Abstract
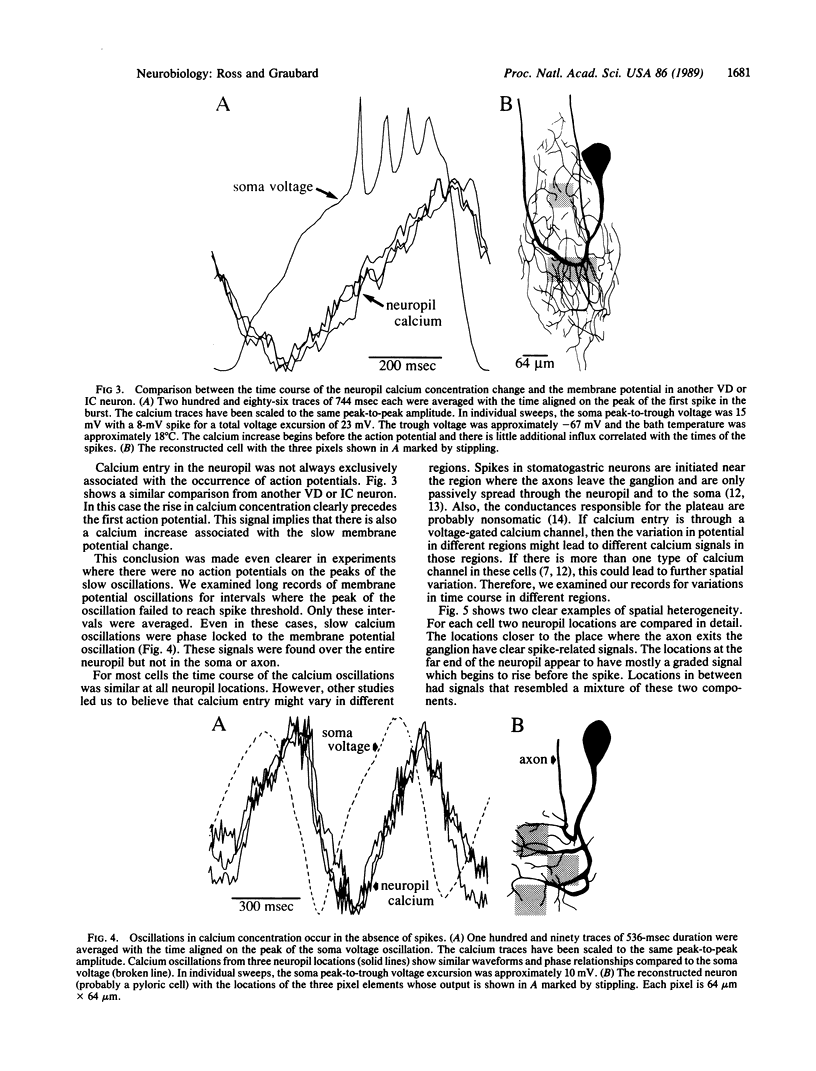
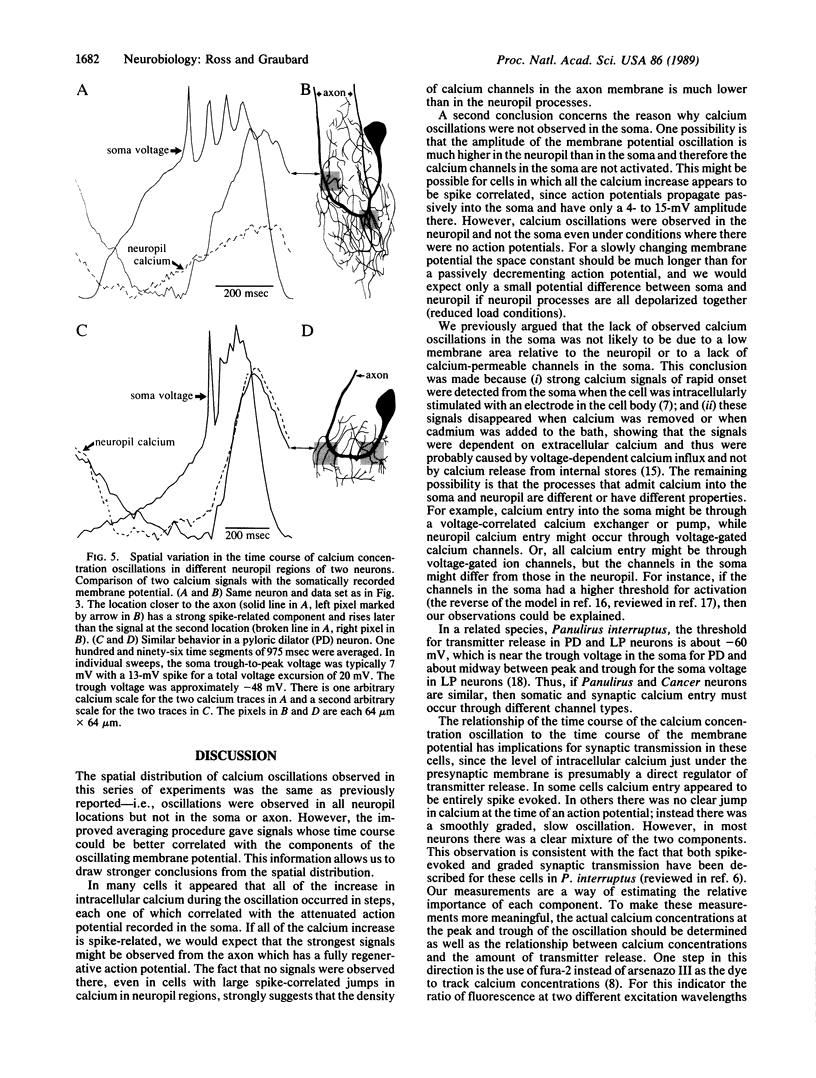
Calcium concentration changes during oscillations of the membrane potential of crab (Cancer irroratus or Cancer borealis) stomatogastric neurons were monitored at many positions by using the calcium indicator dye arsenazo III and a photodiode array. Data analysis algorithms using signal averaging techniques were developed to improve the time resolution of the measured calcium changes. As previously reported, calcium oscillations were detected from all regions of the neuropil but not from the soma or axon. In some cells step increases in intracellular neuropil calcium were correlated with each of the action potentials in the burst (on the peak of the voltage oscillation). In other cells we observed calcium oscillations phase-locked to the membrane potential with no spike-related component. A few cells had both spike-evoked and graded potential components to the calcium oscillations. In those cells, the spatial distribution of the spike-correlated calcium influx differed from that of the voltage-oscillation-correlated calcium influx, suggesting that different neurites might interact with their postsynaptic targets with different mixtures of graded and spike-correlated transmitter release.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Graubard K., Raper J. A., Hartline D. K. Graded synaptic transmission between identified spiking neurons. J Neurophysiol. 1983 Aug;50(2):508–521. doi: 10.1152/jn.1983.50.2.508. [DOI] [PubMed] [Google Scholar]
- Graubard K., Ross W. N. Regional distribution of calcium influx into bursting neurons detected with arsenazo III. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5565–5569. doi: 10.1073/pnas.82.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hooper S. L., O'Neil M. B., Wagner R., Ewer J., Golowasch J., Marder E. The innervation of the pyloric region of the crab, Cancer borealis: homologous muscles in decapod species are differently innervated. J Comp Physiol A. 1986 Aug;159(2):227–240. doi: 10.1007/BF00612305. [DOI] [PubMed] [Google Scholar]
- King D. G. Organization of crustacean neuropil. I. Patterns of synaptic connections in lobster stomatogastric ganglion. J Neurocytol. 1976 Apr;5(2):207–237. doi: 10.1007/BF01181657. [DOI] [PubMed] [Google Scholar]
- King D. G. Organization of crustacean neuropil. II. Distribution of synaptic contacts on identified motor neurons in lobster stomatogastric ganglion. J Neurocytol. 1976 Apr;5(2):239–266. doi: 10.1007/BF01181658. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]


