Abstract
The neurofibromatosis type 2 (NF2) tumor-suppressor protein Merlin is a member of the ERM family of proteins that links the cytoskeleton to the plasma membrane. In humans, mutations in the NF2 gene cause neurofibromatosis type-2 (NF2), a cancer syndrome characterized by the development of tumors of the nervous system. Previous reports have suggested that the subcellular distribution of Merlin is critical to its function, and that several NF2 mutants that lack tumor-suppressor activity present improper localization. Here we used a Drosophila cell culture model to study the distribution and mechanism of intracellular transport of Merlin and its mutants. We found that Drosophila Merlin formed cytoplasmic particles that move bidirectionally along microtubules. A single NF2-causing amino acid substitution in the FERM domain dramatically inhibited Merlin particle movement. Surprisingly, the presence of this immotile Merlin mutant also inhibited trafficking of the WT protein. Analysis of the movement of WT protein using RNAi and pull-downs showed that Merlin particles are associated with and moved by microtubule motors (kinesin-1 and cytoplasmic dynein), and that binding of motors and movement is regulated by Merlin phosphorylation. Inhibition of Merlin transport by expression of the dominant-negative mutant or depletion of kinesin-1 results in increased nuclear accumulation of the transcriptional coactivator Yorkie. These results demonstrate the requirement of microtubule-dependent transport for Merlin function.
Keywords: neurofibromatosis, intracellular transport, dynein, kinesin, microtubules
Merlin is a tumor-suppressor protein of the ERM family encoded by the NF2 gene that controls cell growth and contact-dependent inhibition of proliferation. Mutations in the NF2 gene are the underlying cause of neurofibromatosis type 2 (NF2), a familial cancer syndrome characterized by the development of sporadic tumors of the nervous system (1–3). To gain insight into Merlin’s cellular functions, we have studied a Drosophila Merlin homolog and its disease-causing mutant. Drosophila Merlin shares the same fundamental domain composition as its mammalian counterpart, and several groups have obtained comparable results using the mammalian and Drosophila proteins (4).
Merlin functions by organizing membrane domains that connect signals coming from the extracellular environment to cytoplasmic factors to ultimately regulate cellular proliferation (5–7). Consistent with this role, Merlin is concentrated in actin-rich structures, such as membrane ruffles in isolated cells and at cell–cell contacts in dense cultures (8–10). Merlin also exhibits a punctate distribution that has been attributed to localization to intracellular vesicles (7, 11, 12). Merlin has a dynamic distribution in Drosophila S2 cells. Studies have shown that the protein is initially localized along the cell cortical region and is then internalized in the form of particles (12, 13).
Merlin mislocalization is associated with abnormal tissue growth and proliferation (12, 13). Many NF2 mutations of Merlin are abnormally distributed in the cells and lack tumor-suppressor activity (14, 15). Moreover, when overexpressed, these mutant versions of Merlin exhibit oncogenic properties, act in a dominant-negative manner, and interfere with the activity of the WT protein (13, 16, 17). How the NF2 mutant protein alters the functional properties of WT Merlin remains unclear, however.
Several studies have demonstrated that phosphorylation regulates Merlin function and affects its intracellular localization (18–20). In Drosophila, mutation of a single threonine residue (T616) changes its distribution and association with various ligands, such as Moesin, another member of the ERM family (20). Both in vivo and in cultured cells, Merlin together with Expanded, have been shown to regulate cell growth and differentiation by promoting phosphorylation and cytoplasmic retention of the transcriptional coactivator Yorkie/YAP1 (12, 21–23). Moreover, recent studies suggest that through localization at the cortical actin network, Merlin controls the internalization and signaling of certain membrane receptors, including the epidermal growth factor (6, 9, 24–27). These results highlight the importance of proper Merlin targeting to this region. Here we explore the mechanism of Merlin intracellular trafficking. Our work shows that a Drosophila Merlin homolog forms particles that move along microtubules through a coordinated action of two microtubule motors, kinesin-1 and cytoplasmic dynein. This movement is phosphorylation-dependent and is inhibited by a tumor-causing mutation in the FERM domain of the protein.
Results
Subcellular Distribution of Merlin-GFP Particles in S2 Cells.
Previous studies in Drosophila embryos have demonstrated that Merlin is localized at the apical plasma membrane and in cytoplasmic puncta (12, 13). To study the mechanisms of Merlin intracellular transport, we used Drosophila S2 cells, which have a consistent morphology and spread well on substrates coated with Concanavalin A (Con A). We first analyzed the localization of endogenous Merlin by immunofluorescence using an antibody against Drosophila Merlin. Our results showed that endogenous Merlin forms clusters that are distributed underneath the plasma membrane as well as in the perinuclear region (Fig.1A). To characterize Merlin clusters and their dynamic behavior, we generated an S2 cell line stably expressing a Merlin-GFP fusion protein (MerGFP) under the control of a heat-shock promoter. The expression level and inducible nature of MerGFP in the stable cell line were confirmed by immunoblotting with both anti-GFP and anti-Merlin antibodies (Fig. S1). Similar to untagged Merlin, MerGFP forms particles in the cytoplasm and near the plasma membrane (Fig. 1B and Movie S1). To examine whether Merlin particles are associated with an endocytic compartment, we incubated S2 cells plated in Con A with rhodamine-dextran. Merlin particles did not colocalize with the rhodamine-dextran–containing endosomes after a 4-h postincubation (Fig.1 E–G and Movie S1). Similarly, MerGFP particles did not colocalize with the endosomal marker Rab5 (Fig. S2).
Fig. 1.
Distribution of Merlin and endocytic vesicles in Drosophila S2 cells. (A–D) Drosophila S2 cells plated in Con A without or with Cyto-D treatment; and expressing either endogenous Merwt (A and C) or MerGFP under a heat-shock promoter (B and D). (A and B) Spread S2 cells were fixed and stained with anti-Merlin antibody. Merlin particles are found in the proximity of cell membrane (Inset) and in the perinuclear region (arrow). The distribution is identical for both Merwt (A) and MerGFP (B). (C and D) Cyto-D MerGFP S2 cells form processes that are filled with particles. This distribution is identical for both Merwt (C) and MerGFP (D). (E–G) MerGFP cells incubated with rhodamine-dextran and analyzed by fluorescence microscopy. (E) DIC image of S2 cells plated in Con A. (F) Distribution of MerGFP particles. (G) Rhodamine-dextran–labeled endosomes. See Movie S1.
MerGFP Moves Bidirectionally Along Microtubules.
S2 cells can be induced to form long, thin processes when treated with cytochalasin-D (Cyto-D) and plated on Con A (Fig.1 C and D) (28). Cyto-D–induced processes contain parallel arrays of microtubules of uniform polarity with their plus-end directed toward the tips and are an ideal model for assessing microtubule-based transport. Both endogenous Merlin and MerGFP particles were found in the processes and the perinuclear region of Cyto-D–treated S2 cells (Fig.1 C and D). MerGFP particles move bidirectionally in these processes (Fig. 2 A and B and Movie S2). Approximately 20% of particles undergo fast and repeated bidirectional movements characteristic of microtubule motor–driven transport (Fig. 2C). MerGFP particles exhibited an unbiased bidirectional motion and displayed similar distributions of velocities for plus-end and minus-end movement, with an average plus-end velocity of 0.50 ± 0.22 μm/s and an average minus-end velocity of 0.49 ± 0.21 μm/s (Fig. 2C). Together, these results indicate that MerGFP particles engaged in bidirectional transport are likely to be moved by the microtubule-based transport system.
Fig. 2.
Movement of MerGFP particles along processes of S2 cells. (A and B) MerGFP particles move bidirectionally in the processes of Cyto-D–treated S2 cells (Movie S2). Frames in B correspond to the boxed area in A. Time 0 indicates time-lapse start point (1 h after heat shock), and particle plus and minus end movement is shown in the first and second columns respectively (white arrowheads). (C) Histogram of velocities for MerGFP particles shows unbiased bidirectional movement.
Disease-Causing Mutants of Merlin Are Defective in Transport.
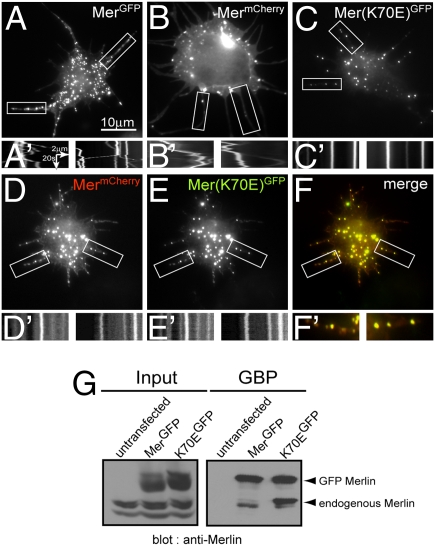
Several groups have used disease-causing mutants of the NF2/Merlin tumor suppressor to elucidate the molecular mechanisms of Merlin function. Certain point mutations in the NF2 gene are known to produce a full-length, stable protein that is functionally inactive. Consequently, we examined the intracellular transport of a disease-causing mutant of Merlin known to lack tumor-suppressor activity and found that it is distributed abnormally compared with WT protein. The Merlin mutant K70E contains a replacement of a single conserved Lys to Glu at position 70 within the FERM domain, which is equivalent to residue K79 in human NF2 (Fig. S3) (14). To study the intracellular transport of this mutant in Drosophila S2 cells, we generated a stable cell line expressing a GFP-tagged mutant, Mer(K70E)GFP, under a heat-shock promoter and observed its distribution and behavior in live cells. Similar to the WT protein, Mer(K70E)GFP formed particles within the perinuclear region and processes of S2 cells (Fig. 3 A and C); however, analysis of time-lapse movies of Mer(K70E)GFP revealed a dramatic difference between the behavior of the WT and mutant protein (Fig. 3 A′–C′). Unlike normal Merlin particles, the clusters formed by Mer(K70E)GFP protein were completely stationary (Fig. 3C′ and Movie S3).
Fig. 3.
An NF2 mutant of Merlin, Mer(K70E)GFP, is defective in intracellular transport. (A and B) Cyto-D–treated S2 cells stably expressing MerGFP (A) or MermCherry (B) (Movie S4). (A′ and B′) Kymographs of the boxed areas showing particles moving bidirectionally along processes. (C) Expression of the NF2-mutant Mer(K70E)GFP in S2 cells. (C′) Kymographs of the boxed areas showing absence of movement (Movie S3). (D–F) Coexpression of MermCherry (D) and Mer(K70E)GFP (E) in S2 cells. (F and F′) Colocalization of the mutant and the WT protein. (D′ and E′) Kymographs of the boxed areas. (G) Pull-down using GBP. Input, crude cell extract from WT cells (untransfected) and cells expressing MerGFP or Mer(K70E)GFP after heat shock. GBP, pull-down from WT extracts (untransfected) or extracts from S2 cells expressing MerGFP or Mer(K70E)GFP. Blots were probed with antibodies against Merlin.
We then tested whether overexpression of WT Merlin could rescue bidirectional transport of the disease-causing mutant. For this purpose, we coexpressed mCherry-tagged Merlin (MermCherry) under a constitutively active promoter in Mer(K70E)GFP cells. Before heat shock, Mer(K70E)GFP was not expressed, and MermCherry formed particles that move bidirectionally along processes, as was seen in its GFP counterpart (Fig. 3 B and B′ and Movie S4). After induction of NF2 mutant expression by heat shock, MermCherry Mer(K70E)GFP colocalized with Mer(K70E)GFP (Fig. 3 D–F). Remarkably, the particles containing both MermCherry and Mer(K70E)GFP were completely immotile (Fig. 3 D′ and E′ and Movie S5). This result could be explained if Mer(K70E)GFP associated in a heterocomplex with WT Merlin. We performed a pull-down of Mer(K70E)GFP using immobilized GFP binder protein (GBP) (29). Blotting with anti-Merlin antibody revealed that endogenous Merlin forms a complex with Mer(K70E)GFP (Fig. 3G). Note that anti-Merlin antibody recognizes both slow-migrating and fast-migrating bands of endogenous Merlin (hyperphosphorylated and hypophosphorylated forms, respectively). Interestingly, K70E, the disease-causing mutant of Merlin, was bound preferentially to the hyperphosphorylated form of the endogeneous protein (Fig. 3G, last lane).
We then tested whether expression of the NF2 mutant caused a global inhibition of transport along microtubules, including cargoes unrelated to Merlin. We selected mitochondria movement for this analysis because mitochondria, like Merlin particles (see below), are moved by conventional kinesin (30). Time-lapse movies of mitochondria labeled with MitoTracker Red show that expression of Mer(K70E)GFP did not affect movement of mitochondria (Movie S6). This finding demonstrates that the inability of Mer(K70E)GFP to move in the cell does not result from global suppression of motor-driven motility, but is likely explained by the intrinsic inability of mutant Merlin to interact with microtubule transport system.
Expression of the NF2 Mutant Mer(K70E)GFP Relocalizes Yorkie to the Nucleus.
Merlin regulates cell growth by signaling through the Hippo pathway to inhibit the function of the transcriptional coactivator Yorkie (Yki) (22). We used Yki translocation into the nucleus of S2 cells as readout to test whether Merlin’s function requires a motile protein (Fig. 4A). In control cells, expression of YkimCherry alone or in coexpression with MerGFP resulted in ∼10% of mCherry fluorescence in the nucleus. Knock-down of Merlin using specific RNAi probes or expression of immotile Mer(K70E)GFP resulted in an almost 3-fold increase in nuclear fluorescence (Fig. 4 A and B and Fig. S4). We hypothesized that immobilization of Merlin by Mer(K70E)GFP is the cause of Yki redistribution, and thus directly tested the physiological relevance of Merlin transport in another series of experiments.
Fig. 4.
Expression of Merlin mutant affects Yorkie distribution. (A) From left to right, expression of YkimCherry alone, coexpression with MerGFP, Merlin RNAi, coexpression with Mer(K70E)GFP, and KHC RNAi. (B) Quantification of Yorkie fluorescence in the nucleus. Error bar indicates SD (ANOVA). P = 0.01.
Merlin Transport Machinery Includes Kinesin-1 and Cytoplasmic Dynein.
To gain insight into the mechanism of Merlin transport along microtubules, we used a combined approach including RNA interference (RNAi), fluorescence microscopy, and biochemical analysis. Merlin has been shown to coimmunoprecipitate with kinesin in cultured HeLa cells (31), which suggests its association with microtubule motors. Direct functional evidence of the involvement of kinesin-1 or dynein in Merlin transport is lacking, however. Therefore, to identify components of the microtubule transport machinery for Merlin particles, MerGFP cells were treated with dsRNA for various motors, and the specificity and efficiency of RNAi knockdown was examined by immunoblot analysis (Fig. S4). When the heavy chain of kinesin-1 (KHC) was knocked down, the motility of MerGFP particles was dramatically reduced (Fig. 5 A and B). Tracking and kymographic analysis of particle movement clearly showed that KHC-RNAi treatment reduced the number of movements by >10-fold (Fig. 5 A and B). As a control for motor specificity, we treated S2 cells with RNAi against another member of the kinesin superfamily, Klp68D (a subunit of Drosophila kinesin-2). As shown in Fig. 5A, depletion of kinesin-2 had no effect on MerGFP movement.
Fig. 5.
Bidirectional movement of Merlin clusters depends on kinesin-1, dynein motors, and adaptor proteins KLC and dynactin. (A) Frequencies of plus-end (white bars) and minus-end (black bars) movements of MerGFP particles. Bars represent the percentage of vectors of length >0.35 μm. Depletion of either KHC or DHC was sufficient to inhibit bidirectional movement of Merlin particles. (B) Kymographs of MerGFP particles moving in the processes. RNAi targets are listed above the corresponding panels. Error bar indicates SD (ANOVA). P = 0.005.
The bidirectional nature of MerGFP transport implies that a minus-end–directed microtubule motor is also involved in the observed retrograde movement. Indeed, depletion of dynein heavy chain (DHC) inhibited the bidirectional movement of particles by >4-fold, as demonstrated by both the number of moving particles (Fig. 5A) and kymographic analysis of time-lapse movies (Fig. 5B). Similar to KHC knockdown, depletion of DHC inhibited both plus-end and minus-end transport. This bidirectional transport inhibition cannot be explained by the simultaneous knockdown of DHC and KHC, because the expression of one of these motors was not affected when the other motor was knocked down (Fig. S4). Based on these results, we conclude that both kinesin-1 and cytoplasmic dynein are required for transport of the Merlin particles along microtubules.
We then tested whether kinesin light chain (KLC) and dynactin, known adapters for kinesin-1 and cytoplasmic dynein, respectively (32, 33) are required for motor-driven transport of Merlin particles. For this test, we depleted S2 cells of KLC or p150glued, the key subunit of the dynactin complex, using specific RNAi probes, and analyzed MerGFP particle movement. The anti-KLC and anti-p150 antibodies used to examine the depletion efficiency decreased dramatically after RNAi treatment (Fig. S4). Knockdown of KLC and p150glued inhibited the movement of MerGFP particles (Fig. 5A and B). In conclusion, our functional data indicate that Merlin intracellular transport is driven by kinesin-1 and cytoplasmic dynein, and that these motors use KLC and dynactin, respectively, as their adapters.
To test the functional significance of the microtubule-dependent transport of Merlin, we tested the effect of kinesin-1 depletion on the distribution of YkimCherry between the nucleus and the cytoplasm. Remarkably, inhibition of kinesin-driven transport of Merlin particles caused a 3-fold increase in the nuclear accumulation of Yorkie. This result is similar to the effect of either depletion of endogenous Merlin or WT Merlin immobilization by expressing the Mer(K70E) mutant (Fig. 4 A and B and Fig. S4).
Merlin Binds Kinesin-1 and Cytoplasmic Dynein.
When endogenous Merlin or MerGFP were pulled-down with either anti-Merlin antibodies or GBP, respectively, both KHC and DHC were detected by immunoblotting (Fig. 6 A and B). As a control for endogenous Merlin immunoprecipitation, we substituted the Merlin antibody with preimmune IgG (Fig. 6A), and to test GBP specificity, we used extracts from untransfected S2 cells that do not express MerGFP (Fig. 6B). Neither KHC nor DHC could be detected in any of these controls, demonstrating that the interactions of both endogenous Merlin and MerGFP with KHC and DHC are specific. Thus, the biochemical data support the functional evidence that Merlin indeed forms a complex with KHC and DHC.
Fig. 6.
Merlin forms a complex with kinesin-1 and dynein motors. (A) Immunoprecipitation of endogenous Merlin from WT S2 cell extracts with anti-Merlin antibody. Input, crude cell extract; IgG, control IgG preimmune serum; anti-Mer, anti-Merlin antibody. (B) Pull-down using GBP. Input, crude cell extract from WT cells (untransfected) and cells expressing MerGFP or Mer(K70E)GFP after heat shock; GBP, pull-down from WT extracts (untransfected) or extracts from S2 cells expressing MerGFP or Mer(K70E)GFP. Blots were probed with antibodies against kinesin (KHC) or dynein (DHC).
The foregoing results led us to hypothesize that the observed lack of transport of Mer(K70E)GFP could be due to a defect in the association with a motor complex. Therefore, we examined whether the Mer(K70E)GFP mutant was able to associate with KHC and DHC. Pull-down of cell extracts from Mer(K70E)GFP using GBP shows that the disease-causing protein did not interact with microtubule motors (Fig. 6B). These data indicate that Merlin clusters are associated with kinesin-1 and dynein molecular motors, and these motors are responsible for movement of the tumor-suppressor protein along microtubules.
Phosphorylation Regulates Association of Merlin With Molecular Motors.
Previous studies demonstrated that in Drosophila the subcellular localization of Merlin is regulated by phosphorylation. A single mutation in a threonine residue—a putative phosphorylation site, Thr616—was shown to be responsible for changes in the protein distribution presumably affecting intramolecular interactions between FERM and C-terminal domains (20). Thus, we tested whether phosphorylation of Thr616 serves as a regulatory mechanism for Merlin transport. We used stable S2 cell lines expressing a nonphosphorylatable mutant of Merlin, Mer(T616A)GFP, or a phosphomimetic mutant, Mer(T616D)GFP, under a heat-shock promoter (Fig. S3). These mutants have been previously shown to behave like the dephosphorylated and phosphorylated forms of Merlin, respectively (20). To analyze the distribution of MerGFP and mutant Merlin particles in 3D, we used confocal microscopy and built Z-stacks (Fig. S5 A–F). In control MerGFP cells, at 4 h postinduction, particles were found at the cell cortex, in processes, and in the perinuclear region of S2 cells (Fig. S5 A, A′′, and D). Expression of Mer(T616A)GFP results in the formation of rather large particles, but unlike MerGFP, Z-stacks show that these particles were preferentially accumulated in the perinuclear region (Fig. 7D and Fig. S5 B, B′′, E, and G). Conversely, a Mer(T616D)GFP phosphomimetic mutant formed smaller particles primarily at the cell cortex (Fig. S5 C, C′′, F, and G). The dramatic effect of a single amino acid substitution at Thr616 on the subcellular localization of Merlin suggests that phosphorylation of Thr616 plays an important regulatory role in protein transport.
Fig. 7.
Thr-616 phosphorylation regulates Merlin bidirectional transport and motor association. (A and A′) S2 cells expressing MerGFP particles move bidirectionally in processes (Inset) as shown by kymograph analysis (A′). (B and B′) Expression of Mer(T616A)GFP, a nonphosphorylatable mutant, produces particles that move bidirectionally (Inset and B′), but mostly accumulate at the perinuclear region (Movie S7). (C and C′) Expression of Mer(T616D)GFP, a phosphomimetic mutant, generates small particles that lack bidirectional movement (C′ and Movie S8) and are located at the cortex (Inset). (D) Comparison of particle distributions in MerGFP and its phosphomutants. The bar graph displays the percentage of particles found in the processes. (E) Immunoprecipitation of cell extracts with anti-GFP antibody. Input, crude cell extract; MerGFP, IP from extracts of cells expressing Merlin-GFP after heat shock; induction, IP from extracts from MerGFP cells before heat shock (control); T616DGFP, IP from extracts from cells expressing Mer(T616D)GFP; T616AGFP, IP from extracts from cells expressing Mer(T616A)GFP. Blots were done with anti-dynein and anti–kinesin-1 antibodies. The bottom part of the gel was stained with Coomassie blue for loading control (load).
To further address the effect of phosphorylation on Merlin particle movement and subcellular distribution, we analyzed time-lapse images of Merlin particles for each individual mutant (Fig. 7 A–C). In agreement with the confocal microscopy data, Mer(T616A)GFP particles were rarely found in processes and mostly accumulated in the perinuclear region (Fig. 7 B and D); however, a few particles found in processes showed fast bidirectional movements (Fig. 7B′ and Movie S7). These movements were comparable to the movements of particles in control MerGFP cells characterized by kymographic analysis (Fig.7 A, A′, B, and B′). In contrast, Mer(T616D)GFP phosphomutant formed smaller and immotile particles distributed beneath the cell boundaries shown in Fig. 7C (Inset) and Fig. S5 C, C′′, F, and G. These observations suggest that phosphorylation of Merlin in Thr616 might affect Merlin’s ability to assemble into a motile complex (Fig. 7C′ and Movie S8). Consistent with these findings, immunoprecipitation studies showed that Mer(T616A)GFP interacted with both kinesin-1 and dynein, whereas Mer(T616D)GFP did not (Fig. 7E). Taken together, our functional and biochemical data suggest that the association of Merlin with a microtubule transport system is dependent on the phosphorylation state of Thr616, and that phosphorylation of this residue is critical to regulation of targeting and distribution of Merlin to specific cell regions.
Discussion
Merlin is a tumor-suppressor protein found both at the plasma membrane and in clusters in the cell. The subcellular distribution of the Merlin protein is critical for its tumor-suppression activity. Merlin has been shown to inhibit mitogenic signaling by binding to the E3 ubiquitin ligase CRL4DCAF1 in the nucleus (34). How the protein translocates between different cellular compartments and reaches the nucleus remains unknown, however. Here we provide evidence that Merlin forms clusters that move along microtubules, and that microtubule-dependent transport of Merlin is an essential part of the regulatory mechanism of the tumor suppressor.
Our data demonstrate that Merlin is bidirectionally transported in the cytoplasm along microtubules guided by the microtubule motors kinesin-1 and cytoplasmic dynein. Movement of Merlin-containing particles also requires the cofactors KLC and dynactin (Fig. S6A). These results agree with previous studies showing that Merlin associates with a 400-KDa complex containing KHC (31). In addition, we show that Merlin transport affects its function. We found a marked increase in nuclear accumulation of the transcriptional coactivator Yorkie, a downstream effector of Merlin, under conditions in which Merlin bidirectional transport is inhibited by KHC knockdown or coexpression with an NF2 mutant (Fig. 4).
Our results showing that K70E colocalizes with WT Merlin in immotile particles agree with previous studies demonstrating that the subcellular distribution of the NF2-mutants of Merlin with FERM domain abnormalities was primarily perinuclear, without accumulation at the membrane (35, 36). These results clearly indicate a defect in protein trafficking with the potential to affect membrane-cytoskeleton signaling and block growth-inhibiting responses, leading to tumor development. Our observations on the effect of coexpression of the NF2 mutant protein with WT Merlin resulted in an ideal model to study the physiological effect of Merlin intracellular transport. The nuclear accumulation of Yorkie is consistent with a loss of function in these experiments. Yorkie is the substrate of the Hippo signaling pathway regulated by Merlin and Expanded (12, 21–23). Therefore, restriction of motor-driven transport of an otherwise normal WT Merlin protein by an NF2 mutant protein results in the loss of function.
A possible mechanism that can explain the targeting of WT Merlin to particles carrying the mutant Merlin protein is the heterodimerization of WT Merlin with Merlin mutant proteins (Fig. S6B) (14, 37). Our findings indicate that NF2 mutants of Merlin defective in transport act as dominant negative forms of Merlin and could lead to loss of function of the WT protein (38). In addition, our results correlate with the fact that the K70E mutation occurs in exon 3 within the FERM domain known to generate an inactive mutant protein with dominant-negative properties (15, 39).
Phosphorylation modulates Merlin subcellular localization and growth suppression, as well as Merlin function. Our data show that phosphorylation of Merlin in Thr616 eliminates its association with the motor complex and leaves Merlin unable to move from the cell membrane. Conversely, Merlin T616A forms particles that associate with microtubule motors; however, these particles accumulate primarily at the perinuclear region. These data correlate with previously reported findings in Drosophila showing that T616D phosphomimetic mutant remains membrane-associated and is internalized at a much slower rate, whereas the nonphosphorylatable T616A is rapidly internalized (20). Phosphorylation has been shown to regulate intramolecular head-to-tail interactions of Merlin. It is possible then that either phosphorylation of Merlin or NF2 mutations that disrupt the folding of Merlin not only affect protein retention at a particular location within the cell, but also disrupt Merlin’s ability to reach its location by interfering with its microtubule-dependent transport.
Human Merlin is also regulated by phosphorylation of both serine and threonine residues at the C terminus of the protein. In particular, the phosphorylation state of the regulatory serine residue (Ser518) in the human protein is known to affect intracellular distribution of the protein and its association with other proteins (14, 18, 19). This serine residue is not conserved in Drosophila, although phosphorylation of Thr616, which is conserved in ERM proteins and has well-described regulatory functions, retains the same regulatory characteristics. Based on our data, it is possible that the corresponding Thr616 residue in Drosophila Merlin acts as the regulatory phosphorylation site controlling the intracellular transport of Merlin (Fig. S6A). We predict that, similar to Thr616 in Drosophila, phosphorylation of Ser518 in human Merlin regulates its association with microtubule motors.
Collectively, our findings reveal the mechanism of intracellular transport of Merlin particles by molecular motors, and show that Merlin trafficking is essential for its function. Our results call into question whether Merlin’s intracellular transport also regulates its function as a growth regulator. We speculate that this indeed is the case, based on our findings on the translocation of the transcriptional coactivator Yorkie.
Finally, point mutations of critical NF2 residues occur naturally in patients. Therefore, we believe that our results form the foundation for further experiments aimed at defining how mutations of Merlin affect Merlin’s association with other proteins that are important for its transport and intracellular signaling.
Materials and Methods
Cell Imaging, Particle Tracking, and Image Analysis.
Images were captured every 1 s for a period of 1 min. Particle movement was analyzed with Diatrack version 3.01 (Semasopht) using vector analysis. Vectors are defined as displacement between two consecutive frames. A threshold of 0.25 μm was used, and vectors with a length below this threshold were excluded from the velocity calculations. The number of vectors was normalized by the total number of particles. At least 10 independent experiments were performed for each condition, and 5–10 cells from each experiment were chosen randomly for analysis. Kymographic analyses of time-lapse movies were obtained using ImageJ software (National Institutes of Health). To quantify particles in the cell body and processes (Fig. 7D), fluorescent images were subjected to processing with the ImageJ Auto-Local Threshold plug-in (Bernsen algorithm), and the distribution of threshold particles in the cell body and processes (outlined using phase-contrast images) was quantified. The same filter was used to analyze the particle size distributions.
Detailed information on the equipment, reagents and conditions, endosome and mitochondria labeling, RNAi, transfection, pull-down assays, immunofluorescence, DNA constructs, and antibodies used in this study is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
L.B. is the recipient of an American Heart Association Postdoctoral Fellowship (Midwest Affiliate). This work was funded by the National Institutes of Health (Grant GM52111 to V.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907389107/DCSupplemental.
References
- 1.Rouleau GA, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neurofibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 2.Hara T, Bianchi AB, Seizinger BR, Kley N. Molecular cloning and characterization of alternatively spliced transcripts of the mouse neurofibromatosis 2 gene. Cancer Res. 1994;54:330–335. [PubMed] [Google Scholar]
- 3.Bianchi AB, et al. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet. 1994;6:185–192. doi: 10.1038/ng0294-185. [DOI] [PubMed] [Google Scholar]
- 4.McClatchey AI. Merlin and ERM proteins: Unappreciated roles in cancer development? Nat Rev Cancer. 2003;3:877–883. doi: 10.1038/nrc1213. [DOI] [PubMed] [Google Scholar]
- 5.Gutmann DH, et al. Increased expression of the NF2 tumor-suppressor gene product, merlin, impairs cell motility, adhesion and spreading. Hum Mol Genet. 1999;8:267–275. doi: 10.1093/hmg/8.2.267. [DOI] [PubMed] [Google Scholar]
- 6.Lallemand D, et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009;28:854–865. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- 7.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis—the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 8.Brault E, et al. Normal membrane localization and actin association of the NF2 tumor suppressor protein are dependent on folding of its N-terminal domain. J Cell Sci. 2001;114:1901–1912. doi: 10.1242/jcs.114.10.1901. [DOI] [PubMed] [Google Scholar]
- 9.Cole BK, Curto M, Chan AW, McClatchey AI. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28:1274–1284. doi: 10.1128/MCB.01139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James MF, Manchanda N, Gonzalez-Agosti C, Hartwig JH, Ramesh V. The neurofibromatosis 2 protein product merlin selectively binds F-actin but not G-actin, and stabilizes the filaments through a lateral association. Biochem J. 2001;356:377–386. doi: 10.1042/0264-6021:3560377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stickney JT, Bacon WC, Rojas M, Ratner N, Ip W. Activation of the tumor suppressor merlin modulates its interaction with lipid rafts. Cancer Res. 2004;64:2717–2724. doi: 10.1158/0008-5472.can-03-3798. [DOI] [PubMed] [Google Scholar]
- 12.McCartney BM, Fehon RG. Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J Cell Biol. 1996;133:843–852. doi: 10.1083/jcb.133.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaJeunesse DR, McCartney BM, Fehon RG. Structural analysis of Drosophila merlin reveals functional domains important for growth control and subcellular localization. J Cell Biol. 1998;141:1589–1599. doi: 10.1083/jcb.141.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stokowski RP, Cox DR. Functional analysis of the neurofibromatosis type 2 protein by means of disease-causing point mutations. Am J Hum Genet. 2000;66:873–891. doi: 10.1086/302812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutmann DH, Hirbe AC, Haipek CA. Functional analysis of neurofibromatosis 2 (NF2) missense mutations. Hum Mol Genet. 2001;10:1519–1529. doi: 10.1093/hmg/10.14.1519. [DOI] [PubMed] [Google Scholar]
- 16.Giovannini M, et al. Schwann cell hyperplasia and tumors in transgenic mice expressing a naturally occurring mutant NF2 protein. Genes Dev. 1999;13:978–986. doi: 10.1101/gad.13.8.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson KC, Kissil JL, Fry JL, Jacks T. Cellular transformation by a FERM domain mutant of the Nf2 tumor suppressor gene. Oncogene. 2002;21:5990–5997. doi: 10.1038/sj.onc.1205693. [DOI] [PubMed] [Google Scholar]
- 18.Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;23:8447–8454. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- 19.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10394–10399. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 20.Hughes SC, Fehon RG. Phosphorylation and activity of the tumor suppressor Merlin and the ERM protein Moesin are coordinately regulated by the Slik kinase. J Cell Biol. 2006;175:305–313. doi: 10.1083/jcb.200608009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badouel C, et al. The FERM-domain protein Expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie. Dev Cell. 2009;16:411–420. doi: 10.1016/j.devcel.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Hamaratoglu F, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 23.McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- 24.Fraenzer JT, et al. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int J Oncol. 2003;23:1493–1500. [PubMed] [Google Scholar]
- 25.McClatchey AI, Fehon RG. Merlin and the ERM proteins: regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19:198–206. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 27.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc Natl Acad Sci USA. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothbauer U, et al. A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol Cell Proteomics. 2008;7:282–289. doi: 10.1074/mcp.M700342-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hakimi MA, Speicher DW, Shiekhattar R. The motor protein kinesin-1 links neurofibromin and merlin in a common cellular pathway of neurofibromatosis. J Biol Chem. 2002;277:36909–36912. doi: 10.1074/jbc.C200434200. [DOI] [PubMed] [Google Scholar]
- 32.Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 33.Gauger AK, Goldstein LSB. The Drosophila kinesin light chain: Primary structure and interaction with kinesin heavy chain. J Biol Chem. 1993;268:13657–13666. [PubMed] [Google Scholar]
- 34.Li W, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–490. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga H, et al. Impairment of cell adhesion by expression of the mutant neurofibromatosis type 2 (NF2) genes which lack exons in the ERM-homology domain. Oncogene. 1998;17:801–810. doi: 10.1038/sj.onc.1202010. [DOI] [PubMed] [Google Scholar]
- 36.Dorogova NV, et al. The role of Drosophila Merlin in spermatogenesis. BMC Cell Biol. 2008;9:1–15. doi: 10.1186/1471-2121-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grönholm M, et al. Homotypic and heterotypic interaction of the neurofibromatosis 2 tumor suppressor protein merlin and the ERM protein ezrin. J Cell Sci. 1999;112:895–904. doi: 10.1242/jcs.112.6.895. [DOI] [PubMed] [Google Scholar]
- 38.Gautreau A, et al. Isolation and characterization of an aggresome determinant in the NF2 tumor suppressor. J Biol Chem. 2003;278:6235–6242. doi: 10.1074/jbc.M210639200. [DOI] [PubMed] [Google Scholar]
- 39.Golovnina K, Blinov A, Akhmametyeva EM, Omelyanchuk LV, Chang LS. Evolution and origin of merlin, the product of the Neurofibromatosis type 2 (NF2) tumor-suppressor gene. BMC Evol Biol. 2005;5:69–86. doi: 10.1186/1471-2148-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.