehabilitation strategies involving sensory deprivation and stimulation take advantage of the activity-dependent plasticity in the somatosensory whisker system to alleviate the observed morbidity (Frostig, 2006). Modulation of whisker barrel circuit activity through sensory deprivation or stimulation was initiated to affect the course of neurodegenerative and neuroplastic responses, and thus alleviate the nature of post-traumatic behavioral deficits. Preliminary neurorehabilitative interventions were designed with an intermittent, clinical outpatient perspective, as observed with other chronic rehabilitative paradigms and outcome measures (Hamm et al., 1996; Kline et al., 2007; Kozlowski et al., 2004). To this end, the whiskers of brain-injured and uninjured rats were shaved to prevent circuit activation, repeated activation during manual stimulation, and use during tactile exploration of an enriched environment.
The whisker nuisance task remains effective in mild brain injury, but loses efficacy with massed whisker stimulation and tactile exploration. Even in humans, rehabilitation strategies can be met with adversity. Although post-injury housing in an enriched environment improves some injury-induced behavioral deficits (Hamm et al., 1996; Maegele et al., 2005; Passineau et al., 2001; Wagner et al., 2002), but not all (Kozlowski et al., 2004; Wagner et al., 2002), experimental control of whisker stimulation becomes problematic. Yet whisker circuit neuronal responses peak and attenuate within an hour of exposure to an enriched environment (Bisler et al., 2002), supporting an outpatient therapy paradigm. By comparing repeated whisker stimulation to voluntary stimulation, the effects of the enriched environment become dissociated from the whisker stimulation. Forced use paradigms have been studied in CNS injury models to alleviate forelimb motor and cognitive deficits (Kleim et al., 2003), but may not translate to whisker somatosensation. The current neurorehabilitation paradigms may suffer further from issues regarding stress, motivation, and massed neurorehabilitation sessions. Refinement of the rehabilitation strategy in combination with appropriate post-injury timing is necessary to effectively intervene and alleviate morbidity. It is possible that delayed rehabilitation strategies will mitigate post-traumatic morbidity, as demonstrated with delayed access to voluntary wheel-running therapy (Griesbach et al., 2007). Alternate rehabilitation approaches could include the use of Elizabethan collars to prevent grooming. Others have employed tactile discrimination tasks with the sensitivity to detect somatosensory dysfunction (Krupa et al., 2004). However, post-traumatic cognitive impairments (Hamm, 2001) would interfere with the conduct of such tasks.
Structural basis for post-traumatic morbidity
TBI is a complex interwoven sequence of ionic and metabolic events from which damaged cells can either recover or degenerate and die. Diffuse brain injury results in synaptic deafferentation followed by synaptic plasticity (Christman et al., 1997; Emery et al., 2003; Povlishock et al., 1992; Povlishock and Katz, 2005), similar to stroke (Carmichael et al., 2001). The neuroplastic growth may be essential to re-establish the trophic support provided by synaptic connectivity and thereby ensure neuronal survival (Emery et al., 2003; Franklin and Johnson, 1998; Gold et al., 1991). However, a by-product of the recovery process would be circuit reorganization, which has long been examined in the fields of peripheral nerve injury, sensory plasticity, and memory consolidation (Chklovskii et al., 2004; Jones, 2000).
Most, if not all, diffusely-injured brain regions likely mount a regenerative response. Regenerative responses in the diffusely-injured brain include frank regeneration, collateral sprouting, and synaptic plasticity. However, in the absence of regulated spatial and temporal guidance cues, regenerative responses likely result in maladaptive restructuring of injured circuits. Moreover, the diffusely-injured brain cannot rely on redundant circuits to compensate for functional impairments. Ultimately, the injury-induced reorganization in response to diffuse brain injury may deplete the future capacity for subsequent adaptive change (Kolb et al., 1998), thereby contributing to persistent post-traumatic morbidity. Therefore the structural plasticity-dependent survival of the injured and axotomized neurons can scramble the wiring diagram and cause gain-of-function morbidity.
Conclusion
For the first time we report a robust, reproducible, and late-onset neurological deficit that emerges after midline FPI that can be used to identify cellular processes associated with post-traumatic morbidity and recovery. We contend that circuit restructuring is responsible for the observed morbidity and thereby provides likely therapeutic targets. Continued exploration of the whisker-barrel circuit may facilitate advancements in our understanding of post-traumatic morbidity, and guide rational therapeutic strategies.
Acknowledgments
We are grateful to Dr. Mark A. Prendergast and his laboratory for assistance with the corticosterone assays. We thank Dr. Karin Westlund High and Dr. Fei Ma for their assistance in evaluating pressure sensitivity in the whisker pad. We are grateful to Dr. Michael T. Bardo and Ms. Emily D. Denehy for conducting the open-field behavioral analysis. Also, we wish to thank Experiences in Undergraduate Research and Kreative Activities (eUreka!), Students Promoting Undergraduate Research (SPUR), Kentucky Yang Researchers Program (KYRP), and National Conference for Undergraduate Research (NCUR) at the University of Kentucky as well as Experience Based Career Education (EBCE) for their continued support of undergraduate research. Supported, in part, by University of Kentucky College of Medicine, NIH NINDS R01 NS065052 and NIH NINDS p30 NS051220.
Author Disclosure Statement
No competing financial interests exist.
References
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J, Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bernardo K.L. Woolsey T.A. Axonal trajectories between mouse somatosensory thalamus and cortex. J. Comp. Neurol. 1987;258:542–564. doi: 10.1002/cne.902580406. [DOI] [PubMed] [Google Scholar]
- Bisler S. Schleicher A. Gass P. Stehle J.H. Zilles K. Staiger J.F. Expression of c-Fos, ICER, Krox-24 and JunB in the whisker-to-barrel pathway of rats: time course of induction upon whisker stimulation by tactile exploration of an enriched environment. J. Chem. Neuroanat. 2002;23:187–198. doi: 10.1016/s0891-0618(01)00155-7. [DOI] [PubMed] [Google Scholar]
- Bohnen N. Twijnstra A. Wijnen G. Jolles J. Tolerance for light and sound of patients with persistent post-concussional symptoms 6 months after mild head injury. J. Neurol. 1991;238:443–446. doi: 10.1007/BF00314651. [DOI] [PubMed] [Google Scholar]
- Bothwell S. Meredith G.E. Phillips J. Staunton H. Doherty C. Grigorenko E. Glazier S. Deadwyler S.A. O'Donovan C.A. Farrell M. Neuronal hypertrophy in the neocortex of patients with temporal lobe epilepsy. J. Neurosci. 2001;21:4789–4800. doi: 10.1523/JNEUROSCI.21-13-04789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M. Preilowski B. Merzenich M.M. Functional architecture of the mystacial vibrissae. Behav. Brain Res. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T. Wei L. Rovainen C.M. Woolsey T.A. New patterns of intracortical projections after focal cortical stroke. Neurobiol. Dis. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- Carvell G.E. Simons D.J. Biometric analyses of vibrissal tactile discrimination in the rat. J. Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii D.B. Mel B.W. Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Chmielowska J. Carvell G.E. Simons D.J. Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J. Comp. Neurol. 1989;285:325–338. doi: 10.1002/cne.902850304. [DOI] [PubMed] [Google Scholar]
- Choi Y. Yoon Y.W. Na H.S. Kim S.H. Chung J.M. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Christman C.W. Salvant J.B., Jr. Walker S.A. Povlishock J.T. Characterization of a prolonged regenerative attempt by diffusely injured axons following traumatic brain injury in adult cat: a light and electron microscopic immunocytochemical study. Acta Neuropathol. (Berl.) 1997;94:329–337. doi: 10.1007/s004010050715. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Cromwell H.C. Mears R.P. Wan L. Boutros N.N. Sensory gating: a translational effort from basic to clinical science. Clin. EEG Neurosci. 2008;39:69–72. doi: 10.1177/155005940803900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Busto R. Ginsberg M.D. Widespread metabolic depression and reduced somatosensory circuit activation following traumatic brain injury in rats. J. Neurotrauma. 1994;11:629–640. doi: 10.1089/neu.1994.11.629. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell A.A. Levin B.E. Lateralized effect of unilateral somatosensory cortex contusion on behavior and cortical reorganization. Brain Res. 1995;675:143–156. doi: 10.1016/0006-8993(95)00050-z. [DOI] [PubMed] [Google Scholar]
- Dyck R.H. Vibrissae. In: Whishaw I.Q., editor; Kolb B., editor. The Behavior of the Laboratory Rat. Oxford University Press; New York: 2004. [Google Scholar]
- Emery D.L. Raghupathi R. Saatman K.E. Fischer I. Grady M.S. McIntosh T.K. Bilateral growth-related protein expression suggests a transient increase in regenerative potential following brain trauma. J. Comp. Neurol. 2000;424:521–531. [PubMed] [Google Scholar]
- Emery D.L. Royo N.C. Fischer I. Saatman K.E. McIntosh T.K. Plasticity following injury to the adult central nervous system: is recapitulation of a developmental state worth promoting? J. Neurotrauma. 2003;20:1271–1292. doi: 10.1089/089771503322686085. [DOI] [PubMed] [Google Scholar]
- Faisal A.A. Selen L.P. Wolpert D.M. Noise in the nervous system. Nat. Rev. Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas O. Povlishock J.T. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog. Brain Res. 2007;161:43–59. doi: 10.1016/S0079-6123(06)61004-2. [DOI] [PubMed] [Google Scholar]
- Faul F. Erdfelder E. Lang A.G. Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Feldman D.E. Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Franklin J.L. Johnson E.M. Control of neuronal size homeostasis by trophic factor-mediated coupling of protein degradation to protein synthesis. J. Cell Biol. 1998;142:1313–1324. doi: 10.1083/jcb.142.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig R.D. Functional organization and plasticity in the adult rat barrel cortex: moving out-of-the-box. Curr. Opin. Neurobiol. 2006;16:445–450. doi: 10.1016/j.conb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Fujimoto S.T. Longhi L. Saatman K.E. McIntosh T.K. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gold B.G. Mobley W.C. Matheson S.F. Regulation of axonal caliber, neurofilament content, and nuclear localization in mature sensory neurons by nerve growth factor. J. Neurosci. 1991;11:943–955. doi: 10.1523/JNEUROSCI.11-04-00943.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M.F. Sharp F.R. Vibrissae tactile stimulation: (14C) 2-deoxyglucose uptake in rat brainstem, thalamus, and cortex. J. Comp. Neurol. 1985;231:457–472. doi: 10.1002/cne.902310405. [DOI] [PubMed] [Google Scholar]
- Griesbach G.S. Gomez-Pinilla F. Hovda D.A. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J. Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- Guic-Robles E. Valdivieso C. Guajardo G. Rats can learn a roughness discrimination using only their vibrissal system. Behav. Brain Res. 1989;31:285–289. doi: 10.1016/0166-4328(89)90011-9. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Temple M.D. O'Dell D.M. Pike B.R. Lyeth B.G. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J. Neurotrauma. 1996;13:41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- Henderson T.A. Jacquin M.F. What makes subcortical barrels? In: Jones E.G., editor; Diamond I.T., editor. Cerebral Cortex. Plenum; New York: 1995. pp. 123–198. [Google Scholar]
- Hogg S. Moser P.C. Sanger D.J. Mild traumatic lesion of the right parietal cortex of the rat: selective behavioural deficits in the absence of neurological impairment. Behav. Brain Res. 1998;93:143–155. doi: 10.1016/s0166-4328(97)00146-0. [DOI] [PubMed] [Google Scholar]
- Hosseini A.H. Lifshitz J. Brain injury forces of moderate magnitude elicit the fencing response. Med. Sci. Sports Exerc. 2009;41:1687–1697. doi: 10.1249/MSS.0b013e31819fcd1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch C.E. DeWitt D.S. Jenkins L.W. Prough D.S. Traumatic brain injury in rats results in increased expression of Gap-43 that correlates with behavioral recovery. Neurosci. Lett. 1998;255:83–86. doi: 10.1016/s0304-3940(98)00712-5. [DOI] [PubMed] [Google Scholar]
- Iarocci G. McDonald J. Sensory integration and the perceptual experience of persons with autism. J. Autism Dev. Disord. 2006;36:77–90. doi: 10.1007/s10803-005-0044-3. [DOI] [PubMed] [Google Scholar]
- Ip E.Y. Zanier E.R. Moore A.H. Lee S.M. Hovda D.A. Metabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injury. J. Cereb. Blood Flow Metab. 2003;23:900–910. doi: 10.1097/01.WCB.0000076702.71231.F2. [DOI] [PubMed] [Google Scholar]
- Isaac W.L. Nonneman A.J. Neisewander J. Landers T. Bardo M.T. Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behav. Neurosci. 1989;103:345–355. doi: 10.1037//0735-7044.103.2.345. [DOI] [PubMed] [Google Scholar]
- Jones E.G. Cortical and subcortical contributions to activity-dependent plasticity in primate somatosensory cortex. Annu. Rev. Neurosci. 2000;23:1–37. doi: 10.1146/annurev.neuro.23.1.1. [DOI] [PubMed] [Google Scholar]
- Jourdan D. Ardid D. Chapuy E. Eschalier A. Le B.D. Audible and ultrasonic vocalization elicited by single electrical nociceptive stimuli to the tail in the rat. Pain. 1995;63:237–249. doi: 10.1016/0304-3959(95)00049-X. [DOI] [PubMed] [Google Scholar]
- Kelley B.J. Farkas O. Lifshitz J. Povlishock J.T. Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and Wallerian degeneration. Exp. Neurol. 2006;198:350–360. doi: 10.1016/j.expneurol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Kelley B.J. Lifshitz J. Povlishock J.T. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J. Neuropathol. Exp. Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Kleim J.A. Jones T.A. Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem. Res. 2003;28:1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- Kleindienst A. Brabant G. Bock C. Maser-Gluth C. Buchfelder M. Neuroendocrine function following traumatic brain injury and subsequent intensive care treatment: A prospective longitudinal evaluation. J. Neurotrauma. 2009;26:1435–1446. doi: 10.1089/neu.2008.0601. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Massucci J.L. Zafonte R.D. Dixon C.E. DeFeo J.R. Rogers E.H. Differential effects of single versus multiple administrations of haloperidol and risperidone on functional outcome after experimental brain trauma. Crit. Care Med. 2007;35:919–924. doi: 10.1097/01.CCM.0000256722.88854.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B. Forgie M. Gibb R. Gorny G. Rowntree S. Age, experience and the changing brain. Neurosci. Biobehav Rev. 1998;22:143–159. doi: 10.1016/s0149-7634(97)00008-0. [DOI] [PubMed] [Google Scholar]
- Kozlowski D.A. Nahed B.V. Hovda D.A. Lee S.M. Paradoxical effects of cortical impact injury on environmentally enriched rats. J. Neurotrauma. 2004;21:513–519. doi: 10.1089/089771504774129856. [DOI] [PubMed] [Google Scholar]
- Krupa D.J. Wiest M.C. Shuler M.G. Laubach M. Nicolelis M.A. Layer-specific somatosensory cortical activation during active tactile discrimination. Science. 2004;304:1989–1992. doi: 10.1126/science.1093318. [DOI] [PubMed] [Google Scholar]
- Land P.W. Buffer S.A., Jr. Yaskosky J.D. Barreloids in adult rat thalamus: three-dimensional architecture and relationship to somatosensory cortical barrels. J. Comp. Neurol. 1995;355:573–588. doi: 10.1002/cne.903550407. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Thomas K.E. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2004. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. [Google Scholar]
- Lifshitz J. Fluid percussion injury. In: Chen J., editor; Xu Z., editor; Xu X.-M., editor; Zhang J., editor. Animal Models of Acute Neurological Injuries. The Humana Press, Inc.; Totowa, NJ: 2008. [Google Scholar]
- Lifshitz J. Kelley B.J. Povlishock J.T. Perisomatic thalamic axotomy after diffuse traumatic brain injury is associated with atrophy rather than cell death. J. Neuropathol. Exp. Neurol. 2007a;66:218–229. doi: 10.1097/01.jnen.0000248558.75950.4d. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Witgen B.M. Grady M.S. Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: evaluation by conditioned fear response. Behav. Brain Res. 2007b;177:347–357. doi: 10.1016/j.bbr.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegele M. Lippert-Gruener M. Ester-Bode T. Sauerland S. Schafer U. Molcanyi M. Lefering R. Bouillon B. Neiss W.F. Angelov D.N. Klug N. McIntosh T.K. Neugebauer E.A. Reversal of neuromotor and cognitive dysfunction in an enriched environment combined with multimodal early onset stimulation after traumatic brain injury in rats. J. Neurotrauma. 2005;22:772–782. doi: 10.1089/neu.2005.22.772. [DOI] [PubMed] [Google Scholar]
- McAllister T.W. Neuropsychiatric sequelae of head injuries. Psychiatr. Clin. North Am. 1992;15:395–413. [PubMed] [Google Scholar]
- McGinn M.J. Kelley B.J. Akinyi L. Oli M.W. Liu M.C. Hayes R.L. Wang K.K. Povlishock J.T. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J. Neuropathol Exp. Neurol. 2009;68:241–249. doi: 10.1097/NEN.0b013e3181996bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T.K. Vink R. Noble L. Yamakami I. Fernyak S. Soares H. Faden A.I. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Melzer P. Van der L.H. Dorfl J. Welker E. Robert P. Emery D. Berrini J.C. A magnetic device to stimulate selected whiskers of freely moving or restrained small rodents: its application in a deoxyglucose study. Brain Res. 1985;348:229–240. doi: 10.1016/0006-8993(85)90441-x. [DOI] [PubMed] [Google Scholar]
- Nesic O. Lee J. Johnson K.M. Ye Z. Xu G.Y. Unabia G.C. Wood T.G. McAdoo D.J. Westlund K.N. Hulsebosch C.E. Regino Perez-Polo J. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J. Neurochem. 2005;95:998–1014. doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- Ordyan N.E. Zhukov D.A. Effects of controllable and uncontrollable stresses on the receptor binding of dexamethasone in the hypophysis and hippocampus of rats with different behavior strategies. Neurosci. Behav. Physiol. 1998;28:22–25. doi: 10.1007/BF02461907. [DOI] [PubMed] [Google Scholar]
- Passineau M.J. Green E.J. Dietrich W.D. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp. Neurol. 2001;168:373–384. doi: 10.1006/exnr.2000.7623. [DOI] [PubMed] [Google Scholar]
- Passineau M.J. Zhao W. Busto R. Dietrich W.D. Alonso O. Loor J.Y. Bramlett H.M. Ginsberg M.D. Chronic metabolic sequelae of traumatic brain injury: prolonged suppression of somatosensory activation. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H924–H931. doi: 10.1152/ajpheart.2000.279.3.H924. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Stone J.R. Traumatic axonal injury. In: Miller L.P., editor; Hayes R.L., editor; Newcomb J.K., editor. Head Trauma: Basic, Preclinical and Clinical Directions. Wiley; Hoboken, NJ: 2001. pp. 281–302. [Google Scholar]
- Povlishock J.T. Erb D.E. Astruc J. Axonal response to traumatic brain injury: reactive axonal change, deafferentation, and neuroplasticity. J. Neurotrauma. 1992;9(Suppl. 1):S189–S200. [PubMed] [Google Scholar]
- Povlishock J.T. Katz D.I. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol. Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Riess P. Bareyre F.M. Saatman K.E. Cheney J.A. Lifshitz J. Raghupathi R. Grady M.S. Neugebauer E. McIntosh T.K. Effects of chronic, post-injury cyclosporin A administration on motor and sensorimotor function following severe, experimental traumatic brain injury. Restor. Neurol. Neurosci. 2001;18:1–8. [PubMed] [Google Scholar]
- Sanders M.J. Dietrich W.D. Green E.J. Behavioral, electrophysiological, and histopathological consequences of mild fluid-percussion injury in the rat. Brain Res. 2001;904:141–144. doi: 10.1016/s0006-8993(01)02424-6. [DOI] [PubMed] [Google Scholar]
- Scholz J. Woolf C.J. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Shaw N.A. The neurophysiology of concussion. Prog. Neurobiol. 2002;67:281–344. doi: 10.1016/s0301-0082(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Singleton R.H. Zhu J. Stone J.R. Povlishock J.T. Traumatically induced axotomy adjacent to the soma does not result in acute neuronal death. J. Neurosci. 2002;22:791–802. doi: 10.1523/JNEUROSCI.22-03-00791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouras E. Merkel D. Grosheva M. Angelova S.K. Schiffer G. Thelen U. Kaidoglou K. Sinis N. Igelmund P. Dunlop S.A. Pavlov S. Irintchev A. Angelov D.N. Manual stimulation, but not acute electrical stimulation prior to reconstructive surgery, improves functional recovery after facial nerve injury in rats. Restor. Neurol. Neurosci. 2009;27:237–251. doi: 10.3233/RNN-2009-0474. [DOI] [PubMed] [Google Scholar]
- Vierck C.J., Jr. Light A.R. Allodynia and hyperalgesia within dermatomes caudal to a spinal cord injury in primates and rodents. Prog. Brain Res. 2000;129:411–428. doi: 10.1016/S0079-6123(00)29032-8. [DOI] [PubMed] [Google Scholar]
- Vos B.P. Strassman A.M. Maciewicz R.J. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J. Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell P.A. Gronwall D.M. Sensitivity to light and sound following minor head injury. Acta Neurol. Scand. 1984;69:270–276. doi: 10.1111/j.1600-0404.1984.tb07812.x. [DOI] [PubMed] [Google Scholar]
- Wagner A.K. Kline A.E. Sokoloski J. Zafonte R.D. Capulong E. Dixon C.E. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci. Lett. 2002;334:165–168. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- Wang G. Thompson S.M. Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J. Neurosci. 2008;28:11959–11969. doi: 10.1523/JNEUROSCI.3296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey T.A. Van der L.H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Wooters T.E. Bardo M.T. Nicotinic receptors differentially modulate the induction and expression of behavioral sensitization to methylphenidate in rats. Psychopharmacology (Berl.) 2009;204:551–562. doi: 10.1007/s00213-009-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]