Abstract
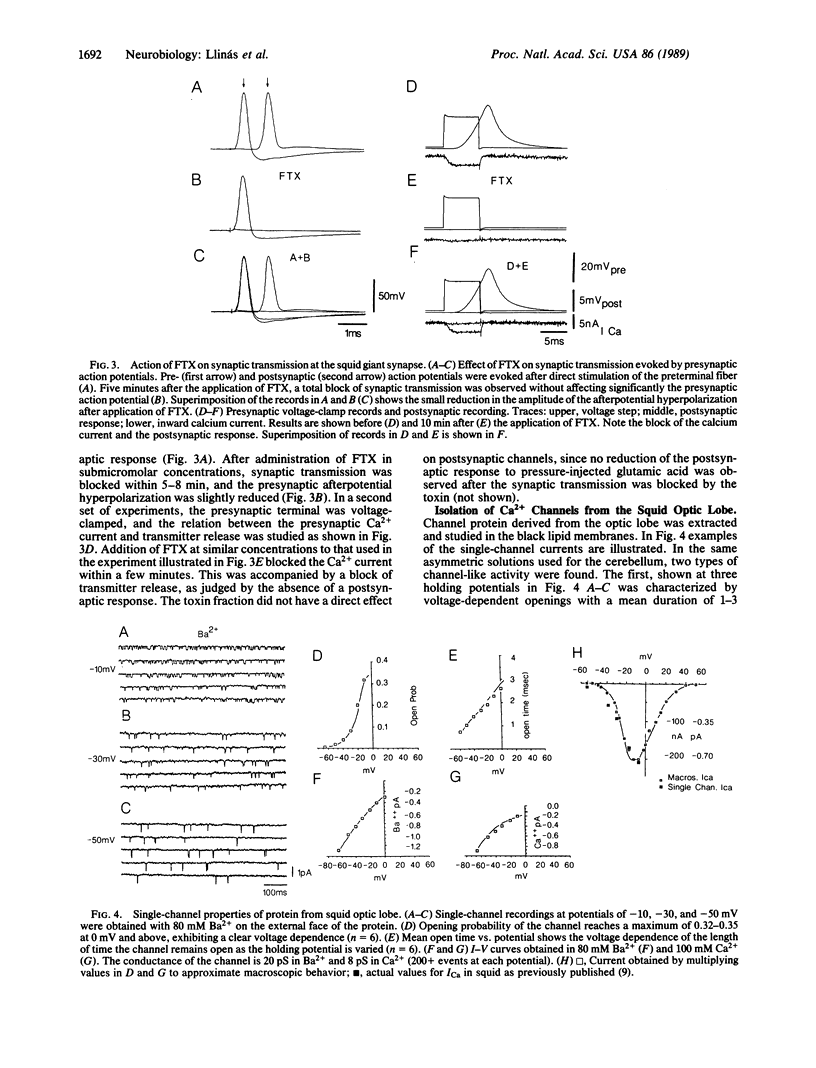
A Ca2+-channel blocker derived from funnel-web spider toxin (FTX) has made it possible to define and study the ionic channels responsible for the Ca2+ conductance in mammalian Purkinje cell neurons and the preterminal in squid giant synapse. In cerebellar slices, FTX blocked Ca2+-dependent spikes in Purkinje cells, reduced the spike afterpotential hyperpolarization, and increased the Na+-dependent plateau potential. In the squid giant synapse, FTX blocked synaptic transmission without affecting the presynaptic action potential. Presynaptic voltage-clamp results show blockage of the inward Ca2+ current and of transmitter release. FTX was used to isolate channels from cerebellum and squid optic lobe. The isolated product was incorporated into black lipid membranes and was analyzed by using patch-clamp techniques. The channel from cerebellum exhibited a 10- to 12-pS conductance in 80 mM Ba2+ and 5-8 pS in 100 mM Ca2+ with voltage-dependent open probabilities and kinetics. High Ba2+ concentrations at the cytoplasmic side of the channel increased the average open time from 1 to 3 msec to more than 1 sec. A similar channel was also isolated from squid optic lobe. However, its conductance was higher in Ba2+, and the maximum opening probability was about half of that derived from cerebellar tissue and also was sensitive to high cytoplasmic Ba2+. Both channels were blocked by FTX, Cd2+, and Co2+ but were not blocked by omega-conotoxin or dihydropyridines. These results suggest that one of the main Ca2+ conductances in mammalian neurons and in the squid preterminal represents the activation of a previously undefined class of Ca2+ channel. We propose that it be termed the "P" channel, as it was first described in Purkinje cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cherksey B. D. Functional reconstitution of an isolated sodium channel from bovine trachea. Comp Biochem Physiol A Comp Physiol. 1988;90(4):771–773. doi: 10.1016/0300-9629(88)90697-4. [DOI] [PubMed] [Google Scholar]
- Cherksey B. D., Zeuthen T. A membrane protein with a K+ and a Cl- channel. Acta Physiol Scand. 1987 Jan;129(1):137–138. doi: 10.1111/j.1748-1716.1987.tb08048.x. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Cameron R., Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983 May;96(5):1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. R., Luque A., Olivera B. M., Barrett J., Cruz L. J. Peptide toxins from Conus geographus venom. J Biol Chem. 1981 May 25;256(10):4734–4740. [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., McIntosh J. M., Cruz L. J., Luque F. A., Gray W. R. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984 Oct 23;23(22):5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Racker E., Knowles A. F., Eytan E. Resolution and reconstitution of ion-transport systems. Ann N Y Acad Sci. 1975 Dec 30;264:17–33. doi: 10.1111/j.1749-6632.1975.tb31473.x. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Sugimori M., Connor J. A., Llinás R. R. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988 Nov 4;242(4879):773–777. doi: 10.1126/science.2847315. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]




