Abstract
Mesenchymal stem/stromal cells (MSC) are typically used to generate bone tissue by a process resembling intramembranous ossification, i.e., by direct osteoblastic differentiation. However, most bones develop by endochondral ossification, i.e., via remodeling of hypertrophic cartilaginous templates. To date, endochondral bone formation has not been reproduced using human, clinically compliant cell sources. Here, we aimed at engineering tissues from bone marrow-derived, adult human MSC with an intrinsic capacity to undergo endochondral ossification. By analogy to embryonic limb development, we hypothesized that successful execution of the endochondral program depends on the initial formation of hypertrophic cartilaginous templates. Human MSC, subcutaneously implanted into nude mice at various stages of chondrogenic differentiation, formed bone trabeculae only when they had developed in vitro hypertrophic tissue structures. Advanced maturation in vitro resulted in accelerated formation of larger bony tissues. The underlying morphogenetic process was structurally and molecularly similar to the temporal and spatial progression of limb bone development in embryos. In particular, Indian hedgehog signaling was activated at early stages and required for the in vitro formation of hypertrophic cartilage. Subsequent development of a bony collar in vivo was followed by vascularization, osteoclastic resorption of the cartilage template, and appearance of hematopoietic foci. This study reveals the capacity of human MSC to generate bone tissue via an endochondral program and provides a valid model to study mechanisms governing bone development. Most importantly, this process could generate advanced grafts for bone regeneration by invoking a “developmental engineering” paradigm.
Keywords: bone repair, endochondral ossification, hypertrophic chondrocytes, regenerative medicine, tissue engineering
Long bones and the axial skeleton in the developing embryo are formed by endochondral ossification, namely, by remodeling of cartilage templates (1). This process relies on the specialized morphoregulatory functions of hypertrophic chondrocytes (2). Hypertrophic chondrocytes derive from the condensation of mesenchymal precursors and produce a type X collagen-rich avascular cartilaginous matrix. At the periphery of this cartilage tissue, the so-called “borderline” hypertrophic chondrocytes (3) instruct surrounding mesenchymal cells to differentiate into osteoblasts, which results in the formation of a “bony collar.” In parallel, chondrocytes in the central regions direct mineralization of the hypertrophic cartilage by initiating remodeling via the production of specific matrix metalloproteinases (MMP) and attract blood vessels by releasing vascular-endothelial growth factor (VEGF). The in-growing blood vessels deliver osteoblastic, osteoclastic, and hematopoietic precursors, which mediate resorption of the cartilaginous template and formation of vascularized bone containing the so-called stromal sinusoids, which provide the microenvironment for hematopoiesis (1).
Mesenchymal stem/stromal cells (MSC) from human adults have the ability to generate bone tissue in a variety of experimental models (4), and hold great potential in regenerative medicine. Thus far, MSC have been shown to generate bone exclusively through direct osteogenic differentiation (i.e., in a manner akin to intramembranous ossification), using a mineralized surface as “priming” substrate, with the exception of implantation in confined environments that prevent blood vessel invasion (e.g., diffusion chambers; refs. 5 and 6). This approach has already been used for a few clinical trials (7) but has not been effective for widespread clinical application (4). The possibility to engineer MSC-based grafts recapitulating the morphogenetic processes of endochondral ossification of embryonic skeletogenesis would represent an important step forward for bone repair, in line with the recently defined “developmental engineering” concepts (8). In particular, the proposed route to bone formation through cartilage remodeling and vascularization, which is also activated during fracture repair, would potentially overcome issues critical to the physiological functioning of engineered bone grafts, such as osteogenic performance, resistance to hypoxic conditions, and efficiency of engraftment. So far, evidences of ectopic bone tissue morphogenesis via formation of hypertrophic cartilage templates have been reported for murine embryonic stem cells (ESC) (9) and chick embryonic mesenchymal cells (10), but the paradigm has thus far not been reproduced using human ESC or clinically potentially more relevant sources such as adult human MSC (9). The present study aimed at establishing and characterizing an endochondral bone tissue engineering approach, which uses bone marrow-derived, human adult MSC. By analogy to the development of long bones, we hypothesized that the stage of hypertrophy reached in vitro by MSC would critically impact on the process of endochondral ossification following implantation. Our results demonstrate that engineered human hypertrophic cartilaginous tissue has the potential to undergo developmental changes similar to the ones during limb formation. Therefore, this study paves the way toward a “developmental engineering” approach for the regeneration of bone.
Results
In Vitro Maturation of Engineered Hypertrophic Cartilage Tissues.
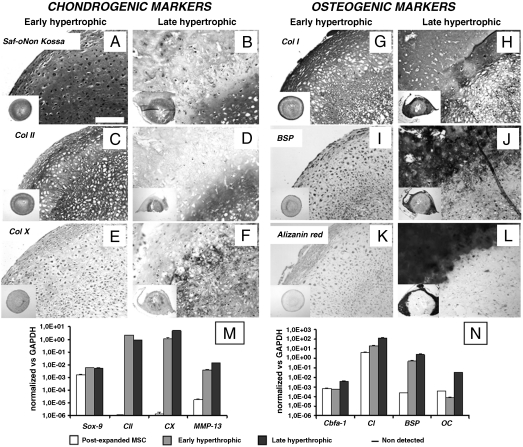
Since endochondral bone formation is initiated by condensation and chondrogenic differentiation of mesenchymal cells, we cultured human adult MSC in a transwell, scaffold-free system to maximize cell–cell interactions (11), using a serum-free medium supplemented with TGFβ1 as a potent inducer of chondrogenesis (12). After one week, the resulting tissues had deposited a loose extracellular matrix, which was faintly positive for glycosaminoglycans (GAG). These specimens will be hereafter referred to as “prechondrogenic” tissues. After 2 weeks, the resulting tissues displayed clear cartilaginous features, including positive Safranin-O staining for GAG (Fig. 1A) and large cells in lacunae embedded in abundant matrix (positive for type II collagen; Fig. 1C). As only low and localized levels of type X collagen, which marks hypertrophic chondrocytes, were detected (Fig. 1E), these specimens will be hereafter referred to as “early hypertrophic” tissues. At this stage, type I collagen was detected throughout the tissue (Fig. 1G), with staining intensity increased in the outer rim. In this outer rim, low levels of bone sialoprotein (BSP) were also detected (Fig. 1I), but no sign of matrix mineralization was apparent (Fig. 1K). In order to induce a more mature hypertrophic phenotype in human MSC, cells were cultured for 3 weeks in chondrogenic medium as described above and then for a further 2 weeks in medium lacking TGFβ1, but supplemented with β-glycerophosphate and l-thyroxin (13, 14). This culture protocol maintained the chondrogenic features, namely, GAG and type II collagen expression (Fig. 1 B and D), but also promoted abundant and widespread accumulation of type X collagen (Fig. 1F). The outer rim of the specimens was uniformly positive for type I collagen (Fig. 1H) and developed a distinct mineralized collar (Fig. 1L), strongly positive for BSP (Fig. 1J). This histological pattern is characteristic of what was previously defined as “chondro-osseous rudiment” (15), and therefore specimens at this stage will be hereafter referred to as “late hypertrophic” tissues. The progression of chondrogenesis through hypertrophy and mineralization between 2 and 5 weeks of in vitro culture was paralleled by increased expression of type X collagen (CX; 4.2-fold), MMP13 (3.7-fold), core-binding factor alpha subunit 1 (Cbfa1; 7.2-fold), osteocalcin (OC; 430-fold), and BSP (BSP; 5.7-fold) transcripts (Fig. 1 M and N). Following hypertrophic differentiation, phenotypic analysis by cell flow cytometry demonstrated an overall decrease in the expression of markers typical for undifferentiated MSC (CD73, CD90, CD105, CD146) and an increase of alkaline phosphatase (ALP; Fig. S1). VEGF transcript, released protein, and matrix-bound protein were detected at similar levels throughout the culture stages (Fig. S2).
Fig. 1.
In vitro maturation of hypertrophic cartilage tissues engineered from human adult MSC. In vitro culture conditions determined the composition and structure of the tissues generated. (A, C, E, and G) Early hypertrophic samples displayed a cartilaginous ECM rich in GAG and Col II with deposition of Col X and Col I in defined regions. (I and K) In the periphery of early hypertrophic samples, low BSP levels were detected, but no calcium was deposited. (B, D, F, H, J, and L) Late hypertrophic samples underwent further maturation in vitro and developed two distinct regions: an inner hypertrophic core (B, D, and F) rich in GAG, Col II, and Col X, and an outer mineralized rim (B, H, J, and L) with a high mineral content, Col I, and BSP. All pictures were taken at the same magnification. (Scale bar: 200 μm.) The insets display low magnification overviews of the entire tissues. (M and N) Quantitative real-time RT-PCR demonstrated an up-regulation of hypertrophic (Col X and MMP-13) and osteogenic (cbfa-1, OC, BSP) markers when comparing late with early hypertrophic tissues. Postexpanded MSC remained in an undifferentiated state but expressed both SOX-9 and Cbfa-1 in combination with high type I collagen and low type II collagen levels.
Progression of Bone Development Following in Vivo Implantation of Hypertrophic Cartilage Constructs.
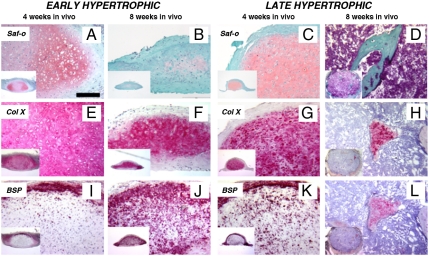
Prechondrogenic, early hypertrophic, and late hypertrophic constructs were implanted subcutaneously into nude mice and harvested after 4 and 8 weeks. Prechondrogenic constructs could not be recovered following implantation, as they were likely resorbed by the host environment. Following 4 weeks in vivo, early hypertrophic samples developed into structures containing cells embedded in large lacunae within an extracellular matrix rich in GAG (Fig. 2A) and type X collagen (Fig. 2E), while BSP deposition was confined in the outer rim (Fig. 2I). Eight weeks after implantation, the extracellular matrix was extensively remodeled and displayed reduced GAG levels (Fig. 2B) and diffused deposition of BSP (including the central regions, Fig. 2J). In the central region BSP overlapped with type X collagen (compare Fig. 2J to Fig. 2F). Four weeks after implantation, late hypertrophic samples displayed two distinct regions: an outer osteoid tissue, which resembled a bony collar, and an inner cartilaginous region, which was faintly positive for GAG (Fig. 2C). Type X collagen was present predominantly in the pericellular space (Fig. 2G), partially overlapping the accumulation of BSP (Fig. 2K). After 8 weeks in vivo, the cartilaginous template was almost completely resorbed, and bone ossicles appeared also in the central region (Fig. 2 D, H, and L). Taken together, these results indicate that (i) a hypertrophic cartilaginous template is required to prime the endochondral process, and (ii) the maturation of early hypertrophic samples toward the endochondral route progresses upon in vivo implantation, albeit it being delayed in comparison to implantation of late hypertrophic tissues.
Fig. 2.
Development of the hypertrophic cartilage tissues following in vivo implantation. The differentiation of cartilaginous constructs in vivo progressed according to their stage of in vitro maturation. (A, E, and I) Four weeks after implantation, early hypertrophic samples had differentiated further toward hypertrophy, displaying larger lacunae, Col X accumulation, and initiated BSP deposition in the outer rim. (B, F, and J) Eight weeks after implantation, early hypertrophic samples had differentiated even further. This was evidenced by a decrease in GAG accumulation, while Col X was maintained and BSP had also been deposited within the cartilaginous core. (C, G, and K) After 4 weeks, late hypertrophic specimens had undergone more intense remodeling, such that GAG and Col X levels were reduced, while BSP had already been deposited within the cartilaginous core. (D, H, and L) After 8 weeks, the cartilaginous template was almost completely resorbed: Bone structures substituted the GAG positive areas in the central region, while Col X and BSP positive areas were restricted to scattered islands. All the pictures were taken at the same magnification. (Scale bar: 200 μm.)
In Vivo Remodeling and Vascularization of Late Hypertrophic Cartilage.
To assess if the progression of tissue morphogenesis in vivo closely resembled normal endochondral ossification, late hypertrophic specimens were analyzed in more detail. Four weeks after implantation, the outer regions corresponding to the bony collar were positive for MMP-13 (Fig. 3A), which is typically expressed by late hypertrophic chondrocytes or osteoblasts and acts upstream of initiating angiogenesis (16). Indeed, capillary vessels positive for CD31 (PECAM-1) began to penetrate the outer matrix (Fig. 3 B and C), consistent with the requirement to transport host osteoclasts, nutrients and proapoptotic signals to the internal hypertrophic cartilaginous template (1, 17). After 8 weeks in vivo, cartilaginous regions undergoing remodeling were surrounded by multinucleated cells of the osteoclastic lineage, as revealed by their positive staining for two markers typically expressed at this stage of resorption (18), namely, tartrate-resistant acid phosphatase (TRAP; Fig. 3D) and MMP-9 (Fig. 3E). The ongoing, active matrix digestion process was confirmed by abundant expression of the so-called cryptic epitope of aggrecan (DIPEN; Fig. 3F), which is exposed specifically upon MMP-mediated cleavage of aggrecan.
Fig. 3.
In vivo remodeling and vascularization of late hypertrophic cartilage implants. (A) The observed remodeling resembles the temporal and spatial changes indicative of ongoing endochondral ossification. Hypertrophic chondrocytes located in the bony collar (BC) synthesized MMP13, which is known to prepare the ECM for vascular invasion during endochondral ossification. (B and C) Newly formed vessels, identified by CD31+ endothelial cells, penetrated the outer matrix and reached the inner core in close proximity to the cartilaginous areas undergoing remodeling (arrows). (D and E) The cartilaginous regions were colonized by TRAP-positive cells synthesizing MMP9. (F) These regions were also positive for the DIPEN, which is produced by MMP-mediated cleavage of aggrecan. (Scale bar: 100 μm.)
Activation of Pathways Involved in Normal Endochondral Ossification.
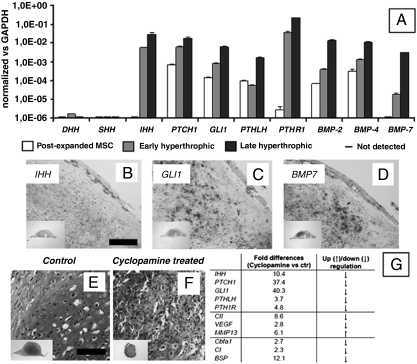
We next performed a real-time RT-PCR and in situ hybridization (ISH) analysis of the expression of key components of pathways that are known to be required for normal endochondral ossification (19–21). In particular, the expression of Indian hedgehog (IHH) as upstream signal, its receptor Patched1 (PTCH1), and GLI1 as mediator of IHH signal transduction was assessed. As expected, all these genes were either not expressed or expressed at very low levels in postexpanded MSC, while their expression levels were markedly increased in the early hypertrophic and even more in the late hypertrophic constructs (IHH 5.2-fold, PTCH1 3.0-fold, GLI1 7.8-fold—when comparing late to early hypertrophic tissues; Fig. 4A). In contrast, the expression of the other two hedgehog ligands present in vertebrate species including humans (sonic hedgehog SHH and desert hedgehog DHH) was not activated (Fig. 4A). Similar to IHH, bone morphogenetic proteins (BMPs) (BMP-2 36.5-fold, BMP-4 7.8-fold, BMP-7 169.4-fold), Parathyroid hormone-related protein (PTHLH 30.8-fold), and its receptor (PTHR1 6.2-fold) were significantly up-regulated in late as compared to early hyperthrophic tissues (Fig. 4A). This shows that the progression toward endochondral ossification, both in vitro and in vivo, is paralleled by the activation and/or up-regulation of the key signaling pathways involved in endochondral bone formation during embryonic limb skeletal morphogenesis (Fig. 4 A–D and Fig. S3). The human specificity of the ISH probes further allows one to conclude that implanted cartilaginous templates included not only cells that provided signals initiating endochondral ossification (i.e., IHH), but also cells which responded to such signals (i.e., by expressing GLI1), and thus activated the required morphogenetic pathways (i.e., by expression of BMP-7). The functional importance of IHH signal transduction was further evidenced by the fact that its selective inhibition by cyclopamine administration (22) to in vitro cultured cartilaginous templates derived from human MSC completely blocked their morphogenesis and maturation (Fig. 4 E–G).
Fig. 4.
Activation of signaling pathways involved in endochondral bone formation in embryos. Signaling pathways typically involved in endochondral ossification were activated in the engineered samples. (A) Real-time RT-PCR analysis indicated that MSC cultured under hypertrophic conditions up-regulated the expression of genes in the IHH signaling pathway (involving IHH, GLI1, and PTCH1), BMPs and parathyroid hormone-related protein signaling (PTHLH, PTHR1). Note that all fold changes in transcript levels are shown in logarithmic scale. (B–D) Four weeks after implantation, the expression of representative genes was assessed by ISH (IHH, GLI1, and BMP7). (E–G) Functional inhibition of the IHH pathway by cyclopamine treatment significantly reduced the expression of genes involved in IHH signaling (IHH, GLI1, PTCH1), PTH signaling (PTHLH, PTHR1), as well as chondrogenic/hypertrophic genes (Col II, VEGF), and osteogenic genes (Cbfa-1, Col I, BSP). Cyclopamine also blocked the differentiation and maturation of the cartilaginous templates in vitro, as assessed by Safranin-O stain. [Scale bar: 200 μm (B–D).] [Scale bar: 400 μm (E and F)].
Characterization and Quantification of the Engineered Endochondral Bone.
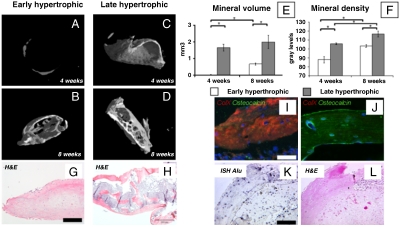
Quantitative microtomography (μCT) of explants confirmed that deposition of mineralized matrix in early hypertrophic samples was negligible at 4 weeks after implantation and remained confined to the outer rim at 8 weeks (Fig. 5 A and B). In contrast, late hypertrophic constructs contained abundant peripheral mineral deposits already at 4 weeks and displayed an interconnected network of trabeculae throughout the core at 8 weeks after implantation (Fig. 5 C and D). Not only the quantity (Fig. 5E) but also the stage of maturation of the mineralized matrix was more advanced in late hypertrophic tissues, as revealed by the significantly higher mineral density (Fig. 5F) at both time points and by the lamellar morphology of the osteoid (Fig. 5 G and H). The complete loss of type X collagen in the lamellar structures of late hypertrophic tissues after 8 weeks in vivo contrasted with the remaining expression and partial overlap with osteocalcin in the early hypertrophic constructs (Fig. 5 I and J). This result indicates that shortened maturation in vitro may delay progression of endochondral ossification in vivo. To further investigate whether prolonged maturation in vivo would be able to compensate for shorter periods of in vitro culture, early hypertrophic constructs were analyzed 11 weeks after implantation, which results in a total period of in vitro and in vivo development equal to late hypertrophic samples analyzed 8 weeks after implantation. Interestingly, early hypertrophic samples analyzed after 11 weeks displayed features of mature bone formation, although they remained significantly smaller than late hypertrophic constructs after 8 weeks of implantation (Fig. S4). It is currently debated whether the bone formed by endochondral ossification is exclusively generated by osteoblastic progenitors delivered by the vasculature or also by cells within the hypertrophic cartilaginous template (15). We thus assessed our explants by ISH for the presence of human Alu repeat sequences. The presence of positive cells within the trabeculae of the bone and the surrounding soft tissue demonstrates an active contribution of the human MSC in the newly formed bone tissue and thus an effective intrinsic osteogenic property of the engineered grafts (Fig. 5 K and L). Due to the nonclonality of the original human MSC population, our results are, however, not conclusive with respect to the controversial issue of whether bone cells derive by direct phenotypic conversion of hypertrophic chondrocytes or by direct osteoblastic differentiation of different MSC subsets (15). Endochondral skeletogenesis is known to generate a marrow microenvironment, which is prerequisite for hematopoiesis (23). Morphological analysis provided evidence for hematopoietic foci in some areas neighboring the bone matrix generated by the human adult MSC (Fig. S5A), which further validates the endochondral ossification process. Support for possibly ongoing hematopoiesis in these foci was provided by the presence of CD146+ stromal cells (24), which were detected in the tissue constructs (Fig. S5B).
Fig. 5.
Morphometric analysis of the engineered bone tissue. (A–D) Three-dimensional μCT reconstructions and (E and F) quantitative histomorphometric data (n = 4) of mineral volume and density indicate higher bone quantity and more advanced maturation of late hypertrophic samples (* indicates significant differences; p < 0.01). (G and H) Trabecular-like structures were found both in the outer bony collar and in the inner core of late, but not early, hypertrophic samples. (Scale bar: 200 μm.) (I and J) Fluorescence characterization for Col X (red) and osteocalcin (green) demonstrated the presence of mature lamellar bone only in late hypertrophic samples. (Scale bar: 50 μm.) (K and L) ISH to detect human Alu repeat sequences and hematoxylin/eosin staining of serial sections indicate that cells derived from the human adult MSC participated in the endochondral ossification process. (Scale bar: 100 μm.)
Discussion
In this study, we report a hitherto not described capacity of adult expanded human MSC to generate de novo bone tissue in vivo through endochondral ossification. The observed endochondral morphogenesis bears striking features of normal endochondral ossification as is typical for limb skeletal development, namely, (i) cellular condensation and hypertrophic chondrogenesis, (ii) functional dependence on IHH signaling, (iii) formation of a bony collar by perichondral ossification, (iv) MMP-mediated matrix remodeling, vascularization, and osteoclastic activity, (v) bone matrix deposition over the resorbed cartilaginous template, and (vi) formation of complete bone tissue, which likely includes functional hematopoietic foci.
Our study shows that activation of the endochondral ossification program requires a mature hypertrophic cartilaginous template obtained in vitro (late hypertrophic constructs) or in vivo (early hypertrophic constructs), in which cells expressing high levels of type X collagen are surrounded by osteoblastic cells expressing high levels of BSP. These observations are consistent with the fact that the development of long bones is triggered by the formation of a vis-à-vis pattern between hypertrophic chondrocytes at the lateral aspects of the rudiment and differentiating osteoblasts in the surrounding mesenchymal tissues (25). Interestingly, the progression of bone formation in vivo was regulated by the developmental stage and size of the hypertrophic cartilaginous constructs generated in vitro for implantation. The need for a fine coordination between the stage of hypertrophy reached in vitro and the time required to achieve endochondral ossification in vivo is further confirmed by the reported absence of “frank” trabecular bone tissue in a previous study where chondrogenically differentiated human MSC were implanted ectopically (26).
The ectopic, “inert” subcutaneous implantation model used for this study allowed us to demonstrate that the hypertrophic cartilaginous constructs contained all necessary “biological instructions” to initiate a developmental process with similarities to what is observed during limb development (i.e., activation and up-regulation of IHH, BMP, and PTHLH signaling, and self-organization of the tissue in a spatially and temporally coordinated manner). The experimental concept described here is in line with the ideas of “developmental engineering,” in that an engineered construct is able to progress through development and differentiation into a structured tissue in a manner comparable to normal progression of embryonic development (8). In particular, no instructive external signals seem required after implantation, and MSC were able to form bone tissue without a ceramic/mineral substrate, which is typically necessary to “prime” differentiation into functional osteoblasts (6, 27). This may open the possibility either to use the tissue itself as a scaffold (this study) or—in case a predefined size and shape is required—to introduce different types of materials (e.g., synthetic polymers) that can be easier tailored with respect to their physical and/or mechanical properties and could be better biodegradable than ceramics (28).
By mimicking normal developmental processes during bone formation and repair, the endochondral route we describe here could provide significant biological and practical advantages in comparison to engineering approaches relying on intramembranous ossification. For example, implantation of a hypertrophic cartilage template, in contrast to a construct delivering undifferentiated MSC or osteoblasts, may be better suited to overcome the initial lack of vascularization and associated hypoxia. In fact, hypertrophic chondrocytes can accelerate graft invasion by blood vessels (e.g., by release of VEGF and production of MMPs) and are physiologically functional even at reduced oxygen tension (29). Moreover, considering the different composition and metabolism of bone tissues formed by intramembranous and endochondral morphogenesis (30), and the concept that bone repair processes should avoid major differences with normal developmental programs (31), MSC-driven bone formation via endochondral ossification could lead to more successful engraftment. Our study warrants further investigations toward a clinical implementation of the developed paradigm, including (i) scaling-up of the constructs implanted and (ii) orthotopic implantation in an immunocompetent animal model as biomechanical and inflammatory/immune mechanisms likely participate in regulating the bone forming potential of the hypertrophic templates. Finally, it will be interesting to assess the contribution of host cells in the observed processes, possibly in immunocompetent models (32), and if devitalized hypertrophic cartilage constructs might be able to initiate endochondral ossification by recruiting host progenitors. Experiments in this direction would open the attractive possibility to manufacture biological off-the-shelf grafts relying on the properties of a cell-laid extracellular matrix.
In conclusion, we describe a model based on human adult MSC, which recapitulates certain aspects of normal endochondral bone formation during embryonic development. The model system described is of clinical relevance for bone engineering and/or regeneration. Furthermore, it can be used to study the cellular/molecular mechanisms that control endochondral bone formation by human adult MSC isolated from both normal individuals and patients affected by specific diseases.
Materials and Methods
All human samples were collected with informed consent of the involved individuals and all mouse experiments were performed in accordance with Swiss law. All studies were approved by the responsible veterinary and ethics authorities. MSC were expanded for two passages and cultured in transwell (5 × 105 cells/insert) for 1 week or 2 weeks in a chondrogenic medium (with TGFβ1; prechondrogenic, and early hypertrophic, respectively), or for 3 weeks in chondrogenic medium followed by 2 weeks in a hypertrophic medium (without TGFβ1 and with beta-glycerophosphate and thyroxine; late hypertrophic). During in vitro culture, selected transwells were supplemented with 3-keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl)-cyclopamine and cultured for 5 weeks. The resulting tissues were analyzed histologically, immunohistochemically, biochemically (GAG and DNA), and by real-time RT-PCR. Prechondrogenic, early hypertrophic, and late hypertrophic tissues were implanted subcutaneously in nude mice and retrieved after 4 or 8 weeks. Tissue development in vivo was evaluated histologically, immunohistochemically, and by μCT. The survival and contribution to bone formation by MSC was evaluated with ISH for human Alu sequences. Nonradioactive RNA ISH on paraffin sections (in vitro and in vivo constructs) was also performed using human antisense riboprobes specific for IHH, GLI1, and BMP7. A more complete and detailed description of the methods is included in SI Text.
Supplementary Material
Acknowledgments.
We are grateful to Silvia Reginato, Dr. Roberto Gianni-Barrera, and Dr. Andrea Banfi for the expert assistance with fluorescence and VEGF quantification; to Prof. Lee Ann Laurent Applegate and Prof. Dominique Pioletti for kindly providing human positive controls for ISH; and to Prof. Bert Mueller for the help in obtaining and interpreting μCT data. The work was partially funded by the Swiss National Science Foundation (Grants 310030-120432 to A. Scherberich and 31003A-113866 to R.Z.), by the European Space Agency (Grant “ERISTO” to I.M.) and by a European Marie Curie Reintegration Grant (PERG05-GA-2009-246576 to J.L.-R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000302107/DCSupplemental.
References
- 1.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 2.Noonan KJ, Hunziker EB, Nessler J, Buckwalter JA. Changes in cell, matrix compartment, and fibrillar collagen volumes between growth-plate zones. J Orthop Res. 1998;16:500–508. doi: 10.1002/jor.1100160416. [DOI] [PubMed] [Google Scholar]
- 3.Bianco P, Cancedda FD, Riminucci M, Cancedda R. Bone formation via cartilage models: The “borderline” chondrocyte. Matrix Biol. 1998;17:185–192. doi: 10.1016/s0945-053x(98)90057-9. [DOI] [PubMed] [Google Scholar]
- 4.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. 2007;4:e9. doi: 10.1371/journal.pmed.0040009. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton BA, et al. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980;151:294–307. [PubMed] [Google Scholar]
- 6.Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138:4456–4462. doi: 10.1210/endo.138.10.5425. [DOI] [PubMed] [Google Scholar]
- 7.Quarto R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 8.Lenas P, Moos MJ, Luyten F. Developmental Engineering: A new paradigm for the design and manufacturing of cell based products. Part I: From three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Pt B-Rev. 2009;15:381–394. doi: 10.1089/ten.TEB.2008.0575. [DOI] [PubMed] [Google Scholar]
- 9.Jukes JM, et al. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:6840–6845. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira SM, et al. Engineering endochondral bone: In vivo studies. Tissue Eng Pt A. 2009;15:635–643. doi: 10.1089/ten.tea.2008.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murdoch AD, et al. Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: Generation of scaffold-free cartilage. Stem Cells. 2007;25:2786–2796. doi: 10.1634/stemcells.2007-0374. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 13.Mackay AM, et al. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 14.Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;8:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muraglia A, et al. Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J Cell Sci. 2003;116:2949–2955. doi: 10.1242/jcs.00527. [DOI] [PubMed] [Google Scholar]
- 16.Stickens D, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber H-P, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:617–618. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 18.Ortega N, Behonick D, Stickens D, Werb Z. How proteases regulate bone morphogenesis. Ann NY Acad Sci. 2003;995:109–116. doi: 10.1111/j.1749-6632.2003.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 19.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karaplis AC, et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–89. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- 21.Yoon BS, et al. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 23.Chan CK, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Riminucci M, et al. Vis-à-vis cells and the priming of bone formation. J Bone Miner Res. 1998;13:1852–1861. doi: 10.1359/jbmr.1998.13.12.1852. [DOI] [PubMed] [Google Scholar]
- 26.Pelttari K, et al. Premature induction of hyperthrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 27.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 28.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 29.Pfander D, Gelse K. Hypoxia and osteoarthritis: How chondrocytes survive hypoxic environments. Curr Opin Rheumatol. 2007;9:457–462. doi: 10.1097/BOR.0b013e3282ba5693. [DOI] [PubMed] [Google Scholar]
- 30.Van den Bos T, Speijer D, Bank RA, Brömme D, Everts V. Differences in matrix composition between calvaria and long bone in mice suggest differences in biomechanical properties and resorption: Special emphasis on collagen. Bone. 2008;43:459–468. doi: 10.1016/j.bone.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Leucht P, et al. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 2008;135:2845–2854. doi: 10.1242/dev.023788. [DOI] [PubMed] [Google Scholar]
- 32.Tasso R, Fais F, Reverberi D, Tortelli F, Cancedda R. The recruitment of two consecutive and different waves of host stem/progenitor cells during the development of tissue-engineered bone in a murine model. Biomaterials. 2009;31:2121–2129. doi: 10.1016/j.biomaterials.2009.11.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.