Abstract
Accurate segregation of duplicated chromosomes ensures that daughter cells get one and only one copy of each chromosome. Errors in chromosome segregation result in aneuploidy and have severe consequences on human health. Incorrect chromosome number and chromosomal instability are hallmarks of tumor cells. Hence, segregation errors are thought to be a major cause of tumorigenesis. A study of the physical mechanical basis of chromosome segregation is essential to understand the processes that can lead to errors. Tremendous progress has been made in recent years in identifying the proteins necessary for chromosome movement and segregation, but the mechanism and structure of critical force generating components and the molecular basis of centromere stiffness remain poorly understood.
Keywords: mitosis, chromosome segregation, microtubule, kinetochore, centromere
INTRODUCTION
Accurate chromosome segregation is essential for the propagation of genetic information to daughter cells during cell division. From the earliest observations of cell division, it has been considered that an intracellular machine facilitates the equal segregation of replicated chromosomes during mitosis. The study of cell division has progressed from images of fixed, stained cells to live-cell analysis of the entire dynamic process. Advances in microscopy, molecular biology, and biophysics have enabled study of the mitotic spindle apparatus as a dynamic structure in living cells at the molecular level. As we complete the “parts list” of the mitotic spindle (i.e., DNA, RNA, and proteins) obtained through genetic and biochemical approaches, the mechanical features of this exquisite machine begin to unfold. The segregation apparatus is a composite of rigid microtubule (MT) struts that are strong in compression, elastic pericentric chromatin that is strong in tension, and the proteinaceous kinetochore that bridges these two polymers. The mitotic spindle is a remarkably weak machine that does an equally remarkable job of high-fidelity partitioning of duplicated chromosomes to daughter cells. This review details the properties of the major polymers and discusses how these protein assemblies function and interact with each other to assemble the spindle by prophase, maintain a stable structure through metaphase, and complete chromosome segregation through spindle elongation in anaphase. We focus on budding yeast as the primary organism in which to describe mitosis for the simplicity in its design. The entire yeast spindle is comprised of approximately 40 MTs (92), vs up to 100 times that in Ptk1 (mammalian) cells (see Figure 1). One nuclear microtubule attaches to each chromosome (kinetochore microtubule), four nuclear microtubules from each spindle pole extend toward the opposite pole, comprising interpolar microtubules, and two or three cytoplasmic microtubules direct spindle positioning. This extraordinary simplicity of the budding yeast spindle allows the role of chromatin as a mechanical element of the mitotic spindle to be directly observed with striking clarity. From a mechanical perspective, we have an opportunity to understand the relative contributions of each of the major components in a system where the connections between these structures are streamlined to unit values. The basic principles deduced from the function of this primitive spindle are remarkably conserved throughout phylogeny and are applicable to understanding form and function of mitotic spindles in all eukaryotes.
Figure 1.
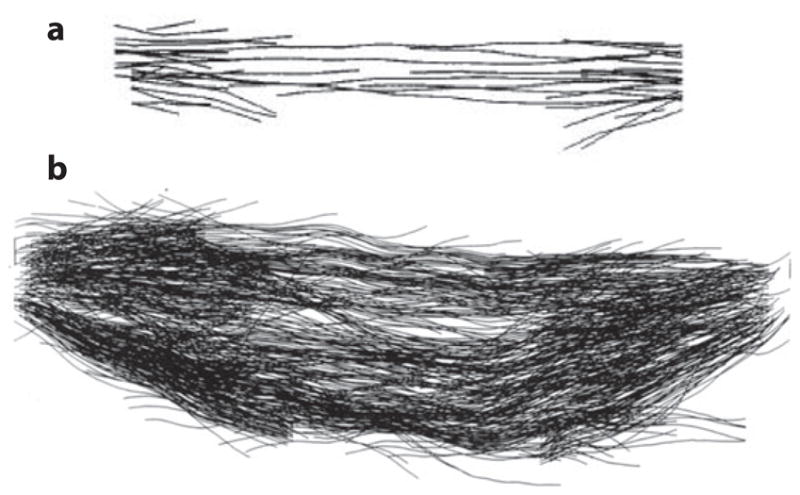
Reconstructions of two mitotic spindles. (a) Saccharomyces cerevisiae (budding yeast). (b) Potorous tridactylus (PtK2, rat kangaroo kidney). There are 40 microtubules in the yeast spindle, 32 kinetochore microtubules, and 8 interpolar microtubules versus hundreds in PtK2 (25–30/chromosome and ~ 115 ipMT from each pole).
YEAST MITOSIS: GENETICS TO CELL BIOLOGY TO BIOPHYSICS
Budding yeast divide as haploids or diploids, bearing 16 or 32 chromosomes, respectively. In the G1 phase of the cell cycle, cells are unbudded and contain one microtubule organizing center [denoted the spindle pole body (SPB)] and one copy of the genome (1 × 107 bp/haploid cell). Commitment to cell division occurs at the G1/S transition known as START (41). START initiates three separate, parallel pathways: bud formation, DNA replication, and SPB duplication (41). S phase cells are apparent by their small bud size. While DNA is replicated, the bud continues to grow, and spindle pole bodies separate from each other to form a bipolar spindle.
Transition from S phase to G2/M is characterized by the completion of DNA replication, formation of a 2 μm bipolar spindle, and attachment of sister chromatids to the mitotic spindle. Sister chromatids can become attached to the spindle prior to the completion of DNA replication due to the close proximity of centromeres to early firing origins of replication. This suggests that S phase and M phase may partially overlap in normally dividing budding yeast (39, 70).
The budding yeast spindle reaches a length of approximately 7–9 μm in late anaphase, spanning the mother-daughter axis. This distance is sufficient to segregate kinetochores and the centromeres to which they are bound; however, the segregation of chromosome arms is spatially and temporally distinct from centromeres due to the extreme length of the arms. A typical yeast chromosome (~1.0 MB) is 340 μm in its B-form configuration, approximately two orders of magnitude longer than the half-spindle. Several mechanisms are likely to contribute to the accurate segregation of chromosome arms preceding cell separation. First is chromatin compaction. The packaging of DNA into a 30-nm fiber folds B-DNA about 42 times (7X-B-DNA to nucleosomal, 6X-nucleosomal to 30-nm solenoid). We therefore consider segregating an 8 μm 30-nm fiber rather than a 340 μm 2-nm fiber. A second compaction mechanism is the tendency for DNA to adopt a random coil. Chromosomes are very soft structures with a modulus of elasticity (Young’s modulus) comparable to soft rubber (~400 Pa) (68). A prominent feature of soft materials is that their behavior is dictated by entropic forces. The entropic elasticity of chromosomal DNA acts to “reel” the arms in to the spindle pole, just as one end of a spring recoils when the other end is pulled to a fixed point (97). This entropic recoil of chromosomal DNA has recently been demonstrated as a potential mechanism for the segregation of replicated DNA in bacteria (55). A third potential force for compaction is entropic contraction that can be generated by an osmotically swollen polyelectrolyte gel such as the chromosome. Mammalian mitotic chromosomes are compacted to ~1 μm diameter by 10 μm length. Recent studies have shown that mitotic chromosomes behave as cross-linked chromatin networks with respect to their bending modulus, rather than as loops tethered to a mechanically contiguous internal scaffold (104, 105). As the chromosome swells and contracts throughout mitosis, this contractile gel provides a potential source of force generation in the spindle (77, 135).
Completion of chromosome segregation is marked by the movement of telomeres and the nucleolus to the daughter cells. Cytokinesis follows, separating the cytoplasm into two discrete compartments. Cell division is complete when cell abscission, dissolution of cell wall material joining the cells, is completed. The newly formed cells may remain senescent or, given sufficient nutrients, enter into another cycle of cell division.
THE MITOTIC SPINDLE APPARATUS: THE PARTS LIST
Spindle Pole Bodies
The budding yeast spindle contains two microtubule organizing centers known as spindle pole bodies (15, 133). As yeast carries out a closed mitosis (no nuclear envelope breakdown), the SPBs are embedded in the nuclear envelope. Electron microscopy reveals that the SPB consists of six plate-like structures or plaques that are approximately 150 nm in diameter with a total thickness of 200 nm (13, 81, 91). The layers exposed to nucleoplasm and cytoplasm, nucleate nuclear and cytoplasmic (astral) microtubules, respectively. Besides their obvious structural role, the spindle pole bodies also serve as a physical platform for regulatory mechanisms during mitosis (80).
Microtubules
Microtubules are polar, dynamic polymers (50). Heterodimers of alpha and beta tubulin are added or removed from the polymer, leading to microtubule lengthening or shortening, respectively (78, 131). Owing to the closed mitosis, the budding yeast spindle consists of two classes of microtubules: cytoplasmic microtubules nucleated by the outer plaque of the SPB, and nuclear microtubules that are nucleated by the inner plaque within the nucleus. The minus ends of nuclear as well as cytoplasmic microtubules are stably anchored to the SPB, and these ends do not exhibit any polymerization dynamics. Both classes of microtubules contribute to the fidelity of chromosome segregation in roles specific to their compartmentalization. Cytoplasmic microtubules interact with the cell cortex via the minus end-directed microtubule-based motor, dynein, to position the nucleus at the future site of cell division (bud neck) (98). During chromosome segregation, cytoplasmic microtubules contribute to the alignment of the elongating spindle along the mother-bud axis. Nuclear microtubules perform three primary functions: spindle formation, kinetochore attachment, and chromosome segregation (see below) (49).
In budding yeast, the minus ends of micro-tubules are embedded in the spindle pole body, and microtubule plus ends are oriented away from spindle pole bodies (92). Minus ends are static (no tubulin subunit turnover) in yeast, whereas plus ends are dynamic (63). Thus, flux mechanisms for microtubule transport are unlikely to contribute to yeast mitosis (63, 65). Microtubule dynamics are described in terms of the velocity of microtubule growth and shortening and the frequency of switching between these two states (rescue is the transition from shortening to growth; catastrophe is the switching from growth to shortening) (75, 130). These parameters are influenced by the local environment of the microtubule plus end, i.e., the constellation of microtubule-associated proteins, insertion into the centromere kinetochore, or proximity to the cell cortex.
There are two subgroups of nuclear microtubules: interpolar and kinetochore microtubules (134). Interpolar microtubules are nucleated from opposing poles, and these two sets of antiparallel microtubules interact with each other to provide a linkage between the two halves of the spindle. These microtubules do not interact directly with kinetochores or sister chromatids. Electron microscopy of nuclear microtubules reveals that approximately four microtubules from each spindle pole body form a bridge spanning the nucleus (134). These microtubules are antiparallel (opposite polarity), and are regularly spaced from each other, suggesting they are cross-linked by microtubule-associated proteins. The entire spindle is 250 nm in diameter, with the nuclear microtubules flared approximately 10° from the spindle axis as they extend away from the spindle pole and toward the opposite pole. By fluorescence microscopy, the group of 16 kinetochore microtubules in each half-spindle appears (using Tub1-GFP) as short tufts, 350 nm in length. Thus the bipolar spindle appears as a bi-lobed structure with kinetochore microtubule tufts emanating from each pole, leaving a gap of about 800 nm in the spindle midzone where overlapping interpolar microtubules are visible. Although the kinetochore microtubules are dynamically unstable, they rarely shrink to the pole; instead they exhibit frequent short excursions of shortening followed by growth (96).
Microtubule Motor Proteins
Budding yeast has six kinesin motors (Kip1p, Kip2p, Kip3p, Cin8p, Kar3p, and Smy1p) and one dynein motor (Dhc1p) (44). All of these, except Smy1p, function during mitosis (60). Microtubule motors are required for the essential mitotic processes of bipolar spindle formation, spindle positioning, metaphase spindle stability, and anaphase (i.e., spindle elongation). The kinesins relevant in the metaphase spindle are Kip1p, Cin8p, and Kar3p.
Cin8p and Kip1p belong to the BimC/kinesin-5 family of motors. Neither is essential, indicative of the redundancy in their functions (47). The Drosophila Kinesin-5 homolog, KLP61F, forms a homotetrameric complex that allows for the cross-linking of microtubules (21). Cin8p and Kip1 are localized to the spindle where they may act as microtubule cross-linking intermediates. The Xenopus homolog Eg5 exhibits plus end–directed motility, suggesting that Cin8 and Kip1p would likewise have plus end–directed motility (36, 113). Molecular motors can develop forces in the range of a few piconewtons (pN). Using quantitative, ratiometric measurements of Cin8p-GFP, we estimate that ~20 Cin8 homotetramers populate each half-spindle (A. Joglekar & K. Bloom, unpublished). Assuming these motors cross-link antiparallel microtubules, they can generate on the order of 120–140 pN of spindle force. These motors may also contribute to the fusiform shape of the spindle via interactions between parallel microtubules.
Kar3p is in the kinesin-14 family of motors, which have the motor domain at the C-terminus and exhibit minus end–directed motility. In contrast to the BimC motors, Kar3p forms heterodimers with the accessory proteins Cik1p or Vik1p that exhibit minus end–directed motility (67, 73). These accessory proteins provide key functionality to Kar3p’s function. Cik1p targets Kar3p to microtubule plus ends (118), whereas Vik1p has surprisingly similar structural features to Kar3p, which provides an opportunity for cooperative binding of Kar3p to the microtubule lattice (1). Vik1p lacks an active site for ATP hydrolysis and promises to yield important insights into the structure and evolution of motors and their accessory proteins. Cells lacking both Cin8p and Kip1p are not viable but deletion of KAR3 suppresses this lethality, suggesting that the minus end–directed motor Kar3p provides an inward force that opposes the outward force generated by Cin8p and Kip1p (112). In support of the prediction that Kar3p provides an inwardly directed spindle force, overexpression of Kar3p produces shorter spindles (111). However, in contrast to this prediction, spindles in kar3Δ mutants are short (94, 118, 137). Thus, the role of Kar3p in the balance of spindle forces that determine mitotic spindle length and stability was unclear.
To understand the site of Kar3p’s function in the spindle, a dicentric chromosome has been used to physically restrain spindle elongation in anaphase (32). When the two centromeres from the same sister chromatid attach to opposite poles, anaphase spindle elongation is delayed and a DNA breakage-fusion-bridge cycle ensues that is dependent on DNA repair proteins (12). Cell survival after dicentric chromosome activation requires Kar3p (and the microtubule-associated proteins, Bim1p and Ase1p) (32). In the absence of Kar3p, anaphase spindles are prone to collapse and buckle in the presence of a dicentric chromosome. Kar3p contributes to spindle stability by cross-linking spindle MTs. kar3Δ mutants show splaying of anaphase ipMTs, likely because of improper ipMT bundling as mediated by Kar3p–Cik1p complexes at the plus ends of ipMTs. Poor ipMT bundling in kar3Δ mutants prevents proper antiparallel binding of the kinesin-5 motors and therefore results in reduced outwardly directed spindle forces. This explains the longstanding enigma of how spindle lengths could be shorter in kar3Δ mutants even though Kar3p could act to resist outwardly directed spindle forces when ipMTs are properly bundled.
Microtubule-Associated Proteins
Microtubule plus ends as well as the lattice are decorated by several plus end–binding proteins that regulate microtubule polymerization dynamics, as well as the duration of growth and shortening events. With respect to the spindle and kinetochore function, these proteins are critical for spindle structure and stability as well as for the microtubule attachment site at the kinetochore. CLIP-170 is an MT-binding protein that was originally characterized as a linker between MTs and membranes in metazoan cells (129). Bik1p, the CLIP-170 ortholog in budding yeast, contributes to the maintenance of depolymerizing MTs at sites of cortical growth in mating yeast (5, 61, 79) and may provide a similar function at the kinetochore. Recent evidence for critical functionality of Bik1p at kinetochores is the demonstration that yeast cells with multiple sets of genomes (increased ploidy) require Bik1p function for survival (61, 120). This study revealed a unique subset of chromosome segregation genes essential for the survival of polyploid cells including several other MAPs (microtubule-associated protein) (e.g., Bim1p and Sli15p). Bim1p, the EB1p ortholog, decorates microtubule-growing plus ends in vegetative (127) and mating cell growth (64). Both Bik1p and Bim1p are also required for cell survival following activation of a dicentric chromosome, as described above for Kar3p (32). Thus two different assays that increase load on the spindle in different ways reveal critical roles for accessory MAPs in spindle/kinetochore functionality. Ase1p is another MAP that is critical for maintaining a stable bundle of overlapping antiparallel microtubules. The molecular mechanisms of the function of the fission yeast homolog of Ase1p have been characterized through computer modeling and high-resolution microscopy (51). The Dam1/DASH complex (an oligomer with 10 subunits) has also been shown to be critical for spindle integrity, although it is also a critical linker protein that facilitates kinetochore-microtubule attachment (see below).
DNA
In vertebrates, chromatin plays a direct role in initiating spindle assembly through the RAN pathway that promotes nucleation of microtubules in the vicinity of the chromosomes. Along with the pre-existing microtubule arrays emanating from the duplicated centrosomes in mitotic cells these newly nucleated microtubules are integrated to form and stabilize the bipolar array seen in a mitotic spindle. In most studies of the mitotic spindle, the chromosomes are thought of as passive cargo for the spindle apparatus without much direct influence on its mechanical properties. However, from a design perspective, DNA is an important mechanical element of the architecture of the spindle. The chromatin architecture in budding yeast is just beginning to be dissected, largely due to the efforts of Straight and colleagues to apply GFP-tagging techniques for chromosome visualization (121). Although yeast chromosomes may not be compacted to the extent observed in metazoa (40), changes in the nucleosomal compaction have a dramatic effect on spindle length control in budding yeast (10). Furthermore, chromatin is an elastic element in the spindle, as evidenced by the antagonism observed between chromatin-based inward force and Kinesin-5-based outward force (Cin8p, Kip1) (10). Thus, not only microtubules (38) but also DNA contribute to metaphase spindle length control, and the basic mechanical properties of chromatin become relevant in describing the contributions of chromatin to spindle stability in budding yeast.
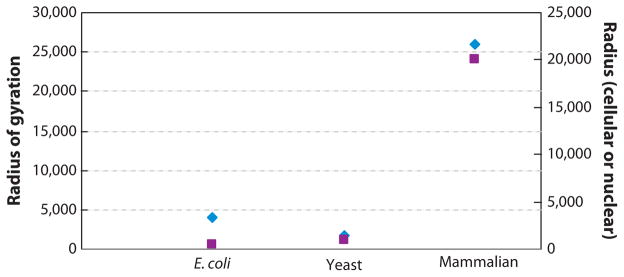
The mechanical properties of DNA can be understood from the perspective of a long-chained polymer. DNA can be thought of as a stiff, but jointed spring (23). It is jointed in the sense that the chain of nucleotides is long and flexible over the length of the chromosome. However, rather than being jointed between each base along the phosphate backbone, the joints are roughly every 50 nm (~150 bp) (see Figure 2). A 150-bp segment is on average linear and defines the persistence length of DNA. DNA is also very long, such that an average chromosome in yeast contains 3333 (500,000 bp/150 bp) of these “straight” segments, known as the contour length. Because the segments are freely jointed, each segment is free to swivel in three dimensions. A polymer chain of this composition behaves as an entropic spring. The polymer will adopt a conformation where each segment has the largest range of motion, or highest entropy. One can calculate the dimension of the theoretical sphere such a chain will occupy if there are no forces beyond thermal motion. The radius of this sphere is the radius of gyration (Rg) and is equal to R(end-end distance)/√6 Rg (Re2 = nb2, = 3333 × 1002 = 5773 nm or ~6 μm). For a typical yeast chromosome Rg = 6/2.45 = 2.45 μm. This is over twice the radius of the nucleus in yeast (see Figure 3). For a typical human chromosome Re = 800,000 × 1002 = 90,000 nm = 90 um = 90/2.45 = 36 μm. The chain can be stretched with very little force, in the range of thermal forces.
Figure 2.
Radius of gyration (nm) for random polymers with persistence length of DNA (50 nm) for E. coli, yeast, and mammalian chromosome (blue diamonds). Radius of the cell or nucleus (nm) for E. coli (cellular), yeast, and mammalian cell (nuclear) ( purple squares). Radius of gyration = Ree/√6; end-end radius Ree2 = nb2; n = number of segments, b = 2 × persistence length.
Figure 3.
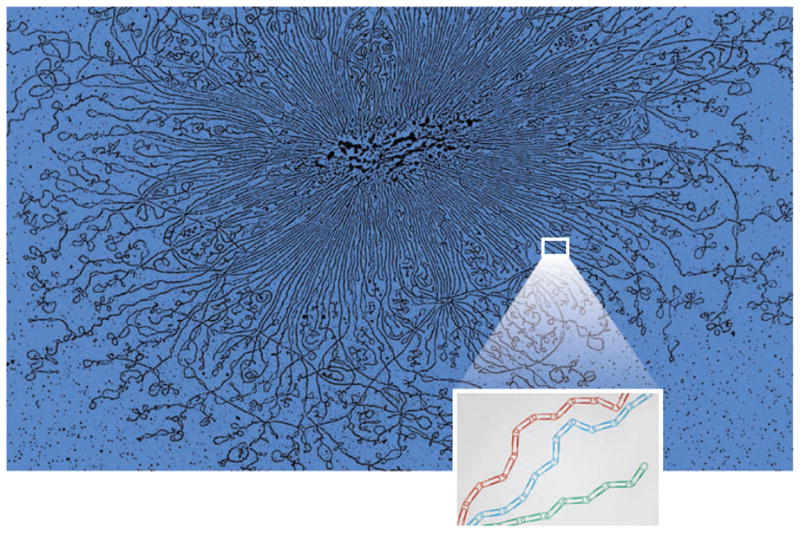
Entropic DNA spring (reprinted from Reference 64a). DNA prepared from Escherichia coli reveals supercoiled loops emanating from a central core (55a). Eukaryotic chromosomes are organized as loops of loops emanating from a nonhistone protein scaffold (29a). The inset is a biophysical representation of the physical nature of DNA. DNA has a persistence length of 50 nm (= 150 bp), depicted by stiff paper clips (in color). The contour length of DNA in a typical eukaryotic chromosome is on the order of hundreds to thousands of kilobase pairs. DNA in the chromosome is a freely jointed chain of many “straight” paper clips linked together.
One can estimate the spring constant of this polymer using the general theorem (Langevin function) that relates thermal fluctuations at equilibrium to the rate of approach to equilibrium. When a polymer such as DNA is stretched to its B-form length it exerts a force proportional to 3 KBT/nb2. Typical entropic spring constants are fractions of pN/nm. We can use this equation to calculate the spring constant for 10 kb of naked DNA as 3 * (4 pn nm)/33 *1002 = 0.036 fn/nm. Upon stretching (to B-form) and release, DNA will recoil with this spring constant. Future experiments must consider and evaluate the mechanical properties of chromatin (i.e., the track) to fully understand how polymerases (i.e., the engines) perform their biochemical function. In the case of the mitotic spindle, we must also consider how the tendency to adopt a random coil (Figure 3) contributes to the balance of forces between DNA, microtubules, and motor proteins achieved in mitosis.
Structure and Function of the Inner Centromere DNA
The budding yeast centromere consists of 3 centromere DNA elements (CDEI, CDEII, and CDEIII), spanning 125 bp, which are conserved across all 16 chromosomes (31). Because of its remarkably small size, the centromere is commonly referred to as a point centromere, whereas the centromeres of other eukaryotes are considered regional centromeres, spanning anywhere from a few kilobases to several megabases (20).
Although the sequence or composition of centromeres in many organisms has been identified, the physical structure and organization of this region of chromatin DNA in vivo is not well understood. Centromeres in all eukaryotes have at least one nucleosome containing a histone H3 variant (Cse4p in budding yeast; CenpA in mammals). This specialized nucleosome appears to be critical in defining the centromere functionally, possibly through facilitating the deposition of kinetochore proteins at the centromere. It is also the case that this specialized nucleosome adopts a unique conformation, perhaps even split into two hemisomes (26), with its own chaperone and assembly and disassembly pathways [Cac1, Hir1 (114), and Scm3 (16, 76, 119)].
The replacement of H3 with Cse4p results in a significant difference in the trajectory of histone tails, which have the greatest interactions with the DNA (8). Cse4p tails are predicted to guide centromeric DNA in a specific path as it enters and exits this nucleosome. This path aligns centromere-flanking sequences in close proximity to each other near the centromere. Assuming nucleosomal compaction of pericentric chromatin, the width of the chromatin fiber entering/exiting the kinetochore would be approximately 22 nm, roughly that of the 25-nm diameter of a microtubule.
The proposal that pericentric chromatin is paired via intramolecular linkage provides a mechanical basis for the inner centromere (8, 136). The inner centromere is a 14–20-kb region that is paired through intramolecular interaction. The apex of this loop is a conserved Cse4 nucleosome that is the base of a microtubule attachment. From a mechanical perspective, a twofold increase in the radius of a filament increases its stiffness 16-fold (r4). Thus a pericentric chromatin loop confers strength to the region of the chromosome subject to mitotic force. In budding yeast, this apex is demarcated by the 125-bp core sequence. In higher eukaryotes chromatin loops have been reported by Zinkowski & Brinkley (138).
Pericentric chromatin is composed of a highly ordered array of nucleosomes, as assayed by micrococcal nuclease mapping of nucleosome position (7). When tension is applied to the sister chromatid pair, pericentric chromatin structure may be altered through the dissociation of nucleosomes, or partial unraveling of DNA around nucleosomes (97). The pericentric chromatin loop is a source of flexibility in the spindle. Kinetochore oscillation has been observed in mammalian cells (115) and, more recently, in yeast over a dynamic range of 50–800 nm (97). A change in the fraction of inter- vs intrachromatid cohesin of pericentric chromatin will displace the centromere, and thus the kinetochore, toward or away from the spindle midpoint. Alternatively, the fraction of pericentric chromatin paired via intrachromatid cohesin does not vary, but the chromatin is elastic because of nucleosome release or assembly. Release of a single nucleosome results in a 65-nm extension (from nucleosomal to B-form DNA). Loss of 20 nucleosomes (from each side of the centromere) increases sister centromere separation by 650 nm. On the basis of centromere DNA dynamics in live cells (97), we estimate that the transition between intra- and interchromatid cohesion is on average 7 kb from the centromere. This translates to ~90 nucleosomes in the C-loop (2 × 7000 bp/160 bp of nucleosomal + linker DNA). Loss of ~20% of the nucleosomes in the area of intrachromatid cohesion is enough to provide the full dynamic range of separation observed in living cells. This model makes two important predictions. One is that chromatin remodeling complexes found at the centromere (114) function in nucleosome reassembly upon loss (or decrease) of tension. Second, the amount of DNA in the C-loop is restricted. If the transition between intra- vs intermolecular pairing is fluid, increased force at the kinetochore will promote flow of DNA into the C-loop. In this situation, there will be little or no opportunity for change in tension between sister chromatids as a function of chromatid separation. Alternatively, the transition is not fluid, and the amount chromatin in the C-loop is invariant. In this case, chromatin compaction and/or nucleosome density may change as a function of change in tension. This model identifies an important site for tension-sensing in the inner centromere, and predicts that proteins at the pericentric chromatin/chromosome arm junction are important for segregation function.
The localization of passenger proteins to the inner centromere in mammalian cells and their role in correcting improper microtubule attachments to the kinetochore further suggest that the inner centromere is the site of tension sensing required to satisfy the spindle checkpoint (22). A change in pericentric chromatin structure, due to the mechanical strain placed on it, may lead to inactivation of regulatory kinases such as Ipl1p/Aurora B. Alternatively, a lack of strain (tension) in the chromatin would activate Ipl1p/Aurora B to destabilize the microtubule attachment through phosphorylation of outer kinetochore proteins.
Kinetochores
The plus ends of kinetochore microtubules are associated with a large, multiprotein, DNA-bound complex known as the kinetochore [see (17, 18) for a recent review]. Over 70 proteins have been characterized as kinetochore proteins based on their localization, interaction with the centromere (whether direct or indirect) as detected by chromatin immunoprecipitation, and copurification with other known kinetochore proteins (42, 69). Additionally, deletion of nonessential kinetochore genes and conditional alleles of essential kinetochore genes exhibit increased rates of chromosome loss (57). Biochemical analysis of kinetochore proteins purified from yeast extracts reveals the presence of five major subcomplexes within the kinetochore. Kinetochore proteins have been assigned to “inner,” “mid,” or “outer” complexes. Inner kinetochore proteins are most closely associated with centromeric DNA, outer kinetochore proteins most closely associated with microtubule plus ends, and the mid-kinetochore class constitutes the balance of kinetochore proteins that likely link the inner and outer kinetochore protein complexes.
The inner kinetochore proteins include the CBF3 complex, a centromere-specific nucleosome containing the H3 variant Cse4p (see above), and another DNA-binding protein Mif2p (homolog of vertebrate CENP-C) (59, 72, 109). These complexes associate directly with the budding yeast point centromere. The single Cse4p-containing nucleosome at the budding yeast centromere raises the possibility that only one such Cse4p nucleosome is required per microtubule attachment (since only one microtubule attaches at each centromere) (54). Determination of the number of CENP-A nucleosomes, and their positioning, in other eukaryotes may clarify the relationship between CENP-A number and the number of microtubule attachments (54). In fission yeast, despite a large central centromere core (4–7 kb) where the CENP-A homolog (Cnp1p) can bind, there are only three nucleosomes of Cnp1p per chromosome, equal to the number of kinetochore microtubules (53). This remarkable conservation of the kinetochore protein architecture between point and regional centromeres strongly suggests that the each microtubule attachment is likely supported by a single CENP-A nucleosome, perhaps even in metazoan centromeres.
The CBF3 complex is composed of three core proteins, Cep3p, Ndc10p, Ctf13p, and two regulatory subunits, Skp1p and Sgt1p. Binding of this complex to the yeast centromere introduces a 60° bend in the pericentric DNA (100). This bend may promote the formation of an intramolecular loop (136), which increases the rigidity of pericentric chromatin (see above). The structure of one of the CBF3 proteins, Cep3p, has recently been determined (4, 106). CEP3 weakly binds to a small region of the centromere containing the highly conserved CDEIII DNA sequence element. A model to reconcile Cep3p binding and the presence of the Cse4 nucleosome at this locus can be deduced from recent studies indicating the instability of the Cse4 nucleosome (26). The Cse4 nucleosome is unstable and readily splits into two hemi-nucleosomes. Micrococcal nuclease digestion indicates that the CDEIII DNA element is midway around the nucleosomal DNA (9). Upon splitting of the nucleosome, CDEIII is predicted to be exposed, thus available for Cep3p binding (see Figure 4). This configuration predicts that the linkage for stable attachment of the kinetochore to the DNA is provided by a clasp-like structure that encircles a small region of the centromere. Other members of the CBF3 complex likely make additional contacts with centromere DNA as well as with histones, thereby strengthening this connection.
Figure 4.
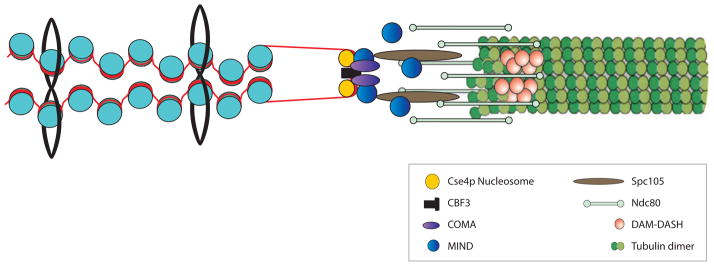
A schematic representation of the interface between kinetochore microtubule, kinetochore and pericentric chromatin. The microtubule ( green, right) is a 25-nm tubule comprised of 13 protofilaments. Pericentric chromatin (blue nucleosomes and red DNA) is organized into an intramolecular loop in mitosis. The dimensions of a single nucleosome are 5 × 11.5 nm. The dimension of an intramolecular loop would be approximately 23 nm. The two major polymers (nucleosomal DNA and microtubules) are similar in cross-sectional dimension. The kinetochore is a proteinaceous structure linking these two polymers in mitosis.
The three DNA-binding components of the kinetochore recruit the “mid-kinetochore” or linker protein complexes, Ctf19p and Mtw1p. In higher eukaryotes, these complexes along with the inner kinetochore proteins exhibit a constitutive localization to the kinetochore, whereas the outer kinetochore proteins are recruited to the kinetochore only during mitosis. In addition to serving as linkers between the inner and outer kinetochore components, these proteins also play a role in the spindle assembly checkpoint signaling. Another protein complex, Spc105p, also serves as a linker protein, although it is not a constitutive component of the kinetochores in fission yeast and in higher eukaryotes. Furthermore, the homolog of this protein, KNL-1 possesses a weak microtubule-binding activity (17a).
The outer kinetochore protein complexes NDC80 and Dam1/DASH form the core microtubule attachment site at the kinetochore. The NDC80 complex is a bona fide kinetochore protein complex, with localization that is exclusive to the kinetochores. It is subject to cell cycle–dependent phosphoregulation, and it likely plays an important role in regulating the behavior of the kinetochore (27, 29). The Dam1/DASH complex, on the other hand, is a microtubule-associated protein (MAP) complex. It localizes stably to the kinetochores in metaphase, but migrates along the spindle microtubules in anaphase. Recent studies using electron microscopy have revealed that the Dam1/DASH complex can form rings that encircle the microtubule lattice in vitro (74, 132). This striking observation opens up the possibility that the kinetochore uses a novel coupling mechanism to harness the lattice energy that is released as a microtubule plus end depolymerizes. Further in vitro studies of the microtubule plus end-coupled motility supported by recombinant Dam1/DASH complex also reveal that this motility can be supported by Dam1/DASH complex rings as well as by other configurations of the Dam1/DASH complex (such as patches or nonencircling helical oligomers) (35). However, theoretical studies indicate that the various binding modes will exhibit distinctly different motility and force generation characteristics (30). Along with microtubule depolymerization-coupled force generation, the Dam1/DASH complex may also provide the critical function of increasing the rescue frequency for the microtubule plus end in a tension-dependent manner.
Chromatin
Although DNA is a flexible, yet stiff spring, its compaction into the chromosome results in a hydrated, protein-DNA complex that is more like a soft, elastic gel (68, 103). In addition, chromosomes have a dynamic structure that is constantly changing. Our challenge is to deduce how the structure of the chromosome influences processes such as tension sensing, anaphase force generation at centromeres, and chromosome arm recoil. A typical mammalian chromosome is compacted into a cylinder that is 1 μm in diameter by approx. 10 μm in length. In cells, chromosomes exhibit mechanical properties consistent with the elastic core being organized throughout the entire cross-sectional area of the chromosome rather than a thin proteinaceous scaffold at the base of floppy DNA loops (2, 56, 102, 105). Among the major proteins that contribute to the mechanical properties of the chromosomes are SMCs (structural maintenance of chromosomes) (58). SMC proteins exist in the cell as large multisubunit complexes. The condensin complex is essential for maintaining chromosome axis elasticity and chromosome shape (2). This complex is comprised of five major proteins, Smc2p-Smc4p-Ycs4p-Brn1p-Ycg1p (45, 46). The SMC complex that holds sister chromatids together prior to anaphase onset is cohesin (84, 93). Cohesin consists of two members of the SMC family of ATPases, Smc1p and Smc3p, and two kleisin subunits, Mcd1p/Scc1p and Scc3p. At anaphase onset, Esp1, a protease inhibited by Pds1p binding, is liberated and cleaves Scc1p. This cleavage dissolves the physical linkage between sister chromatids and allows them to separate from each other. The cohesin-dependent linkage of sister chromatids is critical to their biorientation, and the generation of force at the kinetochore required to satisfy the spindle checkpoint (65). During mitosis, cohesin removal must take place if chromatid segregation is to occur. In vertebrate systems, cohesin removal from sister chromatids is regulated by two pathways (62, 123). One pathway consists of the bulk of cohesin dissociating from chromosome arms during prophase and is dependent on the polo-like kinase Plk1 (124). This process, termed chromatid individualization, causes sister sequences on chromatid arms to appear separated by up to 0.5 microns (83). The second pathway of cohesin removal from chromosomes depends on the proteolytic cleavage of the cohesin subunit Scc1 (Rad21, Mcd1) (128). Cleavage of Scc1 is mediated by the cysteine protease separase at the onset of anaphase and is thought to remove the remaining cohesin from sister chromatids prior to segregation. The study of chromatid individualization in vertebrate systems has been aided by the ability to directly visualize a single chromosome. Work in live grasshopper cells has shown that sister chromatid arms can appear as closely associated yet visibly distinct rods in metaphase. Using microneedles to physically pull sister chromatid arms apart, chromosome arms return to their original positions adjacent to each other after their release from the microneedles (95). Additionally, cohesion is released gradually along the length of a chromosome arm with centromeres separating first in anaphase. These experiments demonstrate that, along with chemical activity of separase, mechanical forces play an important role in separating the paired sister chromatids. Thus sister chromatid arms are still mechanically linked throughout metaphase even though they appear morphologically distinct under the light microscope. This mechanical linkage persists until anaphase in these cells.
The mechanical link between individualized chromosome arms is thought to be composed of residual cohesin complexes that are invulnerable to the prophase pathway, as well as residual catenation between sister chromatid strands. How these cohesin complexes differ from those removed during prophase is not yet clear. A possible component of this mechanism is Sgo1 (48, 71). Originally shown to be at the centromeres of meiotic chromosomes in fission yeast, mammalian Sgo1 has recently been shown to be present along chromosome arms from mitotic prophase on into metaphase (37, 82). Additionally, chromosome spreads from mammalian cells depleted of Sgo1 using RNAi and subsequently arrested in metaphase display an increase in completely separated sister chromatids (126). Sgo1 may play an essential role in cohesion maintenance along the length of sister chromatids throughout prometaphase and metaphase.
Although individualization has been studied in higher eukaryotes, such a phenomenon has not been observed in budding yeast. Unlike vertebrate systems, direct visualization of single chromosomes in yeast is not possible using light microscopy. Yeast chromosome visualization has been limited to the integration of lac operator (E. coli lacO) arrays that are bound by GFP-tagged lac repressors (lacI-GFP) (121, 122). In live cells these arrays appear as spots under the fluorescent microscope. However, using quantitative high-resolution digital microscopy, we have recently shown that chromatid individualization, prior to anaphase, is a feature of yeast mitosis as well (B. Harrison & K. Bloom, unpublished).
BUILDING A MITOTIC SPINDLE AND SPINDLE FUNCTION
Building Spindles
Spindle formation requires the duplication of the spindle pole body inherited from the previous cell cycle. By electron microscopy, the “old” spindle pole body forms a half-bridge in early G1 that elongates and accumulates additional material. This additional material forms a satellite plaque resembling a spindle pole body, except that it is located in the cell’s cytoplasm. Eventually, this plaque is inserted into the nuclear envelope, where it matures and eventually nucleates microtubules into the nucleus (3).
Duplication of the spindle pole body appears to be a conservative process. In nearly all cells, the “old” pole is inherited by the bud during mitosis (99). It is unclear whether this pattern of inheritance is the result of an epigenetic mark on the spindle pole body, or if it is itself an epigenetic marker for other processes within the cell.
Immediately following duplication and insertion into the nuclear envelope, the two spindle pole bodies are nearly adjacent. This positioning means that their nuclear microtubules will be nearly parallel to each other, rather than the antiparallel orientation of the metaphase and anaphase spindles. The duplicated SPBs move away from each other in a microtubule-dependent fashion with help from motor proteins and MAPs (52). Treatment of spindles with the microtubule poison, nocodazole, results in the collapse of spindle pole bodies. The poles do not randomly diffuse around the nuclear envelope in the absence of microtubules, suggesting that organized movement is required for proper spindle pole body orientation. Spindle pole bodies are able to orient themselves into a bipolar spindle in the absence of DNA replication, absence of sister chromatid cohesion, and in kinetochore mutants, suggesting that proper kinetochore attachments are not required to facilitate spindle formation and the orientation of poles to opposite ends of the nucleus.
Establishing Correct Attachments
Shortly after spindle pole body duplication, nuclear microtubules engage in two processes: establishment of a bipolar spindle and capture of sister chromatids. Electron microscopy of this process suggests that microtubules are cross-linked during this time, while spindle pole bodies are oriented to opposing sides of the nucleus. It remains unclear whether kinetochore attachments are established at this time or if a bipolar spindle must first be formed.
During a search-and-capture process, microtubules plus ends interact with centromeres through association of kinetochore proteins. Although the kinetochores in metazoa possess the ability to induce microtubule nucleation (directly at the kinetochores or in the vicinity) (66), this ability is absent in budding yeast and fission yeast (125). The assembly order of the kinetochore complex is not clearly established. Models can be divided into centromere-centric and microtubule-centric classes. The centromere-centric model is based on the result that the vast majority of kinetochore proteins that are found to associate with the centromere by chromatin immunoprecipitation in nocodazole-treated cells. This suggests that the kinetochore can assemble at the centromere and persist until chance encounters with a microtubule.
While several kinetochore proteins can associate with the CEN in a microtubule-independent manner, it is not clear if this is the normal course of events in cells. In fact, a number of kinetochore proteins have been found to associate with microtubules in a centromere-independent manner, giving rise to a microtubule-centric model for kinetochore assembly. Among these proteins are members of the inner kinetochore complex CBF3, suggesting that the formation of a partial or possibly complete kinetochore may be possible in the absence of centromere binding (11). The possibility remains that the kinetochore may form in a centromere-independent manner. In this case, the centromere would then be found by kinetochore proteins already associated with dynamic microtubules.
A third model explaining kinetochore formation embraces both models. It proposes that part of the kinetochore is assembled at the centromere and part at the microtubule. A kinetochore is formed when these two halves find each other. This model is supported by data describing how improper attachments are corrected. Phosphorylation of Dam1p by the Ipl1p (Aurora B) kinase leads to weakened interaction between the DAM/DASH complex and the Ndc80/Nuf2 complex. Accordingly, kinetochore assembly would require the centromere-associated kinetochore proteins to be found by the microtubule-associated kinetochore proteins.
Recognizing and Correcting Incorrect Attachments
Kinetochore attachments are only productive if they result in the equal segregation of chromosomes in anaphase. Therefore, the cell must recognize attachments that are not amphitelic. In budding yeast, where only one microtubule binds at each kinetochore, the most frequent attachment error is syntelic attachment. In other eukaryotes, merotelic attachments may also occur and must be recognized and corrected by the cell.
Proper attachments bear two hallmarks, both necessary to satisfy the spindle checkpoint before a cell enters anaphase. First, a microtubule must be associated with the kinetochore. Second, that microtubule attachment must generate tension. Sister chromatids having mono-attachments or syntelic attachments will not have tension at their kinetochores. In both cases, the cell cycle is delayed until the error is corrected (6, 48).
Correction of mono-attachment is carried out by simply delaying anaphase, giving the cell more time to establish attachments at the unattached kinetochore. Syntelic attachments are corrected by Ipl1p-dependent destabilization of the attachment. Recently, it was shown that Ipl1-dependent destabilization of kinetochore attachments results in cell cycle delay by the creation of mono-oriented sisters, rather than a direct tension-dependent signal to the spindle checkpoint (101).
The question of how tension is sensed at a kinetochore attachment remains unanswered. It has been proposed that Ipl1p is a part of a tension-sensing complex with Sli15p (INCENP) and Bir1p (Survivin) (110). If this complex directly senses tension, then it also positions Ipl1p to immediately act when tension is not sensed. The mechanical strain applied to this complex might result in the regulation of Ipl1 activity. This regulation could occur through control of kinase activity, or spatial regulation of the complex. Ipl1p kinase activity is modulated through Sli15p association, suggesting a possible means of regulation. Additionally, Ipl1p has been reported to dissociate from the kinetochore upon biorientation (14).
The Role of Microtubule Dynamics Regulation in Spindle Function
During most of the cell cycle, nuclear microtubules are dynamic. Microtubule dynamics are important for the formation of attachments to sister chromatid centromeres, and the production of tension at kinetochores. Unlike many other eukaryotes, in budding yeast only microtubule plus ends are dynamic. Turnover at the minus end, which appears embedded in the spindle pole body, has never been observed. Stability of microtubule minus ends simplifies the study of microtubule dynamics in this organism.
The metaphase spindle maintains a stable spindle length in budding yeast (like most other eukaryotes). It had long been thought that spindle stability was a result of the stable crosslinking of interpolar microtubules. This model suggests that interpolar microtubules would also be stable, whereas kinetochore microtubules are dynamic. More recently, the dynamics of all nuclear microtubules has been observed in pre-anaphase cells. These findings raise the question of how interpolar microtubules contribute to spindle stability when they are themselves dynamic is not clear. At anaphase onset, Cdc14p phosphatase activity is required for nuclear microtubule stabilization (43). Cdc14p is released as part of the FEAR and MEN pathways, and contributes to a number of events at the completion of mitosis (25).
Anaphase A (the shortening of kinetochore microtubules) happens concurrently or shortly after anaphase B (spindle elongation) in budding yeast (97). This infers that kinetochore microtubules are only able depolymerize after anaphase onset, but polymerization is inhibited. The stability of interpolar microtubules during anaphase suggests that both depolymerization and polymerization are inhibited. This difference is likely due to other proteins associated with the plus ends of these two classes of nuclear microtubules. In the case of kinetochore microtubules, kinetochore (or kinetochore-associated) proteins may regulate microtubule dynamics in metaphase and anaphase. At interpolar microtubules, midzone proteins including Ase1p, Slk19p, and passenger proteins might actually stabilize plus ends and inhibit depolymerization. In this case, the primary function of Cdc14p would be the inhibition of microtubule polymerization.
The budding yeast spindle is a prime candidate for computer modeling because of its relative simplicity compared to other eukaryotes (see Figure 1). The low number of microtubules (about 20) in each half-spindle makes it possible to model the contribution of each microtubule polymer (34, 117). Since only one microtubule attaches at each kinetochore, the complication of dealing with microtubule bundles is alleviated. Furthermore, the overall similarity of the yeast spindle to other eukaryotes suggests that a functional model of spindle mechanics in yeast could be translated to understand the mechanics of more complicated spindles.
To date, the most comprehensive modeling of spindle dynamics has focused on the regulation of kinetochore microtubules. Kinetochore microtubules contribute to the alignment of sister kinetochores on the metaphase plate, generate the tension needed to satisfy the spindle checkpoint, and are ultimately required for the segregation of the genome. Thus the regulation of kinetochore microtubules is fundamental to understanding the mitotic spindle apparatus as a cellular machine.
Regulation of kinetochore microtubules has been described in terms of a microtubule catastrophe gradient centered at the spindle midpoint (34). The catastrophe gradient results in the depolymerization of kinetochore microtubules that attempt to grow across the midpoint of the spindle. This activity organizes the kinetochore microtubules of each half-spindle. Additionally, the catastrophe gradient leads to the separation of sister chromatids during metaphase as the kinetochore microtubules attached to a sister chromatid pair depolymerize away from the gradient’s center at the spindle midpoint.
The catastrophe gradient does not result in complete depolymerization of kinetochore microtubules. The effects of the gradient are opposed by tension-dependent microtubule rescue. In other words, as kinetochore microtubules depolymerize the force at kinetochores increases (assuming sisters are bioriented). This tension results in microtubule rescue (i.e., a switch to microtubule growth). Regulation of kinetochore microtubule length is therefore a result of the balance of these two factors, which likewise defines the positioning of kinetochores during metaphase.
The modeling of kinetochore microtubules in terms of a catastrophe gradient and tension-dependent rescue is an elegant description of what may transpire in the cell. The combination of computer simulations based on this model with actual data from wild-type and mutant yeast cells offer validity to this approach. Complete confidence in the model will require the identification of the catastrophe gradient, and an understanding of how kinetochore and interpolar microtubules are differentially regulated (e.g., why do interpolar microtubules grow through the catastrophe gradient?).
The next question to be addressed through computer simulation is the regulation of spindle length. The answer will require increased understanding of the contributions of individual motor proteins in sliding interpolar microtubules apart to generate outward spindle force. Likewise, the opposing inward force requires further characterization. This force appears to be a composed of contributions from minus end–directed motors as well as the stretching apart of sister chromatids. As further in vitro studies of motor function and chromatin’s biophysical properties unveil new details about these molecules, these parameters can be fed into models until computer-simulated data begin to match experimental observations. These models can then be further tested through the quantitative analysis of spindle changes in mutant cells.
Contributions of Chromatin to Spindle Stability and Chromosome Segregation
Microtubules, microtubule-based motors, and MAPs together form the active, force-generating components of the spindle machine. Therefore, extensive experimentation in a variety of systems that focuses on individual components as well as interplay among various components has yielded a wealth of quantitative data. Furthermore, the well-described biophysical properties of these prime movers have allowed scientists to build a theoretical framework that attempts to describe the principles of spindle assembly and maintenance in terms of the known properties of these active components (19, 24, 33, 85, 96). Chromatin, especially in the centromeric regions, is an important mechanical element in mitotic spindles. The biochemical role of chromatin in establishing a bipolar structure has been extensively studied. Studies in meiotic Xenopus egg extracts show that chromatin is the primary activator of the RAN pathway for nucleating new microtubules in the vicinity of the chromosomes. In acentric systems, this is a crucial step in the establishment of a bipolar spindle. Chromatin also acts as a passive element in resisting the forces generated by the opposing action at the sister kinetochores. The influence of this role of chromatin as well as its mechanical properties in spindle length regulation is evident in metazoan mitotic spindles. If chromatin as a passive resistive element is completely removed from the mitotic spindle apparatus (by deactivating kinetochores) in HeLa cells, the mitotic spindle achieves lengths that are 60% longer than control spindles (28). Furthermore, studies in Drosophila S2 cells reveal that RNAi of Rad21, a cohesin complex subunit (Scc1), results in precocious separation of sister kinetochores, along with a 25% increase in the stable length of the mitotic spindle. In this case, although centromeric chromatin is still a part of the spindle, its mechanical properties are now altered due to defective cohesion between sister chromosomes.
The mechanical properties of pericentric chromatin remain virtually undefined. The simplicity of the mitotic spindle and centromere architecture in budding yeast provides the ideal opportunity to discover and measure the mechanical properties of pericentric chromatin most relevant to spindle mechanics. Recent experimentation in this direction (10) has revealed some striking, quantitative information about the importance of DNA compaction in regulating spindle length. Importantly, this work also gauged the role of DNA properties directly against microtubule-based motors in deciding the steady-state spindle length in budding yeast. In combination with direct in vitro measurements of the biophysical properties of yeast chromatin, such in vivo experimentation constitutes a promising approach for defining the role of chromatin as an essential mechanical element of the mitotic spindle.
WEAK MACHINES AND ENTROPIC MACHINES
How powerful is the spindle from a mechanical perspective and how does it compare to other nanomachines? The performance of a machine over a range of sizes can be compared by power output/volume (88, 89). This measure relates force and velocity to volume to reveal how concentrated force generators are. The bacterial flagellum has a power output per volume of 108 erg sec−1/cm3. Muscle is 106 erg sec−1/cm3 and a eukaryotic flagellum is 105 erg sec−1/cm3. The grasshopper spindle is 6 erg sec−1/cm3. The power output of the spindle is five orders of magnitude less than muscle and eight orders of magnitude weaker than the bacteria flagellum. Why is the spindle so weak? What must we consider in understanding the biological role of such a relatively powerless machine?
Although relatively weak, the force-generating systems are sufficient to deform mitotic chromosomes into their characteristic “V” upon anaphase onset. This likely reflects the relatively soft nature of the chromosome (easily deformable) relative to their viscous surroundings. The rate of chromosome movement is more diagnostic of the strength of the spindle. Chromosomes move to spindle poles on the order of microns/minute (86, 97). Motor proteins such as kinesin or dynein translocate microtubules on the order of microns/sec. The spindle sacrifices speed to prevent shear force that might damage DNA as well as for accuracy of segregation (88, 89). These early mechanical manipulations revealed that velocity was independent of chromosome size, or load-independent, and led to influential models that invoked a “velocity-governor” regulating chromosome movement. This velocity-governor could be produced by microtubule depolymerization.
An alternative hypothesis proposes that chromosome flexibility regulates the rate of chromosome movement (107). In this view, the second major polymer of the spindle, namely DNA/chromatin, contributes more directly to mechanism of force generation. Using a model of the chromosome as a segmented chain (see above and Figure 2), a soft Young’s modulus and different chromosome lengths, Raj & Peskin (107) found that for stiffer chromosomes, velocity is not independent of length. Although these studies have no direct experimental correlate, they promote the idea from the modeling side to consider additional sources of force in the spindle. Studies examining chromosome breakage in mutants lacking topoisomerase II have indicated that short chromosomes are less entangled than long chromosomes (116). Perhaps there is a difference in the stiffness of short vs long chromosomes as well.
Several observations are indicative of forces of unknown origin in the spindle. One is hinged mitosis (90). Stress on one bundle of kinetochore microtubules results in bending of kinetochore microtubules on adjacent chromosomes. Thus kinetochore microtubules of adjacent chromosomes are mechanically linked (90). Another recent finding is a radial expulsion force in the spindle (136). The pericentric chromatin is radially displaced from the central spindle and kinetochore microtubules by 40 nm in budding yeast (136). The idea that microtubules generate a force that is linearly transduced through sister chromatids is not tenable. This machine is working not just through a viscous milieu, but more likely through a complex network of cross-linked entropic springs (chromatin) and cross-linked stiff compression elements (microtubules). Non-kinetochore proteins, such as SMCs, MAPs, and motors undoubtedly contribute to the mechanical properties of the mitotic spindle.
ADDITIONAL ROLES FOR MICROTUBULES: PROTEIN TRANSPORT CONDUITS
While microtubules clearly play an essential role in the structure of the spindle, they also provide conduits to transmit information. This happens mechanically through the transmission of forces. For instance, when one end of a microtubule is pulled, the opposing end “senses” a tug. The strain associated with that pulling force may result in partial deformation of proteins, resulting in changes in binding sites and/or binding affinity of associated proteins. In this way, a signaling pathway is transduced through mechanical force.
Additionally, microtubules may also function as roadways that allow for directed transport of proteins functioning in signaling roles. Signaling pathways can occur through interactions of soluble, diffuse proteins in the cell, but the transport of signaling molecules along a microtubule provides for a more highly regulated method of directing a signal to a particular site.
One example is found in the passenger proteins, which relocalize from the inner centromere to the spindle midzone following anaphase onset. In mammalian cells, INCENP, Aurora B kinase, Survivin, and Borealin are passenger proteins that play multiple critical functions during mitosis (108). Mutations in these proteins result in errors in chromosome attachment, spindle abnormalities, and errors in cytokinesis. The dramatic relocalization of these proteins from the inner centromere to the spindle midzone likely reflects different functions of passenger proteins during mitosis, and highlights the importance of microtubules for the directed transport of these proteins to the mid-zone in late mitosis, where they function in cytokinesis.
In budding yeast, the passenger protein homologs also localize to the spindle following anaphase onset. Additionally, the inner kinetochore complex CBF3 has been found along microtubules during anaphase and at the midzone in late anaphase. Deletion or mutation of passenger proteins has resulted in phenotypes including spindle stability defects and delayed spindle disassembly (11). Mutation of NDC10, a CBF3 component that localizes the spindle in a Survivin- (Bir1p) dependent manner, results in defects in both spindle stability and cell division. Thus the transport of these proteins to the midzone in yeast is similarly important for proper completion of mitosis.
CONCLUSIONS: SPECULATION AND FUTURE DIRECTIONS
A unifying way to relate lessons learned from studying mitosis in budding yeast is to consider the yeast mitotic apparatus as one mammalian kinetochore (see Figure 5). The kinetochores from 16 chromosomes are clustered into a single diffraction-limited spot in mitosis; with sister kinetochores separated on average by approximately 800 nm, similar to the arrangement sister kinetochores of one mammalian kinetochore. Multiple attachment sites may be clustered whether they are on separate chromosomes (as in yeast) or within a single chromosome (as in mammals). In budding yeast, the path of pericentric DNA is an intramolecular loop that extends from the longitudinal axis of the chromosome, axially to the plus end of the kinetochore microtubule (Figure 5) (136). While we do not know whether the path of pericentric DNA in yeast informs us to the path taken in other organisms, we must clearly consider the chromosome segregation apparatus as a composite structure of two biopolymers, DNA loops and microtubules. C-loops provide the compliant linkage between stiffer kinetochore microtubules, as well as a physical mechanism for biorientation of sister kinetochores.
Figure 5.
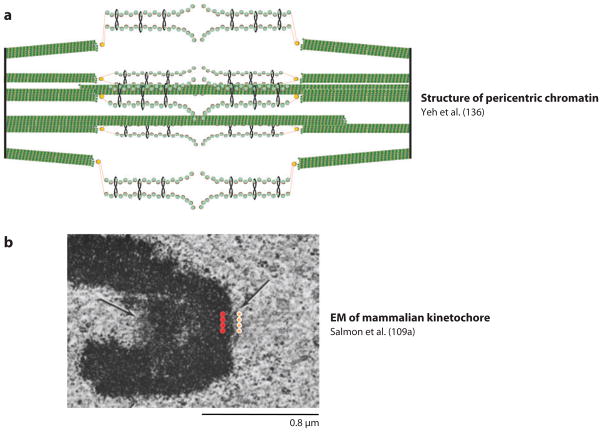
Is the yeast spindle comparable to one mammalian kinetochore? (a) The segregation apparatus in budding yeast is composed of kinetochore and interpolar microtubules ( green) and pericentric chromatin organized into C-loops of intramolecularly paired chromatids (136). Sister centromeres are separated by an average of 800 nm in mitosis (97), and are clustered into a single diffraction-limited spot in mitosis. (b) Longitudinal section through a HeLa cell in prometaphase (109a). The trilaminar structure of a mammalian kinetochore is marked by the orange and red dots. Multiple attachment sites may be clustered whether they are on separate chromosomes (as in budding yeast) or within a single chromosome (as in the Hela cell shown here).
The forces generated upon relaxation of DNA to a random coil are small indeed. However, the forces that move chromosomes are also very small (87, 88). In proposing new perspectives from a composite mitotic segregation apparatus of DNA and microtubules, one lesson from the physical properties of these polymers is the role of entropic forces. Entropy is sufficient to drive the segregation of highly confined polymers, and has been postulated to contribute to chromosome segregation in E. coli (55). Entropy may contribute to segregation of chromosome arms in eukaryotic cells. Perhaps the spindle is weak because its job is to provide just enough force to overcome thermal motion and bias centromeres to opposite spindle poles. Once centromeres reach their pole, dissolution of the remaining mechanical linkages between sister chromatids allows each strand to reptate toward its respective centromere.
As we delve into the underlying principles of complex functions, we must consider the physical properties of the molecules involved. In the case of chromosome segregation, elaborate signal transduction mechanisms have evolved to ensure high-fidelity segregation. The forces to move chromosomes are extremely weak and very likely involve entropic elasticity of freely jointed chains (i.e., DNA).
SUMMARY POINTS
The mitotic spindle is a composite structure of rigid microtubule struts that are strong in compression and elastic pericentric chromatin that is strong in tension.
The kinetochore is a proteinaceous structure bridging the two major polymers of the mitotic segregation apparatus.
Microtubule-based motor proteins can act as microtubule cross-linking proteins and microtubule depolymerases to generate force and regulate microtubule length and dynamics in the mitotic spindle.
DNA is an entropic spring. In the absence of force, DNA will adopt a random coil whose dimensions are dictated by the persistence length and contour length of the DNA molecule.
Pericentric chromatin is organized into an intramolecular loop in mitosis. This loop reflects intrastrand interactions within a single sister chromatid.
Both chromatin and microtubules contribute to spindle stability and length control.
- MT
microtubule
- SPB
spindle pole body
- Young’s modulus
a physical measure of the material properties of a substance; the relation of stress (distribution of force per unit area;F/A) to strain (a geometric expression of deformation, Δ length/total length)
- MAP
microtubule-associate protein
- Persistence length
a description of a filament’s resistance to thermal force; the distance over which the correlation of the direction of the two ends of a polymer is lost
- Contour length
the total length of a polymer
- Radius of gyration
the radius of random coil that a polymer adopts in the absence of external force; dictated by the persistence length and contour length of a polymer
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Contributor Information
David C. Bouck, Email: bouck@unc.edu.
Ajit P. Joglekar, Email: ajitj@unc.edu.
Kerry S. Bloom, Email: kerry_bloom@unc.edu.
LITERATURE CITED
- 1.Allingham JS, Sproul LR, Rayment I, Gilbert SP. Vik1 modulates microtubule-Kar3 interactions through a motor domain that lacks an active site. Cell. 2007;128:1161–72. doi: 10.1016/j.cell.2006.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almagro S, Riveline D, Hirano T, Houchmandzadeh B, Dimitrov S. The mitotic chromosome is an assembly of rigid elastic axes organized by structural maintenance of chromosomes (SMC) proteins and surrounded by a soft chromatin envelope. J Biol Chem. 2004;279:5118–26. doi: 10.1074/jbc.M307221200. [DOI] [PubMed] [Google Scholar]
- 3.Araki Y, Lau CK, Maekawa H, Jaspersen SL, Giddings TH, Jr, et al. The Saccharomyces cerevisiae spindle pole body (SPB) component Nbp1p is required for SPB membrane insertion and interacts with the integral membrane proteins Ndc1p and Mps2p. Mol Biol Cell. 2006;17:1959–70. doi: 10.1091/mbc.E05-07-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellizzi JJ, 3rd, Sorger PK, Harrison SC. Crystal structure of the yeast inner kinetochore subunit Cep3p. Structure. 2007;15:1422–30. doi: 10.1016/j.str.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlin V, Styles CA, Fink GR. BIK1, a protein required for microtubule function during mating and mitosis in Saccharomyces cerevisiae, colocalizes with tubulin. J Cell Biol. 1990;111:2573–86. doi: 10.1083/jcb.111.6.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15:3118–29. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom K, Carbon J. Yeast centromere DNA is in a unique and highly ordered structure in chromosomes and small circular minichromosomes. Cell. 1982;29:305–17. doi: 10.1016/0092-8674(82)90147-7. [DOI] [PubMed] [Google Scholar]
- 8.Bloom K, Sharma S, Dokholyan NV. The path of DNA in the kinetochore. Curr Biol. 2006;16:R276–78. doi: 10.1016/j.cub.2006.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom KS, Carbon J. Yeast centromere DNA is in a unique and highly ordered structure in chromosomes and small circular minichromosomes. Cell. 1982;29:305–17. doi: 10.1016/0092-8674(82)90147-7. [DOI] [PubMed] [Google Scholar]
- 10.Bouck DC, Bloom K. Pericentric chromatin is an elastic component of the mitotic spindle. Curr Biol. 2007;17:741–48. doi: 10.1016/j.cub.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouck DC, Bloom KS. The kinetochore protein Ndc10p is required for spindle stability and cytokinesis in yeast. Proc Natl Acad Sci USA. 2005;102:5408–13. doi: 10.1073/pnas.0405925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brock JA, Bloom K. A chromosome breakage assay to monitor mitotic forces in budding yeast. J Cell Sci. 1994;107(Part 4):891–902. doi: 10.1242/jcs.107.4.891. [DOI] [PubMed] [Google Scholar]
- 13.Bullitt E, Rout MP, Kilmartin JV, Akey CW. The yeast spindle pole body is assembled around a central crystal of Spc42p. Cell. 1997;89:1077–86. doi: 10.1016/s0092-8674(00)80295-0. [DOI] [PubMed] [Google Scholar]
- 14.Buvelot S, Tatsutani SY, Vermaak D, Biggins S. The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. J Cell Biol. 2003;160:329–39. doi: 10.1083/jcb.200209018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byers B, Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975;124:511–23. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camahort R, Li B, Florens L, Swanson SK, Washburn MP, Gerton JL. Scm3 is essential to recruit the histone h3 variant cse4 to centromeres and to maintain a functional kinetochore. Mol Cell. 2007;26:853–65. doi: 10.1016/j.molcel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Chan GK, Liu ST, Yen TJ. Kinetochore structure and function. Trends Cell Biol. 2005;15:589–98. doi: 10.1016/j.tcb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 17a.Cheesman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–97. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 18.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 19.Civelekoglu-Scholey G, Sharp DJ, Mogilner A, Scholey JM. Model of chromosome motility in Drosophila embryos: adaptation of a general mechanism for rapid mitosis. Biophys J. 2006;90:3966–82. doi: 10.1529/biophysj.105.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleveland DW, Mao Y, Sullivan KF. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–21. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 21.Cole DG, Saxton WM, Sheehan KB, Scholey JM. A “slow” homotetrameric kinesin-related motor protein purified from Drosophila embryos. J Biol Chem. 1994;269:22913–16. [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105:2053–67. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y, Bustamante C. Pulling a single chromatin fiber reveals the forces that maintain its higher-order structure. Proc Natl Acad Sci USA. 2000;97:127–32. doi: 10.1073/pnas.97.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cytrynbaum EN, Scholey JM, Mogilner A. A force balance model of early spindle pole separation in Drosophila embryos. Biophys J. 2003;84:757–69. doi: 10.1016/S0006-3495(03)74895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Amours D, Amon A. At the interface between signaling and executing anaphase–Cdc14 and the FEAR network. Genes Dev. 2004;18:2581–95. doi: 10.1101/gad.1247304. [DOI] [PubMed] [Google Scholar]
- 26.Dalal Y, Wang H, Lindsay S, Henikoff S. Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol. 2007;5:e218. doi: 10.1371/journal.pbio.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–82. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 28.DeLuca JG, Moree B, Hickey JM, Kilmartin JV, Salmon ED. hNuf2 inhibition blocks stable kinetochore-microtubule attachment and induces mitotic cell death in HeLa cells. J Cell Biol. 2002;159:549–55. doi: 10.1083/jcb.200208159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draviam VM, Shapiro I, Aldridge B, Sorger PK. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 2006;25:2814–27. doi: 10.1038/sj.emboj.7601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Earnshaw WC, Laemmli UK. Architecture of metaphase chromosomes and chromosome scaffolds. J Cell Biol. 1983;96:84. doi: 10.1083/jcb.96.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Efremov A, Grishchuk EL, McIntosh JR, Ataullakhanov FI. In search of an optimal ring to couple microtubule depolymerization to processive chromosome motions. Proc Natl Acad Sci USA. 2007;104:19017–22. doi: 10.1073/pnas.0709524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzgerald-Hayes M, Clarke L, Carbon J. Nucleotide sequence comparisons and functional analysis of yeast centromere DNAs. Cell. 1982;29:235–44. doi: 10.1016/0092-8674(82)90108-8. [DOI] [PubMed] [Google Scholar]
- 32.Gardner MK, Haase J, Mythreye K, Molk JN, Anderson M, et al. The microtubule-based motor Kar3 and plus end-binding protein Bim1 provide structural support for the anaphase spindle. J Cell Biol. 2008;180:91–100. doi: 10.1083/jcb.200710164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner MK, Odde DJ, Bloom K. Hypothesis testing via integrated computer modeling and digital fluorescence microscopy. Methods. 2007;41:232–37. doi: 10.1016/j.ymeth.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Gardner MK, Pearson CG, Sprague BL, Zarzar TR, Bloom K, et al. Tension-dependent regulation of microtubule dynamics at kinetochores can explain metaphase congression in yeast. Mol Biol Cell. 2005;16:3764–75. doi: 10.1091/mbc.E05-04-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gestaut DR, Graczyk B, Cooper J, Widlund PO, Zelter A, et al. Phosphoregulation and depolymerization-driven movement of the Dam1 complex do not require ring formation. Nat Cell Biol. 2008;10:407–14. doi: 10.1038/ncb1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gheber L, Kuo SC, Hoyt MA. Motile properties of the kinesin-related Cin8p spindle motor extracted from Saccharomyces cerevisiae cells. J Biol Chem. 1999;274:9564–72. doi: 10.1074/jbc.274.14.9564. [DOI] [PubMed] [Google Scholar]
- 37.Gimenez-Abian JF, Diaz-Martinez LA, Wirth KG, Andrews CA, Gimenez-Martin G, Clarke DJ. Regulated separation of sister centromeres depends on the spindle assembly checkpoint but not on the anaphase promoting complex/cyclosome. Cell Cycle. 2005;4:11. doi: 10.4161/cc.4.11.2146. [DOI] [PubMed] [Google Scholar]
- 38.Goshima G, Wollman R, Stuurman N, Scholey JM, Vale RD. Length control of the metaphase spindle. Curr Biol. 2005;15:1979–88. doi: 10.1016/j.cub.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 39.Goshima G, Yanagida M. Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell. 2000;100:619–33. doi: 10.1016/s0092-8674(00)80699-6. [DOI] [PubMed] [Google Scholar]
- 40.Guacci V, Yamamoto A, Strunnikov A, Kingsbury J, Hogan E, et al. Structure and function of chromosomes in mitosis of budding yeast. Cold Spring Harbor Symp Quant Biol. 1993;58:677–85. doi: 10.1101/sqb.1993.058.01.075. [DOI] [PubMed] [Google Scholar]
- 41.Hartwell LH, Culotti J, Pringle JR, Reid BJ. Genetic control of the cell division cycle in yeast. Science. 1974;183:46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- 42.He X, Rines DR, Espelin CW, Sorger PK. Molecular analysis of kinetochore-microtubule attachment in budding yeast. Cell. 2001;106:195–206. doi: 10.1016/s0092-8674(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 43.Higuchi T, Uhlmann F. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature. 2005;433:171–76. doi: 10.1038/nature03240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hildebrandt ER, Hoyt MA. Mitotic motors in Saccharomyces cerevisiae. Biochim Biophys Acta. 2000;1496:99–116. doi: 10.1016/s0167-4889(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 45.Hirano T. The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev. 2002;16:399–414. doi: 10.1101/gad.955102. [DOI] [PubMed] [Google Scholar]
- 46.Hirano T, Kobayashi R, Hirano M. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 1997;89:511–21. doi: 10.1016/s0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- 47.Hoyt MA, He L, Loo KK, Saunders WS. Two Saccharomyces cerevisiae kinesin-related gene products required for mitotic spindle assembly. J Cell Biol. 1992;118:109–20. doi: 10.1083/jcb.118.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Indjeian VB, Murray AW. Budding yeast mitotic chromosomes have an intrinsic bias to biorient on the spindle. Curr Biol. 2007;17:1837–46. doi: 10.1016/j.cub.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 49.Inoue S. Polarization optical studies of the mitotic spindle. I. The demonstration of spindle fibers in living cells. Chromosoma. 1953;5:487–500. doi: 10.1007/BF01271498. [DOI] [PubMed] [Google Scholar]
- 50.Inoue S, Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967;50(Suppl):259–92. [PMC free article] [PubMed] [Google Scholar]
- 51.Janson ME, Loughlin R, Loiodice I, Fu C, Brunner D, et al. Crosslinkers and motors organize dynamic microtubules to form stable bipolar arrays in fission yeast. Cell. 2007;128:357–68. doi: 10.1016/j.cell.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 52.Jaspersen SL, Winey M. The budding yeast spindle pole body: structure, duplication, and function. Annu Rev Cell Dev Biol. 2004;20:1–28. doi: 10.1146/annurev.cellbio.20.022003.114106. [DOI] [PubMed] [Google Scholar]
- 53.Joglekar AP, Bouck D, Finley K, Liu X, Wan Y, et al. Molecular architecture of the kinetochore-microtubule attachment site is conserved between point and regional centromeres. J Cell Biol. 2008 doi: 10.1083/jcb.200803027. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joglekar AP, Bouck DC, Molk JN, Bloom KS, Salmon ED. Molecular architecture of a kinetochore-microtubule attachment site. Nat Cell Biol. 2006;8:581–85. doi: 10.1038/ncb1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jun S, Mulder B. Entropy-driven spatial organization of highly confined polymers: lessons for the bacterial chromosome. Proc Natl Acad Sci USA. 2006;103:12388–93. doi: 10.1073/pnas.0605305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55a.Kavenoff R, Bowen BC. Electron microscopy of membrane-free folded chromosomes from Escherichia coli. Chromosoma. 1976;59:89–101. doi: 10.1007/BF00328479. [DOI] [PubMed] [Google Scholar]
- 56.Kireeva N, Lakonishok M, Kireev I, Hirano T, Belmont AS. Visualization of early chromosome condensation: a hierarchical folding, axial glue model of chromosome structure. J Cell Biol. 2004;166:775–85. doi: 10.1083/jcb.200406049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koshland D, Hieter P. Visual assay for chromosome ploidy. Methods Enzymol. 1987;155:351–72. doi: 10.1016/0076-6879(87)55024-8. [DOI] [PubMed] [Google Scholar]
- 58.Koshland D, Strunnikov A. Mitotic chromosome condensation. Annu Rev Cell Dev Biol. 1996;12:305–33. doi: 10.1146/annurev.cellbio.12.1.305. [DOI] [PubMed] [Google Scholar]
- 59.Lechner J, Carbon J. A 240 kd multisubunit protein complex, CBF3, is a major component of the budding yeast centromere. Cell. 1991;64:717–25. doi: 10.1016/0092-8674(91)90501-o. [DOI] [PubMed] [Google Scholar]
- 60.Lillie SH, Brown SS. Suppression of a myosin defect by a kinesin-related gene. Nature. 1992;356:358–61. doi: 10.1038/356358a0. [DOI] [PubMed] [Google Scholar]
- 61.Lin H, de Carvalho P, Kho D, Tai CY, Pierre P, et al. Polyploids require Bik1 for kinetochore-microtubule attachment. J Cell Biol. 2001;155:1173–84. doi: 10.1083/jcb.200108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Losada A, Hirano M, Hirano T. Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev. 1998;12:1986–97. doi: 10.1101/gad.12.13.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maddox PS, Bloom KS, Salmon ED. The polarity and dynamics of microtubule assembly in the budding yeast Saccharomyces cerevisiae. Nat Cell Biol. 2000;2:36–41. doi: 10.1038/71357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maddox PS, Stemple JK, Satterwhite L, Salmon ED, Bloom K. The minus end-directed motor Kar3 is required for coupling dynamic microtubule plus ends to the cortical shmoo tip in budding yeast. Curr Biol. 2003;13:1423–28. doi: 10.1016/s0960-9822(03)00547-5. [DOI] [PubMed] [Google Scholar]
- 64a.Maher B. Spring theory. Nature. 2007;448:984–86. doi: 10.1038/448984a. [DOI] [PubMed] [Google Scholar]
- 65.Maiato H, DeLuca J, Salmon ED, Earnshaw WC. The dynamic kinetochore-microtubule interface. J Cell Sci. 2004;117:5461–77. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- 66.Maiato H, Rieder CL, Khodjakov A. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J Cell Biol. 2004;167:831–40. doi: 10.1083/jcb.200407090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manning BD, Barrett JG, Wallace JA, Granok H, Snyder M. Differential regulation of the Kar3p kinesin-related protein by two associated proteins, Cik1p and Vik1p. J Cell Biol. 1999;144:1219–33. doi: 10.1083/jcb.144.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marshall WF, Marko JF, Agard DA, Sedat JW. Chromosome elasticity and mitotic polar ejection force measured in living Drosophila embryos by four-dimensional microscopy-based motion analysis. Curr Biol. 2001;11:569–78. doi: 10.1016/s0960-9822(01)00180-4. [DOI] [PubMed] [Google Scholar]
- 69.McAinsh AD, Tytell JD, Sorger PK. Structure, function, and regulation of budding yeast kinetochores. Annu Rev Cell Dev Biol. 2003;19:519–39. doi: 10.1146/annurev.cellbio.19.111301.155607. [DOI] [PubMed] [Google Scholar]
- 70.McCarroll RM, Fangman WL. Time of replication of yeast centromeres and telomeres. Cell. 1988;54:505–13. doi: 10.1016/0092-8674(88)90072-4. [DOI] [PubMed] [Google Scholar]
- 71.McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 2005;3:e86. doi: 10.1371/journal.pbio.0030086. Epub 2005 March 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meluh PB, Yang P, Glowczewski L, Koshland D, Smith MM. Cse4p is a component of the core centromere of Saccharomyces cerevisiae. Cell. 1998;94:607–13. doi: 10.1016/s0092-8674(00)81602-5. [DOI] [PubMed] [Google Scholar]
- 73.Middleton K, Carbon J. KAR3-encoded kinesin is a minus-end-directed motor that functions with centromere binding proteins (CBF3) on an in vitro yeast kinetochore. Proc Natl Acad Sci USA. 1994;91:7212–16. doi: 10.1073/pnas.91.15.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miranda JJ, De Wulf P, Sorger PK, Harrison SC. The yeast DASH complex forms closed rings on microtubules. Nat Struct Mol Biol. 2005;12:138–43. doi: 10.1038/nsmb896. [DOI] [PubMed] [Google Scholar]
- 75.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–42. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 76.Mizuguchi G, Xiao H, Wisniewski J, Smith MM, Wu C. Nonhistone Scm3 and histones CenH3-H4 assemble the core of centromere-specific nucleosomes. Cell. 2007;129:1153–64. doi: 10.1016/j.cell.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 77.Mogilner A, Oster G. Polymer motors: pushing out the front and pulling up the back. Curr Biol. 2003;13:R721–33. doi: 10.1016/j.cub.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 78.Mohri H. Amino-acid composition of “Tubulin” constituting microtubules of sperm flagella. Nature. 1968;217:1053–54. doi: 10.1038/2171053a0. [DOI] [PubMed] [Google Scholar]
- 79.Molk JN, Salmon ED, Bloom K. Nuclear congression is driven by cytoplasmic microtubule plus end interactions in S. cerevisiae. J Cell Biol. 2006;172:27–39. doi: 10.1083/jcb.200510032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molk JN, Schuyler SC, Liu JY, Evans JG, Salmon ED, et al. The differential roles of budding yeast Tem1p, Cdc15p, and Bub2p protein dynamics in mitotic exit. Mol Biol Cell. 2004;15:1519–32. doi: 10.1091/mbc.E03-09-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muller EG, Snydsman BE, Novik I, Hailey DW, Gestaut DR, et al. The organization of the core proteins of the yeast spindle pole body. Mol Biol Cell. 2005;16:3341–52. doi: 10.1091/mbc.E05-03-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakajima M, Kumada K, Hatakeyama K, Noda T, Peters JM, Hirota T. The complete removal of cohesin from chromosome arms depends on separase. J Cell Sci. 2007;120:4188–96. doi: 10.1242/jcs.011528. [DOI] [PubMed] [Google Scholar]
- 83.Nasmyth K. Segregating sister genomes: the molecular biology of chromosome separation. Science. 2002;297:559–65. doi: 10.1126/science.1074757. [DOI] [PubMed] [Google Scholar]
- 84.Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- 85.Nedelec F, Surrey T, Maggs AC. Dynamic concentration of motors in microtubule arrays. Phys Rev Lett. 2001;86:3192–95. doi: 10.1103/PhysRevLett.86.3192. [DOI] [PubMed] [Google Scholar]
- 86.Nicklas RB. Chromosome velocity during mitosis as a function of chromosome size and position. J Cell Biol. 1965;25(Suppl):119–35. doi: 10.1083/jcb.25.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicklas RB. Measurements of the force produced by the mitotic spindle in anaphase. J Cell Biol. 1983;97:542–48. doi: 10.1083/jcb.97.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nicklas RB. A quantitative comparison of cellular motile systems. Cell Motil. 1984;4:1–5. doi: 10.1002/cm.970040102. [DOI] [PubMed] [Google Scholar]
- 89.Nicklas RB. The forces that move chromosomes in mitosis. Annu Rev Biophys Biophys Chem. 1988;17:431–49. doi: 10.1146/annurev.bb.17.060188.002243. [DOI] [PubMed] [Google Scholar]
- 90.Nicklas RB, Kubai DF, Hays TS. Spindle microtubules and their mechanical associations after micromanipulation in anaphase. J Cell Biol. 1982;95:91–104. doi: 10.1083/jcb.95.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Toole ET, Mastronarde DN, Giddings TH, Jr, Winey M, Burke DJ, McIntosh JR. Three-dimensional analysis and ultrastructural design of mitotic spindles from the cdc20 mutant of Saccharomyces cerevisiae. Mol Biol Cell. 1997;8:1–11. doi: 10.1091/mbc.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Toole ET, Winey M, McIntosh JR. High-voltage electron tomography of spindle pole bodies and early mitotic spindles in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:2017–31. doi: 10.1091/mbc.10.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Onn I, Heidinger-Pauli J, Guacci V, Ünal E, Koshland DE. Sister chromatid cohesion: a simple concept with a complex reality. Annu Rev Cell Dev Biol. 2008 doi: 10.1146/annurev.cellbio.24.110707.175350. In press. [DOI] [PubMed] [Google Scholar]
- 94.Page BD, Snyder M. CIK1: a developmentally regulated spindle pole body-associated protein important for microtubule functions in Saccharomyces cerevisiae. Genes Dev. 1992;6:1414–29. doi: 10.1101/gad.6.8.1414. [DOI] [PubMed] [Google Scholar]
- 95.Paliulis LV, Nicklas RB. Micromanipulation of chromosomes reveals that cohesion release during cell division is gradual and does not require tension. Curr Biol. 2004;14:2124–29. doi: 10.1016/j.cub.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 96.Pearson CG, Gardner MK, Paliulis LV, Salmon ED, Odde DJ, Bloom K. Measuring nanometer scale gradients in spindle microtubule dynamics using model convolution microscopy. Mol Biol Cell. 2006;17:4069–79. doi: 10.1091/mbc.E06-04-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pearson CG, Maddox PS, Salmon ED, Bloom K. Budding yeast chromosome structure and dynamics during mitosis. J Cell Biol. 2001;152:1255–66. doi: 10.1083/jcb.152.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pearson CG, Yeh E, Gardner M, Odde D, Salmon ED, Bloom K. Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase. Curr Biol. 2004;14:1962–67. doi: 10.1016/j.cub.2004.09.086. [DOI] [PubMed] [Google Scholar]
- 99.Pereira G, Tanaka TU, Nasmyth K, Schiebel E. Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. EMBO J. 2001;20:6359–70. doi: 10.1093/emboj/20.22.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pietrasanta LI, Thrower D, Hsieh W, Rao S, Stemmann O, et al. Probing the Saccharomyces cerevisiae centromeric DNA (CEN DNA)-binding factor 3 (CBF3) kinetochore complex by using atomic force microscopy. Proc Natl Acad Sci USA. 1999;96:3757–62. doi: 10.1073/pnas.96.7.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinsky BA, Tatsutani SY, Collins KA, Biggins S. An Mtw1 complex promotes kinetochore biorientation that is monitored by the Ipl1/Aurora protein kinase. Dev Cell. 2003;5:735–45. doi: 10.1016/s1534-5807(03)00322-8. [DOI] [PubMed] [Google Scholar]
- 102.Poirier MG, Eroglu S, Marko JF. The bending rigidity of mitotic chromosomes. Mol Biol Cell. 2002;13:2170–79. doi: 10.1091/mbc.01-08-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poirier MG, Marko JF. Micromechanical studies of mitotic chromosomes. J Muscle Res Cell Motil. 2002;23:409–31. [PubMed] [Google Scholar]
- 104.Poirier MG, Marko JF. Mitotic chromosomes are chromatin networks without a mechanically contiguous protein scaffold. Proc Natl Acad Sci USA. 2002;99:15393–97. doi: 10.1073/pnas.232442599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poirier MG, Nemani A, Gupta P, Eroglu S, Marko JF. Probing chromosome structure with dynamic force relaxation. Phys Rev Lett. 2001;86:360–63. doi: 10.1103/PhysRevLett.86.360. [DOI] [PubMed] [Google Scholar]
- 106.Purvis A, Singleton MR. Insights into kinetochore-DNA interactions from the structure of Cep3Delta. EMBO Rep. 2008;9:56–62. doi: 10.1038/sj.embor.7401139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raj A, Peskin CS. The influence of chromosome flexibility on chromosome transport during anaphase A. Proc Natl Acad Sci USA. 2006;103:5349–54. doi: 10.1073/pnas.0601215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 109.Russell ID, Grancell AS, Sorger PK. The unstable F-box protein p58-Ctf13 forms the structural core of the CBF3 kinetochore complex. J Cell Biol. 1999;145:933–50. doi: 10.1083/jcb.145.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109a.Salmon ED, Goode D, Maugel TK, Bonar DB. Pressure-induced depolymerization of spindle microtubules. III. Differential stability in HeLa cells. J Cell Biol. 1976;69:443–54. doi: 10.1083/jcb.69.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sandall S, Severin F, McLeod IX, Yates JR, 3rd, Oegema K, et al. A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell. 2006;127:1179–91. doi: 10.1016/j.cell.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saunders W, Lengyel V, Hoyt MA. Mitotic spindle function in Saccharomyces cerevisiae requires a balance between different types of kinesin-related motors. Mol Biol Cell. 1997;8:1025–33. doi: 10.1091/mbc.8.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saunders WS, Hoyt MA. Kinesin-related proteins required for structural integrity of the mitotic spindle. Cell. 1992;70:451–58. doi: 10.1016/0092-8674(92)90169-d. [DOI] [PubMed] [Google Scholar]
- 113.Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–43. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- 114.Sharp JA, Franco AA, Osley MA, Kaufman PD. Chromatin assembly factor I and Hir proteins contribute to building functional kinetochores in S. cerevisiae. Genes Dev. 2002;16:85–100. doi: 10.1101/gad.925302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Skibbens RV, Skeen VP, Salmon ED. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J Cell Biol. 1993;122:859–75. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spell RM, Holm C. Nature and distribution of chromosomal intertwinings in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:1465–76. doi: 10.1128/mcb.14.2.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sprague BL, Pearson CG, Maddox PS, Bloom KS, Salmon ED, Odde DJ. Mechanisms of microtubule-based kinetochore positioning in the yeast metaphase spindle. Biophys J. 2003;84:3529–46. doi: 10.1016/S0006-3495(03)75087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sproul LR, Anderson DJ, Mackey AT, Saunders WS, Gilbert SP. Cik1 targets the minus-end kinesin depolymerase kar3 to microtubule plus ends. Curr Biol. 2005;15:1420–27. doi: 10.1016/j.cub.2005.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stoler S, Rogers K, Weitze S, Morey L, Fitzgerald-Hayes M, Baker RE. Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization. Proc Natl Acad Sci USA. 2007;104:10571–76. doi: 10.1073/pnas.0703178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Storchova Z, Breneman A, Cande J, Dunn J, Burbank K, et al. Genome-wide genetic analysis of polyploidy in yeast. Nature. 2006;443:541–47. doi: 10.1038/nature05178. [DOI] [PubMed] [Google Scholar]
- 121.Straight AF, Belmont AS, Robinett CC, Murray AW. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr Biol. 1996;6:1599–608. doi: 10.1016/s0960-9822(02)70783-5. [DOI] [PubMed] [Google Scholar]
- 122.Straight AF, Marshall WF, Sedat JW, Murray AW. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science. 1997;277:574–78. doi: 10.1126/science.277.5325.574. [DOI] [PubMed] [Google Scholar]
- 123.Sumara I, Vorlaufer E, Gieffers C, Peters BH, Peters JM. Characterization of vertebrate cohesin complexes and their regulation in prophase. J Cell Biol. 2000;151:749–62. doi: 10.1083/jcb.151.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, et al. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9:515–25. doi: 10.1016/s1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- 125.Tanaka K, Mukae N, Dewar H, van Breugel M, James EK, et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434:987–94. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- 126.Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC, Yu H. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell. 2006;10:575–85. doi: 10.1016/j.devcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 127.Tirnauer JS, O’Toole E, Berrueta L, Bierer BE, Pellman D. Yeast Bim1p promotes the G1-specific dynamics of microtubules. J Cell Biol. 1999;145:993–1007. doi: 10.1083/jcb.145.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uhlmann F, Lottspeich F, Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400:37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- 129.Vaughan KT. Microtubule plus ends, motors, and traffic of Golgi membranes. Biochim Biophys Acta. 2005;1744:316–24. doi: 10.1016/j.bbamcr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 130.Walker RA, O’Brien ET, Pryer NK, Soboeiro MF, Voter WA, et al. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988;107:1437–48. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weisenberg RC, Borisy GG, Taylor EW. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968;7:4466–79. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- 132.Westermann S, Avila-Sakar A, Wang HW, Niederstrasser H, Wong J, et al. Formation of a dynamic kinetochore- microtubule interface through assembly of the Dam1 ring complex. Mol Cell. 2005;17:277–90. doi: 10.1016/j.molcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 133.Winey M, Byers B. Assembly and functions of the spindle pole body in budding yeast. Trends Genet. 1993;9:300–4. doi: 10.1016/0168-9525(93)90247-f. [DOI] [PubMed] [Google Scholar]
- 134.Winey M, Mamay CL, O’Toole ET, Mastronarde DN, Giddings TH, Jr, et al. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J Cell Biol. 1995;129:1601–15. doi: 10.1083/jcb.129.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wolgemuth CW, Mogilner A, Oster G. The hydration dynamics of polyelectrolyte gels with applications to cell motility and drug delivery. Eur Biophys J. 2004;33:146–58. doi: 10.1007/s00249-003-0344-5. [DOI] [PubMed] [Google Scholar]
- 136.Yeh E, Haase J, Paliulis LV, Joglekar A, Bond L, et al. Pericentric chromatin is organized into an intramolecular loop in mitosis. Curr Biol. 2008;18:81–90. doi: 10.1016/j.cub.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zeng X, Kahana JA, Silver PA, Morphew MK, McIntosh JR, et al. Slk19p is a centromere protein that functions to stabilize mitotic spindles. J Cell Biol. 1999;146:415–25. doi: 10.1083/jcb.146.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zinkowski RP, Meyne J, Brinkley BR. The centromere-kinetochore complex: a repeat subunit model. J Cell Biol. 1991;113:1091–110. doi: 10.1083/jcb.113.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]