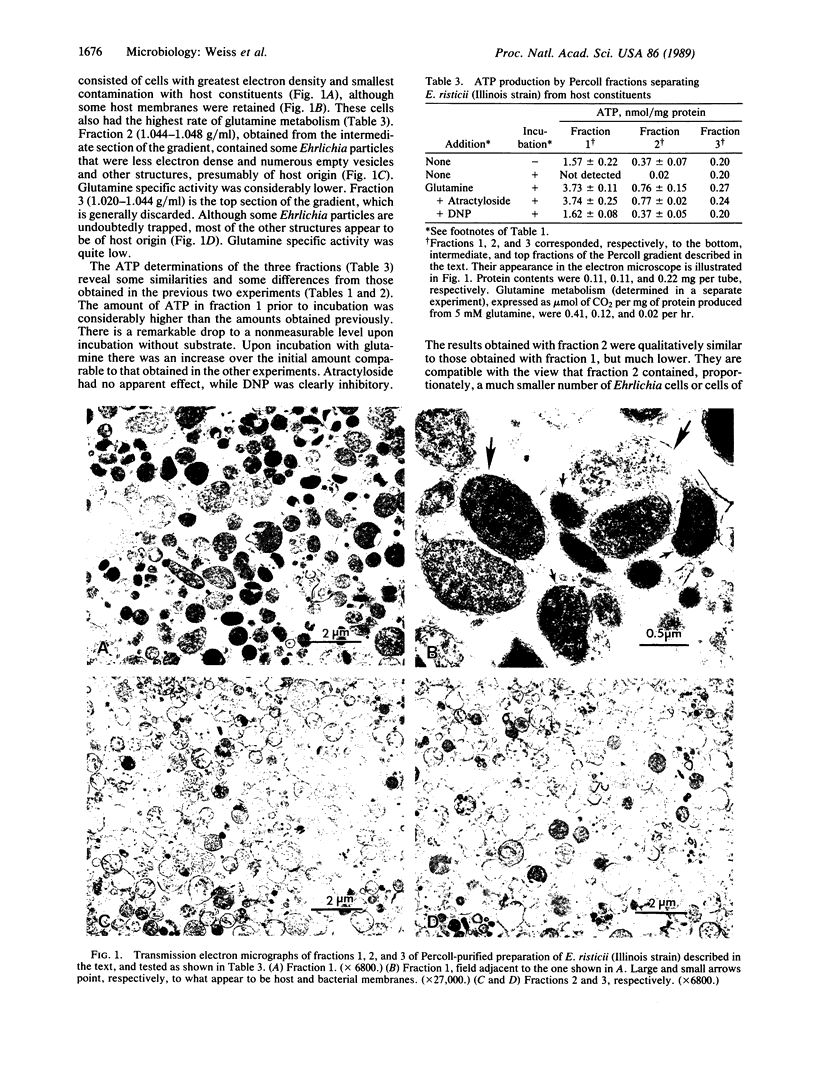
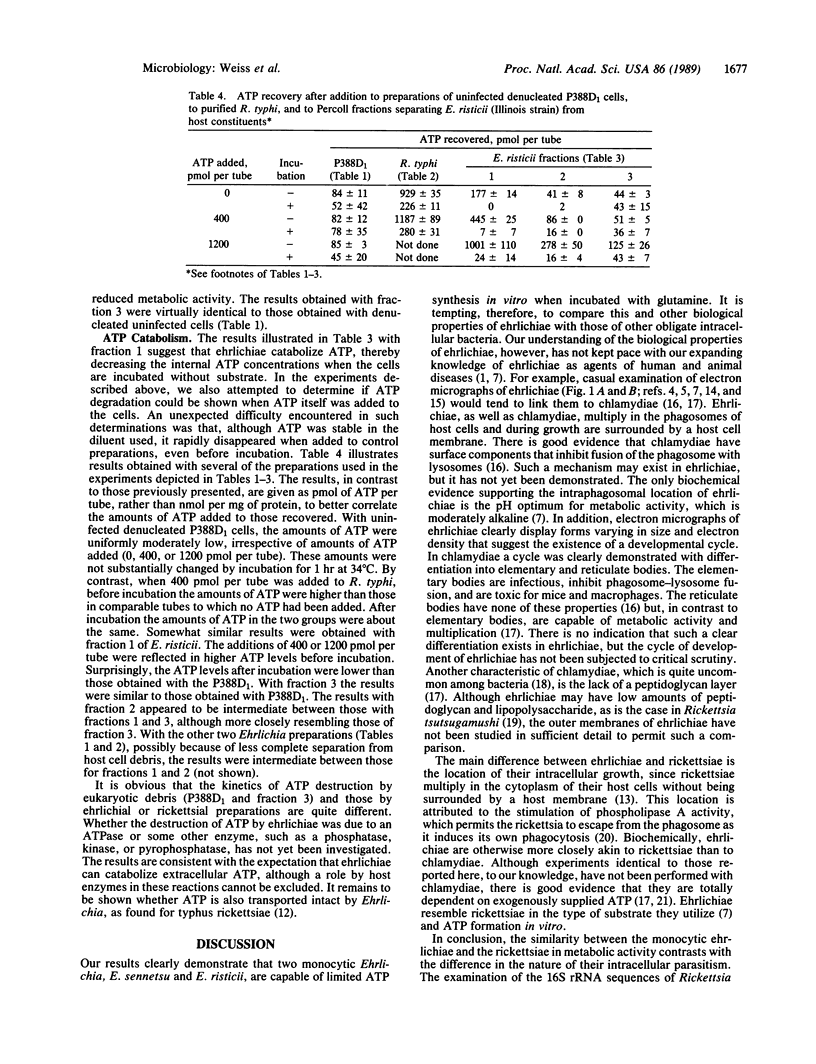
Abstract
We investigated if the monocytic Ehrlichia are totally dependent on their host cells for energy, or, as Rickettsia, are capable of some ATP synthesis in vitro. The Miyayama strain of Ehrlichia sennetsu and the Maryland and Illinois strains of Ehrlichia risticii were cultivated in a mouse macrophage cell line, separated from host cell constituents by procedures that included Renografin or Percoll gradient centrifugation, and tested after cryopreservation. Cells incubated without a metabolizing substrate contained little, if any, ATP. When the Ehrlichia cells were incubated for 1 hr at 34 degrees C with glutamine, significant amounts of ATP were detected. The amounts of ATP attained with glutamine were decreased in some instances by the addition of atractyloside, an inhibitor of adenine nucleotide translocase in mitochondria, and were decreased consistently and to a greater extent by 2,4-dinitrophenol. When ATP, instead of glutamine, was added to the ehrlichiae, upon incubation the amount of ATP was markedly decreased. Comparable responses under all these conditions were obtained with Rickettsia typhi, although the final ATP levels were higher. Control preparations derived from uninfected mouse macrophages or from the discards of the Ehrlichia purification procedures contained negligible amounts of ATP, which were not increased by incubation with glutamine. We conclude that with respect to ATP metabolism, the monocytic Ehrlichia resemble Rickettsia more closely than Chlamydia, even though Ehrlichia resemble Chlamydia in their intracellular location in the phagosomes and in possibly having a developmental cycle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Tamura A., Ohashi N., Urakami H., Kaya S., Fukushi K. Deficiency of peptidoglycan and lipopolysaccharide components in Rickettsia tsutsugamushi. Infect Immun. 1987 Sep;55(9):2290–2292. doi: 10.1128/iai.55.9.2290-2292.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Holland C. J., Ristic M., Cole A. I., Johnson P., Baker G., Goetz T. Isolation, experimental transmission, and characterization of causative agent of Potomac horse fever. Science. 1985 Feb 1;227(4686):522–524. doi: 10.1126/science.3880925. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapmund G. Rickettsial diseases of the Far East: new perspectives. J Infect Dis. 1984 Mar;149(3):330–338. doi: 10.1093/infdis/149.3.330. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Hatch T. P., Woese C. R. Eubacterial origin of chlamydiae. J Bacteriol. 1986 Aug;167(2):570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Dasch G. A., Kang Y. H., Westfall H. N. Substrate utilization by Ehrlichia sennetsu and Ehrlichia risticii separated from host constituents by renografin gradient centrifugation. J Bacteriol. 1988 Nov;170(11):5012–5017. doi: 10.1128/jb.170.11.5012-5017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. The biology of rickettsiae. Annu Rev Microbiol. 1982;36:345–370. doi: 10.1146/annurev.mi.36.100182.002021. [DOI] [PubMed] [Google Scholar]
- Weiss E., Wilson N. N. Role of exogenous adenosine triphosphate in catabolic and synthetic activities of Chlamydia psittaci. J Bacteriol. 1969 Feb;97(2):719–724. doi: 10.1128/jb.97.2.719-724.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. Y., Rikihisa Y. Lack of lysosomal fusion with phagosomes containing Ehrlichia risticii in P388D1 cells: abrogation of inhibition with oxytetracycline. Infect Immun. 1988 Dec;56(12):3209–3215. doi: 10.1128/iai.56.12.3209-3215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Weiss E. Energy metabolism of Rickettsia typhi: pools of adenine nucleotides and energy charge in the presence and absence of glutamate. J Bacteriol. 1978 Jun;134(3):884–892. doi: 10.1128/jb.134.3.884-892.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Early events in the interaction of the obligate intracytoplasmic parasite, Rickettsia prowazekii, with eucaryotic cells: entry and lysis. Ann Inst Pasteur Microbiol. 1986 May-Jun;137A(3):333–336. doi: 10.1016/s0769-2609(86)80044-8. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]