Abstract
Ghrelin O-acyltransferase (GOAT) attaches octanoate to proghrelin, which is processed to ghrelin, an octanoylated peptide hormone that stimulates release of growth hormone (GH) from pituitary cells. Elimination of the gene encoding ghrelin or its receptor produces only mild phenotypes in mice. Thus, the essential function of ghrelin is obscure. Here, we eliminate the Goat gene in mice, thereby eliminating all octanoylated ghrelin from blood. On normal or high fat diets, Goat−/− mice grew and maintained the same weights as wild-type (WT) littermates. When subjected to 60% calorie restriction, WT and Goat−/− mice both lost 30% of body weight and 75% of body fat within 4 days. In both lines, fasting blood glucose initially declined equally. After 4 days, glucose stabilized in WT mice at 58–76 mg/dL. In Goat−/− mice, glucose continued to decline, reaching 12–36 mg/dL on day 7. At this point, WT mice showed normal physical activity, whereas Goat−/− mice were moribund. GH rose progressively in calorie-restricted WT mice and less in Goat−/− mice. Infusion of either ghrelin or GH normalized blood glucose in Goat−/− mice and prevented death. Thus, an essential function of ghrelin in mice is elevation of GH levels during severe calorie restriction, thereby preserving blood glucose and preventing death.
Keywords: blood glucose, ghrelin, hypoglycemia, knockout mice, octanoate
Ghrelin is an octanoylated, 28-amino acid peptide hormone secreted by the stomach. It was identified through its ability to stimulate release of growth hormone (GH) from pituitary cells (1). Plasma levels of ghrelin rise before meals, and ghrelin injection enhances food intake in rodents and humans (2, 3). These studies define the pharmacologic effects of ghrelin. It has been much more difficult to define the effects of ghrelin deficiency. When fed a normal chow diet, gene-manipulated mice lacking ghrelin (4, 5) or its receptor (6, 7) show a normal growth pattern without signs of GH deficiency. They may or may not exhibit a slight resistance to obesity induced by high fat diets and a slight improvement in insulin secretion and glucose tolerance under these circumstances. When fed a 50% calorie-restricted diet for 40 days, ghrelin and ghrelin receptor knockout mice lost the same amount of weight as wild-type (WT) mice. Their fasting blood glucose levels were slightly reduced on some, but not all, days (7).
The GH-releasing activities of ghrelin are entirely dependent on the attachment of an octanoate residue to serine-3 of ghrelin (8). Ghrelin is the only peptide known to contain an eight-carbon fatty acid, a modification that has been conserved in evolution as far back as fish (8). Recently, our laboratory (9) and others (10) identified a membrane-bound enzyme that transfers octanoate from octanoyl CoA to serine-3 of ghrelin, forming an acyl ester. This enzyme is referred to as GOAT, which stands for ghrelin O-acyltransferase.
In an attempt to explain the evolutionary conservation of the octanoate residue on ghrelin, in the current studies we sought to define the metabolic consequences in mice that lack the Goat gene as a result of homologous recombination. In the one previous report on Goat−/− mice, the only positive finding was a minor reduction in weight when fed a diet rich in octanoate (11). In the current study, we tested the requirement for GOAT under the opposite condition—namely, under conditions of calorie restriction. When faced with a 60% calorie-restricted diet (40% of normal intake), Goat −/− mice failed to maintain their blood sugar. Unlike WT mice, Goat−/− mice became moribund after 7 days. These data reveal an essential function of ghrelin, perhaps accounting for its evolutionary conservation—namely, maintenance of viability during periods of famine.
Results
Fig. S1A shows the strategy by which VelociGene technology was used to replace the coding region of Goat with a cassette containing a lacZ reporter gene and a Neomycin resistance gene (neo). Genomic PCR analysis showed the bands predicted for WT and disrupted alleles of Goat (Fig. S1B). Quantitative RT-PCR analysis of mRNA from gastric mucosa showed an elimination of the GOAT transcript in Goat−/− mice, whereas the amount of mRNA encoding preproghrelin was unaffected (Fig. S1C).
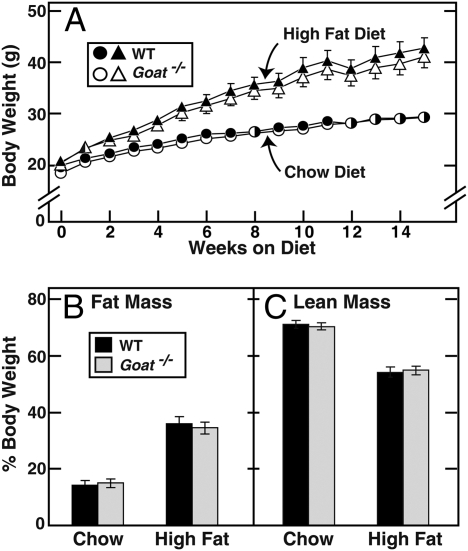
The Goat−/− mice had a normal appearance through adulthood, and they produced normal numbers of offspring. Their body weights were indistinguishable from littermate WT controls when fed a normal chow diet (18% calories from fat) or a high fat diet (45% calories from fat) (Fig. 1A). Fat mass and lean mass, measured by NMR, were identical in the chow-fed WT and Goat−/− mice (Figs. 1 B and C). After 14 weeks on the high fat diet, fat mass increased by the same amount in both lines. Daily food intake was the same in WT and Goat−/− mice when fed ad libitum.
Fig. 1.
Body composition of WT and Goat−/− mice fed a chow or high fat diet. Male littermates (4-wk-old) were fed ad libitum either the chow diet or the high fat diet for the indicated time. (A) Mice were weighed at weekly intervals. (B and C) Fourteen weeks after diet treatments, the total fat (B) and lean (C) body masses were measured by NMR. Each value represents mean ± SEM of data from six mice.
Oral glucose tolerance tests showed that the chow-fed Goat−/− mice had modestly enhanced glucose tolerance (Fig. S2A), which was associated with a slight but significant increase in plasma insulin at the 30-min time point (Fig. S2C). On the high fat diet, glucose tolerance worsened in both mouse lines, but the Goat−/− mice continued to show lower glucose levels than WT mice (Fig. S2B). The reduction in blood glucose in Goat−/− mice was accompanied by a marked elevation in plasma insulin levels, especially at the 30-min time point (2-fold) (Fig. S2D). This result is consistent with previous findings in mice lacking ghrelin (12).
Previous observations showing that ghrelin levels increase in the fasted state (13–15) suggested that ghrelin might play a metabolic role during calorie restriction. To test this hypothesis, we first placed mice in individual cages and measured the food intake of each mouse when fed ad libitum. WT and Goat−/− mice consumed the same amount of food (WT, 4.4 ± 0.08 g/day; Goat−/−, 4.5 ± 0.08 g/day). We then switched each mouse to a calorie-restricted diet that contained only 40% of the daily amount of calories that the same mouse consumed when fed ad libitum. The mice were fed at 6 p.m. each day (3 h before the beginning of the dark cycle). After 3 days, WT and Goat−/− mice both consumed all of their food within 1 h after feeding, a pattern that continued throughout the experiment.
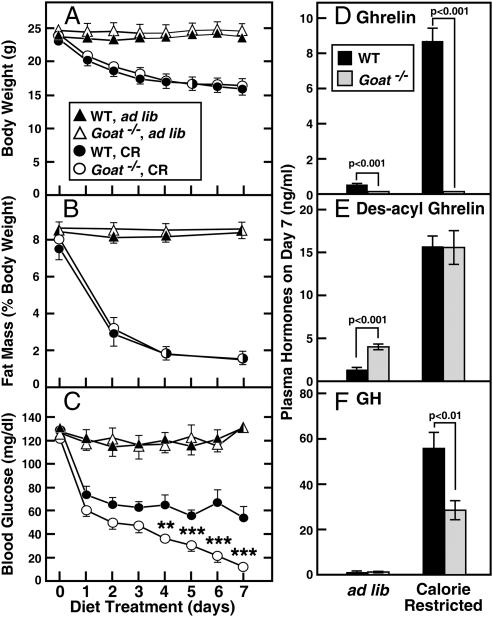
Fig. 2 shows the results of the calorie restriction study. WT and Goat−/− mice both lost ≈28% of their body weight in the first 4 days, after which both groups stabilized (Fig. 2A). Both groups lost 75% of their fat mass, as determined by NMR (Fig. 2B). Fasting blood glucose was measured at 5:30 p.m., 30 min before feeding (Fig. 2C). In both groups, the fasting blood glucose declined within the first 2 days. In WT mice, fasting blood glucose remained relatively constant at 54–65 mg/dL through day 7. In marked contrast, fasting blood glucose in the Goat−/− mice never stabilized. It continued to decline until it reached a nadir of 12 mg/dL on day 7. This decline occurred even though the Goat−/− mice continued to consume their food as rapidly as WT mice, and even though the body weights of the two groups were the same. After 7 days, WT mice appeared active and healthy. In contrast, Goat−/− mice were lethargic and nearly moribund. At this point, both groups of mice were euthanized at 5:30 p.m., and plasma was obtained for hormone measurements. Plasma ghrelin was elevated by 18-fold in plasma of calorie-restricted WT mice as compared with ad libitum-fed WT mice (Fig. 2D). As expected, ghrelin was not detectable in the plasma of Goat−/− mice that consumed either diet. On the ad libitum diet, the level of des-acyl ghrelin was elevated by 3-fold in Goat−/− mice as compared with WT (Fig. 2E). Levels of des-acyl ghrelin rose markedly in both groups on the calorie-restricted diet, and there was no longer any difference between Goat−/− and WT mice. On the ad libitum diet, plasma GH levels were similarly low in Goat−/− and WT mice (1.4 ± 0.4 and 1.3 ± 0.6 ng/mL) (Fig. 2F). On the calorie-restricted diet, GH levels rose in both groups, but WT mice attained levels that were 2-fold higher than Goat−/− mice.
Fig. 2.
Comparison of WT and Goat−/− mice fed the chow diet ad libitum or subjected to 60% calorie restriction. Male littermates (8-wk-old) were housed in individual cages and fed ad libitum with the chow diet or subjected to 60% calorie restriction as described in Materials and Methods. Body weight (A) was measured daily at 5:30 p.m., and total fat mass (B) was measured by NMR every 2 or 3 days at 5 p.m. (C) Blood glucose was measured daily with a Bayer Glucometer at 5:30 p.m. (30 min before feeding). (D–F) the mice were euthanized at 5:30 p.m. (before feeding) on the seventh day of calorie restriction, and plasma levels of ghrelin (D), des-acyl ghrelin (E), and GH (F) were determined. Each value represents mean ± SEM of data from six mice. Asterisks (*) denote level of statistical significance (Student's t test) between the WT and Goat−/− mice under calorie-restricted conditions. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Table 1 summarizes various metabolic parameters that were measured at 5:30 p.m. on day 7 of the ad libitum-fed and calorie-restricted mice studied in Fig. 2. Body weight, fat mass, and blood levels of glucose, ghrelin, and GH were as described above. Plasma IGF1 levels were reduced by >90% in both mouse lines despite the elevations in GH. Plasma-free fatty acids were extremely low in calorie-restricted WT as well as Goat−/− mice despite the elevation in GH, which is known to elevate plasma-free fatty acids (16). The latter discordancy is likely attributable to the severe depletion of fat mass, which limits the source of fatty acids. The adipose deficiency also likely explains the fall in plasma β-hydroxybutyrate, the product of fatty acid oxidation, which occurred in both lines. Plasma insulin fell to levels that were nearly undetectable in both lines, and plasma glucagon rose by ≈2-fold in Goat−/− as well as WT mice. Neither plasma corticosterone nor cortisol levels were significantly different between WT mice and Goat−/− mice after calorie restriction, but it should be noted that the levels observed are those normally associated with stress and some of this elevation may be caused by stress induced by animal handling.
Table 1.
Response of WT and Goat−/− mice to calorie restriction for 7 days: Metabolic parameters
| Parameter | Ad libitum fed | Calorie-restricted | ||
| WT | Goat−/− | WT | Goat−/− | |
| Body weight, g | 23.7 ± 0.6 | 24.7 ± 1.0 | 15.9 ± 0.6 | 16.4 ± 0.5 |
| Fat mass, % body weight | 8.4 ± 0.3 | 8.6 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.1 |
| Lean mass, % body weight | 74.2 ± 0.7 | 73.1 ± 0.7 | 78.1 ± 0.1 | 78.6 ± 0.2 |
| Blood glucose, mg/dL | 131 ± 4 | 133 ± 7 | 54 ± 10 | 12 ± 1*** |
| Ghrelin, ng/mL | 0.5 ± 0.1 | <0.004*** | 8.7 ± 0.7 | <0.004*** |
| Des-acyl ghrelin, ng/mL | 1.3 ± 0.1 | 4.0 ± 0.3*** | 15.7 ± 1.2 | 15.6 ± 2.0 |
| Growth hormone, ng/dL | 1.3 ± 0.6 | 1.4 ± 0.4 | 66 ± 8 | 34 ± 5** |
| IGF1, ng/mL | 32 ± 3 | 37 ± 3 | 3.1 ± 1.5 | 1.1 ± 0.7 |
| Free fatty acids, mM | 0.44 ± 0.06 | 0.46 ± 0.05 | 0.07 ± 0.02 | 0.04 ± 0.02 |
| β-Hydroxybutyrate, mM | 0.25 ± 0.02 | 0.27 ± 0.04 | 0.12 ± 0.01 | 0.10 ± 0.01 |
| Insulin, ng/mL | 0.38 ± 0.04 | 0.66 ± 0.09* | 0.006 ± 0.005 | 0.016 ± 0.016 |
| Glucagon, pg/mL | 46 ± 7 | 59 ± 4 | 83 ± 16 | 106 ± 19 |
| Corticosterone, ng/mL | 158 ± 62 | 189 ± 45 | 225 ± 28 | 172 ± 23 |
| Cortisol, pg/mL | 531 ± 117 | 556 ± 128 | 844 ± 71 | 620 ± 73 |
Male wild-type and Goat−/− littermates (8-wk old) were fed a chow diet ad libitum or subjected to 60% calorie restriction for 7 days before study (same animals that were used in Fig. 2). Each value represents mean ± SEM of data from six mice. Concentrations of blood glucose and plasma parameters were determined as described in Materials and Methods. Asterisks (*) denote level of statistical significance (Student's t test) between WT and Goat−/− mice under the same fed or calorie-restricted condition.
*, P < 0.05;
**, P < 0.01;
***, P < 0.001.
To determine whether the liver was making an appropriate transcriptional response to calorie restriction, we measured the levels of various mRNAs in liver extracts (Table 2). All of the changes in the calorie-restricted WT and Goat−/− mice were similar to each other and consistent with a low insulin/high glucagon state. Thus, SREBP-1c mRNA was reduced by 90% and this was associated with reductions in mRNAs encoding all of the measured lipogenic proteins, which are SREBP-1c targets. On the other hand, two gluconeogenic mRNAs, PEPCK and glucose-6-phosphatase, were elevated, consistent with the reduced insulin/glucagon ratio. Also consistent with this state was the IGFBP-1 mRNA, which was markedly elevated in the calorie-restricted livers. The mRNA encoding IGF1 was reduced despite the elevated plasma GH levels.
Table 2.
Relative amounts of mRNAs in livers of calorie-restricted WT and Goat−/− mice
| mRNA | ad libitum fed | Calorie-restricted | ||
| WT | Goat−/− | WT | Goat−/− | |
| SREBP pathway | ||||
| SREBP-1c | 1.0 | 0.85 | 0.08 | 0.08 |
| SREBP-2 | 1.0 | 1.07 | 0.48 | 0.38 |
| Lipogenesis | ||||
| ATP citrate lyase | 1.0 | 1.32 | 0.13 | 0.13 |
| ACC-1 | 1.0 | 1.10 | 0.50 | 0.51 |
| FAS | 1.0 | 1.33 | 0.14 | 0.14 |
| SCD-1 | 1.0 | 1.25 | 0.40 | 0.52 |
| Gluconeogenesis | ||||
| PEPCK | 1.0 | 1.08 | 4.46 | 4.78 |
| G6Pase | 1.0 | 1.05 | 2.84 | 3.82 |
| Others | ||||
| IGF1 | 1.0 | 1.12 | 0.41 | 0.38 |
| IGFBP-1 | 1.0 | 0.89 | 36.7 | 74.5 |
| IGFBP-3 | 1.0 | 1.65 | 0.76 | 0.66 |
Male WT and Goat−/− littermates (8-wk-old) were fed a chow diet ad libitum or subjected to 60% calorie restriction for 7 days and euthanized at 5:30 p.m. Total RNA from four to six mouse livers per group were pooled and quantified by real-time PCR as described in Materials and Methods. Glyceraldehyde 3-phosphate dehydrogenase mRNA was used as the invariant control. Each value represents the amount of mRNA relative to that in fed WT mice, which is arbitrarily defined as 1.0. Primers for real-time PCR analysis were as described in Materials and Methods except for the following: IGF1 (NM_010512): 5′-CCACACTGACATGCCCAAGA-3′ and 5′-TCCTTTGCAGCTTCGTTTTCT-3′; IGFBP-3 (NM_008343): 5′-GAGTGTGGAAAGCCAGGTT-GTC-3′ and 5′-GCATGGAGTGGATGGAACTTG-3′. SREBP, sterol regulatory element-binding protein; ACC-1, acetyl-CoA carboxylase-1; FAS, fatty acid synthase; SCD-1, stearoyl-CoA desaturase-1; PEPCK, phosphoenolpyruvate carboxylase; G6Pase, glucose-6-phosphatase.
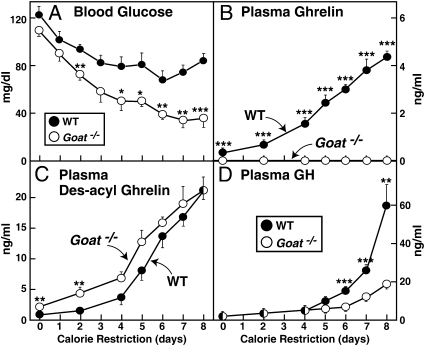
Fig. 3 shows an experiment in which we measured glucose and hormone levels throughout the period in which the animals were subjected to calorie restriction. Again, blood glucose levels in WT mice stabilized after 4 days, whereas in Goat−/− mice, they continued to decline (Fig. 3A). In WT mice, plasma ghrelin rose by the second day, and it continued to rise linearly throughout the experiment (Fig. 3B). Ghrelin was not detectable in plasma of the Goat−/− mice. Plasma des-acyl ghrelin rose in both mouse strains (Fig. 3C). Plasma GH levels rose modestly in both mouse strains through day 4 (Fig. 3D). Thereafter, the levels rose much more rapidly in WT mice in a pattern that coincided with stabilization of the plasma glucose. In contrast, in Goat−/− mice, GH levels rose much more slowly so that by day 8, they were only one-third of the levels seen in WT mice. Fig. S3 shows a glucose tolerance test carried out in WT and Goat−/− mice after 5 days of calorie restriction. Blood glucose levels were significantly lower in the Goat−/− mice at 15 and 30 min after glucose gavage.
Fig. 3.
Differential changes in blood glucose and GH levels in WT and Goat−/− mice subjected to calorie restriction. Male WT and Goat−/− littermates (8-wk-old) were subjected to a 60% calorie restriction as described in Materials and Methods . The concentrations of blood glucose (A), plasma ghrelin (B), plasma des-acyl ghrelin (C), and plasma GH (D) were measured at 5:30 p.m. (30 min before feeding) on the indicated day. Each value represents mean ± SEM of data from five mice. Asterisks (*) denote level of statistical significance (Student's t test) between WT and Goat−/− mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
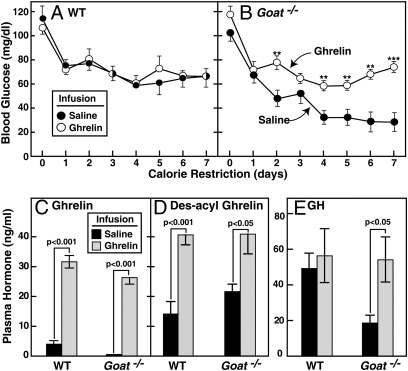
To determine whether ghrelin administration would prevent the hypoglycemia in Goat−/− mice, we used an osmotic minipump to deliver a continuous infusion of ghrelin or saline beginning 3 days before initiation of calorie restriction. Infusion of ghrelin had no effect on the WT mice; their plasma glucose levels were the same as seen in WT mice infused with saline (Fig. 4A). In Goat−/− mice infused with saline, the expected hypoglycemia was seen, whereas infusion of ghrelin abolished the hypoglycemia, restoring blood glucose levels to those seen in WT mice (Fig. 4B). Plasma hormone levels were measured at 5:30 p.m. on day 7. After ghrelin infusion, plasma levels of ghrelin and des-acyl ghrelin were elevated to the same extent in WT and Goat−/− mice (Fig. 4 C and D). Importantly, ghrelin infusion normalized the GH level in the Goat−/− mice (Fig. 4E). Despite this GH increase, plasma IGF1 remained low on day 7 in calorie-restricted WT and Goat−/− mice (2.2 ± 0.8 and 2.6 ± 0.9 ng/mL, respectively; n = 6 mice in each group). Plasma free fatty acids in WT (0.22 ± 0.04 mM) and Goat−/− (0.23 ± 0.09 mM) mice remained low. Plasma β-hydroxybutyrate levels in WT (0.34 ± 0.10 mM) and Goat−/− (0.35 ± 0.13 mM) also remained low.
Fig. 4.
Comparison of calorie-restricted WT and Goat−/− mice undergoing continuous s.c. infusion of saline or ghrelin. Alzet osmotic pumps (delivery rate of 0.25 μL/h) filled with saline or saline (●) containing 5 mg/mL ghrelin (○) were inserted s.c. in the interscapular regions of 8-wk-old male littermate WT and Goat−/− mice. Three days after initiation of the infusion, both groups of mice were placed under 60% calorie restriction that was continued for 7 days. (A and B) Blood glucose levels were measured daily at 5:30 p.m. (30 min before feeding). (C–E) Mice were euthanized at 5:30 p.m. on the seventh day of calorie restriction. Plasma levels of ghrelin (C), des-acyl ghrelin (D), and GH (E) were determined. Each value represents mean ± SEM of data from 6 mice. Asterisks (*) denote level of statistical significance (Student's t test) between WT and Goat−/− mice infused with saline or ghrelin. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
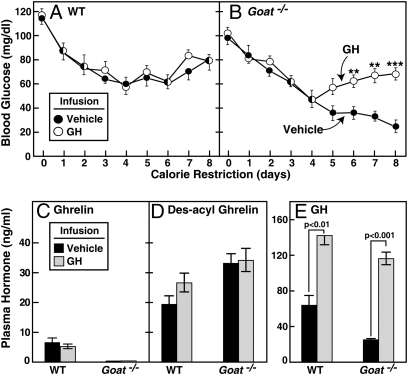
The preceding data raised the possibility that calorie-restricted Goat−/− mice develop hypoglycemia because their ghrelin deficiency prevents a maximal increase in plasma GH, which is known to counteract hypoglycemia (16). To test this hypothesis, we used an osmotic minipump to deliver a continuous infusion of GH or vehicle into calorie-restricted WT and Goat−/− mice. Infusions were started 3 days before initiation of calorie restriction. In vehicle-infused Goat−/− mice, blood glucose declined as before (Fig. 5B). This decline was prevented by the GH infusion. The GH infusion had no effect on blood glucose levels in calorie-restricted WT mice (Fig. 5A). After GH infusion, plasma levels of GH increased significantly and were elevated to the same extent in WT and Goat−/− mice (Fig. 5E). No significant change was seen in plasma levels of ghrelin (Fig. 5C) or des-acyl ghrelin (Fig. 5D). This experiment was repeated with nearly identical results (Fig. S4). As with the ghrelin infusion, GH infusion corrected the hypoglycemia in Goat−/− mice without significant elevations in IGF1 as measured on day 8 (2.2 ± 1.0 and 2.4 ± 1.0 ng/mL in WT and Goat−/− mice, respectively; n = 5 mice in each group).
Fig. 5.
Comparison of calorie-restricted WT and Goat−/− mice undergoing continuous s.c. infusion of vehicle or GH. Alzet osmotic pumps (delivery rate of 0.25 μL/h) filled with vehicle (●) or vehicle containing 2.5 mg/mL GH (○) were inserted s.c. in the interscapular regions of 8-wk-old male littermate WT and Goat−/− mice. Three days after initiation of the infusion, both groups of mice were placed under 60% calorie restriction that was continued for 8 days. (A and B) Blood glucose levels were measured daily at 5:30 p.m. (30 min before feeding). (C–E) Mice were euthanized at 5:30 p.m. on the eighth day of calorie restriction. Plasma levels of ghrelin (C), des-acyl ghrelin (D), and GH (E) were determined. Each value represents mean ± SEM of data from five mice. Asterisks (*) denote level of statistical significance (Student's t test) between WT and Goat−/− mice infused with vehicle or GH. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
The current data reveal one essential function of ghrelin in mice—namely, maintenance of elevated plasma GH levels during periods of severe calorie restriction, thereby assuring sufficient blood glucose to permit survival. The metabolic difference between WT and Goat−/− mice became apparent after 4 days of a severe 60% calorie restriction (40% of normal food consumption), at which point both lines of mice had lost ≈30% of body weight and 75% of body fat (Fig. 2). Thereafter, in five experiments, WT mice maintained blood glucose in the range of 58–76 mg/dL, which was sufficient for viability (Figs. 2–5 and Fig. S4). In contrast, Goat−/− mice exhibited a fall in blood glucose to the range of 12–36 mg/dL. By day 7–8, Goat−/− mice appeared moribund, at which time they were killed.
Calorie-restricted WT mice developed progressive elevations in plasma ghrelin levels. By day 7, plasma ghrelin was 12- to 18-fold higher than observed in ad libitum-fed mice (Figs. 2 and 3). Calorie-restricted Goat−/− mice showed no detectable ghrelin. Both lines of mice exhibited marked increases in des-acyl ghrelin (Figs. 2 and 3). Importantly, despite their ghrelin deficiency, Goat−/− mice became hungry as indicated by their ingestion of all food within 1 h of delivery. Thus, ghrelin is not essential for hunger during periods of starvation.
Throughout our study, we noted that hypoglycemia in Goat−/− mice was observed only when we collected the blood at 5:30 p.m., one-half hour before feeding. Owing to their rapid food consumption, the calorie-restricted mice had been without food for ≈22 h. When we measured blood glucose at 9 a.m. on day 7 (after 14 h without food), we found similar values in WT and Goat−/− mice (76 ± 6.2 and 65 ± 6.5 mg/dL, respectively; n = 10 mice per group). The glycogen content in livers of WT and Goat−/− mice at 9 a.m. on day 7 were similar (17 ± 0.3 and 18 ± 1.0 μg/mg tissue; 5 mice per group); 8.5 h later (23.5 h after feeding), these values fell to 3.6 ± 0.7 and 2.0 ± 0.6 μg/mg of tissue, respectively (23 mice per group). Moreover, we did not find hypoglycemia in Goat−/− mice that were fed a normal diet and then fasted for 24 h. These data indicate that hypoglycemia in Goat−/− mice requires two conditions: (i) severe prolonged calorie restriction, leading to a loss of nearly all body fat stores; and (ii) acute food deprivation, leading to reduced liver glycogen.
Hypoglycemia in Goat−/− mice was not caused by excess insulin, or by deficiency of glucagon (Table 1). Indeed, at 5:30 p.m., insulin was barely detectable, and glucagon was elevated to the same degree as in WT mice. Moreover, the liver responded normally to these hormones, at least at the transcriptional level, by producing elevated mRNAs for gluconeogenic enzymes and reduced mRNAs for lipogenic enzymes (Table 2). After 5 days of calorie restriction, an oral glucose tolerance test, performed at 5 p.m. just before feeding, showed a lower peak of blood glucose in calorie-restricted Goat−/− mice as compared with calorie-restricted WT mice. Peak blood glucose levels in WT mice averaged ≈300 mg/dL. The corresponding value in Goat−/− mice was 150 mg/dL (P < 0.001). These data raise the possibility that Goat−/− mice have an elevated glucose disposal rate (Fig. S3). The livers of Goat−/− mice may lack the substrate to permit the increased gluconeogenesis that would be required to maintain blood glucose in the face of increased glucose removal from blood. Tests of this hypothesis will require studies of glucose production and disposal.
Hypoglycemia in Goat−/− mice was associated with relative, but not absolute, GH deficiency (Figs. 2–5, Fig. S4, and Table 1). In WT mice, GH levels began to rise dramatically at day 5, coincident with the stabilization of blood glucose (Fig. 3D). In Goat−/− mice GH also rose with calorie restriction, but the rise was delayed and not as pronounced as in WT (Fig. 3D). These data indicate that other signals, in addition to ghrelin, can elevate GH in calorie-restricted animals, but these signals are not strong enough to produce maximal GH levels, and they cannot prevent hypoglycemia and death. Infusion of ghrelin via an osmotic minipump restored the GH level in calorie-restricted Goat−/− mice and restored blood glucose to the same levels seen in WT (Fig. 4). The importance of GH deficiency was confirmed when we administered GH by an osmotic minipump and found that it prevented hypoglycemia and death in Goat−/− mice (Fig. 5 and Fig. S4). It must be noted that plasma GH was not reduced in Goat−/− mice when they consumed a chow diet (Table 1). Ghrelin appears to be required for GH secretion only when elevated GH levels are demanded during calorie restriction. This finding explains the normal growth pattern of Goat−/− mice as well as the previously reported Ghrelin −/− mice (4, 5, 7).
GH maintained blood glucose in calorie-restricted WT mice, even though IGF1 levels were depressed (Table 1). Moreover, IGF1 levels did not rise significantly with GH infusion in either WT or Goat−/− mice (measured on day 8). These data indicate that the glucose-elevating actions of GH occur independently of IGF1. This finding makes teleologic sense because IGF1 would lower glucose, not raise it.
The standard view of GH action states that GH maintains blood sugar by enhancing lipolysis in adipose tissue, thereby releasing fatty acids that spare glucose utilization in muscle (16). It seems unlikely that this mechanism prevails in the calorie-restricted mice because they have very little adipose tissue from which to release fatty acids. Consistent with the reduction in adipose tissue, plasma-free fatty acid levels were low in calorie-restricted WT and Goat−/− mice. Moreover, they were not elevated significantly by ghrelin or GH infusion. After 7 days of these infusions, mean fatty acid levels in calorie-restricted Goat−/− mice were 0.23 ± 0.08 mM (ghrelin infusion) and 0.20 ± 0.06 mM (GH infusion).
Although GOAT is essential for survival during periods of famine in mice, the enzyme is not essential for energy storage in times of plenty. Thus, Goat−/− mice gained weight and stored fat normally when fed a high fat diet (Fig. 1). These findings are in general consistent with previous observations in mice lacking ghrelin or its receptor (4–7). Thus, ghrelin should not be considered a hormone that enhances food intake or fat storage during times of plenty, but rather one that maintains blood glucose during times of famine. The hypoglycemia in our Goat−/− mice was much more profound than observed previously by Sun et al. (7) in their ghrelin and ghrelin receptor knockout mice. Sun et al. fed a diet containing 50% of normal calories as opposed to the 40% diet used in the current studies. We observed that hypoglycemia in Goat−/− mice did not occur until total body fat had declined below 2% of body mass. It is possible that the mice studied by Sun et al. retained sufficient fat mass to avoid severe hypoglycemia. Despite these differences, the conclusion of our study is similar to that reached by Sun et al., namely, that the major actions of ghrelin involve maintenance of blood sugar levels in the undernourished state. A similar hypothesis was advanced by Cummings et al. (17).
The current findings in calorie-restricted mice have parallels in humans with calorie restriction secondary to anorexia nervosa. Like calorie-restricted mice, anorexic humans have elevated ghrelin and GH levels (18). They also have reduced IGF1 levels despite the elevation in plasma GH. It is tempting to speculate that the elevated ghrelin in these humans is essential in maintaining maximal GH and, thus, maintaining blood glucose, as it is in calorie-restricted mice.
Materials and Methods
Mice.
All mice were housed in colony cages with 12-h light/12-h dark cycles. The dark cycle began at 9:00 or 10:00 p.m. The chow diet consisted of Teklad Mouse/Rat Diet 7002 from Harlan Teklad Global Diets. This diet contains 3.0 kcal/g of metabolizable energy, of which 18% of calories are from fat, 49% from carbohydrates, and 33% from protein. All animal experiments were performed with the approval of the Institutional Animal Care and Research Advisory Committee at University of Texas Southwestern Medical Center at Dallas.
Metabolic Parameters.
Mice were anesthetized with isoflurane, after which blood was drawn from the retro-orbital sinus and collected in EDTA-coated tubes containing p-hydroxymercuribenzoic acid (final concentration, 1 mM). The plasma was separated immediately and stored without treatment at −80 °C except for samples used for measurement of ghrelin and IGF1, which were treated before freezing with HCl (final concentration, 0.1 M) and MIX diluent (Assaypro), respectively. Plasma glucose, cholesterol, and triglycerides were measured with the Vitros 250 Chemistry Analyzer (Ortho Clinical Diagnostics) by the Mouse Metabolic Phenotyping Core at the University of Texas Southwestern. Octanoylated ghrelin and des-acyl ghrelin were measured by immunoassay kits that distinguished both forms of the peptide (Cat. No. A05117 and A05118; Cayman Chemical). Plasma levels of GH, corticosterone, and cortisol were measured with commercial kits from Cayman. Plasma levels of the following compounds were measured with commercial kits from the indicated vendor: free fatty acids (Wako Chemicals), β-hydroxybutyrate (Pointe Scientific, Inc.), IGF1 (Assaypro), and glucagon (Millipore). Glycogen content of liver was measured with an assay kit from Biovision.
High Fat Diet.
For high fat feeding studies, mice were fed a diet containing 45% calories from fat. The diet was composed of 24 gm% fat, 41 gm% carbohydrate, and 24 gm% protein (Cat No. D12451; Research Diets).
Calorie Restriction and Infusion of Hormones.
One week before initiation of calorie restriction, 8-wk-old male WT and Goat−/− littermates were placed in individual cages and fed the chow diet ad libitum. During this week of acclimation, food intake was monitored to determine the average amount of food consumed daily by each mouse. Thereafter, the mice were randomly separated into two groups. One group continued to receive the chow diet ad libitum. The other group was subjected to 60% calorie restriction such that each mouse was fed at 6 p.m. every day with an amount of food equal to 40% of the daily amount consumed by the same mouse during the week of acclimation. Body weight and blood glucose were measured daily at 5:30 p.m. before feeding; total body fat was measured every 2 or 3 days at 5 p.m. by NMR (Bruker Minispec mq7.5 NMR analyzer). The mice were euthanized with isoflurane at 5:30 p.m. (before feeding) on the seventh or eighth day of calorie restriction to collect blood and various tissues for analyses.
For calorie restriction studies in which mice received a continuous s.c. infusion of recombinant ghrelin or GH, an Alzet osmotic pump (Cat. No. 1002; DURECT) was inserted s.c. in the interscapular region of each mouse 3 days before the initiation of calorie restriction. For ghrelin infusion studies, the pump was filled with saline or saline containing 5 mg/mL rat recombinant ghrelin (Cat. No. RP10781; GenScript). The octanoylated ghrelin was delivered at a rate of 0.25 μL/h (30 μg/24 h). For GH infusion, the pump was filled with vehicle (70 mM sodium bicarbonate at pH 9.5, 137 mM NaCl, and 100 μg/mL rat albumin) or vehicle containing 2.5 mg/mL recombinant rat GH (obtained from the National Hormone and Peptide Program of National Institute of Diabetes and Digestive and Kidney Diseases through A.F. Parlow). The GH was delivered at a rate of 0.25 μL/h (15 μg/24 h).
Quantitative Real-Time PCR.
Total RNA was isolated, and real-time PCR measurements were made on pooled RNA samples from 4 to 6 mice in each group using primers as described (9, 19, 20). All reactions were done in triplicate where the mean range of variation for all values was 0.40 ± 0.03%. The relative amount of all mRNAs was calculated by using the comparative threshold cycle (CT) method. Cyclophilin or GAPDH mRNA was used as the invariant control.
Other Methods.
Additional information is described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Monica Mendoza, Isis Soto, and Richard Gibson for invaluable help with animal studies; and Liz Lummus and Robert Hammer (University of Texas Southwestern Transgenic Core Facility) for ES cell injections. This work was supported by grants for the National Institute of Health (HL20948), Perot Family Foundation, and the Moss Heart Foundation. G.L. is a recipient of an Individual Biomedical Research Award from The Hartwell Foundation. R.L.L. is supported by Medical Scientist Training Program Grant ST32GM08014.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1002271107/DCSupplemental.
References
- 1.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Small CJ, Bloom SR. Gut hormones and the control of appetite. Trends Endocrinol Metab. 2004;15:259–263. doi: 10.1016/j.tem.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wortley KE, et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA. 2004;101:8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zigman JM, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirchner H, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3:379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 14.Toshinai K, et al. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93:1980–1987. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Møller N, Jørgensen JOL. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 17.Cummings DE, Foster-Schubert KE, Overduin J. Ghrelin and energy balance: focus on current controversies. Curr Drug Targets. 2005;6:153–169. doi: 10.2174/1389450053174569. [DOI] [PubMed] [Google Scholar]
- 18.Misra M, Klibanski A. Neuroendocrine consequences of anorexia nervosa in adolescents. Endocr Dev. 2010;17:197–214. doi: 10.1159/000262540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, et al. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc Natl Acad Sci USA. 2001;98:13607–13612. doi: 10.1073/pnas.201524598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang G, et al. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.