Abstract
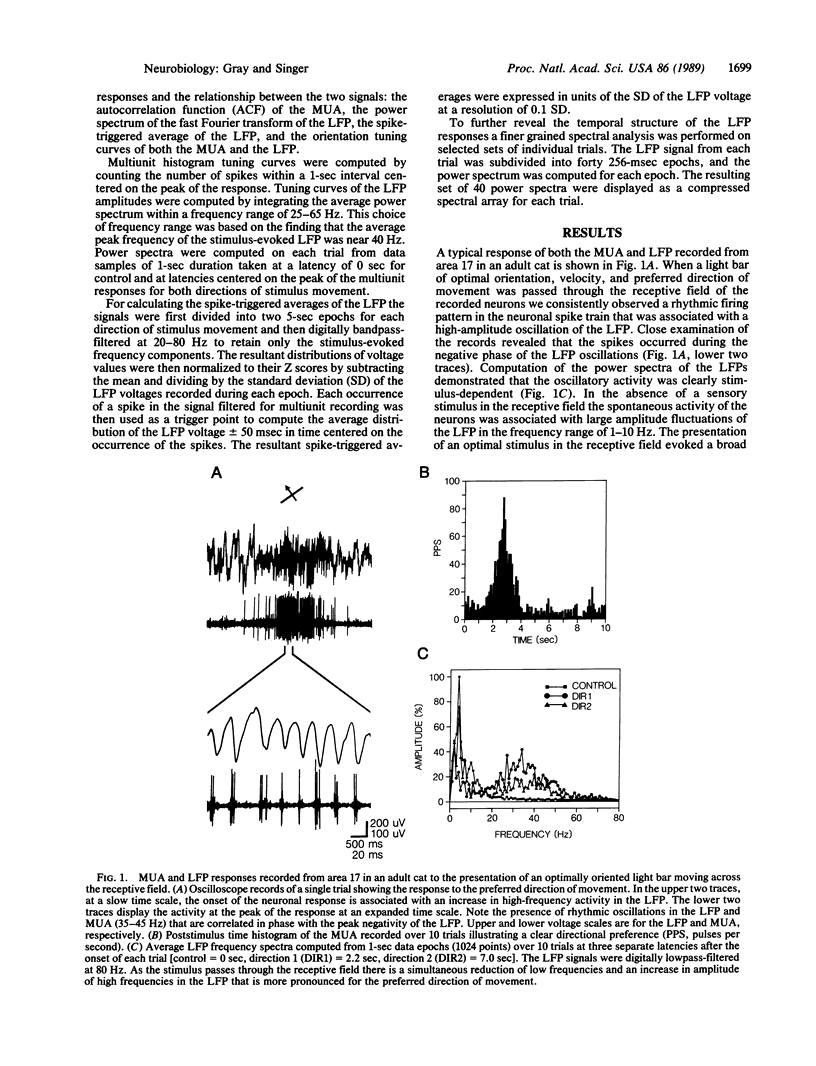
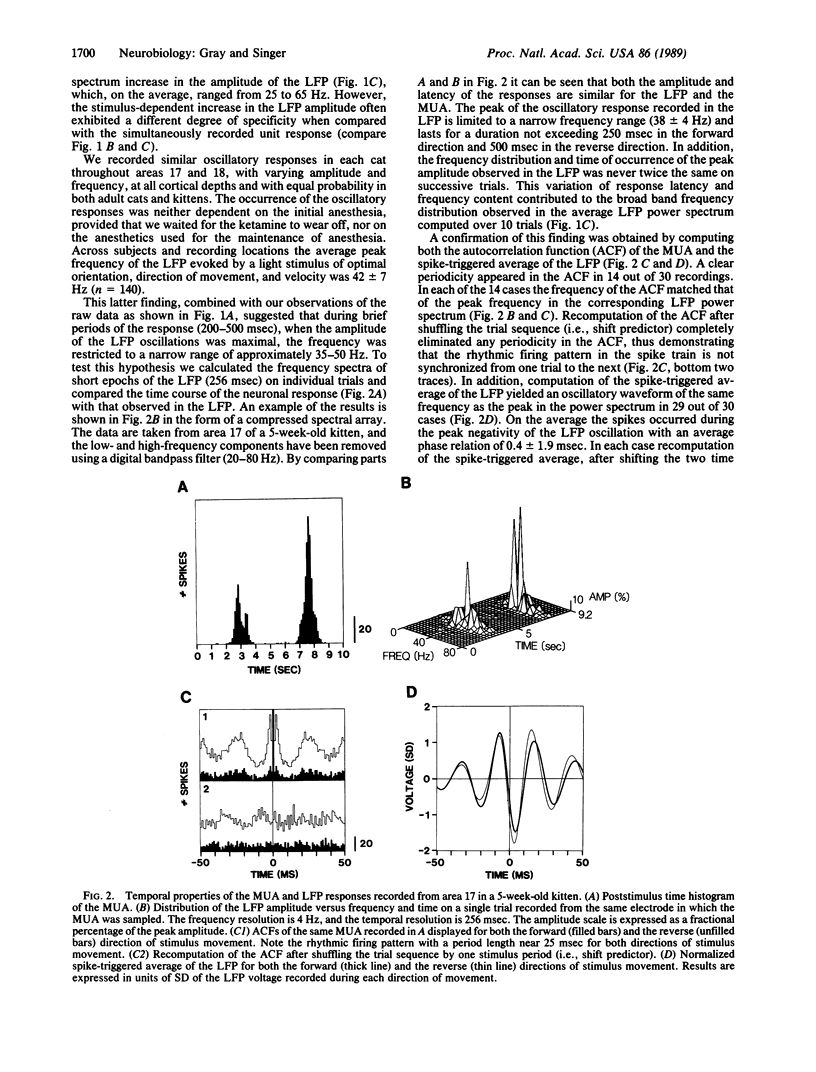
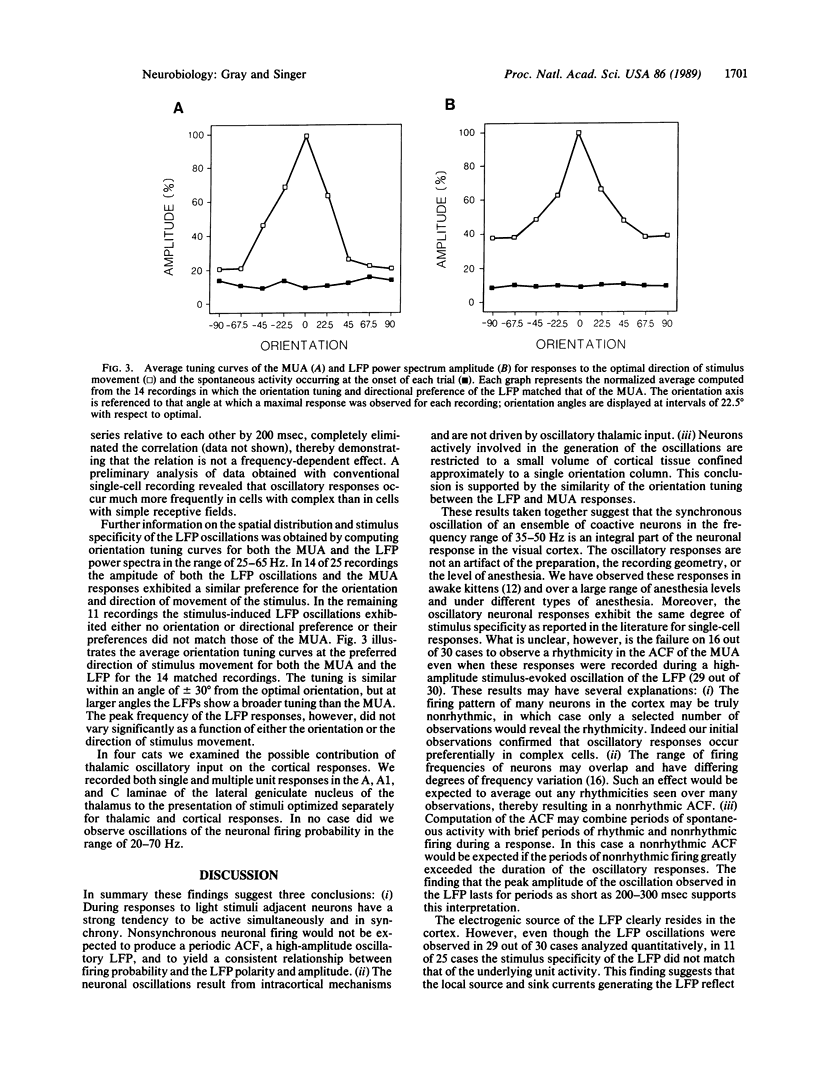
In areas 17 and 18 of the cat visual cortex the firing probability of neurons, in response to the presentation of optimally aligned light bars within their receptive field, oscillates with a peak frequency near 40 Hz. The neuronal firing pattern is tightly correlated with the phase and amplitude of an oscillatory local field potential recorded through the same electrode. The amplitude of the local field-potential oscillations are maximal in response to stimuli that match the orientation and direction preference of the local cluster of neurons. Single and multiunit recordings from the dorsal lateral geniculate nucleus of the thalamus showed no evidence of oscillations of the neuronal firing probability in the range of 20-70 Hz. The results demonstrate that local neuronal populations in the visual cortex engage in stimulus-specific synchronous oscillations resulting from an intracortical mechanism. The oscillatory responses may provide a general mechanism by which activity patterns in spatially separate regions of the cortex are temporally coordinated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN E. D. Sensory discrimination with some recent evidence from the olfactory organ. Br Med Bull. 1950;6(4):330–333. doi: 10.1093/oxfordjournals.bmb.a073625. [DOI] [PubMed] [Google Scholar]
- Bouyer J. J., Montaron M. F., Rougeul A. Fast fronto-parietal rhythms during combined focused attentive behaviour and immobility in cat: cortical and thalamic localizations. Electroencephalogr Clin Neurophysiol. 1981 Mar;51(3):244–252. doi: 10.1016/0013-4694(81)90138-3. [DOI] [PubMed] [Google Scholar]
- Bouyer J. J., Montaron M. F., Vahnée J. M., Albert M. P., Rougeul A. Anatomical localization of cortical beta rhythms in cat. Neuroscience. 1987 Sep;22(3):863–869. doi: 10.1016/0306-4522(87)92965-4. [DOI] [PubMed] [Google Scholar]
- Di Prisco G. V., Freeman W. J. Odor-related bulbar EEG spatial pattern analysis during appetitive conditioning in rabbits. Behav Neurosci. 1985 Oct;99(5):964–978. doi: 10.1037//0735-7044.99.5.964. [DOI] [PubMed] [Google Scholar]
- Ferster D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J Neurosci. 1986 May;6(5):1284–1301. doi: 10.1523/JNEUROSCI.06-05-01284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finette S., Harth E., Csermely T. J. Anistropic connectivity and cooperative phenomena as a basis for orientation sensitivity in the visual cortex. Biol Cybern. 1978 Sep 28;30(4):231–240. doi: 10.1007/BF00361044. [DOI] [PubMed] [Google Scholar]
- Freeman W. J. Nonlinear dynamics of paleocortex manifested in the olfactory EEG. Biol Cybern. 1979 Nov;35(1):21–37. doi: 10.1007/BF01845841. [DOI] [PubMed] [Google Scholar]
- Freeman W. J., van Dijk B. W. Spatial patterns of visual cortical fast EEG during conditioned reflex in a rhesus monkey. Brain Res. 1987 Oct 6;422(2):267–276. doi: 10.1016/0006-8993(87)90933-4. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- Gray C. M., Skinner J. E. Centrifugal regulation of neuronal activity in the olfactory bulb of the waking rabbit as revealed by reversible cryogenic blockade. Exp Brain Res. 1988;69(2):378–386. doi: 10.1007/BF00247583. [DOI] [PubMed] [Google Scholar]
- Greuel J. M., Luhmann H. J., Singer W. Evidence for a threshold in experience-dependent long-term changes of kitten visual cortex. Brain Res. 1987 Jul;431(1):141–149. doi: 10.1016/0165-3806(87)90203-3. [DOI] [PubMed] [Google Scholar]
- Haberly L. B., Bower J. M. Analysis of association fiber system in piriform cortex with intracellular recording and staining techniques. J Neurophysiol. 1984 Jan;51(1):90–112. doi: 10.1152/jn.1984.51.1.90. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. H., van Rotterdam A., Storm van Leeuwen W., Tielen A. M. Dynamic characteristics of visual evoked potentials in the dog. II. Beta frequency selectivity in evoked potentials and background activity. Electroencephalogr Clin Neurophysiol. 1970 Sep;29(3):260–268. doi: 10.1016/0013-4694(70)90138-0. [DOI] [PubMed] [Google Scholar]
- Machleidt C., Rose P., Mittmann U. Prevention of coronary platelet aggregation with a phosphodiesterase inhibitor RX-RA 69. Thromb Res. 1985 Mar 1;37(5):595–604. doi: 10.1016/0049-3848(85)90092-1. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol. 1984 Aug;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. C., Finkel L. H., Edelman G. M. Plasticity in the organization of adult cerebral cortical maps: a computer simulation based on neuronal group selection. J Neurosci. 1987 Dec;7(12):4209–4223. doi: 10.1523/JNEUROSCI.07-12-04209.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeul A., Bouyer J. J., Dedet L., Debray O. Fast somato-parietal rhythms during combined focal attention and immobility in baboon and squirrel monkey. Electroencephalogr Clin Neurophysiol. 1979 Mar;46(3):310–319. doi: 10.1016/0013-4694(79)90205-0. [DOI] [PubMed] [Google Scholar]
- Toyama K., Kimura M., Tanaka K. Organization of cat visual cortex as investigated by cross-correlation technique. J Neurophysiol. 1981 Aug;46(2):202–214. doi: 10.1152/jn.1981.46.2.202. [DOI] [PubMed] [Google Scholar]
- von Seelen W., Mallot H. A., Giannakopoulos F. Characteristics of neuronal systems in the visual cortex. Biol Cybern. 1987;56(1):37–49. doi: 10.1007/BF00333066. [DOI] [PubMed] [Google Scholar]



