Abstract
We have developed procedures that permit isolation and propagation of clonal cell cultures from the olfactory epithelium of the 5- to 7-day-old rat that appear to represent the neuroblasts that repopulate the sensory neurons in the olfactory epithelium throughout life. The cell lines we report here synthesize neuron-specific enolase, which is a neuron marker, 43-kDa growth-associated protein, a protein associated with neuronal growth cones, and carnosine, a possible olfactory neurotransmitter. In two of the cell lines we have found dose-dependent cAMP accumulation following exposure to submicromolar concentrations of chemical odorants in the medium. These two cell lines show different patterns of odorant specificity when tested against a panel of six chemicals commonly used as test odorants. We anticipate that these and similarly derived cell lines will prove valuable in studying aspects of neurogenesis and olfaction.
Full text
PDF

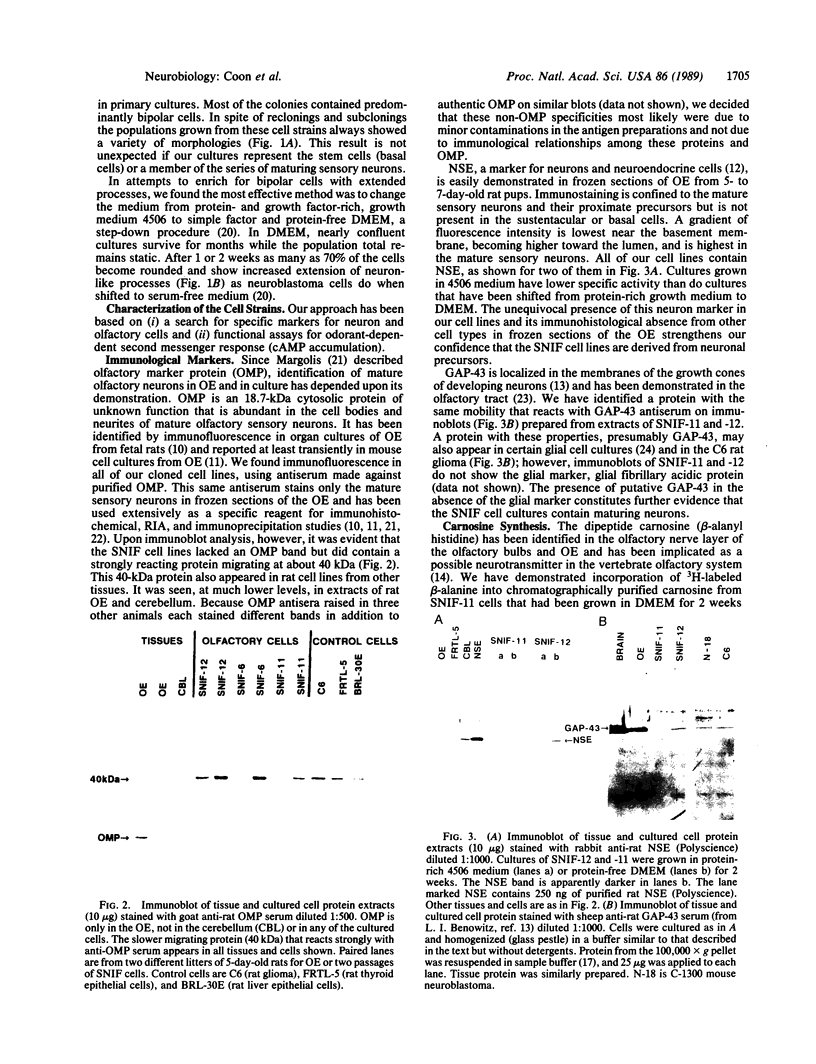
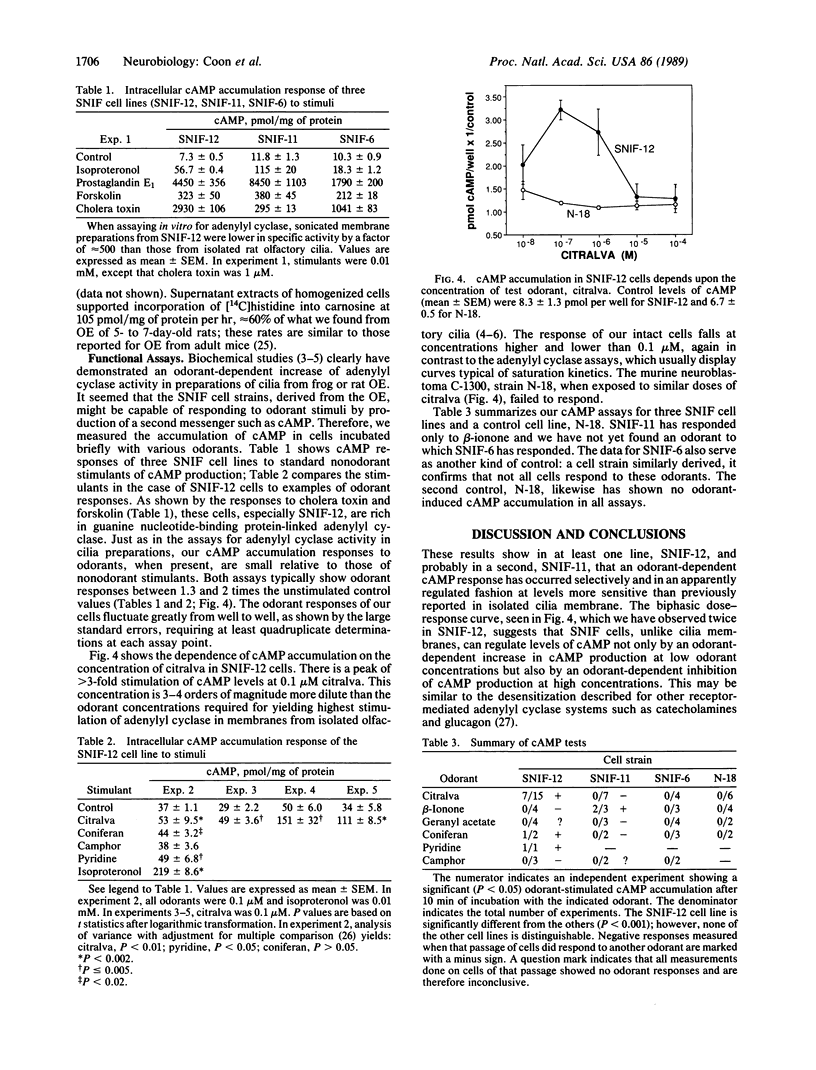

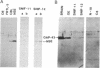
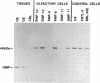
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein N. I., Quinn M. R. A novel cell line isolated from the murine olfactory mucosa. In Vitro. 1981 Jul;17(7):593–598. doi: 10.1007/BF02618457. [DOI] [PubMed] [Google Scholar]
- Gonzales F., Farbman A. I., Gesteland R. C. Cell and explant culture of olfactory chemoreceptor cells. J Neurosci Methods. 1985 Jul;14(2):77–90. doi: 10.1016/0165-0270(85)90118-9. [DOI] [PubMed] [Google Scholar]
- Grady T., Fickova M., Tager H. S., Trivedi D., Hruby V. J. Stimulation and inhibition of cAMP accumulation by glucagon in canine hepatocytes. J Biol Chem. 1987 Nov 15;262(32):15514–15520. [PubMed] [Google Scholar]
- Graziadei P. P. Cell dynamics in the olfactory mucosa. Tissue Cell. 1973;5(1):113–131. doi: 10.1016/s0040-8166(73)80010-2. [DOI] [PubMed] [Google Scholar]
- Harding J., Margolis F. L. Denervation in the primary olfactory pathway of mice. III. Effect on enzymes of carnosine metabolism. Brain Res. 1976 Jul 9;110(2):351–360. doi: 10.1016/0006-8993(76)90407-8. [DOI] [PubMed] [Google Scholar]
- Jacobson R. D., Virág I., Skene J. H. A protein associated with axon growth, GAP-43, is widely distributed and developmentally regulated in rat CNS. J Neurosci. 1986 Jun;6(6):1843–1855. doi: 10.1523/JNEUROSCI.06-06-01843.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwayanagi M., Kurihara K. Neuroblastoma cell as model for olfactory cell: mechanism of depolarization in response to various odorants. Brain Res. 1984 Feb 20;293(2):251–258. doi: 10.1016/0006-8993(84)91232-0. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Labarca P., Simon S. A., Anholt R. R. Activation by odorants of a multistate cation channel from olfactory cilia. Proc Natl Acad Sci U S A. 1988 Feb;85(3):944–947. doi: 10.1073/pnas.85.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Reagan J., Gyorgyi T., Roby A. Olfaction by melanophores: what does it mean? Proc Natl Acad Sci U S A. 1988 Jan;85(1):261–264. doi: 10.1073/pnas.85.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis F. L. A brain protein unique to the olfactory bulb. Proc Natl Acad Sci U S A. 1972 May;69(5):1221–1224. doi: 10.1073/pnas.69.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis F. L. Carnosine in the primary olfactory pathway. Science. 1974 May 24;184(4139):909–911. doi: 10.1126/science.184.4139.909. [DOI] [PubMed] [Google Scholar]
- Meiri K. F., Pfenninger K. H., Willard M. B. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci U S A. 1986 May;83(10):3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton D. G. Dynamics of cell populations in the olfactory epithelium. Ann N Y Acad Sci. 1974 Sep 27;237(0):52–61. doi: 10.1111/j.1749-6632.1974.tb49843.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Stallcup W., LaCorbiere M., Kidokoro Y., Orgel L. Ontogeny of electrically excitable cells in cultured olfactory epithelium. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7782–7786. doi: 10.1073/pnas.82.22.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley S. G., Robinson C. J., Dickinson K., Aujla R., Dodd G. H. Olfactory adenylate cyclase of the rat. Stimulation by odorants and inhibition by Ca2+. Biochem J. 1986 Dec 1;240(2):605–607. doi: 10.1042/bj2400605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard G., Holley A. Receptor cell responses to odorants: similarities and differences among odorants. Brain Res. 1984 Feb 6;292(2):283–296. doi: 10.1016/0006-8993(84)90764-9. [DOI] [PubMed] [Google Scholar]
- Sklar P. B., Anholt R. R., Snyder S. H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986 Nov 25;261(33):15538–15543. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitković L., Steisslinger H. W., Aloyo V. J., Mersel M. The 43-kDa neuronal growth-associated protein (GAP-43) is present in plasma membranes of rat astrocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8296–8300. doi: 10.1073/pnas.85.21.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]