Abstract
Background
Lidocaine and bupivacaine are commonly infiltrated into surgical cutaneous wounds to provide local anaesthesia after surgical procedures. However, very little is known about their effects on cutaneous wound healing. If an inhibitory effect is demonstrated, then the balance between the benefits of postoperative local anaesthesia and the negatives of impaired cutaneous wound healing may affect the decision to use local anaesthesia or not. Furthermore, if a difference in the rate of healing of lidocaine- and bupivacaine-treated cutaneous wounds is revealed, or if an inhibitory effect is found to be dose-dependent, then this may well influence the choice of agent and its concentration for clinical use.
Methods
Immediately before incisional wounding, we administered lidocaine and bupivacaine intradermally to adult female mice, some of which had been ovariectomized to act as a model of post-menopausal women (like post-menopausal women, ovariectomized mice heal wounds poorly, with increased proteolysis and inflammation). Day 3 wound tissue was analysed histologically and tested for expression of inflammatory and proteolytic factors.
Results
On day 3 post-wounding, wound areas and extent of re-epithelialization were comparable between the control and local anaesthetic-treated animals, in both intact and ovariectomized groups. Both tested drugs significantly increased wound activity of the degradative enzyme matrix metalloproteinase-2 relative to controls, while lidocaine also increased wound neutrophil numbers.
Conclusions
Although lidocaine and bupivacaine influenced local inflammatory and proteolytic factors, they did not impair the rate of healing in either of two well-established models (mimicking normal human wound healing and impaired age-related healing).
Keywords: anaesthetics local, bupivacaine; anaesthetics local, lidocaine; mouse
Surgical wound infiltration with local anaesthetic (LA) agents is widely used to provide a cost-effective and relatively low-risk method of localized analgesia. Despite this, its impact on subsequent cutaneous wound healing has not been investigated in detail. Isolated studies have described some of the effects of the commonly used LA lidocaine on healing skin wounds. In rat models of acute wound repair, doses of lidocaine ranging from 0.5% to 2.0% have been shown to reduce wound breaking strength and to impair healing (as assessed by histological scoring).1,2
However, no consensus exists: others have reported beneficial effects of lidocaine-containing jelly and ointment on wound re-epithelialization,3 whereas another study reported that 1% lidocaine failed to influence breaking strengths of sutured wounds 8 days post-wounding.4 Moreover, bupivacaine, another routinely used LA agent, was shown, at a concentration of 0.5%, to have no influence on healing parameters in a clinical model,5 but at higher concentrations to increase breaking strengths of sutured incisional wounds in guinea pigs.6
The aim of the present study was to assess whether concentrations of lidocaine and bupivacaine used in humans to provide anaesthesia and analgesia influence the healing of incisional skin wounds, and associated inflammatory and proteolytic responses, in well-established mouse models of normal and impaired healing. Mice have been shown to heal incisional wounds with similar spatial and temporal profiles to humans. Ovariectomized mice were used as a model of age-impaired wound healing since a lack of oestrogen has been shown to induce the cellular and molecular changes that characterize poor healing in elderly females and males.7
Methods
Animal studies
Twenty seven-week-old female C57BL/6 mice (Charles River, Margate, UK), 10 of which had been bilaterally ovariectomized 2 weeks previously, were anaesthetized with isoflurane 4% for induction, isoflurane 1.5% for maintenance (in nitrous oxide 60% and oxygen). Their dorsa were shaved and cleansed using ethanol. Animals were injected at two points, 1 cm either side of the midline, with 50 µl of lidocaine 0.5/1%, bupivacaine 0.25/0.5%, or vehicle (sterile dH2O). A 1 cm full-thickness incision was then made through each of the resulting blebs.
The two injections each animal received differed in terms of drug, concentration, or both, thus giving an n number of eight per treatment. Group size was selected based on power calculations (P<0.05) and our previous experience that groups of this size (and indeed smaller) yielded positive differences in the same incisional wound model.7,8
After operation, all animals received immediate analgesia in the form of buprenorphine (0.1 mg kg−1; s.c.) and were housed individually. Three days post-wounding, the animals were killed and their wounds excised, bisected, and processed in an 8% formaldehyde-based fixative.
All animal studies were approved by the UK Government Home Office and procedures performed in accordance with Home Office regulations relating to animal care (HO project licence number 40/2526).
Histology and immunohistochemistry
Fixed tissue was embedded in paraffin. Five-micrometre thick histological sections prepared from the centre of each wound were stained with haematoxylin and eosin and analysed immunohistochemically with a rat monoclonal IgG raised against the neutrophil marker Ly-6G (BD Biosciences, Oxford, UK) and a goat polyclonal IgG raised against macrophage migration inhibitory factor (MIF) (R&D Systems, Abingdon, UK). Control sections treated with phosphate-buffered saline (PBS) in place of primary IgG yielded no staining. Bound primary IgG was detected using colorimetric VECTASTAIN ABC peroxidase kits in conjunction with Novared substrate (all Vector Laboratories, Peterborough, UK). Paraffin-embedded tissue sections were subjected to Picrosirius red staining by immersion in 0.1% Sirius red (in picric acid) for 1 h and then washing in acidified water. Stained collagen fibres may be visualized under plane-polarized light.9 Measurement of wound areas and cell numbers/unit area was performed using Image-Pro Plus software (Media Cybernetics, Marlow, UK).10 Picrosirius red (collagen) and MIF (epidermal) staining intensities were analysed by four-grade semi-quantitative scoring: 0, no staining; 1, weak; 2, moderate; 3, strong. Data are presented as box-and-whisker plots.
Immunoblotting
Total protein was extracted from mouse wound tissue using an SDS-based detergent buffer. Extracted protein samples (n=5 per treatment group) were pooled and 1 mg was tested by immunoblotting as described previously.11 A primary IgG raised against MIF (goat polyclonal) (R&D Systems) was used in conjunction with a rabbit anti-goat secondary IgG (both GE Healthcare). As a loading control, β-actin was detected used a mouse monoclonal IgG (Sigma-Aldrich, Poole, UK). Signal intensities were determined densitometrically and MIF:β-actin signal ratios were calculated.
Zymography
Total protein was extracted from unwounded skin and wound tissue using denaturing, non-reducing buffer. Thus-extracted protein samples (n=5 per treatment group) were pooled and 50 µg tested zymographically for gelatinase activities as described previously.12 Wound samples were analysed alongside human matrix metalloproteinase (MMP)-2, purified from stably transfected mouse myeloma cells.13
Neutrophil migration assay
Granulocytes were isolated from heparinized human peripheral blood using Mono-Poly Resolving Medium (MP Biomedicals, Cambridge, UK). The effects of lidocaine on the directional migration of neutrophils to the chemoattractant IL-8 (50 ng ml−1) were tested in a Transwell migration assay, in which 24-well Transwell plates (Corning, Amsterdam, The Netherlands) were used. Isolated neutrophils were resuspended in a culture medium supplemented with lidocaine (0–1 mM) and then seeded into the upper chambers of the wells (2×106 cells per well), each separated from a lower chamber by a polycarbonate membrane (pore size 3 µm). Recombinant human IL-8 (50 ng ml−1 in culture medium) (R&D systems) was added to each lower chamber. After incubation for 1 h at 37°C in 5% CO2, neutrophils that had migrated to the lower chambers were counted using a haemocytometer.
Statistical analysis
Simfit (version 6.7.15) (University of Manchester, Manchester, UK) was used to test for normality by the Shapiro–Wilk test. Statistical significance was then determined by one-way analysis of variance (anova) (for parametric data) and the Kruskal–Wallis test (for non-parametric data). Between-group differences were determined by Bonferroni-corrected Student's t-tests (parametric data) and Mann–Whitney U-tests (non-parametric data). Confidence intervals were calculated for statistically significant differences.
Results
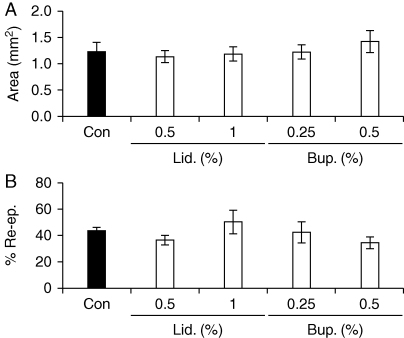
Wounds treated with lidocaine 0.5/1% or bupivacaine 0.25/0.5% healed at similar rates to vehicle-treated wounds, as measured by wound areas and extent of re-epithelialization on day 3 post-wounding (Fig. 1a and b).
Fig 1.
Local anaesthetics, at relevant concentrations, do not influence the rate of healing of acute skin wounds in mice. (a) Day 3 wound areas in intact mice treated perioperatively with lidocaine or bupivacaine (or vehicle). (b) Percentage re-epithelialization of day 3 wounds from LA-treated and control intact animals. Values are expressed as mean (sem). n=7–8 per treatment group. Statistical significance was tested by one-way anova (a) and the Kruskal–Wallis test (b). Bup., bupivacaine; Con, control (vehicle: sterile dH2O); Lid., lidocaine; Re-ep., re-epithelialization.
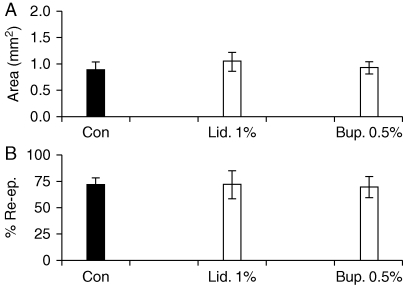
Ovariectomized mice were used to reproduce the impaired cutaneous wound healing state that occurs in elderly humans, an approach that has been previously validated.14 As in the intact animals, both tested concentrations of lidocaine and bupivacaine failed to affect the rate of cutaneous wound healing (Fig. 2a and b).
Fig 2.
Lack of effect of local anaesthetics on repair in a model of impaired healing. (a) Day 3 wound areas in ovariectomized (OVX) mice treated before operation with lidocaine or bupivacaine (or vehicle). (b) Percentage re-epithelialization of day 3 wounds from LA-treated and control OVX animals. Values are expressed as mean (sem). n=7–8 per treatment group. Statistical significance was tested by one-way anova (a) and the Kruskal–Wallis test (b). Bup., bupivacaine; Con, control (vehicle: sterile dH2O); Lid., lidocaine; Re-ep., re-epithelialization.
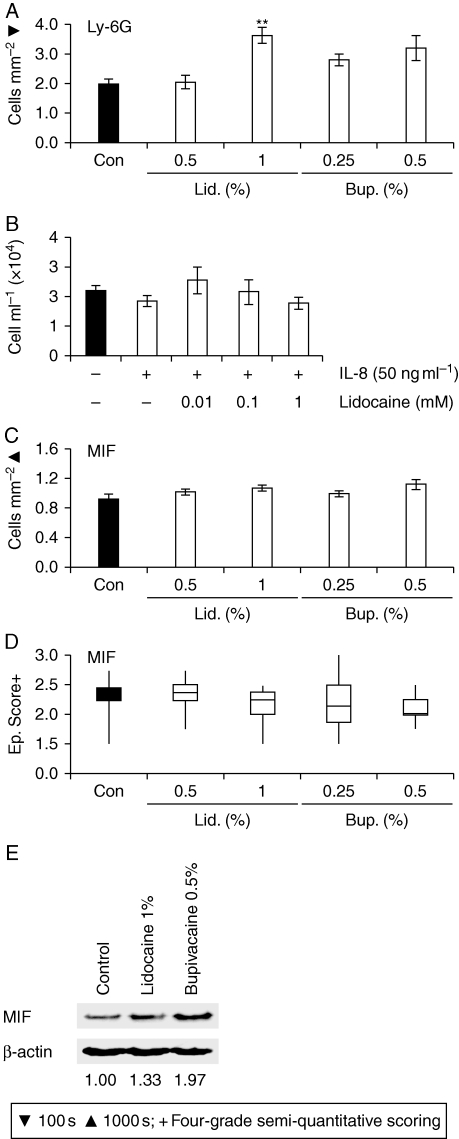
Further analysis highlighted a significant increase in day 3 wound numbers of neutrophils in mice treated with lidocaine 1% (difference between mean number of cells mm−2=166; CI 95.2–238.0) (Fig. 3a). The absence of a comparable response in lidocaine 0.5%-treated animals suggests that the drug may exert its effect in a dose-dependent fashion (although additional doses would need to be tested to confirm this). Subsequently, we revealed that lidocaine decreases the migration of human peripheral blood neutrophils to IL-8 (Fig. 3b), suggesting that increased invasion of LA-treated wounds by neutrophils does not result from direct enhancement of neutrophil migration.
Fig 3.
Local anaesthetics influence wound inflammation. (a) Day 3 wound numbers of Ly-6G-positive neutrophils in intact mice treated perioperatively with LA or vehicle (statistical significance tested by one-way anova [P<0.002], influence of treatment) followed by Bonferroni-corrected Student's t-tests: **P<0.01 (vs Con). (b) Transwell migration of IL-8-primed blood-derived neutrophils co-treated with lidocaine. (c) Day 3 wound numbers of MIF-positive cells (one-way anova). (d) Epidermal MIF staining scores (Kruskal–Wallis test). (e) MIF protein levels (immunoblotting) (values denote MIF:β-actin signal ratios). Values (a and c) are expressed as mean (sem). n=5–8 per treatment group. Arrows (a and c) identify cell immunostaining. Bup., bupivacaine; Con, control (vehicle: sterile dH2O); Ep., epidermal; Lid., lidocaine.
A key feature of delayed healing in the elderly is elevated local production of MIF,7 the master mediator of delayed healing in oestrogen-deprived female mice,8 which has been implicated in a number of other cutaneous pathologies, including eczema15 and psoriasis.16 We attempted to establish whether local MIF levels were affected by LA treatment. We immunolocalized MIF to the neoepidermis and infiltrating inflammatory cells in day 3 wounds (data not shown). However, none of the LA treatments significantly influenced epidermal or inflammatory cell wound MIF levels (Fig. 3c and d), although there was a trend towards increased overall wound MIF levels in animals treated with bupivacaine 0.5% (Fig. 3e).
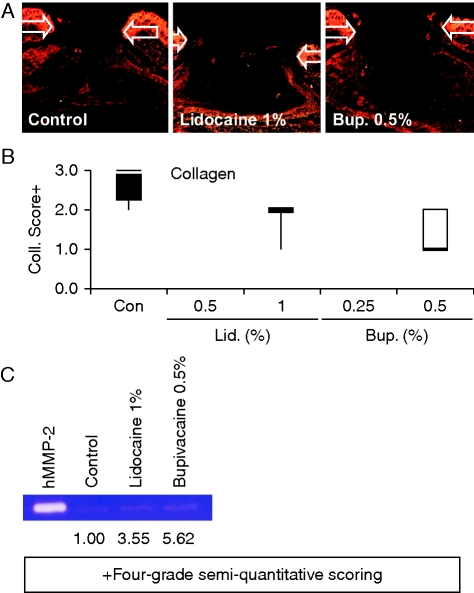
Increased proteolysis and reduced neodermal accumulation of collagen are typical features of delayed healing of acute wounds in elderly humans and aged mice9,17 and chronic venous ulceration.18 Despite our findings that LA agents do not affect wound repair in terms of wound areas and re-epithelialization, we observed a trend towards reduced overall day 3 wound levels of collagen (Fig. 4a and b), and increased activity of the collagen-degrading enzyme MMP-2 (Fig. 4c), in LA-treated animals.
Fig 4.
Effects of LAs on wound collagen levels and proteolysis. (a) Picrosirius red staining of day 3 wounds in intact mice treated with LA or vehicle. (b) Day 3 wound neodermal collagen scores (Kruskal–Wallis test). (c) Zymographic analysis of wound MMP-2 activities in day 3 wounds from control and LA-treated mice (values denote relative signal intensities). n=5–8 per treatment group. Arrows (a) define the wound margins. Bup., bupivacaine; Coll., collagen; Con, control (vehicle: sterile dH2O); hMMP-2, human MMP-2; Lid., lidocaine.
Discussion
Cutaneous wounding activates a co-ordinated set of overlapping responses which together bring about timely repair of the damage sustained.19 These responses include restoration of the epidermal barrier through the migration and proliferation of keratinocytes (re-epithelialization) and the synthesis of matrix proteins to reconstitute the dermis. A controlled inflammatory and proteolytic response, meanwhile, clears the wound of pathogens and debris.
The influence of LAs on these processes has been probed through a limited number of animal studies. Although lidocaine has been reported to enhance wound re-epithelialization in rats,3 the effects of LAs on wound collagen accumulation have been the source of some contention.4,6,20
Collectively, the presented findings suggest that cutaneous infiltration of clinically relevant concentrations of lidocaine or bupivacaine does not significantly delay wound healing in healthy adult mice. These findings corroborate some previous observations,4,5 but contradict others.1–3,6 However, the disparities can be explained, at least in part, by the supra-clinical drug doses used in previous studies,6 or by critical differences in experimental protocols. For example, Morris and Tracey1 administered lidocaine as a high-volume (2 ml) s.c. and intradermal injection and found that injection of vehicle alone impaired healing. Madhuchandra and colleagues3 applied the same drug in the form of a jelly or ointment, whereas Doğan and colleagues2 chose wound breaking strength as their primary measure of healing.
We did, however, find that the tested LAs adversely affect wound proteolytic activities and, in the case of lidocaine, neutrophil numbers. LAs have previously been identified as being modulators of inflammation. Notably, lidocaine has been shown to reduce neutrophil activation in vitro.21 Again, though, the in vivo data are conflicting. Lidocaine has been shown to delay inflammatory resolution of zymosan-induced peritonitis in mice22 but to protect against endotoxic23 and hyperoxic24 lung injury, in all instances through effects on neutrophil populations and cytokine secretion. In studies on guinea pigs, meanwhile, topical application of 1% lidocaine to sutured incisional wounds did not influence the acute inflammatory response.4 Differences between our findings and those of previous studies may be the result of differences in test species,4,23,24 injury model,23,24 and/or drug dose/delivery.4,23,24
Excessive inflammation and proteolysis are detrimental to healing.9,13,18,25 This is most apparent in the elderly, in whom the healing of acute wounds is consequently delayed. Wound re-epithelialization occurs more slowly in post-menopausal women (for whom we used ovariectomized mice as a model) than in premenopausal women.10 Delayed wound closure, combined with an apparent age-associated decrease in the phagocytic activity of wound macrophages,26 elevates the risk of local infection. Indeed, surgical site infection rates reportedly increase as a factor of patient age.27,28
Our observation of a trend towards reduced wound collagen accumulation in LA-treated mice mirrors a previous report of reduced synthesis of collagen in s.c.-implanted stainless steel mesh cages in rats treated with lidocaine.20 This may go some way towards explaining other reports of reduced wound breaking strength in lidocaine-treated rodents,1,3 although the correlation between collagen fibre density and wound breaking strength is not absolute.4
In summary, we have demonstrated that two commonly used LAs, lidocaine and bupivacaine, have no significant effect on repair in rodent models of ‘normal’ or ‘impaired’ wound healing. To our knowledge, this is the first time that the effect of LAs on poorly healing wounds with excessive inflammation and proteolytic activity has been evaluated. Our data further indicate that the tested LAs increase local inflammatory/proteolytic activity, although not to an extent that impairs healing. However, such effects could be important in other clinical scenarios in which LAs are administered continuously or repeatedly over a prolonged time period.
Conflict of interest
None declared.
Funding
This work was supported by the Wellcome Trust (grant number GR064256MA).
References
- 1.Morris T, Tracey J. Lignocaine: its effects on wound healing. Br J Surg. 1977;64:902–3. doi: 10.1002/bjs.1800641219. doi:10.1002/bjs.1800641219. [DOI] [PubMed] [Google Scholar]
- 2.Doğan N, Uçok C, Korkmaz C, Uçok O, Karasu HA. The effects of articaine hydrochloride on wound healing: an experimental study. J Oral Maxillofac Surg. 2003;61:1467–70. doi: 10.1016/j.joms.2003.05.002. doi:10.1016/j.joms.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Madhuchandra SP, Bhat MP, Ramesh KV. Wound healing profile of topical xylocaine preparations in rodents. Indian J Exp Biol. 1991;29:877–8. [PubMed] [Google Scholar]
- 4.Drucker M, Cardenas E, Arizti P, Valenzuela A, Gamboa A. Experimental studies on the effect of lidocaine on wound healing. World J Surg. 1998;22:394–7. doi: 10.1007/s002689900403. doi:10.1007/s002689900403. [DOI] [PubMed] [Google Scholar]
- 5.Bagul A, Taha R, Metcalfe MS, Brook NR, Nicholson ML. Pre-incision infiltration of local anesthetic reduces postoperative pain with no effects on bruising and wound cosmesis after thyroid surgery. Thyroid. 2005;15:1245–8. doi: 10.1089/thy.2005.15.1245. doi:10.1089/thy.2005.15.1245. [DOI] [PubMed] [Google Scholar]
- 6.Dere K, Sen H, Teksoz E, et al. The comparison of the effects of different doses of levobupivacaine infiltration on wound healing. J Invest Surg. 2009;22:112–6. doi: 10.1080/08941930802713019. doi:10.1080/08941930802713019. [DOI] [PubMed] [Google Scholar]
- 7.Hardman MJ, Waite A, Zeef L, Burow M, Nakayama T, Ashcroft GS. Macrophage migration inhibitory factor: a central regulator of wound healing. Am J Pathol. 2005;167:1561–74. doi: 10.1016/S0002-9440(10)61241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashcroft GS, Mills SJ, Lei K, et al. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–18. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junqueira LCU, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–55. doi: 10.1007/BF01002772. doi:10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 10.Ashcroft GS, Dodsworth J, van Boxtel E, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-β1 levels. Nat Med. 1997;3:1209–15. doi: 10.1038/nm1197-1209. doi:10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- 11.Ashcroft GS, Horan MA, Herrick SE, Tarnuzzer RW, Schultz GS, Ferguson MW. Age-related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res. 1997;290:581–91. doi: 10.1007/s004410050963. doi:10.1007/s004410050963. [DOI] [PubMed] [Google Scholar]
- 12.Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. doi:10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 13.Murphy G, Willenbrock F, Ward RV, Cockett MI, Eaton D, Docherty AJ. The C-terminal domain of 72 kDa gelatinase A is not required for catalysis, but is essential for membrane activation and modulates interactions with tissue inhibitors of metalloproteinases. Biochem J. 1992;283:637–41. doi: 10.1042/bj2830637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardman MJ, Ashcroft GS. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol. 2008;9:R80. doi: 10.1186/gb-2008-9-5-r80. doi:10.1186/gb-2008-9-5-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez RS, Diepgen TL, Neumann C, Sorg C. Detection of migration inhibitory factor (MIF) by a monoclonal antibody in the microvasculature of inflamed skin. Arch Dermatol Res. 1990;282:374–8. doi: 10.1007/BF00372087. [DOI] [PubMed] [Google Scholar]
- 16.Steinhoff M, Meinhardt A, Steinhoff A, Gemsa D, Bucala R, Bacher M. Evidence for a role of macrophage migration inhibitory factor in psoriatic skin disease. Br J Dermatol. 1999;141:1061–6. doi: 10.1046/j.1365-2133.1999.03206.x. doi:10.1046/j.1365-2133.1999.03206.x. [DOI] [PubMed] [Google Scholar]
- 17.Ashcroft GS, Horan MA, Ferguson MW. Aging is associated with reduced deposition of specific extracellular matrix components, an upregulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J Invest Dermatol. 1997;108:430–7. doi: 10.1111/1523-1747.ep12289705. doi:10.1111/1523-1747.ep12289705. [DOI] [PubMed] [Google Scholar]
- 18.Wysocki AB, Staiano-Cioco L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–8. doi: 10.1111/1523-1747.ep12359590. doi:10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 19.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. doi:10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 20.Chvapil M, Hameroff SR, O'Dea K, Peacock EE., Jr Local anesthetics and wound healing. J Surg Res. 1979;27:367–71. doi: 10.1016/0022-4804(79)90155-0. doi:10.1016/0022-4804(79)90155-0. [DOI] [PubMed] [Google Scholar]
- 21.Peck SL, Johnston RB, Jr, Horwitz LD. Reduced neutrophil superoxide anion release after prolonged infusions of lidocaine. J Pharmacol Exp Ther. 1985;235:418–22. [PubMed] [Google Scholar]
- 22.Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impact the resolution of inflammation. PLoS ONE. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. doi:10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikawa K, Maekawa N, Nishina K, Takao Y, Yaku H, Obara H. Effect of lidocaine pretreatment on endotoxin-induced lung injury in rabbits. Anesthesiology. 1994;81:689–99. doi: 10.1097/00000542-199409000-00023. doi:10.1097/00000542-199409000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Takao Y, Mikawa K, Nishina K, Maekawa N, Obara H. Lidocaine attenuates hyperoxic lung injury in rabbits. Acta Anaesthesiol Scand. 1996;40:318–25. doi: 10.1111/j.1399-6576.1996.tb04439.x. doi:10.1111/j.1399-6576.1996.tb04439.x. [DOI] [PubMed] [Google Scholar]
- 25.Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest. 1998;78:47–58. [PubMed] [Google Scholar]
- 26.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–35. doi: 10.1046/j.0022-202x.2001.01539.x. doi:10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 27.Bertin ML, Crowe J, Gordon SM. Determinants of surgical site infection after breast surgery. Am J Infect Control. 1998;26:61–5. doi: 10.1016/s0196-6553(98)70062-8. doi:10.1016/S0196-6553(98)70062-8. [DOI] [PubMed] [Google Scholar]
- 28.Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87:844–50. doi: 10.1302/0301-620X.87B6.15121. [DOI] [PubMed] [Google Scholar]