Abstract
The induction of mossy fiber-CA3 long-term potentiation (LTP) and depression (LTD) has been variously described as being dependent on either pre- or postsynaptic factors. Some of the postsynaptic factors for LTP induction include ephrin-B receptor tyrosine kinases and a rise in postsynaptic Ca2+ ([Ca2+]i). Ca2+ is also believed to be involved in the induction of the various forms of LTD at this synapse. We used photolysis of caged Ca2+ compounds to test whether a postsynaptic rise in [Ca2+]i is sufficient to induce changes in synaptic transmission at mossy fiber synapses onto rat hippocampal CA3 pyramidal neurons. We were able to elevate postsynaptic [Ca2+]i to approximately 1 μm for a few seconds in pyramidal cell somata and dendrites. We estimate that CA3 pyramidal neurons have approximately fivefold greater endogenous Ca2+ buffer capacity than CA1 neurons, limiting the rise in [Ca2+]i achievable by photolysis. This [Ca2+]i rise induced either a potentiation or a depression at mossy fiber synapses in different preparations. Neither the potentiation nor the depression was accompanied by consistent changes in paired-pulse facilitation, suggesting that these forms of plasticity may be distinct from synaptically induced LTP and LTD at this synapse. Our results are consistent with a postsynaptic locus for the induction of at least some forms of synaptic plasticity at mossy fiber synapses.
INTRODUCTION
Mossy fiber synapses onto CA3 pyramidal neurons display a form of long-term potentiation (LTP) with properties quite distinct from LTP in CA1 neurons (Nicoll and Malenka 1995). Mossy fiber LTP can be induced in the presence of both N-methyl-D-aspartate receptor (NMDAR) and α-amino-3-hy-droxy-5-methylisoxazole-4-proprionate (AMPA) receptor antagonists (Castillo et al. 1994; Harris and Cotman 1986; Ito and Sugiyama 1991; Yeckel et al. 1999; Zalutsky and Nicoll 1990). Although its expression is generally agreed to be presynaptic, due to an increase in transmitter release, there is less agreement regarding its site of induction (Henze et al. 2000). This may be due to the existence of multiple forms of mossy fiber LTP (Urban and Barrionuevo 1996). At least one of the forms of mossy fiber LTP is blocked by postsynaptic injection of ephrin-B receptor tyrosine kinase inhibitors (Contractor et al. 2002) and Ca2+ chelators (Alle et al. 2001; Williams and Johnston 1989; Yeckel et al. 1999; but see Mellor and Nicoll 2001). The latter result suggests that, under certain conditions, mossy fiber LTP can depend on postsynaptic factors, including a rise in Ca2+ during synaptic stimulation.
Mossy fiber LTP is also reversible (Chen et al. 2001), and mossy fiber synapses can exhibit long-term depression (LTD). As with mossy fiber LTP, there may be multiple forms of mossy fiber LTD. LTD induction does not require activation of NMDARs, but its expression can be either pre- or postsynaptic (Kobayashi et al. 1996; Lei et al. 2003). The membrane-permeant acetoxymethyl esters of EGTA and BAPTA (EGTA-AM and BAPTA-AM) interfere with LTD induction (Huang et al. 2002; Kobayashi et al. 1999), although it is not clear whether the membrane-permeant chelators are acting pre-or postsynaptically. A recent report suggests that a postsynaptically induced and expressed form of LTD exists at this synapse that is dependent on a rise in postsynaptic Ca2+ (Lei et al. 2003).
In this study, we used postsynaptic caged Ca2+ photolysis to determine whether an elevation of [Ca2+]i in CA3 pyramidal cells in brain slices from rat hippocampus was sufficient to induce changes in mossy fiber transmission. Our results indicate that a postsynaptic [Ca2+]i rise can induce long-lasting forms of potentiation and depression at these synapses. Because neither of these forms of plasticity was accompanied by a consistent change in paired-pulse facilitation (PPF), both may be expressed postsynaptically.
METHODS
Slice preparation
Hippocampal slices were cut from Sprague-Dawley rat (22–28 days) brains as described previously (Wang and Zucker 2001), using procedures approved by our Animal Care and Use Committee. After deep anesthesia was achieved by intraperitoneal injection of a mixture (approximately 0.1 ml) of ketamine (42.8 mg/ml), xylazine (8.6 mg/ml), and acepromazine (1.4 mg/ml), animals were placed in a −20°C environment for 5 min (Kapur et al. 2001), followed by transcardiac perfusion with 50 ml ice-cold oxygenated solution containing (in mM) 40 NaCl, 80 cholineCl or 150 sucrose, 4 KCl, 1.25 NaH2PO4, 25 NaHCO3, 7 MgCl2, 0.5 CaCl2, 10 dextrose, and 0.6 ascorbate, saturated with 95% O2-5% CO2, with an osmolarity of approximately 317 mOsm. The whole brain was rapidly removed after decapitation. Both hemispheres were sectioned (350-μm thick slices) using a Ralph glass or sapphire blade mounted on a Vibratome 3000 (Vibratome, St. Louis, MO) with vertical vibration <3 μm. Minimizing the vertical vibration of the cutting blade reduces distortion on cutting slices (Geiger et al. 2002). The blade was advanced at 1 mm/min or slower, producing a horizontal vibration of approximately 1.2 mm, to avoid crushing the sliced tissue. The cutting solution was refrigerated to 0–2°C. Slices were transferred for 30 min to an incubation chamber in the same solution at 35°C, after which they were maintained in the recording solution at room temperature consisting of (in mM) 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 4 MgCl2, 5 CaCl2, and 10 dextrose, saturated with 95% O2-5% CO2, with an osmolarity of approximately 326 mOsm.
Electrophysiological recording
A recording chamber was placed on a modified Nikon Optiphot II microscope. Slices were visualized with a UV-transmitting 40× water immersion objective (n.a. 0.7, Olympus, Tokyo, Japan) using a Dodt Gradient Contrast attachment (Luigs and Neumann, Ratingen, Ger-many) to the 100-W halogen trans-illumination lamp and an RG9 infrared filter, and imaged with a SensiCam CCD camera (PCO Computer Optics, Kelheim, Germany) controlled by Axon Imaging Workbench 4.0 (Axon, Union City, CA). Whole cell recordings from CA3 pyramidal cells were obtained in voltage clamp at −72 mV after correction for liquid junction potentials, using a voltage-clamp amplifier (SEC-05LX, npi Electronic, Tamm, Germany) controlled by Axon’s pClamp 8 or 9. Series resistance, input resistance, and leak current were monitored continuously throughout experiments. Recording pipettes had resistances of 4–6 MΩ when filled with (in mM) 120 K-gluconate, 20 KCl, 25 KHepes, 4 MgCl2, 2 Na2ATP, and 0.3 NaGTP, pH = 7.3 adjusted with HCl. For uncaging experiments, 5 mM (and in 2 instances, 10 mM) nitrophenyl EGTA (NPE; Ellis-Davies 1998) or dimethoxy-nitrophenyl-EGTA-4 (DM-NPE-4; Del-Principe et al. 1999; Maeda et al. 1999), loaded 65% with CaCl2, and 1 mM BTC were included, and K-gluconate was reduced, keeping osmolarity constant. The bathing solution contained 10 μM picrotoxin and 20 μM bicuculine for blocking inhibitory transmission (Yeckel et al. 1999) and was perfused at 30°C and approximately 2 ml/min. Effects of phosphatase inhibitors calyculin A (1 μM) or cyclosporin A (200 μM) were tested after preincubation for 2 h or 30 min, respectively. All chemicals were purchased from Sigma (St. Louis, MO) except for the following: APV, calyculin A, cyclosporin A, DCG-IV, and MK-801 came from Tocris (Ellisville, MO); BTC and NPE came from Molecular Probes (Eugene, OR); DM-nitrophen came from Calbiochem (San Diego, CA); and DM-NPE-4 came from Dr. Graham Ellis-Davies (Univ. of Pennsylvania, Philadelphia, PA).
Afferent stimulation
There is much informal debate about the best way to stimulate mossy fibers. Many authors stimulate the presynaptic granule cell bodies in the dentate gyrus. This has the advantage of minimizing contamination from commissural/associational (C/A) afferents. However, stimulating in the dentate is likely to activate mossy fibers projecting to a wide area of the CA3 region, exciting many CA3 pyramidal cells. If such cells are driven above threshold, their recurrent collaterals to other CA3 pyramidal cells can contribute a disynaptic response to any given recorded CA3 pyramidal neuron (Claiborne et al. 1993; Henze et al. 2000). In addition, stimulation in the dentate is likely to activate CA3 pyramidal axon collaterals antidromically. We attempted to minimize both problems by locally stimulating a small number of mossy fiber afferents in the stratum lucidum, near the recorded neuron. However, this might also result in some contamination of mossy fiber stimulation with C/A afferents.
Bipolar stimulating electrodes consisted of a glass microelectrode (~5 μm OD) filled with bathing solution and having a fine tungsten rod glued to its side. One such electrode was placed in the s. lucidum, typically 30–50 μm from the edge of the stratum pyramidale and 50–200 μm lateral to the recording electrode, for mossy fiber stimulation. Another was placed in the stratum radiatum, typically 100–150 μm from the s. pyramidale, for C/A fiber stimulation. Alternate stimuli were delivered every 10 or 20 s. In most experiments, test stimuli consisted of two 0.2-ms pulses separated by a 30- to 50-ms interval. Excitatory postsynaptic currents (EPSCs) from both pathways were recorded on separate software channels, and their slopes were analyzed online by pClamp 8 or 9.
Identifying mossy fiber responses
We used several criteria for distinguishing mossy fiber responses from C/A responses (Schulz et al. 1994; Yeckel et al. 1999). Mossy fiber EPSCs have a faster rising phase than C/A EPSCs. Mossy fiber synapses also show larger PPF than do C/A synapses (Salin et al. 1996). Finally, only mossy fiber EPSCs are presynaptically blocked by 1 μM of the group II mGluR agonist (2S,1′R,2′R,3′R)-2-(2,3-dicarboxycyclopropyl)glycine (DCG-IV) (Kamiya and Ozawa 1999). At the end of most experiments, DCG-IV (1 μM) was added to the bath perfusion system for 3–5 min and washed out.
We used all of these criteria to distinguish mossy fiber from C/A afferents. Responses classified in this fashion showed the expected differences: mossy fiber rise time (measured from 20 to 80% of the peak) was 1.07 ± 0.04 (SE) ms, while for C/A responses, the rise time was 1.86 ± 0.14 ms; mossy fiber PPF was 2.56 ± 0.17, while C/A PPF was 1.20 ± 0.05. Last, DCG-IV blocked mossy fiber responses by ≥75% in all experiments in which it was applied but never reduced C/A responses by more than 10%.
[Ca2+]i measurement and photolysis of caged Ca2+
Ca2+ imaging and uncaging Ca2+ were performed as described previously (Wang and Zucker 2001). [Ca2+]i was estimated using the visible wavelength ratiometric fluorescent Ca2+ indicator BTC (Iatridou et al. 1994). We chose BTC (Ca2+ affinity of 7.4 μM) because we expected [Ca2+]i elevations of several micromolars, similar to what we observed in CA1 pyramidal cells under similar conditions (Wang and Zucker 2001). BTC fluorescence was excited alternately at 407 ± 5 and 469 ± 5 nm through a 500-nm dichroic mirror, using a T.I.L.L Photonics (Gräfelfing, Germany) Polychorme IV monochromator, and ratiometric images were produced from emission images filtered at 535 ± 20 nm at ≤2 images/s. [Ca2+]i was estimated according to Eq. 5 of Grynkiewicz et al. (1985), using calibration parameters derived as described in Wang and Zucker (2001). Different regions of the neuron, e.g., the soma, proximal dendrite, and somewhat more distal dendrite, were chosen for analysis.
Photolysis light from a 150-W shuttered Cermax xenon arc lamp (ILC technology, Sunnyvale, CA) was focused onto one end of an optical fiber through an f/1.25 40-mm focal length quartz lens. The other end was connected to the epifluorescence port of the microscope with a T.I.L.L dual-port condenser combining photolysis and fluorescence excitation beams with a 400-nm dichroic mirror, which restricted photolysis energy to the near UV. Light duration was controlled with a Vincent Associates (Rochester, NY) Uniblitz electrical shutter. We calibrated our light source by measuring the photolysis rate of light focused through the microscope onto microcuvettes containing the Ca2+ cage DM-nitrophen and the Ca2+ indicator fluo-3 (Zucker 1994), and correcting for the differences in UV absorbance and quantum efficiency between DM-nitrophen and NPE. Three seconds of photolysis should photolyze about 71% of NPE at a depth of 150 μm below the surface of the slice. We expected that a longer photolysis would cause little additional rise in [Ca2+]i because Ca2+ extrusion would eventually outpace photolysis. This prediction was confirmed by preliminary tests showing the largest [Ca2+]i rises were obtained following 3-s exposures. An even higher proportion of DM-NPE-4 than NPE would be photolyzed in this time. We have also checked for photo-damage due to light exposure and found that only after much longer exposures (>6 s) did we begin to notice a drop in input resistance and increase in holding current.
Problem with polysynaptic contamination
As pointed out above, mossy fiber stimulation, even in the s. lucidum, is likely to include polysynaptic responses, where the first synapse is onto a neighboring pyramidal cell and the second synapse is onto the recorded neuron. This poses several problems. One is that any observed potentiation or depression could occur in the second non–mossy fiber synapse by the usual NMDAR-dependent mechanisms. We have already shown such synapses to be subject to LTP and LTD by postsynaptic elevation of [Ca2+]i (Malenka et al. 1988; Neveu and Zucker 1996; Yang et al. 1999). To address this problem, we analyzed only fast rising EPSCs with short latency, which contain a monosynaptic mossy fiber component. Measuring the rising slopes of these EPSCs (from between about 25 and 75% of the peak) minimizes polysynaptic contributions. The large PPF and the sensitivity to DCG-IV assure that mossy fiber synapses are located somewhere in the excited pathway, and focusing on the monosynaptic component assures that changes in any possible second non–mossy fiber synapse are minimized.
Data classification and statistics
In most studies of long-term plasticity, only one type of response is observed in any particular experimental paradigm. Statistical significance of results is then assessed by grouping all responses from all experiments and testing for significant changes before and after stimulation. Often, this is done by comparing histograms of changes in average excitatory postsynaptic potential (EPSP) slope (Yeckel et al. 1999). We used these conventional procedures to analyze tetanically evoked LTP. In the case of photolysis-evoked responses, however, we sometimes observed potentiation, and other times depression, of EPSCs. Grouping all results together would obscure changes that are really present.
We therefore decided to analyze the results of each photolysis experiment separately for statistical significance. Responses were quantified as the rising slope measured from 25 to 75% of the peak of the first of paired EPSCs. These were binned into one 5- to 10-min group before photolysis and successive 5-min groups after photolysis. We performed one-way ANOVA on the data, asking first whether all responses after photolysis were significantly different from all responses before photolysis. If they were (at the 5% level), we performed a Tukey means comparison of each postphotolysis group to the prephotolysis responses. To be counted as an example of potentiation or depression, responses had to meet all of the following criteria: 1) the change in EPSC slope had to be in one direction; 2) it had to be persistent (significantly different in at least 4 of 5, or 5 of 6 5-min periods after photolysis; our records usually lasted 25–30 min after photolysis); and 3) it had to be rapid (reaching significance within the 1st or 2nd 5-min period after photolysis). Data with a nonstable baseline before photolysis, or a continuous trend after photolysis, were discarded. Once results have been so classified, the responses from each class were grouped in the usual way to provide averaged data to show average effects of photolysis inducing potentiation or depression. This procedure eliminates biases that might otherwise invalidate our conclusions.
Facilitation
Statistical analysis of changes in facilitation by ANOVA required that individual facilitation values (the ratio of the 2nd EPSC slope to the 1st one) be calculated for each pair of responses. However, more accurate and less biased measures of facilitation were obtained by taking the ratio of the average of facilitated responses to the average of unfacilitated response (Kim and Alger 2001). After assessing statistical significance, this latter measure was used to estimate the magnitude of facilitation.
Mossy fiber potentiation or depression was operationally defined as a >20% change relative to baseline in the slope of the EPSC rising phase during the period 20–25 min after stimulation (Yeckel et al. 1999). Measured values are expressed as means ± SE.
RESULTS
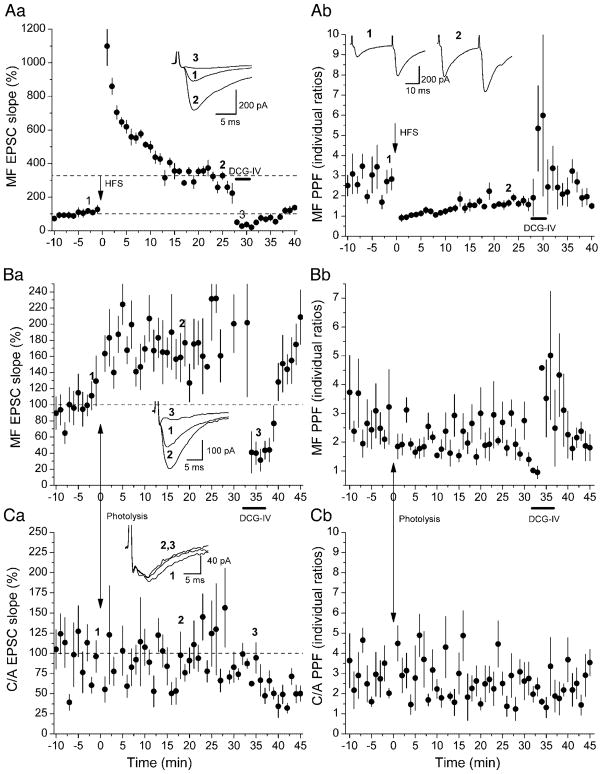
Our initial focus in this study was to see whether we could induce a form of potentiation at mossy fiber synapses onto hippocampal CA3 pyramidal neurons by postsynaptic elevation of [Ca2+]i. We began by assessing the condition of our slices to see if they were capable of generating conventional, tetanically induced mossy fiber LTP. For this purpose, we stimulated mossy fiber afferents and C/A afferents using separate electrodes, identifying the pathways stimulated by the criteria described in METHODS—locus of stimulation, delay, rise time, variability of responses, degree of PPF, and susceptibility to block by DCG-IV. Figure 1Aa illustrates an example of LTP induced by high-frequency stimulation (HFS, 3 trains of 100 pulses each at 100 Hz, separated by 9-s intervals). HFS was delivered to mossy fibers in the presence of NMDAR antagonists dl-2-amino-5-phosphonopentanoic acid (APV, 50 μM) and (+)-MK-801 maleate (20 μM). Stimulation elicited a 200% increase in the maximal slope of the rising phase of EPSCs, measured in the interval between 25 and 75% of reaching the peak. Subsequent addition of DCG-IV (1 μM) to the perfusion medium depressed the EPSCs by 87.1%, confirming their origin from mossy fibers. The depressed responses after DCG-IV showed increased PPF (Fig. 1Ab).
Fig. 1.
Examples of high-frequency stimulation (HFS)-induced long-term potentiation (LTP) and photolysis-induced long-lasting potentiation at mossy fiber (MF) synapses. Rising slopes of the 1st of paired excitatory postsynaptic currents (EPSCs) and corresponding paired-pulse facilitation (PPF) at 30-ms intervals are plotted on left and right panels, respectively. A: HFS of mossy fibers (3 1-s 100-Hz trains separated by 10 s) elicited LTP (Aa), with a reduction in PPF (Ab). (2S,1′R,2′R,3′R)-2-(2,3-dicarboxycyclopropyl)glycine (DCG-IV; 1 μM) was added to the perfusion medium during the bar. B and C: photolysis of caged Ca2+ elicited a long-lasting potentiation in mossy fiber synapses (Ba), but had no effect in commissural/associational (C/A) afferents (Ca: from a different neuron). DCG-IV selectively inhibited mossy fiber response without effect on C/A responses. Uncaging Ca2+ also induced a small but significant reduction in PPF in mossy fiber input (Bb), with no change in C/A input (Cb). Each point plots the average of 6 consecutive EPSCs (±SE). Insets: sample recordings.
This experiment was performed on 23 slices, all of which showed a sudden increase in synaptic transmission right after the tetanus, which decayed within 10–15 min and which we attribute to post-tetanic potentiation (PTP). In 20 of these slices, LTP was also observed, where LTP was defined as a persistent increase in EPSP slope lasting ≥25 min after stimulation and exceeding a 20% increase over pretetanic levels at 20–25 min after stimulation, by which time PTP had dissipated. In nine of these experiments, the perfusion pipette contained 5 mm of NPE loaded 65% with Ca2+, as a test of whether we could obtain tetanically induced LTP in neurons filled with NPE. There was no difference in the distribution of LTP magnitudes between the cells filled with NPE and the cells not filled with NPE by Kolmogorov-Smirnov test (Fig. 2C). Grouping all these slices, the average increase in EPSC slope was to 237 ± 15% of pretetanic slopes (P < 0.05, 2A), which is close to what others have observed (202–221%, Yeckel et al. 1999; 236%, Zalutsky and Nicoll 1990).
Fig. 2.
Summary of HFS- and photolysis-induced MF long-lasting potentiation. A: HFS-induced MF LTP in 20 neurons, 9 of which were filled with nitrophenyl-EGTA (NPE) or dimethoxy-nitrophenyl-EGTA-4 (DM-NPE-4). Data showed no significant difference in EPSC slope or PPF (data not shown) and were therefore grouped together for analysis. B: average responses of 5 experiments in which photolysis induced long-lasting MF potentiation (●). Average responses in 7 cells containing no caged Ca2+ show that light alone has no effect on transmission (○). C: cumulative probability plots summarize results: each point represents the average magnitude of change relative to baseline for a single experiment measured 20–25 min after HFS (○) or photolysis (●). *HFS experiments on cells containing no caged Ca2+. D: HFS- and photolysis-induced MF potentiation differ in PPF. Facilitation was persistently reduced in LTP after HFS, but not after photolysis-induced potentiation. Each bar represents a 5-min average of the 2nd EPSC slope divided by average of the 1st. *Significant differences (P < 0.05) compared with 10 min before stimulation. E: cumulative probability graphs of PPF induced by HFS (○) and photolysis (●) and measured 20–25 min after stimulation. *Experiments showing significant changes in PPF.
In 10 of these 20 experiments, DCG-IV was added to the perfusion medium after LTP induction; it suppressed the potentiated EPSCs to 5.2 ± 1.1% of their magnitudes before adding the drug. This is even greater suppression than the 12.3 ± 3.0% of baseline reported previously, in which DCG-IV was applied without tetanic stimulation (Yeckel et al. 1999).
PPF is due to an increase in transmitter release (Zucker and Regehr 2002), and its change was used to assess the locus of LTP expression (Zalutsky and Nicoll 1990). Figure 1Ab shows an example where individual PPF was significantly reduced from 0 to 25 min after HFS. The estimate of PPF based on the ratio of averaged second responses divided by the average of first responses (Kim and Alger 2001) was significantly decreased 20–25 min after HFS when measured in 17 of the 20 experiments showing mossy fiber LTP (PPF reduced to 81 ± 3% of baseline, P < 0.05, Fig. 2D). However, not all experiments exhibited a persistent reduction in facilitation. The cumulative probability plot reveals that about one-third of the neurons did not show a significant decrease in PPF (Fig. 2E). Our results are similar to those obtained by others at mossy fiber synapses (Staubli et al. 1990; Xiang et al. 1994; Zalutsky and Nicoll 1990).
Are the changes really in mossy fiber synapses?
As pointed out in METHODS, it is possible that C/A afferents are recruited in the s. lucidum. Then any change in “mossy fiber” EPSCs could, in principle, be due to a change in this contaminant. In fact, we usually observe an incomplete block of “mossy fiber” EPSCs by DCG-IV (see Fig. 1).
We have used the following approach to analyze this problem. Consider in particular the results of Fig. 1Aa. The initial response might include responses to commissural/associational fibers (C) as well as responses to mossy fibers (M). Since responses are normalized to their initial magnitude, we may write M + C = 1. After tetanic stimulation, the responses are increased by 228% in this experiment. If we ignore the possibility of C contamination, we would conclude that mossy fiber LTP was 228%. We don’t know, however, whether the increase is in M or C or both. If the new mossy fiber component is M′, and the new C/A component is C′, then M′+ C′ = 3.28. We want to know the effect of stimulation on M; namely, we want to know M′/M − 1, which is the fractional increase in the mossy fiber portion of the signal. Since DCG-IV blocks only M′, the remaining response is C′, which is 0.37, leaving M′ = 2.91. To determine C, we need to know how much potentiation there could be in the C′ component of the responses. The largest level of tetanus-induced LTP observed in CA3 C/A responses is an increase of about 100% (Yeckel et al. 1999; Zalutsky and Nicoll 1990), in which case C′/C = 2.0. To be conservative, let C′/C = 2.5, then C = 0.15, M = 0.85, and M′/M = 3.4. Now the maximally corrected mossy fiber potentiation is 240%, even greater than the uncorrected estimate. This type of analysis was performed on each experiment in which we inferred an effect of photolysis on mossy fiber synapses (see following page). It is important to emphasize, however, that this analysis does not require the assumption that DCG-IV blocks 100% of the mossy fiber response, which, in fact, it is unlikely to do (cf. Alle et al. 2001). Using a 100% block by DCG-IV in the analysis is a worst case scenario because this assumes that the unblocked portion is entirely due to the C/A component. As shown below (see Effect of C/A contamination), if the unblocked component were due to a partially unblocked mossy fiber response, the analysis would result in an even larger component of the LTP being from mossy fiber inputs.
Uncaging Ca2+ can potentiate mossy fiber synapses
We next examined whether we could induce a change in synaptic strength by uncaging Ca2+ postsynaptically. We included NPE or DM-NPE-4 loaded 65% with Ca2+ in the pipette solution and perfused neurons for ≥20 min before photolysis. In five experiments, we observed a long-lasting potentiation of mossy fiber responses following elevation of [Ca2+]i by caged Ca2+ photolysis. Figure 1, Ba and Ca, plots mossy fiber and C/A EPSCs, respectively, from one such experiment. Compared with HFS-induced LTP, photolysis-induced potentiation lacked the large PTP phase. This is expected, however, because PTP is presynaptically induced, and the presynaptic pathway was never tetanized. Statistical analysis indicated that the means of mossy fiber EPSC slopes in the total period from 0 to 25 min and within each 5-min period after photolysis were significantly increased compared with the means of EPSCs in the 10 min before photolysis. While in this particular experiment uncaging Ca2+ induced a significant, but small, reduction in PPF (Fig. 1Bb), there was no significant change in PPF when averaged over all of the experiments in which potentiation was observed (Fig. 2D).
Out of a total of 30 recordings of mossy fiber responses, postsynaptic elevation of [Ca2+]i induced a significant potentiation of responses in five experiments similar to (and including) that of Fig. 1B. In one of these five slices, the C/A responses were also potentiated. In two of them, the C/A responses were unchanged; Fig. 1, B and C, illustrates one of these experiments. In two others, C/A responses were not recorded. The mossy fiber responses in these five datasets were normalized to the average prephotolysis EPSC slopes, and the grouped responses are plotted in Fig. 2B. On average, the EPSC slopes in these five cells were potentiated by 64% (164 ± 11% of baseline, n = 5, P < 0.05). Increase of EPSCs by photolysis is not due to photo-damage, since there is no change in cells where the caged compound was excluded intracellularly (104 ± 3% of baseline, n = 7, P > 0.05, see Fig. 2B). Although this potentiation is significantly smaller than the LTP induced with three trains of HFS, which showed a 137 ± 15% increase (P < 0.05), it is similar to previous results showing a 42% increase (142 ±11% above baseline) when a single long train of HFS is used to induce mossy fiber LTP (Yeckel et al. 1999). Cumulative probability plots also show a significant difference in magnitude between photolysis-and HFS-induced mossy fiber potentiation (P < 0.05 and 0.039, respectively, Kolmogorov-Smirnov test, see Fig. 2C).
Effect of C/A contamination
We used the procedure described above for conventional LTP to estimate the effects of C/A contamination on our measurements of mossy fiber EPSC potentiation. We assumed that the unpotentiated responses were comprised of mossy fiber (M) and C/A (C) components, as were the responses after photolysis (M′, C′), and that in DCG-IV, only C′ is left (a worst case assumption—see following page). For the data from the experiment of Fig. 1B, M + C = 1 (normalization), M′ + C′ = 1.79, and C′ = 0.37. Ignoring C and C′ yields a measure of potentiation of 79%. Next, we need to estimate the amount of potentiation that photolysis is likely to have produced in C, namely C′/C. In experiments on CA1 neurons, caged Ca2+ photolysis typically induced an NMDAR-dependent LTP of 50% (Malenka et al. 1988; Neveu and Zucker 1996; Yang et al. 1999). Taking C′/C = 1.5 gives C = 0.25, M = 0.75, and mossy fiber potentiation of 89%. Even using the most conservative realistic assumption that C′/C = 2.5, then C = 0.15, M = 0.85, and M′/M = 1.67, so mossy fiber potentiation was 67%. This type of analysis was performed on all experiments in which DCG-IV data were available (Table 1). In each case, correction for the likely (or even the most extreme) effects of C/A contamination left mossy fiber potentiation at >25%.
Table 1.
Estimating magnitude of mossy fiber potentiation induced by uncaging Ca2+
| Cell no. | C + M | C′ + M′ | C′* | M′** | If C′/C = 1.5, then M′/M = | If C′/C = 2.5, then M′/M = |
|---|---|---|---|---|---|---|
| 010814c1 | 1 | 1.49 | 0.34 | 1.2 | 1.48 | 1.33 |
| 011002c1 | 1 | 1.29 | 0.1 | 1.2 | 1.28 | 1.25 |
| 011004c2 | 1 | 1.78 | N/A | N/A | N/A | N/A |
| 011022c1 | 1 | 1.79 | 0.37 | 1.4 | 1.89 | 1.67 |
| 020918c1 | 1 | 1.84 | 0.36 | 1.5 | 1.94 | 1.72 |
C + M, average EPSC slope for 10 min before photolysis, normalized to 1; C′ + M′, normalized average EPSC slope 20–25 min after photolysis. C and C′, C/A component before and after photolysis respectively; M and M′, mossy fiber component before and after photolysis respectively.
EPSC slope during DCG-IV application, assuming block of M′.
Calculated from (C′ + M′) − C′.
To carry this analysis even further, we may ask whether there is any amount of potentiation of C/A fibers that could account for the results. To do this, we set M′ = M (assuming no mossy fiber potentiation), with M + C = 1, M′ + C′ = 1.79, and C′= 0.37 as before, to get C = − 0.42. A negative number for C is impossible, so there is no way that our results could arise from a potentiation only in C/A afferents. Applying this analysis to all entries of Table 1 gave similar results—C was always negative, so M′ cannot equal M.
As mentioned above, we may suppose that DCG-IV does not block mossy fiber responses entirely. In that case, the correct value of M′/M would be even larger. For example, if the DCG-IV block of M′ is only 80%, then in the experiment of Fig. 1B, C′ + 0.2M′ = 0.37, while C′ + M′ = 1.79 as before, giving M′ = 1.78, C′ = 0.01, and with C′/C = 2.5, C = 0.004, M = 0.996, and M′/M = 1.79, so mossy fiber potentiation was 79% rather than the 67%, calculated by assuming a complete block of mossy fiber responses by DCG-IV.
Uncaging Ca2+ can also induce a depression of mossy fiber synapses
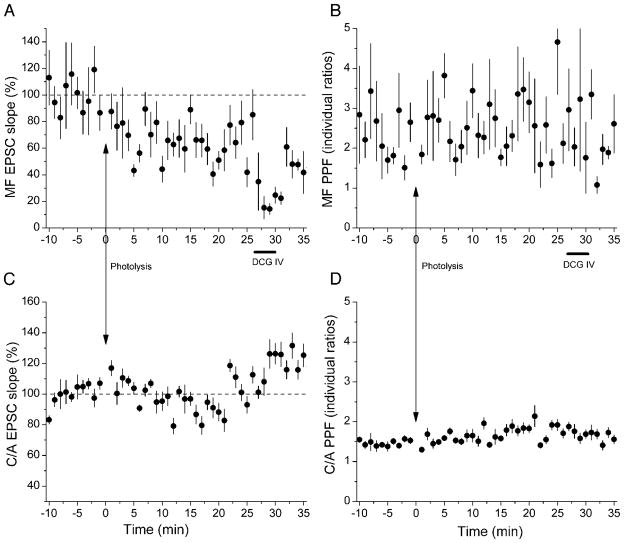
In most of our uncaging experiments, photolysis did not induce potentiation, but rather a long-lasting depression. Photolysis induced a significant potentiation in mossy fiber pathways in 5 experiments and depression in 18, while effects in the remaining 7 experiments were either too small or not persistent enough to be counted as clear examples of potentiation or depression. An example of depression is shown in Fig. 3A, where photolysis induced a persistent decrease in EPSC slope but no change in PPF (Fig. 3B). Simultaneous recording of C/A responses showed no change in either EPSC (Fig. 3C) or facilitation (Fig. 3D).
Fig. 3.
Example of photolysis-induced long-lasting depression in MF synapses. Photolysis elicited a sustained depression in MF EPSC slopes (A), but no significant change in MF PPF (B). Simultaneously recorded C/A EPSCs (C) and their PPFs (D) were unaffected by photolysis. DCG-IV selectively blocked MF EPSCs. Each point plots the average of 6 successive EPSCs (±SE).
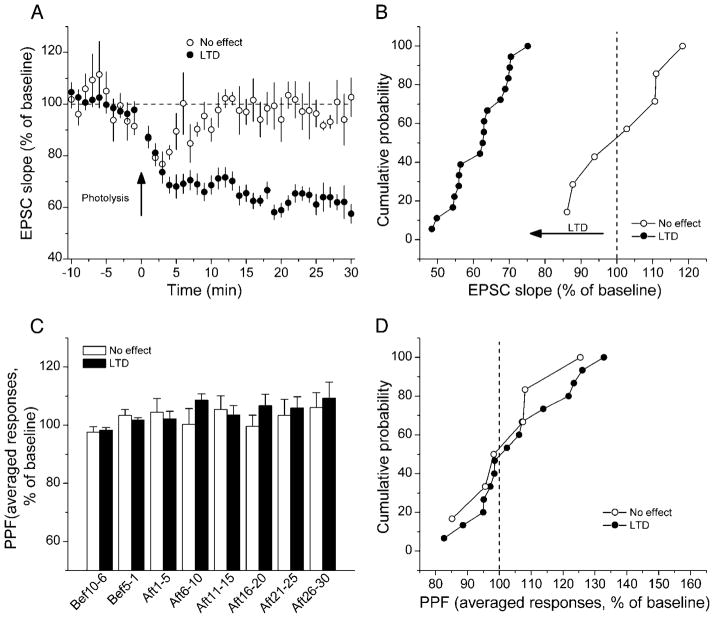
In 18 of our 30 mossy fiber recordings, a statistically significant depression was induced by postsynaptic [Ca2+]i elevation (Fig. 4A). In nine of these slices, effects of DCG-IV allowed us to correct for C/A contamination. We assumed that photolysis produced a 38% reduction in C/A responses comprising the mossy fiber stimulation, typical of the magnitudes of photolysis-induced depression of NMDAR-dependent LTD (Neveu and Zucker 1996). As shown in Table 2, the corrected depression exceeded 25% in all but one experiment.
Fig. 4.
Summary of photolysis-induced long-lasting depression of MF responses. A: uncaging Ca2+ induced long-lasting depression in MF EPSC slopes in 18 neurons (●) and no obvious long-term change in 7 other neurons (○). B: cumulative probability plots of EPSC slopes in cells showing depression or no change in EPSCs 20–25 min after photolysis. C: PPF didn’t change in either group. D: cumulative probability plots show no difference in PPF between groups showing (●) or not showing (○) long-lasting depression at 20–25 min after photolysis.
Table 2.
Estimating magnitude of mossy fiber depression induced by uncaging Ca2+
| Cell no. | C + M | C′ + M′ | C′ | M′ | If C′/C = 0.62, then M′/M = | If M′ = M, then C′/C = |
|---|---|---|---|---|---|---|
| 010717c1 | 1.00 | 0.50 | 0.13 | 0.37 | 0.47 | 0.21 |
| 011008c1 | 1.00 | 0.75 | 0.07 | 0.69 | 0.77 | 0.22 |
| 020215c3 | 1.00 | 0.56 | 0.15 | 0.42 | 0.55 | 0.34 |
| 020226c1 | 1.00 | 0.68 | 0.15 | 0.53 | 0.69 | 0.32 |
| 020304c2 | 1.00 | 0.63 | 0.08 | 0.54 | 0.63 | 0.22 |
| 020320c2 | 1.00 | 0.55 | 0.13 | 0.42 | 0.53 | 0.29 |
| 020530c3 | 1.00 | 0.54 | 0.09 | 0.45 | 0.53 | 0.20 |
| 020625c2a | 1.00 | 0.70 | 0.12 | 0.58 | 0.72 | 0.40 |
| 020625c2b | 1.00 | 0.70 | 0.08 | 0.62 | 0.71 | 0.27 |
| 020709c1 | 1.00 | 0.63 | 0.02 | 0.61 | 0.63 | 0.07 |
Variables defined in Table 1.
As with potentiation, one can ask whether any amount of depression in C/A afferents could account entirely for the results. Assuming M′ = M, one can solve for C = C′ + 1 − (M′ + C′). Applying this to all the data in Table 2, we find that the values of C′/C range from 0.07 to 0.40 (rightmost column of Table 2, 0.25 ± 0.09). Thus for all of the depression to have occurred in a C/A component, that depression would have had to be from 60 to 93%. This explanation is extremely unlikely, because such depression in C/A synapses would be far more depression than has ever been observed for either electrically induced LTD (Dudek and Bear 1992; Mulkey and Malenka 1992) or to photolysis-induced LTD in Shaffer collateral synapses onto CA1 cells (Neveu and Zucker 1996; Yang et al. 1999).
In experiments showing depression, uncorrected EPSC slope decreased to 62 ± 2% of baseline (P < 0.05), which is very close to the reported level of LTD induced by low-frequency synaptic stimulation at mossy fiber synapses (Kobayashi et al. 1996). C/A responses were recorded simultaneously in eight of these slices. In two of them, the C/A EPSCs were potentiated, in two others they were depressed, and in four slices they were unaffected. As in the case of photolysis-induced potentiation, effects on mossy fiber and C/A responses appeared to be independent.
In seven other slices, Ca2+ uncaging induced an initial depression, but with recovery occurring within 15 min after photolysis (to 104 ± 7% of baseline, 20–25 min after photolysis). Averaged results from these experiments are also plotted in Fig. 4A. Cumulative probability plots show a significant difference between these two groups of data (Fig. 4B, P < 0.05, Kolmogorov-Smirnov test). No significant changes in PPF were observed in either group (Fig. 4, C and D).
Postsynaptic [Ca2+]i rise caused by photolysis
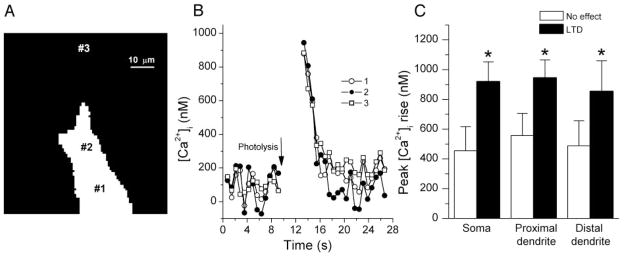
In many experiments, the ratiometric dye BTC was included in the pipette solution, allowing us to monitor the postsynaptic [Ca2+]i change induced by photolysis. Figure 5 shows recordings of BTC fluorescence changes. Photolysis elicited a uniform [Ca2+]i rise in the soma and selected locations in proximal and in somewhat more distal dendrites, decaying fairly rapidly (τ ≈ 2 s) after cessation of illumination. On average, the peak [Ca2+]i rises for these three areas were 919 ± 133, 944 ± 122, 853 ± 206 nM, respectively, in neurons showing depression of mossy fiber responses, and substantially less, 455 ± 162, 557 ± 149, 488 ± 169 nM, respectively, in neurons with no significant change in EPSC on photolysis. The difference between the two groups is significant (P < 0.05), suggesting that a higher [Ca2+]i rise, approaching 1 μM, was required to induce depression. We do not have sufficient data on [Ca2+]i changes in cells showing potentiation from photolysis to permit conclusions about relative [Ca2+ ]i rises in those cells.
Fig. 5.
Measurement of photolysis-induced [Ca2+]i change. A: photograph of BTC fluorescence from a CA3 pyramidal neuron. Numbers mark where postsynaptic [Ca2+]i transients were measured. B: photolysis-induced Ca2+ transients in the cell body and in proximal and distal dendrites (1, 2, 3 as marked in A). C: higher [Ca2+]i elevation was observed in cells showing long-lasting depression than in those showing no change in transmission at MF synapses.
Suppression of photolysis-induced depression by protein phosphatase inhibition
Because NPE and DM-NPE-4 photolysis were relatively successful in producing depression of mossy fiber synapses, we were able to test the pharmacological sensitivity of this effect. Protein phosphatases have been reported to block induction of CA3 LTD (Huang et al. 2002). To examine whether protein phosphatase is involved in the signal transduction of photolysis-induced mossy fiber depression, we incubated slices with the calcineurin antagonist cyclosporin A (200 μM) in five experiments or the PP1/2A antagonist calyculin A (1 μM) in three experiments. A significant effect of photolysis (by ANOVA analysis) was observed in only one of these experiments (with calyculin A). All of these experiments provided data for ≥15 min after photolysis, so the period between 10 and 15 min after photolysis was analyzed. Cumulative probability histograms of these results are plotted in Fig. 6A and are compared with a probability plot of responses measured during the same period in all of our photolysis experiments done without phosphatase inhibition.
Fig. 6.
Dependence of photolysis-induced long-lasting MF depression on protein phosphatases. A: photolysis had no effect on MF responses in 7 of 8 experiments inhibiting phosphatase 1/2A with calyculin A (1 μm) or phosphatase 2B with cyclosporin A (200 μm). Cumulative probability distributions for these results are plotted along with results of 30 photolysis experiments done without phosphatase inhibition. Data symbols distinguish experiments showing statistically significant (by ANOVA) potentiation (○), depression (△), or no change (●). B: PPF was not altered by photolysis in cells treated with phosphatase inhibitors (filled bars). Untreated cells in which photolysis induced long-lasting depression were also unaffected by photolysis (open bars, same data as filled bars in Fig. 4C). C: [Ca2+]i elevation was similar in these 2 groups of cells.
Using Fisher’s exact test, the frequency of occurrence of depression is significantly lower in the presence of phosphatase inhibitors (1 of 8) than in control experiments (18 of 30; P < 0.05). The frequency of obtaining potentiation in control experiments (5 of 30) is too low to allow conclusions to be drawn from the lack of potentiation seen in the eight experiments using phosphatase inhibitors (P = 0.5).
PPF was not altered in cells treated with phosphatase inhibitors (Fig. 6B). However, recall that it was also unchanged in untreated cells in which photolysis did induce depression. There was also no difference in extent of [Ca2+]i elevation between cells showing long-lasting depression and cells treated with phosphatase inhibitors (Fig. 6C), indicating that the block of depression was not due to a reduced level of [Ca2+ ]i caused by photolysis in the cells treated with an inhibitor.
DISCUSSION
Photolysis-induced depression of mossy fiber responses
Our key finding is that elevation of postsynaptic [Ca2+]i to about 1 μM for about 2 s is often effective at inducing a depression in mossy fiber synapses onto CA3 pyramidal neurons. The depression is of similar magnitude to electrically induced LTD and shows a similar sensitivity to protein phosphatase inhibition. However, depotentiation of LTP in CA3 neurons is expressed presynaptically, as shown by an increase in PPF and by assessment of transmitter release probabilities using the NMDAR blocker MK-801 (Huang et al. 2002). In contrast, there was no change in PPF following photolysis-induced depression. One form of LTD that is induced by postsynaptic action potentials alone and that is blocked by postsynaptic Ca2+ chelators (Lei et al. 2003) is also not accompanied by changes in PPF. The photolysis-induced depression observed here may thus be similar to this postsynaptically induced LTD.
Another form of mossy fiber LTD, however, requires activation of presynaptic type 2 metabotropic glutamate receptors (mGluR) (Kobayashi et al. 1996; Yokoi et al. 1996). These receptors may inhibit adenylyl cyclase by G protein coupling (Chen et al. 2001), reducing cAMP production and protein kinase A (PKA) activity, and in turn dephosphorylating target proteins. The available data thus support the existence of both pre- and postsynaptically induced and expressed forms of depression at mossy fiber synapses.
Photolysis-induced potentiation of mossy fiber responses
We also observed that, in some cells, postsynaptic photolysis of caged Ca2+ induced a significant potentiation of mossy fiber– evoked responses. The photolysis-induced potentiation was about one-half as large as the magnitude of tetanic LTP, and it was not accompanied by changes in PPF. Because electrically induced LTP has been shown to produce a decrease in PPF (Zalutsky and Nicoll 1990), our results suggest that photolysis-induced potentiation may be a distinct form of potentiation from tetanically induced LTP.
Different forms of mossy fiber LTP have also been suggested based on different induction protocols. For example, Urban and Barrionuevo (1996) used short and long trains of synaptic stimulation and found that LTP induced by short trains could be blocked by postsynaptic Ca2+ chelators, whereas LTP from long trains could not. Different groups have reported varying abilities to block mossy fiber LTP with postsynaptic Ca2+ chelators (Alle et al. 2001; Mellor and Nicoll 2001; Yeckel et al. 1999), and one possibility for these differences is that there are multiple forms of LTP at this synapse. In support of this possibility is that there have been disagreements regarding the role of presynaptic kainate receptors in LTP (Bortolotto et al. 1999; Contractor et al. 2000, 2001; Lauri et al. 2003; Yeckel et al. 1999; Zalutsky and Nicoll 1990), on postsynaptic (i.e., Hebbian) induction mechanisms for LTP (Contractor et al. 2002; Jaffe and Johnston 1990; Kapur et al. 1998; Zalutsky and Nicoll 1990), on the pre-versus postsynaptic role for cAMP in LTP (Hopkins and Johnston 1988; Wang et al. 2003; Weisskopf et al. 1994), and on the role of opioid receptors in LTP (Derrick and Martinez 1996; Weisskopf et al. 1993; Williams and Johnston 1996). Perhaps an explanation for some of these discrepancies is that there are multiple forms of LTP at this synapse with different mechanisms for both induction and expression.
Buffer capacity of CA3 neurons
It is interesting to compare the effects of photolysis on CA3 neurons to similar experiments on CA1 cells (Wang and Zucker 2001). In those cells, a dimmer light source was used to elevate somatic [Ca2+]i to a level of about 2.5 μM. We have simulated the effect of that light by using a computational model of NPE photolysis in cells (Yang et al. 1999), taking account of the effects of light absorption by tissue above a typical cell (150 μm depth in slice assumed), the presence of a native Ca2+ buffer plus the indicator dye (which acts as an additional buffer), and Ca2+ extrusion. Our simulations matched the [Ca2+]i levels reached experimentally, as well as the [Ca2+]i decay time constants observed (about 4–5 s), with an endogenous buffer ratio of 188, consistent with measurements of Helmchen et al. (1996) and a bulk [Ca2+]i-dependent Ca2+ removal process with intrinsic time constant (in the absence of buffer) of 20 ms. The Ca2+ transients of the cells of Wang and Zucker (2001) decayed more slowly than those measured by Helmchen et al. (1996), but the latter experiments were done at a much higher temperature (37°C rather than 22°C) and in dendritic processes with a much larger surface-to-volume ratio than the somatic responses measured by Wang and Zucker (2001), in which surface pumps should be more effective. In Wang and Zucker (2001), [Ca2+]i decay was also slowed by the unphotolyzed exogenous buffer (NPE) still present after the light exposure.
Using this same computational model, which successfully reproduced the observations of Wang and Zucker (2001) in CA1 cells, we simulated the effects of our now brighter light on CA3 dendrites, but we had to increase the endogenous buffer ratio by 5-fold and the pump rate by 10-fold compared with parameters used in CA1 simulations. The increased removal rate is partly due to the higher temperatures used for the present experiments (30°C rather than 22°C used for the CA1 measurements), and even more to the much smaller processes from which [Ca2+]i was measured in CA3 cells, from which surface membrane pumps will remove Ca2+ much more efficiently. More interesting was the requirement of a substantially higher endogenous buffer ratio needed to account for the much smaller [Ca2+]i transients produced by photolysis in CA3 neurons than in CA1 cells, despite the use of a brighter light source and heavier Ca2+-loading of the perfused Ca2+-cage. We believe the higher buffer capacity of CA3 cells provides one explanation for why more it is so much more difficult to block LTP in CA3 neurons than in CA1 neurons with exogenous Ca2+ buffer (Alle et al. 2001; Mellor and Nicoll 2001; Yeckel et al. 1999).
Differential Ca2+ threshold hypothesis
The relatively modest magnitude of potentiation induced by a postsynaptic [Ca2+]i rise could have any of several causes. Perhaps maximal potentiation requires more than a rise in postsynaptic [Ca2+]i for its full induction. But an equally likely explanation is that the [Ca2+]i elevation in our experiments may have been less than optimal. In cells in which depression was induced, the maximum achievable [Ca2+]i elevation was about 1 μM, due mainly to significantly stronger Ca2+ buffering and more rapid Ca2+ extrusion in CA3 pyramidal cells than in CA1 cells. Similar levels of [Ca2+]i elevation were probably achieved in those experiments using the same protocols in which potentiation was produced. If, as in CA1 cells, reliable induction of LTP requires [Ca2+]i elevation of more like 10 μ for ≥2 s (Malenka et al. 1992; Yang et al. 1999), then even the experiments in which potentiation was observed almost certainly occurred with [Ca2+]i elevations that were far from optimal for inducing the more traditional tetanically induced LTP. Likewise, since reliable induction of CA1 LTD requires a [Ca2+]i elevation lasting ≥1 min, then in the experiments in which depression was observed, the depression was probably also triggered by a less-than-optimal Ca2+ signal. In this regard, the present results resemble those of Neveu and Zucker (1996) and Yang et al. (1999), who found that a brief, modest [Ca2+]i elevation similar to that achieved here could induce either LTP or LTD in synapses onto CA1 neurons, but neither with very high reliability. The simplest explanation is that this signal just “tickles” processes responsible for both LTP and LTD, and either one (or neither one) might be induced. Interestingly, recent simulations of a model of long-lasting changes in synaptic efficacy involving complex interacting phosphorylation and dephosphorylation pathways (D’Alcantara et al. 2003) shows a similar unstable response to a brief modest [Ca2+ ]i elevation. Another equally likely possibility, however, is that the modest elevation of postsynaptic [Ca2+] in the absence of presynaptic stimulation induces forms of both potentiation and depression at mossy fiber synapses that are distinct from synaptically induced LTP and LTD.
Acknowledgments
We thank Dr. Graham Ellis-Davies for the generous gift of DM-NPE-4; Dr. Peter Jonas, R. English, and the Vibratome Company for valuable technical assistance; and Dr. Katsunori Kobayashi for advice on slice preparation.
GRANTS
This work was supported by National Institutes of Neurological Disorders and Stroke Grant NS-15114.
References
- Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber-interneuron synapse. Proc Natl Acad Sci USA. 2001;98:14708–14713. doi: 10.1073/pnas.251610898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Südhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1α is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Chen YL, Huang CC, Hsu KS. Time-dependent reversal of long-term potentiation by low-frequency stimulation at the hippocampal mossy fiber-CA3 synapses. J Neurosci. 2001;21:3705–3714. doi: 10.1523/JNEUROSCI.21-11-03705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne BJ, Xiang Z, Brown TH. Hippocampal circuitry complicates analysis of long-term potentiation in mossy fiber synapses. Hippocampus. 1993;3:115–121. doi: 10.1002/hipo.450030202. [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Sailer A, O’Gorman S, Heinemann SF. Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J Neurosci. 2000;20:8269–8278. doi: 10.1523/JNEUROSCI.20-22-08269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alcantara P, Schiffmann SN, Swillens S. Bidirectional synaptic plasticity as a consequence of interdependent Ca2+-controlled phosphorylation and dephosphorylation pathways. Eur J Neurosci. 2003;17:2521–2528. doi: 10.1046/j.1460-9568.2003.02693.x. [DOI] [PubMed] [Google Scholar]
- DelPrincipe F, Egger M, Ellis-Davies GC, Niggli E. Two-photon and UV-laser flash photolysis of the Ca2+ cage, dimethoxynitrophenyl-EGTA-4. Cell Calcium. 1999;25:85–91. doi: 10.1054/ceca.1998.0009. [DOI] [PubMed] [Google Scholar]
- Derrick BE, Martinez JL., Jr Associative, bidirectional modifications at the hippocampal mossy fibre-CA3 synapse. Nature. 1996;381:429–434. doi: 10.1038/381429a0. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies GCR. Synthesis of photosensitive EGTA derivatives. Tetrahedron Lett. 1998;39:953–956. [Google Scholar]
- Geiger JR, Bischofberger J, Vida I, Fröbe U, Pfitzinger S, Weber HJ, Haverkampf K, Jonas P. Patch-clamp recording in brain slices with improved slicer technology. Pfluegers. 2002;443:491–501. doi: 10.1007/s00424-001-0735-3. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Imoto K, Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys J. 1996;70:1069–1081. doi: 10.1016/S0006-3495(96)79653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Hopkins WF, Johnston D. Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J Neurophysiol. 1988;59:667–687. doi: 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- Huang C-C, Chen Y-L, Liang Y-C, Hsu K-S. Role for cAMP and protein phosphatase in the presynaptic expression of mouse hippocampal mossy fibre depotentiation. J Physiol. 2002;543:767–778. doi: 10.1113/jphysiol.2002.025668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatridou H, Foukaraki E, Kuhn MA, Marcus EM, Haugland RP, Katerinopoulos HE. The development of a new family of intracellular calcium probes. Cell Calcium. 1994;15:190–198. doi: 10.1016/0143-4160(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Ito I, Sugiyama H. Roles of glutamate receptors in long-term potentiation at hippocampal mossy fiber synapses. Neuroreport. 1991;2:333–336. doi: 10.1097/00001756-199106000-00008. [DOI] [PubMed] [Google Scholar]
- Jaffe D, Johnston D. Induction of long-term potentiation at hippocampal mossy-fiber synapses follows a Hebbian rule. J Neurophysiol. 1990;64:948–960. doi: 10.1152/jn.1990.64.3.948. [DOI] [PubMed] [Google Scholar]
- Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Dual mechanism for presynaptic modulation by axonal metabotropic glutamate receptor at the mouse mossy fibre-CA3 synapse. J Physiol. 1999;518:497–506. doi: 10.1111/j.1469-7793.1999.0497p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Umeda K, Ozawa S, Manabe T. Presynaptic Ca2+ entry is unchanged during hippocampal mossy fiber long-term potentiation. J Neurosci. 2002;22:10524–10528. doi: 10.1523/JNEUROSCI.22-24-10524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Yeckel M, Johnston D. Hippocampal mossy fiber activity evokes Ca2+ release in CA3 pyramidal neurons via a metabotropic glutamate receptor pathway. Neuroscience. 2001;107:59–69. doi: 10.1016/s0306-4522(01)00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Yeckel MF, Gray R, Johnston D. L-Type calcium channels are required for one form of hippocampal mossy fiber LTP. J Neurophysiol. 1998;79:2181–2190. doi: 10.1152/jn.1998.79.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci. 2001;21:9608–9618. doi: 10.1523/JNEUROSCI.21-24-09608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Manabe T, Takahashi T. Calcium-dependent mechanisms involved in presynaptic long-term depression at the hippocampal mossy fibre-CA3 synapse. Eur J Neurosci. 1999;11:1633–1638. doi: 10.1046/j.1460-9568.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Manabe T, Takahashi T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron. 2001;32:697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- Lei S, Pelkey KA, Topolnik L, Congar P, Lacaille JC, McBain CJ. Depolarization-induced long-term depression at hippocampal mossy fiber-CA3 pyramidal neuron synapses. J Neurosci. 2003;23:9786–9795. doi: 10.1523/JNEUROSCI.23-30-09786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hough CJ, Frederickson CJ, Sarvey JM. Induction of mossy fiber → CA3 long-term potentiation requires translocation of synaptically released Zn2+ J Neurosci. 2001;21:8015–8025. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Taverna FA, Tu R, Ackerley CA, Wang YT, Roder J. Endogenous Zn2+ is required for the induction of long-term potentiation at rat hippocampal mossy fiber-CA3 synapses. Synapse. 2000;38:187–197. doi: 10.1002/1098-2396(200011)38:2<187::AID-SYN10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Maeda H, Ellis-Davies GC, Ito K, Miyashita Y, Kasai H. Supralinear Ca2+ signaling by cooperative and mobile Ca2+ buffering in Purkinje neurons. Neuron. 1999;24:989–1002. doi: 10.1016/s0896-6273(00)81045-4. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Lancaster B, Zucker RS. Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron. 1992;9:121–128. doi: 10.1016/0896-6273(92)90227-5. [DOI] [PubMed] [Google Scholar]
- Mellor J, Nicoll RA. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nat Neurosci. 2001;4:125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homo-synaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Neveu D, Zucker RS. Postsynaptic levels of [Ca2+]i needed to trigger LTD and LTP. Neuron. 1996;16:619–629. doi: 10.1016/s0896-6273(00)80081-1. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Mellor J, Frerking M, Schmitz D. Kainate receptors and synaptic plasticity. Nature. 2000;406:957. doi: 10.1038/35023075. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Tank DW. The maintenance of LTP at hippocampal mossy fiber synapses is independent of sustained presynaptic calcium. Neuron. 1991;7:451–459. doi: 10.1016/0896-6273(91)90297-d. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Larson J, Lynch G. Mossy fiber potentiation and long-term potentiation involve different expression mechanisms. Synapse. 1990;5:333–335. doi: 10.1002/syn.890050410. [DOI] [PubMed] [Google Scholar]
- Urban NN, Barrionuevo G. Induction of hebbian and non-hebbian mossy fiber long-term potentiation by distinct patterns of high-frequency stimulation. J Neurosci. 1996;16:4293–4299. doi: 10.1523/JNEUROSCI.16-13-04293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Pineda VV, Chan GC, Wong ST, Muglia LJ, Storm DR. Type 8 adenylyl cyclase is targeted to excitatory synapses and required for mossy fiber long-term potentiation. J Neurosci. 2003;23:9710–9718. doi: 10.1523/JNEUROSCI.23-30-09710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zucker RS. Photolysis-induced suppression of inhibition in rat hippocampal CA1 pyramidal neurons. J Physiol. 2001;533:757–763. doi: 10.1111/j.1469-7793.2001.t01-1-00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993;362:423–427. doi: 10.1038/362423a0. [DOI] [PubMed] [Google Scholar]
- Williams S, Johnston D. Long-term potentiation of hippocampal mossy fiber synapses is blocked by postsynaptic injection of calcium chelators. Neuron. 1989;3:583–588. doi: 10.1016/0896-6273(89)90268-7. [DOI] [PubMed] [Google Scholar]
- Williams SH, Johnston D. Actions of endogenous opioids on NMDA receptor-independent long-term potentiation in area CA3 of the hippocampus. J Neurosci. 1996;16:3652–3660. doi: 10.1523/JNEUROSCI.16-11-03652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Greenwood AC, Kairiss EW, Brown TH. Quantal mechanism of long-term potentiation in hippocampal mossy-fiber synapses. J Neurophysiol. 1994;71:2552–2556. doi: 10.1152/jn.1994.71.6.2552. [DOI] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zucker R. Photorelease techniques for raising or lowering intracellular Ca2+ Methods Cell Biol. 1994;40:31–63. doi: 10.1016/s0091-679x(08)61109-7. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]